Abstract
Matcha and green tea catechins such as (−)-epicatechin (EC), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG) have long been studied for their antioxidant and health-promoting effects. Using specific fluorophores for H2S (AzMC) and polysulfides (SSP4) as well as IC-MS and UPLC-MS/MS-based techniques we here show that popular Japanese and Chinese green teas and select catechins all catalytically oxidize hydrogen sulfide (H2S) to polysulfides with the potency of EGC > EGCG >> EG. This reaction is accompanied by the formation of sulfite, thiosulfate and sulfate, consumes oxygen and is partially inhibited by the superoxide scavenger, tempol, and superoxide dismutase but not mannitol, trolox, DMPO, or the iron chelator, desferrioxamine. We propose that the reaction proceeds via a one-electron autoxidation process during which one of the OH-groups of the catechin B-ring is autooxidized to a semiquinone radical and oxygen is reduced to superoxide, either of which can then oxidize HS− to thiyl radicals (HS•) which react to form hydrogen persulfide (H2S2). H2S oxidation reduces the B-ring back to the hydroquinone for recycling while the superoxide is reduced to hydrogen peroxide (H2O2). Matcha and catechins also concentration-dependently and rapidly produce polysulfides in HEK293 cells with the potency order EGCG > EGC > EG, an EGCG threshold of ~300 nM, and an EC50 of ~3 μM, suggesting green tea also acts as powerful pro-oxidant in vivo. The resultant polysulfides formed are not only potent antioxidants, but elicit a cascade of secondary cytoprotective effects, and we propose that many of the health benefits of green tea are mediated through these reactions. Remarkably, all green tea leaves constitutively contain small amounts of H2S2.
Keywords: Reactive sulfide species, Reactive oxygen species, Antioxidants
Graphical abstract
1. Introduction
The use of tea as a medicinal drink originated almost 5000 years ago during the Tang Dynasty in China [1] from where it was brought by Buddhist monks to Japan in the 6th century AD. Today, tea is the second most consumed beverage after water [2]. Numerous health benefits have been attributed to the ingestion of tea and its bioactive constituents including anticancer effects [[3], [4], [5], [6], [7], [8], [9]], protection against cardiovascular diseases [10,11] and metabolic syndrome [[12], [13], [14], [15], [16]], protectant activity against Alzheimer's, Parkinson's and other neurodegenerative diseases [[17], [18], [19], [20], [21]] as well as affecting mood and alleviating stress [22,23], antiviral activity [24], protection against infectious diseases [25] and perturbation of the gut microflora [26] and against gastrointestinal inflammatory diseases [27], while also improving fertility [28]. Most of these benefits are attributed specifically to green tea, which comprises twenty percent of total tea consumption.
Right after harvesting, green tea leaves are processed by heating to inactivate polyphenol oxidase, prevent color change and preserve their polyphenol content [4]. Matcha is a speciality grown and finely stoneground green tea which is used in the classical Japanese tea ceremony and particularly rich in catechins, flavanol compounds that account for 60–80% of all polyphenols in green tea [11]). The most prevalent, and extensively studied green tea catechins are (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG) and (−)-epigallocatechin-3-gallate (EGCG). The numerous health benefits, described above, are largely attributed to their ability to act as antioxidants, scavenge reactive oxygen species (ROS) and quench other free radicals, and to activate intracellular antioxidant defenses. However, catechins do not react with hydrogen peroxide (H2O2), for example [29,30]; in fact, they generate it. In certain instances, they may act as pro-oxidants [31,32], form covalent adducts with protein and non-protein thiols [33,34], and many of the biological effects of EGCG have been attributed to binding to or sterically interfering with certain enzymes or other regulatory proteins [35] as well as modulating mitochondrial function and metabolism [36].
We have previously described the chemical and biological similarities between ROS and reactive sulfur species (RSS) and proposed that many of the effects of antioxidant compounds can also be attributed to their effects on RSS metabolism [[37], [38], [39], [40], [41], [42], [43], [44], [45]]. Much of the RSS signaling is due to persulfidation of cysteines on regulatory proteins, a process that is accomplished by transfer of a sulfane sulfur from a persulfide or polysulfide (R2S2 or R2Sn, where R is H, cysteine or glutathione and n > 2) to the protein cysteine (reviewed in Ref. [[46], [47], [48], [49], [50]]. Although there are a number of pathways through which sulfane sulfur can be produced, most sulfane appears to be derived from endogenously-formed hydrogen sulfide (H2S [38]). Persulfides and polysulfides are excellent antioxidants through their ability to directly quench ROS [49] as well persulfidate keap1 which liberates Nrf2 and initiates activation of the nuclear antioxidant response elements. Polysulfides can also act as antioxidants [31,32,49,51,52], which is also consistent with the actions of teas [31,32].
Given the well-known antioxidant effects of both green teas and persulfides, we wondered if some of the effects of tea were mediated through sulfur metabolism. To that effect we examined RSS metabolism by select tea catechins and infusions freshly prepared from several Japanese and Chinese green tea varieties including Matcha, Tencha (the leaves used to produce Matcha; using a reed-covered culture method to block out most direct sunlight), Gyokuro (a variety where bushes are covered for about 3 weeks before harvesting, resulting in the suppression of catechin formation from theanine and a rich, unique aroma), Sencha (the most frequently consumed green tea in Japan), and Huang Shan (a famous green tea from the Yellow Mountain area in China). We demonstrate that green tea and tea catechins catalytically oxidize H2S to polysulfides in aqueous solution, brewed tea and in cells. These polysulfides are potent redox-active constituents with direct antioxidant effects that can also act as pro-oxidants to initiate a variety of indirect cellular antioxidant responses consistent with the reported cytoprotective effects of green tea.
2. Materials and methods
2.1. Chemicals/tea origin and preparations
SSP4 (3′, 6′-di(O-thiosalicyl)fluorescein) was purchased from Dojindo Molecular Technologies Inc. (Rockville, MD). Jade Leaf Matcha green tea powder (Matcha) was purchased from Amazon (Seattle, WA); Gyokuro was from Shohokuen Co, Ltd. (Mitsukoshi, Sendai, Japan), Sencha from ITO EN, Ltd. (Tokyo, Japan), Huang Shan Mao Feng was from MingCha Ltd. (Chai Wan, Hong Kong). Sodium tetra sulfide (Na2S4) was kindly provided by Dojindo Molecular Technologies Inc. All other chemicals were purchased from either ThermoFisher Scientific (Grand Island, NY) or Sigma-Aldrich (St. Louis, MO). Here we use H2S to denote the total sulfide (sum of H2S + HS−) derived from the dissolution of sodium sulfide (Na2S). While S2− is often thought to also be part of the H2S + HS− equilibrium, it actually does not exist under these conditions [53]. Phosphate buffered saline (PBS; in mM): 137 NaCl, 2.7 KCl, 8 Na2HPO4, 2 NaH2PO4; pH was adjusted with 10 mM HCl or NaOH to pH 7.4. Matcha powder was dissolved in distilled water at room temperature (RT). The other Japanese and Chinese green tea infusions were prepared by brewing 2.0 g of leaves in 100 mL of either tap water or MilliQ water kept at 80 °C for 4 min before filtration through a 0.2 μm filter; those aqueous tea extracts were allowed to reach RT before use in experiments. Total phenolic content of green teas infusions was determined using Folin-Ciocalteu reagent (Sigma-Aldrich) and expressed by comparison to a standard curve for gallic acid. All reactions were carried out at RT in closed vials, and tape was placed over well plates to minimize volatilization of H2S.
2.2. Fluorescence measurements
Incubation solutions were aliquoted into black 96-well plates in a darkened room and fluorescence measured with a SpectraMax M5e plate reader (Molecular Devices, Sunnyvale, CA). Excitation/emission wavelengths for 3′,6′-Di(O-thiosalicyl)fluorescein (SSP4), and 7-azido-4-methylcoumarin (AzMC) were 482/515 and 365/450 nm, respectively, as per manufacture's recommendations. These fluorophores have been shown to have sufficient specificity relative to other sulfur compounds and reactive oxygen and nitrogen species (ROS and RNS, respectively) to be effectively employed for the intended analyses, i.e. the detection of polysulfides by SSP4 and H2S for AzMC [41,54,55].
2.3. Cells
Human embryonic kidney epithelial (HEK293) cells were cultured and maintained at 37 °C in a humidified atmosphere of 5% CO2/21% O2 in DMEM (low glucose) supplemented with 10% fetal bovine serum in the presence of 1% penicillin/streptomycin. Fluorescence intensity was measured in cells grown to confluence in 96-well plates and using a SpectraMax M5e plate reader, as described above. Cells were continuously incubated with either AzMC (20 μM) or SSP4 (10 μM) in order to detect as much RSS as possible as H2S freely diffuses across cell membranes and many cells export polysulfides as they are generated [56,57]. Compounds of interest were added after an initial baseline reading, which was subsequently subtracted from the resulting fluorescence readouts.
2.4. Hypoxia
Hypoxia experiments were performed in a model 856-HYPO hypoxia chamber (Plas Labs, Inc. Lansing, MI). For experiments in buffer, the buffer was first purged with 100% N2 and then placed in the hypoxia chamber under 100% N2. This reduced O2 to <0.35% at room temperature (20 °C). The reactants were allowed to incubate in the hypoxia chamber for 90 min, then covered with the plate cover, with sides sealed with parafilm® prior to moving them to the plate reader, which was in room air. For experiments with cells, the cells were incubated in 5% O2/5% CO2 (balance N2) in the hypoxia chamber at 37 °C. The plates were removed at timed intervals, fluorescence measured and returned to their respective environments thereafter.
2.5. Oxygen dependency of catechin reactions
Oxygen was monitored with a FireStingO2 oxygen sensing system (Pyroscience Sensor Technology, Aachen, Germany) using a non-oxygen consuming 3 mm dia OXROB10 fiberoptic probe. The probe was calibrated with room air (21% O2) and 100% nitrogen (0% O2). Buffer (PBS) was purged with 100% nitrogen for 20 min to reduce the O2 content to ~3–5% to increase the sensitivity of the H2S effect. Degassed buffer was placed in a 5 mL glass vial, the probe was inserted into the buffer through a rubber cap and sealed with parafilm®. Headspace was reduced to accommodate subsequent injections but decrease H2S volatilization. In control experiments, four aliquots of H2S (as Na2S) were injected through the parafilm and stopper via a microliter syringe to produce concentration increments of 1 mM per injection; the puncture hole was resealed after each injection with tape. In other experiments, 100 μM EGCG was injected followed by four H2S injections.
2.6. Mass spectrometric detection of polysulfides, sulfite, thiosulfate and sulfate
Ultrahigh performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS) detection was used to identify and quantify polysulfides formed during the incubation of select catechins or aqueous green tea extracts with H2S (added as Na2S). Polysulfides formed were derivatized by incubation with iodoacetamide (IAM) for 60 min at room temperature. Due to the lack of authentic reference standards for IAM-derivatized polysulfides, which prevented us from constructing concentration/response curves for individual polysulfides and determine their ionization efficiencies, no exact concentrations could be determined. However, results expressed in the form of ‘peak areas’ are often difficult to interpret and to compare, in terms of relative concentrations, to other metabolites. We therefore adopted the following approach, using two different compounds for internal reference: sodium tetrasulfide (Na2S4; Dojindo) and potassium polysulfide (K2Sx; Sigma). Spectra of aqueous buffer solutions of Na2S4 and K2Sx showed identical chromatographic behavior but differed in speciation, as reported earlier [58]. K2Sx has been used widely as fertilizer and fungicide as well as a source of sulfur in electrochemical and preparative inorganic studies; yet, its chemical composition is poorly defined (>42% as K2Sx), subject to change upon storage and therefore unsuited for calibration purposes. Thus, “calibrations” were performed with iodoacteamide (IAM)-derivatized aqueous solutions of Na2S4, a substance with a more refined chemical composition (≥90.0% as tetrasulfide). The peak areas for individual polysulfides were converted into concentration estimates using a theoretical ‘response factor’ by relating the sum of all sulfide/polysulfide peak areas (S1–S5) detected in Na2S4 solutions to the peak area for sulfide (S1) of an equimolar Na2S solution, assuming no losses.
Reaction mixtures were analyzed using a Waters Aquity UPLC system hyphenated to a tandem quadrupole mass spectrometer (Xevo TQ-S, Waters) using a mixed mode column (Modus Aqua, 1.6 μm, 100 × 2.2 mm; Chromatography Direct) kept at 30 °C for separation. Mobile phase A was 5 mM ammonium formate in water with 0.15% formic acid; mobile phase B was 5 mM ammonium formate in 95% acetonitrile with 5% H2O and 0.15% formic acid. The gradient was as follows: 99% A decreasing to 60% A over 4.5 min, afterwards down to 0% A over 0.5 min and maintained at that level for 1.5 min. The column was then equilibrated back to 99% A over 0.5 min and maintained at 99% A for an additional minute. The flow rate was 0.2 mL/min and the injection volume 5 μL. The following mass spectrometry settings were employed: capillary voltage 3.0 kV, source offset 5 V, desolvation gas flow 800 L/h, cone gas flow 150 L/h, nebulizer pressure 7.0 bar, collision gas flow 0.14 mL/min, desolvation temperature 400 °C. The following MRM transitions were used for the detection of IAM-derivatized sulfide and polysulfide species (with cone and collision energies of 8 V and 12 V, respectively): 149 > 104 (IAM2-S1), 181 > 91 (IAM2-S2), 213 > 91 (IAM2-S3), 245 > 91 (IAM2-S4), 277 > 91 (IAM2-S5), 309 > 91 (IAM2-S6) and 341 > 91 (IAM2-S7).
Ion chromatography mass spectrometry (IC-MS) was used to quantify the concentrations of sulfite, thiosulfate and sulfate in green tea incubates with H2S using a dual-channel reagent-free high-pressure Dionex ICS-5000 MSQ system equipped with an AS-AP autosampler (Thermo Scientific) and conductivity, UV/Vis and mass spectrometry detectors in series. Sulfite, thiosulfate and sulfate were separated on a Dionex IonPac AS16 2 × 250 mm analytical column kept at a temperature of 30 °C. An eluent generator with a potassium hydroxide cartridge was used to produce the following conditions: a linear gradient was produced from 20 mM to 30 mM between 0 and 4.0 min, after which it was kept constant at 30 mM until 6.5 min, followed by a steep gradient up to 80 mM and reversal to 20 mM for equilibration until 10 min at a constant flow rate of 0.38 mL/min. Total run time was 10 min with retention times of 4.1, 4.1 and 6.0 min for sulfite, sulfate and thiosulfate. Injection volume was 2.5 μL. The quadrupole detector was coupled to the IC via an electrospray ionization interface (ESI) operated in negative SIM mode at m/z values of 81, 97 and 113 for the monoprotonated anions of sulfite, sulfate and thiosulfate, respectively. Capillary voltage was kept at 2.5 kV, with cone voltages of 66 V, 70 V and 55 V for sulfite, sulfate and thiosulfate, respectively. Probe temperature was kept constant at 500 °C.
For UPLC-MS/MS experiments, 500 μL of tea extract or catechin stock solution was mixed with 500 μL of 2 mM Na2S (all in sealed vials), mixed and incubated for maximally 60 min. 100 μL aliquots were removed at regular time intervals, mixed with 10 μL of 100 mM IAM and incubated for 1 h. For IC-MS experiments, 500 μL of tea extract was mixed with 500 μL of 2 mM Na2S in closed vials, vortexed and directly injected as quickly as possible. The delay between the mixing of solutions and the instrument actually injecting onto the column was 2 min; aliquots of the same reaction mixture were injected an additional 5 times. Run time was 10 min per injection. Representative chromatograms are depicted in Supplemental Fig. S1.
2.7. Statistical analysis
Unless specified otherwise, all experimental runs were carried out at least in duplicate and averaged. Data was analyzed and graphed using QuatroPro (Corel Corporation, Ottawa Ont, Canada) and SigmaPlot 13.0 (Systat Software, Inc., San Jose, CA). Statistical significance was determined using Students’ t-test, paired t-test, or one-way ANOVA and the Holm-Sidak test for multiple comparisons, as appropriate, using SigmaStat (Systat Software, San Jose, CA). Results are given as mean ± SE; significance was assumed when p ≤ 0.05.
3. Results
3.1. Matcha produces polysulfides from H2S
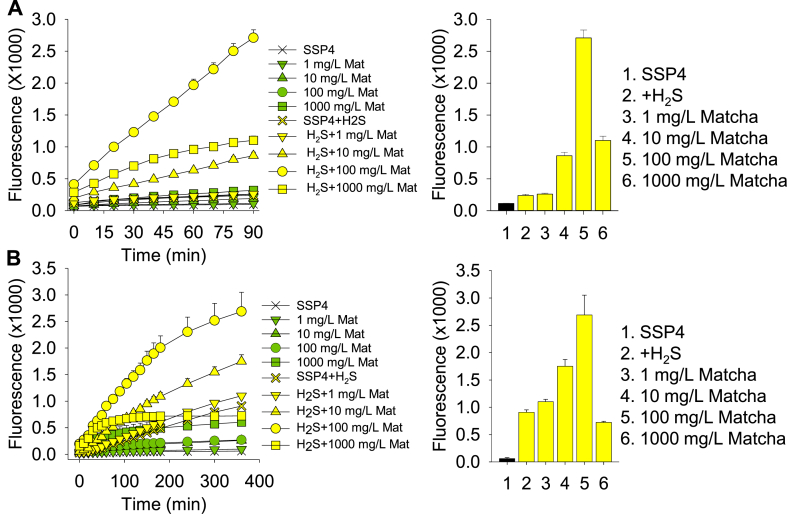
In initial experiments we incubated Matcha with 1 mM H2S in buffer and followed polysulfide production by measuring SSP4 fluorescence (Fig. 1A and B). Matcha produced polysulfides in a time- and concentration-dependent manner up to 100 mg/L. The initial responses appeared to be linear, but began to plateau by 360 min, and the onset of kinetic change was inversely associated with Matcha concentration. Matcha-catalyzed polysulfide production was slower than the direct reaction of a polysulfide (H2S2) with SSP4. SSP4 fluorescence was significantly inhibited by1000 mg/L Matcha due to optical quenching (Supplemental Fig. S2).
Fig. 1.
Matcha concentration-dependently produces polysulfides (SSP4 fluorescence) from 1 mM H2S in buffer (A) short-term study, (B) long term study. Matcha-catalyzed production of polysulfides is considerably slower than the direct reaction of persulfide (H2S2) with SSP4 (A). Mean ± SE; n = 4 wells per treatment; error bars may be hidden by symbols. Bar graphs show effects at 90 min (A) or 360 min (B).
3.2. Catechins produce polysulfides from H2S
3.2.1. SSP4 fluorescence
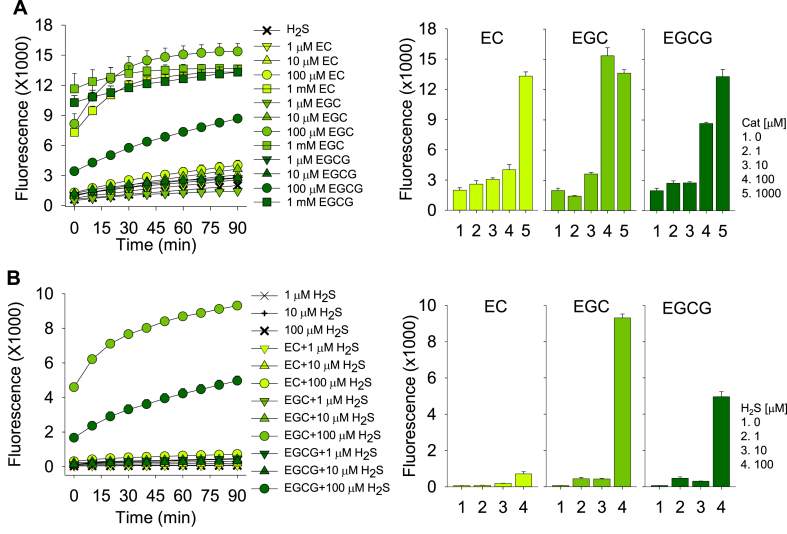
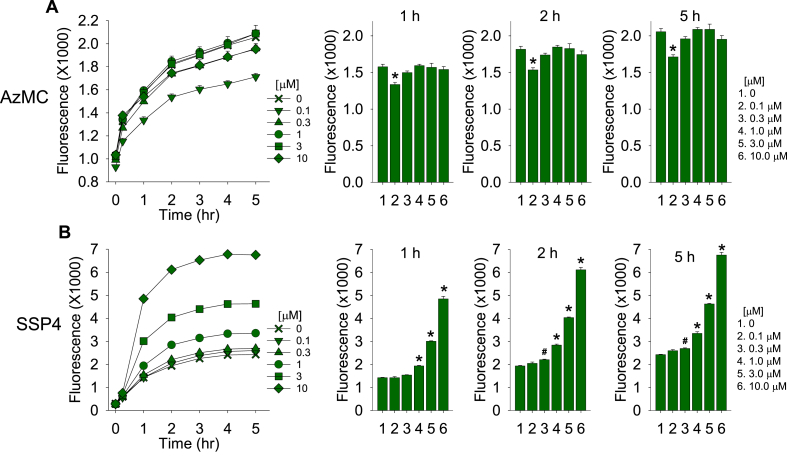
As catechins are among the most bioactive ingredients of green tea, we incubated 1 mM H2S with increasing concentrations of epicatechin (EC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG). All three catechins were found to concentration-dependently generate polysulfides (Fig. 2A). SSP4 fluorescence was similar for all three catechins incubated with 1 mM H2S, whereas at 100 μM H2S, EGC was the most potent and EC the least. The initial increase in SSP4 fluorescence appeared to be limited by the reaction between SSP4 and the polysulfide (Cf. Fig. 1A).
Fig. 2.
Effects of catechins (Cat) on polysulfide production from H2S. (A) Polysulfides are concentration-dependently produced by epicatechin (EC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG) when incubated with 1 mM H2S. (B) In the presence of 100 μM catechins, polysulfides are only produced when H2S = 100 μM. Mean ± SE, n = 4 wells per treatment; error bars may be hidden by symbols. Bar graphs show effects at 90 min.
We then examined the effects of H2S on polysulfide production in the presence of 100 μM catechins (Fig. 2B). Although under these conditions EGC was again the most efficacious and EC the least, there was no clear concentration-dependence below 100 μM H2S.
3.2.2. Mass spectrometric identification of polysulfides produced from H2S by catechins
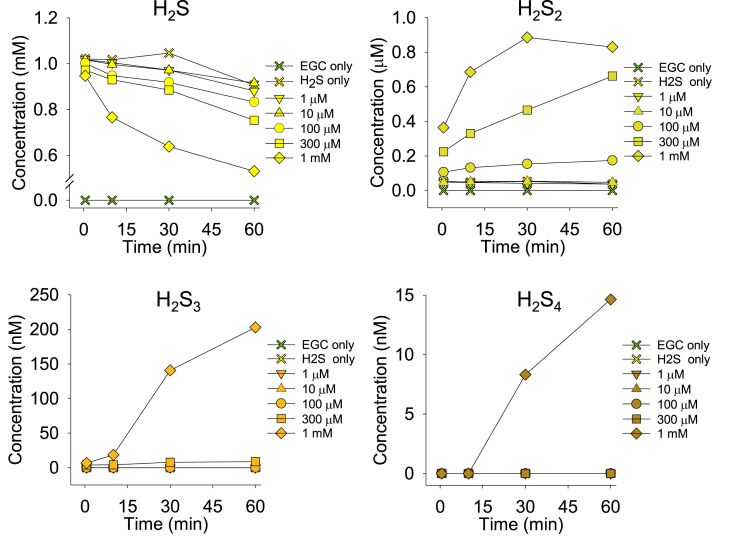
The effects of EGC on H2S and individual polysulfides are shown in Fig. 3, and the effects of EGCG are shown in Supplemental Fig. S3. EGC consumed H2S at faster rates as catechin concentrations increased, and this was accompanied by the formation of polysulfides of different chain-length, in the order of S2 > S3 > S4 (whereas longer-chain polysulfide formation was seen only with the highest catechin concentrations). EGCG produced similar results, although considerably less polysulfides were produced and S4 was not detected.
Fig. 3.
Mass spectrometric characterization of time-dependent changes in concentrations of H2S (S1) and longer-chain polysulfide concentrations (S2–S4; species with sulfur chains >5 were below quantifiable levels) after addition of various concentrations of EGC to 1 mM H2S. Polysulfide concentrations are estimates (see Methods for details).
3.3. Effects of matcha and catechins on cellular sulfur metabolism
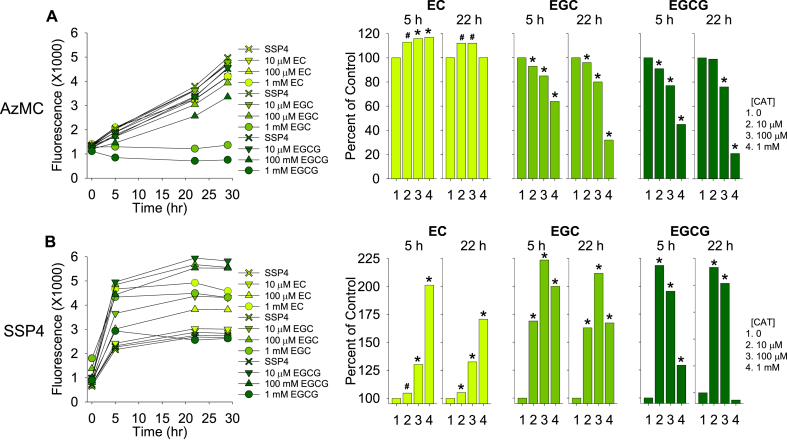
The ability of Matcha and catechins to oxidize H2S to polysulfides in aqueous solution suggested that these compounds might have similar attributes in cells. In initial studies we observed that Matcha time- and concentration-dependently increased SSP4 fluorescence in HEK293 cells (Supplemental Fig. S4). A significant increase in fluorescence was already evident in the time required to add the Matcha and SSP4 to the cells and measure fluorescence in the plate reader (t = 0 h), which took approximately 10–15 min. Fluorescence continued to increase for up to 20 h with all catechins and then plateaued; these levels were maintained for three days and the differences between treatments persisted throughout the duration of the experiment. This prompted a further examination on the effects of individual catechins on cellular H2S and polysulfides.
As shown in Fig. 4, EC slightly but significantly increased cellular H2S-related fluorescence intensity, whereas both EGC and EGCG concentration-dependently decreased it, an effect that became more pronounced at 22 h. All catechins initially increased polysulfides, EGCG was the most potent and EC the least; higher EGC and EGCG concentrations resulted in lower polysulfide-related fluorescence intensities than intermediate concentrations. These results show that low doses of catechins increase intracellular polysulfides and they suggest that this occurs by consuming intracellular H2S.
Fig. 4.
Effects of catechins (CAT), epicatechin (EC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG) on (A) H2S (AzMC fluorescence) and (B) polysulfides (SSP4 fluorescence) in HEK293 cells. Left panels show responses over 29 h; bar graphs show percent change compared to control (100%) at 5 and 20 h. Mean + SE; n = 8 wells per treatment; error bars may be hidden by symbols. Statistical significance; #, p < 0.01; *, p < 0.001 calculated for means at 5 and 22 h are shown on bar graphs for clarity.
The abrupt, and apparently near-maximal, increase in SSP4 fluorescence produced by 10 μM EGCG suggested that the effects of this catechin warranted further examination over shorter increments of time and with lower EGCG concentrations. As shown in Fig. 5, EGCG from 0.1 to 10 μM had little effect on cellular H2S (the lower AzMC fluorescence at 0.1 μM is likely an anomaly). Conversely, polysulfides were rapidly and concentration-dependently produced by EGCG (SSP4 fluorescence) in HEK293 cells with significant responses observed within the first hour of catechin addition; at 2 h, cellular polysulfides were significantly increased by as little as 0.3 μM EGCG, with minimal further increase in fluorescence thereafter. These results show that catechins are potent and rapid effectors of cellular polysulfide production.
Fig. 5.
Short-term effects of epigallocatechin gallate (EGCG) on (A) H2S (AzMC fluorescence) and (B) polysulfides (SSP4 fluorescence) in HEK293 cells. EGCG had little effect on H2S, whereas it rapidly and concentration-dependently increased polysulfide production. SSP4 was added at t = 0 h; EGCG was added 5–10 min later. Bar graphs show effects at 1, 2 and 5 h after SSP4 addition; #, p < 0.00.1; *, p < 0.001.
3.4. Mechanism of H2S oxidation by catechins in buffer
3.4.1. Substrate limitation
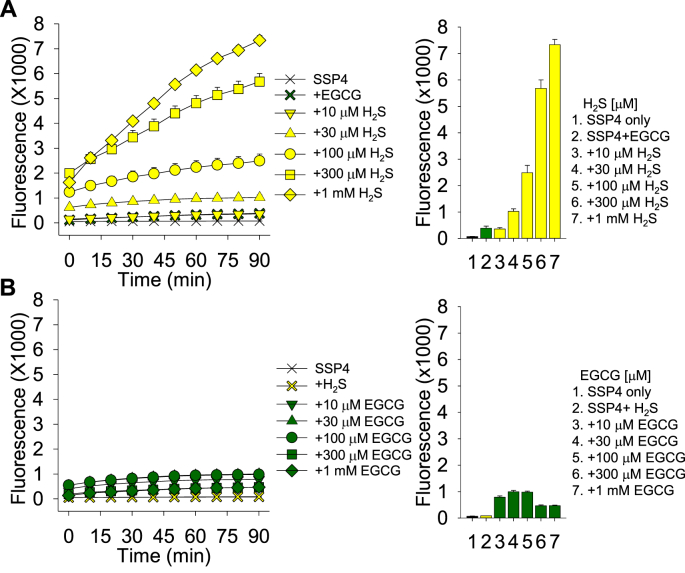
If catechins catalytically oxidize H2S to polysulfides we would expect this reaction to be limited by the availability of H2S rather than by catechin availability. This was examined by comparing polysulfide production by a single concentration of EGCG (30 μM) over a range of H2S concentrations to polysulfide production with a single concentration of H2S (30 μM) over a range of EGCG concentrations. Those concentrations were chosen to allow SSP4 fluorescence to increase without becoming a limiting factor. As shown in Fig. 6, increasing H2S concentration in the presence of 30 μM EGCG produced a H2S concentration-dependent increase in SSP4 fluorescence, whereas increasing EGCG concentration in the presence of 30 μM H2S did not. These results suggest that EGCG catalytically oxidizes H2S to produce polysulfides.
Fig. 6.
Polysulfide production (SSP4 fluorescence) in buffer by 30 μM EGCG is concentration-dependently elevated by increasing H2S concentrations (A), whereas there is no concentration-dependent effect with 30 μM H2S and varying EGCG concentrations (B). Mean ± SE, n = 4 wells per treatment; error bars may be hidden by symbols. Bar graphs show effects at 90 min incubation with SSP4.
3.4.2. Oxygen dependency
Mochizuki et al. [59] showed that EGCG autoxidation ultimately proceeded to oxidize the B-ring of the flavanol, first to a semiquinone radical and then to a quinone whereas oxygen was reduced to superoxide in the process. As any of these products could oxidize H2S, we next determined if this autoxidation reaction initiated or contributed to catechin-mediated polysulfide production.
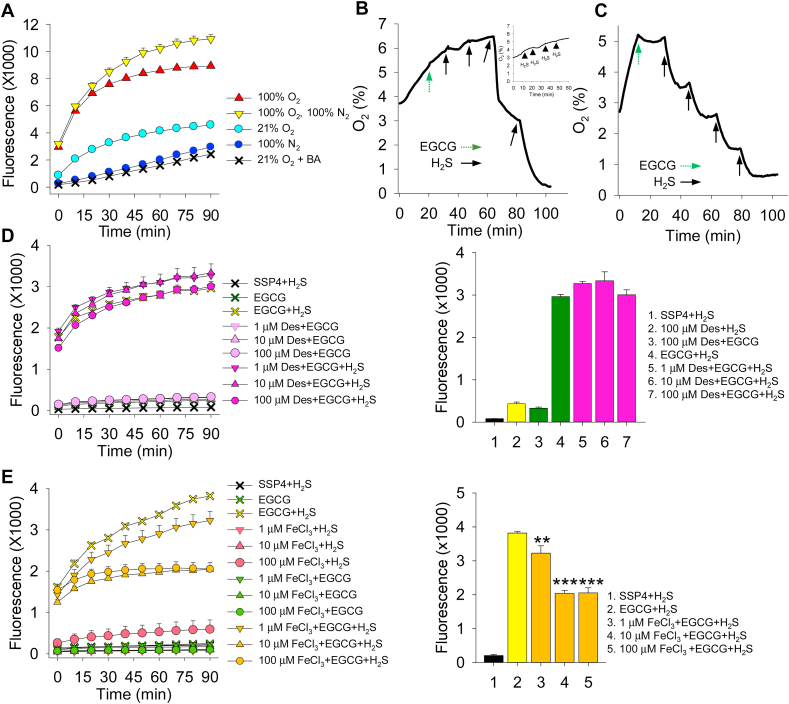
In the first group of experiments we examined the role of oxygen in oxidizing EGCG prior to addition of H2S. EGCG (100 μM) was added to buffer, then bubbled with either 100% O2, room air (21% O2), 100% N2 or 100% O2 for 20 min followed by 100% N2 for 20 min H2S (300 μM) was then added and polysulfide production monitored by SSP4 fluorescence. In these experiments, polysulfide production was lower in buffer bubbled with 21% O2 than it was in 100% O2 and lower still in 100% N2 (Fig. 7A) suggesting that O2 was required to oxidize EGCG prior to H2S oxidation. When EGCG was initially oxygenated with 100% O2 then deoxygenated with 100% N2 prior to H2S addition polysulfides were produced at a slightly greater rate than the 100% O2 samples. The reason for this further increase is unclear, but it is possible that in the continual presence of O2 the polysulfides are further oxidized to products that are not detected by SSP4. Nevertheless, these results suggest that O2 is necessary to oxidize EGCG prior to EGCG oxidation of H2S. Mochizuki et al. [59] also showed that EGCG autooxidation was inhibited by 100 mM boric acid. We confirmed this observation by adding boric acid to EGCG in buffer (titrated to pH 7.4 to eliminate any possible pH effects) and bubbling with 100% O2 prior to addition of H2S (Fig. 7A).
Fig. 7.
Role of oxygen, boric acid (BA) and iron in EGCG oxidation of H2S to polysulfides. (A) EGCG (100 μM) was incubated in buffer bubbled for 2 h with either 100% O2, room air (21% O2), 100% N2 (100% O2), or 100% O2 for 2 h then bubbled with 100% N2 for 10 min (100% O2, 100% N2) and subsequently exposed to 300 μM H2S for 1 h. Oxygen increased polysulfide production; little polysulfide production was observed in buffer bubbled with 100% N2 and incubated with H2S for 1 h or in 21% O2 buffer with BA (100 mM) for 1 h. (B) Typical trace showing O2 consumption after addition of 100 μM EGCG (dashed arrow) and 1 mM H2S (solid arrows) at pH 7.4. EGCG slightly decreased the rate of O2 increase, whereas H2S progressively decreased O2 concentration. Inset shows that H2S alone did not substantially affect O2 levels. (C) Typical trace showing O2 consumption after addition of 100 μM EGCG (dashed arrow) and 1 mM H2S (solid arrows) in buffer at pH 9.0. The effects of EGCG and H2S were greatly amplified compared to pH 7.4 (D) Desferrioxamine from 1 to 100 μM did not affect polysulfide formation by EGCG reaction with 100 μM H2S. (E) FeCl3 decreases polysulfide formation by EGCG and 100 μM H2S. Mean + SE, n = 4 wells per treatment; **, P < 0.01; ***, P < 0.001; error bars may be hidden by symbols. Bar graphs show effects at 90 min incubation with SSP4.
The direct effects of EGCG and H2S on O2 consumption are shown in Fig. 7B and C. In these experiments, there was a slight leak of O2 into the reaction vessel. With buffer pH 7.4, 100 μM EGCG appeared to somewhat decrease the rate of O2 increase and sequential additions of 1 mM H2S progressively decreased O2, resulting in nearly complete O2 depletion by the fourth addition. H2S alone did not substantially affect O2 in the absence of EGCG (Fig. 7B, Inset). Autoxidation of EGCG is greatly enhanced under alkaline conditions [59]. Multiple additions of H2S (as Na2S) will make the buffer progressively more alkaline. We would expect this to increase O2 consumption and this appeared to occur by the third and fourth additions of H2S (Fig. 7B). To confirm the pH effect, we repeated the experiment in buffer at pH 9.0 and the effects of both EGCG and H2S on O2 consumption were more dramatic (Fig. 7C). These results show that EGCG-mediated H2S oxidation consumes O2.
3.4.3. Role of iron-catalyzed reactions
The pro-oxidative properties of catechins have been attributed, in part, to indirect effects mediated by trace metals, especially iron, that are often found in reagents and solvents. Complexing these contaminants with chelating agents such as desferrioxamine has also been shown to effectively eliminate catechin autoxidation [4]. In order to determine if iron-catalyzed oxidized catechins were responsible for oxidizing H2S we examined these reactions in the presence of increasing concentrations of desferrioxamine. As shown in Fig. 7D, EGCG-catalyzed polysulfide formation was unaffected by 1–100 μM desferrioxamine. We then added FeCl3 to determine if EGCG could be oxidized by Fe3+ and then, in turn oxidize H2S. However, FeCl3 actually decreased polysulfide formation (Fig. 7E). These results indicate that trace metal contaminants are unlikely contributors to catechin-catalyzed oxidation of H2S and that high concentrations of Fe3+ actually interferes with these reactions.
3.4.4. Evidence for a free radical mechanism
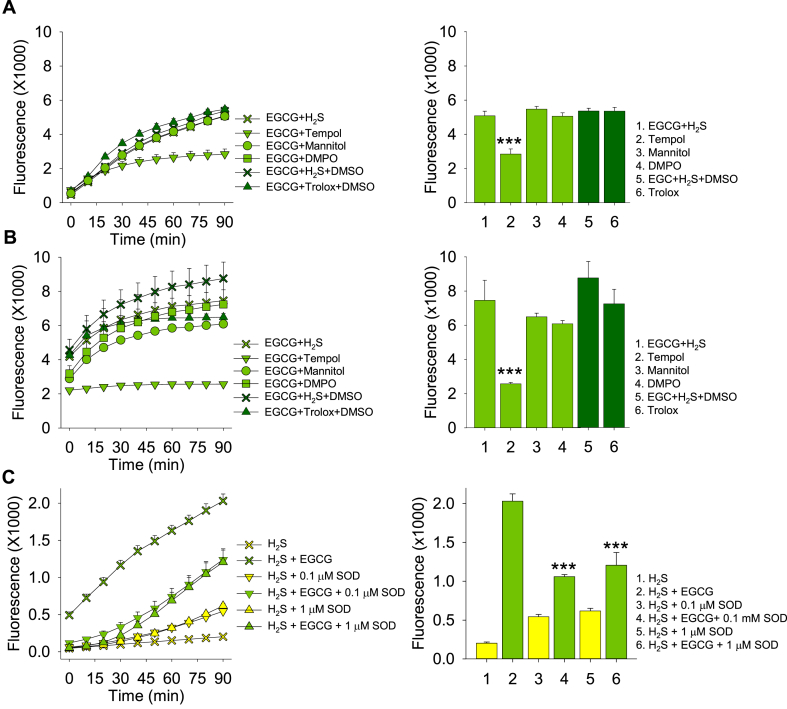
The effects of antioxidants and free radical scavengers on EGCG-mediated polysulfide formation from H2S are shown in Fig. 8A and B. The superoxide scavenger and superoxide dismutase mimetic, 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (Tempol), halved polysulfide formation, whereas the hydroxyl radical scavenger, mannitol, the nitric oxide spin trap, 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and the water-soluble vitamin E analog, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were all ineffective. Mn/Cu superoxide dismutase (SOD) also halved polysulfide formation (Fig. 8C). This suggests that H2S production is partly dependent on superoxide radical formation. It was not possible to determine if H2O2 was involved in the reaction because high concentrations of catalase directly oxidize H2S to polysulfides [43] and low concentrations appear to interfere with SSP4 activation by polysulfides (not shown).
Fig. 8.
Effects of 1 mM tempol, mannitol, DMPO and trolox on 100 μM epigallocatechin gallate (EGCG) formation of polysulfides from 100 μM H2S when SSP4 was added shortly after (A) or 120 min after adding reactants (B). Only tempol affected polysulfide formation. Trolox was dissolved in DMSO and compared to DMSO supplemented EGCG + H2S. (C) Effects of superoxide dismutase (SOD) on 100 μM EGCG oxidation of 300 μm H2S. Both SOD concentrations inhibited polysulfide production. Mean + SE, n = 4 wells per treatment; error bars may be hidden by symbols; ***; p < 0.001 compared to EGCG + H2S.
3.5. Mass spectrometric identification of polysulfide and sulfoxide production from H2S by fresh green tea infusions
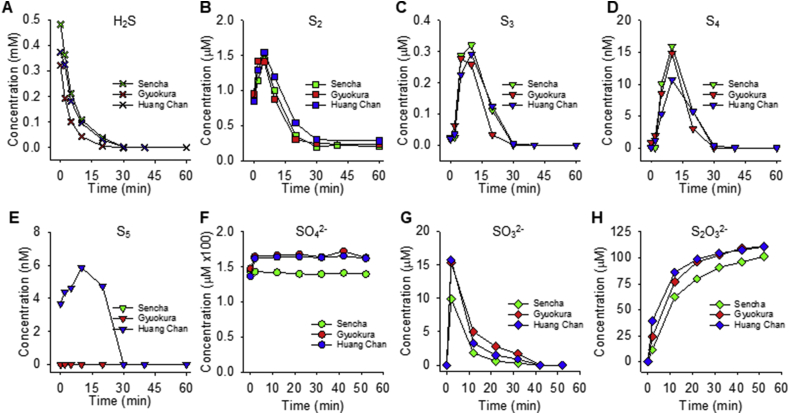
In our final series of experiments, we sought to confirm and extend our findings to freshly brewed green tea infusions. Mass spectrometric analysis of the effects of extract from two other popular Japanese teas and one Chinese green tea brewed in tap water on H2S and individual polysulfides is shown in Fig. 9. As observed with Matcha and select catechins, H2S rapidly decreased after addition of the aqueous green tea extract with a half-time of 3.4 ± 0.16 min (n = 3; not shown). This was accompanied by a rapid generation of polysulfides, initially hydrogen persulfide (H2S2) and, slightly later, lesser amounts of H2S3 and H2S4. Polysulfide production stopped when H2S was exhausted and concentrations declined rapidly thereafter. The maximum amount of S2–S4 formed after 5 min incubation corresponded to approximately 0.43% (S2), 0.08% (S3) and 0.004% (S4) of the amount of Na2S added (estimates based on peak areas). Unexpectedly, we found H2S2 already constitutively in all green tea leave extracts (Sencha: 177 ± 17.8 nM, Gyokuro: 200 ± 10.6 nM, Hung Chan: 230 ± 6.4 nM based on peak area comparisons to sulfide), corresponding to amounts between 8.9 and 11.5 nmol/g of tea. Differences in peak concentrations of polysulfides produced from H2S between the different green tea infusions prepared from Sencha, Gyokuro and Hung Chan leaves were minimal, with very similar kinetics as well, in spite of the considerably lower polyphenol content of the Gyokuro variety (Sencha: 11.0 ± 0.2 mM, Gyukuro: 5.7 ± 0.1 mM, Hung Chan 7.7 ± 0.1 mM, expressed as gallic acid equivalents; n = 3).
Fig. 9.
Mass spectrometric characterization of time-dependent changes in H2S (A), individual polysulfides S2–S5 (B–E) and sulfate (H2SO4; F), sulfite (H2SO3; G) and thiosulfate (H2S2O3; H) after addition of aqueous extracts from three different green teas (Sencha, Gyokuro and Huang Chan) to 1 mM H2S. H2S rapidly declines and is essentially gone by 30 min, whereas there is a rapid but transient increase in persulfide (S2) followed by lesser amounts of S3–S5 over this same period. All tea extracts contained some H2S2 and considerable concentrations of sulfate already before addition of H2S, and sulfate concentrations only slightly increased thereafter. Thiosulfate concentration rapidly increased in the initial 30 min and appeared to be inversely related to H2S; sulfite concentration transiently increased and returned to near baseline by 20 min. Exemplary results from single determinations in which polysulfides and sulfoxides were determined side-by-side using UPLC-MS/MS and IC-MS. While concentrations for sulfite, thiosulfate and sulfate were quantified using authentic standards, polysulfide concentrations are estimates only (see Methods for details). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In parallel to the formation of polysulfides, we also monitored the production of three sulfoxides using ion chromatography-mass spectrometry (IC-MS). Sulfate (SO42-) concentrations of all tea infusions were high to begin with (Sencha: 123.5 μM, Gyokuro: 127.7 μM, Hung Chan: 114.5 μM; corresponding to between 5.7 and 6.4 μmole sulfate/g tea) and, with one exception, slightly increased further between the first and second time points sampled, remaining constant thereafter. Thiosulfate (S2O32-) concentration rapidly increased over the initial 10 min and plateaued after all H2S was consumed. A small amount of sulfite (SO32-) was also produced within the first 20 min but rapidly decreased thereafter; sulfate and thiosulfate were the only stable products in this reaction system. As with polysulfide formation, the profile of changes in sulfoxide concentrations were comparable between the different green tea infusions. We estimate that we only recovered one quarter of the total amount of sulfide added. While we cannot exclude that some sulfur may have been lost due to volatilization, none of the incubation solutions became turbid (excluding the possibility of colloidal sulfur formation) and it is likely that additional sulfur products (e.g., polythionates) were produced that were not detected by our methods.
3.5.1. Effects of incubation conditions on polysulfide production by green tea and EGCG
Lastly, we provided additional evidence that catechins are oxidized prior to polysulfide production from H2S. We compared H2S metabolism by green tea brewed in tap water to the same amount and source of green tea (Sencha) brewed in Milli-Q water to determine if the quality of the water used to prepare infusions affected the reaction. Total polyphenol content was similar between both preparations (11.9 ± 0.06 mM with MilliQ water compared to 11.0 ± 0.16 mM with tap water). As shown in Supplemental Fig. S5A, H2S decreased faster when incubated with extract from tap water-brewed tea than it did when incubated with extract from tea brewed in Milli-Q water (t1/2 = 4.3 and 13.9 min, respectively). Polysulfide concentrations rose sooner, persisted for a shorter period of time and decrease faster with tap water-brewed tea. These results suggest that an impurity in tap water enhanced the catalytic activity of the tea for H2S oxidation and polysulfide formation/metabolism.
We then examined other factors that could potentially affect oxidation and catalytic activity of EGCG (Supplemental Fig. S5B). Tap water at 80 °C substantially decreased H2S and H2S was also decreased by approximately 15% in samples bubbled with air or left open to room air for 1 h. Polysulfide production was increased when EGCG was exposed to or bubbled with air or in tap water at 80 °C. Hydrogen peroxide (H2O2), which is known to be produced and accumulate to concentrations exceeding 100 μM during catechin autoxidation [30], was found to only minimally affect H2S consumption and polysulfide production. We also observed that heating Matcha to simulate brewing tea did not affect polysulfide production from H2S (Supplemental Fig. S5C) suggesting that temperature has little effect on catechin extraction from tea powder or catechin stability. Collectively, these results suggest that prior oxidation of catechins is a requisite for H2S oxidation to polysulfides, and water quality moderates these effects.
4. Discussion
Green tea and tea catechins are well known for a variety of health benefits. While the many cytoprotective effects of these compounds have been attributed to various antioxidant, pro-oxidant, and other molecular interactions, as of 2019, “the exact mechanistic pathways underlying the biological activities of green tea polyphenols remain obscure” [11]. Here we show that, in the presence of oxygen, all tea preparations and tea catechins investigated oxidize H2S to polysulfides, both in aqueous solution and even more effectively in cells, and that production is in the order of S2>S3>S4; in addition, thiosulfate is produced. Moreover, all green tea leaves were found to constitutively contain small amounts of H2S2.
Many of the effects of polysulfides and thiosulfate are consistent with the reported cytoprotective effects of tea polyphenols. Inorganic polysulfides are potent direct antioxidants [49] that also initiate a variety of cytoprotective cellular responses; the latter promoting the dissociation of Nrf2 from Keap1 and triggering antioxidant response element dependent transcriptional responses in the nucleus [[60], [61], [62], [63]], all of which have positive health benefits similar to those produced by classical ‘antioxidants’ [46,47,49,64]. Thiosulfate also has a variety of cytoprotective effects [[65], [66], [67], [68], [69], [70]] and can act as a H2S ‘donor’ under hypoxic conditions [71]. Based on these considerations, we propose that many of the health benefits of green tea are derived from their direct effects on cell sulfur metabolism.
4.1. Catechin oxidation of H2S is a catalytic process
Green tea extract and EGCG have been shown to irreversibly bind to a variety of proteins, in many cases after autoxidation of the catechins and formation of covalent bonds with cysteinyl thiols [33,[72], [73], [74]]. Much of this binding appears to occur at the 2′ carbon in the B ring. Our work suggests that catechins also bind H2S, however, this does not appear to be irreversible because with a fixed low concentration of EGCG (30 μM), polysulfide production continued to increase as H2S concentration was increased up to 1 mM; 33 times the EGCG concentration. Conversely, polysulfide production was unaffected when the H2S concentration was fixed and EGCG was varied (Fig. 6). If H2S was oxidized to a polysulfide and irreversibly bound to EGCG we would have expected SSP4 fluorescence to have plateaued around 30 μM H2S. These experiments suggest catechin oxidation of H2S is a catalytic process.
4.2. Catechin metabolism of H2S is an oxidative process
The sulfur in H2S is in its most reduced state; so, catechins must be oxidants to form polysulfides. This requires an initial oxidation of the catechin and the continual supply of an oxidant for the catalytic cycle. Our experiments show that catechin-mediated polysulfide production is enhanced by pre-exposure of the catechin to oxygen (Fig. 7A), providing this initial oxidative step, and that catechin oxidation of H2S consumes oxygen (Fig. 7B,C), allowing it to catalytically cycle between H2S oxidation and O2 reduction. It is less clear exactly how the catechin is initially oxidized and how it cycles between H2S and O2, but it is possible that transition metals are involved in the process.
It is generally accepted that oxidation of polyphenols such as catechin occurs at the B ring hydroquinone which undergoes a one-electron oxidation to a semiquinone radical or a two-electron oxidation to the quinone. However, while oxygen-mediated oxidation of polyphenol (PhOH) anions is thermodynamically favored, it is kinetically unfavorable [4]. This has been suggested to be overcome by trace-metal catalysis where a metal such as Fe3+oxidizes PhOH forming a polyphenol free radical (PhO∙). Both PhO∙ and the reduced metal can then generate superoxide from O2 which can then form additional PhO∙ while the spontaneous dismutation of superoxide generates hydrogen peroxide (H2O2; [4]). Low-levels of metal ion contaminants, such as iron, are commonly found in many chemical reagents and in all but ultra-pure water and can catalyze these reactions [4]; this is consistent with the much higher concentrations of H2O2 formed from green tea extracts in phosphate buffer compare to distilled water [75]. The effects of iron can be prevented by metal chelators such as desferrioxamine [76,77]. However, we were unable to affect H2S oxidation with desferrioxamine or FeCl3 (Fig. 7D, E) suggesting that iron contamination does not contribute to H2S oxidation in our experiments. However, catechins can also undergo autoxidation in the presence of copper ions [59]. While we did not address the effects of Cu2+ ions directly, we did observe that the rate of H2S oxidation by either green tea extract or EGCG was greatly increased when the tea (or the catechin) solutions were prepared in tap water. This could be explained by the higher copper concentrations in tap water in Southampton, where these experiments were performed, compared to the US due to the predominance of copper piping for drinking water installations in the UK. Mochizuki et al. [59] also showed that catechin autoxidation was inhibited by borate, which we have confirmed in our experiments (Fig. 7A), and borate is an excellent complexing agent for Cu2+ and other transition metals [78].
A number of studies have shown that catechins can be autoxidized by O2 in the absence of metal catalysts, albeit slowly [59], which is consistent with our findings (Supplemental Fig. S4). Increasing pH also increases catechin polyphenol (PhOH) autoxidation [59], which was also observed by us (Fig. 7C). Our ability to partially inhibit polysulfide formation with the superoxide scavenger, tempol, and with superoxide dismutase (Fig. 8) provides additional evidence for a free radical-mediated mechanism.
4.3. Proposed mechanisms of H2S oxidation by catechins
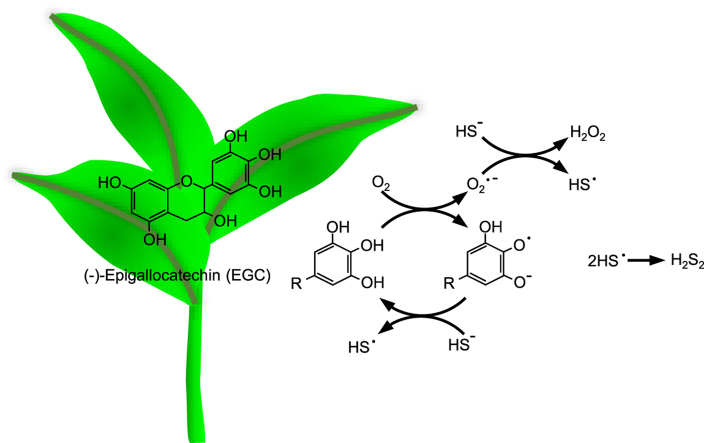
We propose that oxygen-mediated autoxidation of the catechin's B-ring hydroquinone (B–OH) producing a semiquinone radical (B–O•) and superoxide (O2•-) is the first step in the catalytic process (eq. (1));
| B–OH + O2 –> B–O• + O2•- + H+ | (1) |
After this there are a number of mechanisms to oxidize H2S and form polysulfides, all of which include H2S reduction of the catechin to complete the catalytic cycle. In the first scheme, both the semiquinone radical and superoxide react with H2S to form thiyl radicals (HS•) that combine with each other to produce the persulfide (H2S2); this also reduces the semiquinone to the hydroquinone and the superoxide to peroxide (eqs. (2), (3), (4)));
| B–O• + H2S –> B–OH + HS• | (2) |
| O2•- + H2S + H+ –> H2O2 + HS• | (3) |
| 2 HS• –> H2S2 | (4) |
In the second scheme, the superoxide formed in eq. (1) oxidizes a second catechin hydroquinone to form another semiquinone radical (eq. (5)) that oxidizes the second H2S (eq. (2));
| B–OH + O2•- –> B–O• + H2O2 | (5) |
In the third scheme, the superoxide formed in eq. (1) oxidizes the semiquinone radical formed in eq. (1) to a quinone (B O; eq. (6)), and the quinone can undergo two single-electron oxidation reactions with H2S, regenerating the hydroquinone and two thiyl radicals, which immediately react to form disulfane, H2S2 (eq. (7)); or, in the fourth scheme, the quinone can cycle between the quinone and semiquinone by oxidizing H2S (eq. (8)) and reducing superoxide (eq. (6));
| B–O• + O2•- +2H+ –> B O + H2O2 | (6) |
| B=O + 2H2S –> B–OH + H2S2 | (7) |
| B=O + H2S –> B–O• + HS• | (8) |
In the fifth scheme, a hydroquinone and a quinone are in equilibrium with two semiquinones (eq. (9)) and those semiquinones each oxidize H2S (eq. (2));
| B–OH + B O <−> 2B–O• | (9) |
Mochizuki et al. [59] demonstrated the existence of all of the above oxidative reactions, the equilibrium with the hydroquinone and quinone and showed that the peroxide that was produced did not participate in any of the reactions. However, without a reductant, these reactions will end when all of the catechin is oxidized. This will not occur in the presence of H2S and, assuming there is sufficient oxygen, the reaction can catalytically redox cycle until all the H2S is oxidized. The abundance of oxygen in cells relative to H2S [55] ensures that H2S is the limiting factor and that even small amounts of catechin are able to produce significant amounts of polysulfides.
4.4. Importance of the catechin B-ring OH and the D-ring gallate
The two vicinal hydroxyl (catechol) groups of the B-ring of EC, EGC and EGCG and the D-ring of EGCG render these compounds electron rich and enable hydrogen atom transfer (HAT) or single electron transfer (SET) reactions that give catechins their antioxidant function [4]. HAT mechanisms are believed to be more important in breaking the chain of lipid peroxidation reactions than SET reactions. The addition of a third vicinal OH group (pyrogallol) at the 3′ carbon of the B-ring (i.e. going from EC to EGC) increases the antioxidant activity of the catechin [75]. Flavanoids in general scavenge a variety of oxygen, nitrogen and other radical species and the antioxidant efficiency increases as the number of hydroxyl groups increases, i.e., EGCG > EGC > EC [2,4,11].
We also observed that EGC with three OH groups was more efficacious than EC with two OH moieties, in both buffer (Fig. 2) and in cells (Fig. 4). However, polysulfide formation from H2S is an oxidative reaction and how increasing OH groups on the B-ring affects H2S oxidation is not immediately obvious; however, it may be related to their lower one-electron redox potential [79]. We also showed that EGC was somewhat more efficacious than EGCG in buffer suggesting that the D-ring gallate not only did not participate in H2S oxidation, but may have some inhibitory activity as well. Mochizuki et al. [59] also observed that the D-ring gallate did not increase catechin autoxidation. The inhibitory effects we observed remain to be explained.
4.5. Effects of catechins on cellular sulfur metabolism
The effects of catechins on cellular sulfur metabolism are generally consistent with their effects in buffer, albeit more efficacious in the latter (cf Fig. 2, Fig. 5). All catechins initially increased polysulfides, EGCG was the most potent and EC the least. Both ECG and EGCG also decreased cellular H2S, suggesting that polysulfide production is at least partially at the expense of intracellular H2S. However, unlike the situation in buffer, we observed that EGCG was more efficacious in polysulfide production than either EC or EGC in HEK293 cells (Fig. 5). This could be attributable to preferential cellular uptake, or other unique attributes of the gallate D-ring not apparent in buffer.
The effects of catechins in cells could also arise from factors in addition to oxidation of H2S to polysulfides. A number of studies have shown that EGCG, but not EC or EGC, uniquely inhibits proteins, including enzymes, by fitting into the active site pocket via hydrogen bonds and van der Waals forces [33,35,80]. Catechins also react with the sulfur or seleniun group of reduced thioredoxin (Trx) or thoiredoxin reductase (TrxR), respectively, thereby inhibiting the enzymes [[72], [73], [74]]. Regardless, catechin-mediated sulfur metabolism appears to be an integral component of the therapeutic effects of these compounds, and it is not surprising that addition of sulfide salts (NaHS), an H2S donor (GYY4137) or the garlic derivative diallyltrisulfide synergistically enhance the anti-cancer effect of EGCG in multiple myeloma cells and prolong survival in a mouse xenograph model without affecting normal cells [81].
4.6. Polysulfide formation by green tea is accompanied by oxidation of H2S to sulfite, thiosulfate and sulfate
In addition to Matcha, we tested three other freshly prepared green tea infusions for their ability to support this chemistry. This was considered important because – while all leaves and leave buds harvested for tea production originate from the same plant (Camellia sinensis, Theaceae) – many different varieties exists, giving rise to an enormous diversity in flavor/aroma, appearance and molecular composition of green leave constituents (including catechins, alkaloids, amino acids, vitamins, and chlorophyll), depending on geographical variation in soil mineral composition, growth and cultivation conditions (sunlight vs shade, lowland vs highland), leave harvest (manual vs machine), time of harvest and processing (e.g. pan-fired, oven-dried or steamed for heat inactivation, rolled vs non-rolled). Moreover, the composition of their aqueous extracts varies with brewing conditions [[82], [83], [84]]. Chinese green teas are slightly fermented and often sweet and lighter, with a lower polyphenol content and less of the grassy/umami flavor characteristic of Japanese green teas. Due to this compositional heterogeneity we felt it would be of limited value to determine the concentration of specific catechins in Matcha or any other particular tea variety, but more important to demonstrate that a number of widely enjoyed green teas are capable of entertaining the same sulfur chemistry, regardless of their country of origin, growth condition and leaf processing specifics.
In addition to confirming the validity of our findings for different sources and preparations of green tea, we were interested to see whether oxidation products other than polysulfides were formed from H2S in this reaction. Indeed, all of the tested green teas revealed a qualitatively identical pattern of reaction products, with comparable relative yields yet moderate differences in reaction kinetics. Except for H2S2, which remained elevated after H2S was consumed (and was found to be present in the tea extract even before addition of H2S), all other polysulfides were metastable with concentrations rapidly dropping after the initial peaks between 5 and 15 min. This was preceded by a transient rise in sulfite concentration in the first few minutes and gradual formation of thiosulfate until all H2S was consumed. Unsurprisingly, all tea leave varieties investigated contained considerable amounts of sulfate, which is an essential nutrient for growth and the primary source of sulfur in plants. Although we monitored a wealth of different anionic sulfur metabolites in this study, on a molar basis about 75% of the added sulfide remained unaccounted for, indicating the involvement of additional metabolic routes yet to be identified.
In summary, the experimental results of our current study suggest that green tea consumption is associated with the production of thiosulfate and polysulfides, which - in the presence of oxygen - are formed via catalytic reaction with the ubiquitous cellular signaling molecule H2S. Thiosulfate itself has been demonstrated to have cytoprotective effects via modulating sulfide levels in vivo [71,85], whereas persulfides and polysulfides have a variety of unique redox-modulating properties we are just beginning to unravel [49]. The series of non-enzymatic reactions of green tea catechins with H2S described in the current work bears an uncanny resemblance to the oxidative enzymatic removal of H2S in mammalian and invertebrate mitochondria, where sulfide:oxidoreductase converts sulfide to persulfides and transfers the electrons to the ubiquinone pool, a sulfur dioxygenase subsequently oxidizes one persulfide to sulfite, and a sulfur transferase facilitates the reaction of the second persulfide with sulfite to form thiosulfate [86]. We did not expect to also find small amounts of the sulfur analogue of H2O2, H2S2 already constitutively in green tea leaves. We hypothesize that many of the beneficial health effects of green tea are explained by the presence and formation of these reactive sulfur species, which are endowed with direct and indirect antioxidant properties. Our results in cultured cells suggest that this mode of action is likely to operate also in vivo. Whether habitual green tea consumption is associated with intermittent pro-oxidant and thus potentially preconditioning-like, exercise-mimetic effects that have the potential to affect human redox balance warrants further investigation.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgments
This research was supported in part by Indiana University BRG grant and by personal funds (KRO). We are indebted to Dr. Grielof Koster for the kind gift of the Chinese green tea. MF acknowledges support by the SCBR mass spectrometry unit, the Faculty of Medicine and the NIHR Biomedical Research Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101731.
Contributor Information
Kenneth R. Olson, Email: olson.1@nd.edu.
Martin Feelisch, Email: M.Feelisch@soton.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lu Y. Ecco Press; New York, NY: 1995. The Classic of Tea: Origins & Rituals. [Google Scholar]
- 2.Yang C.S., Wang X., Lu G., Picinich S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Canc. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bange E., Timlin C., Kabel C., Svoboda J., Roeker L., Mato A.R. Evidence for and against green tea and turmeric in the management of chronic lymphocytic leukemia. Clin. Lymphoma, Myeloma & Leukemia. 2018;18:e421–e426. doi: 10.1016/j.clml.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert J.D., Elias R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch. Biochem. Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecumberri E., Dupertuis Y.M., Miralbell R., Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013;32:894–903. doi: 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Miyata Y., Matsuo T., Araki K., Nakamura Y., Sagara Y., Ohba K., Sakai H. Anticancer effects of green tea and the underlying molecular mechanisms in bladder cancer. Medicines (Basel) 2018:5. doi: 10.3390/medicines5030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyata Y., Shida Y., Hakariya T., Sakai H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules. 2019:24. doi: 10.3390/molecules24010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao X., Xiao X., Chen D., Yu B., He J. Tea and its components prevent cancer: a review of the redox-related mechanism. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20215249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirakami Y., Shimizu M. Possible mechanisms of green tea and its constituents against cancer. Molecules. 2018:23. doi: 10.3390/molecules23092284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangels D.R., Mohler E.R., 3rd Catechins as potential mediators of cardiovascular health. Arterioscler. Thromb. Vasc. Biol. 2017;37:757–763. doi: 10.1161/ATVBAHA.117.309048. [DOI] [PubMed] [Google Scholar]
- 11.Xing L., Zhang H., Qi R., Tsao R., Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019;67:1029–1043. doi: 10.1021/acs.jafc.8b06146. [DOI] [PubMed] [Google Scholar]
- 12.Asbaghi O., Fouladvand F., Gonzalez M.J., Aghamohammadi V., Choghakhori R., Abbasnezhad A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Compl. Ther. Med. 2019;46:210–216. doi: 10.1016/j.ctim.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Hibi M., Takase H., Iwasaki M., Osaki N., Katsuragi Y. Efficacy of tea catechin-rich beverages to reduce abdominal adiposity and metabolic syndrome risks in obese and overweight subjects: a pooled analysis of 6 human trials. Nutr. Res. 2018;55:1–10. doi: 10.1016/j.nutres.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T., Pervin M., Goto S., Isemura M., Nakamura Y. Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules. 2016:21. doi: 10.3390/molecules21101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu P., Ying L., Hong G., Wang Y. The effects of the aqueous extract and residue of Matcha on the antioxidant status and lipid and glucose levels in mice fed a high-fat diet. Food Funct. 2016;7:294–300. doi: 10.1039/c5fo00828j. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Ho C.T., Long P., Meng Q., Zhang L., Wan X. Preventive efficiency of green tea and its components on nonalcoholic fatty liver disease. J. Agric. Food Chem. 2019;67:5306–5317. doi: 10.1021/acs.jafc.8b05032. [DOI] [PubMed] [Google Scholar]
- 17.Cascella M., Bimonte S., Muzio M.R., Schiavone V., Cuomo A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer's disease: an overview of pre-clinical studies and translational perspectives in clinical practice. Infect. Agents Canc. 2017;12:36. doi: 10.1186/s13027-017-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farzaei M.H., Bahramsoltani R., Abbasabadi Z., Braidy N., Nabavi S.M. Role of green tea catechins in prevention of age-related cognitive decline: pharmacological targets and clinical perspective. J. Cell. Physiol. 2019;234:2447–2459. doi: 10.1002/jcp.27289. [DOI] [PubMed] [Google Scholar]
- 19.Kakutani S., Watanabe H., Murayama N. Green tea intake and risks for dementia, alzheimer's disease, mild cognitive impairment, and cognitive impairment: a systematic review. Nutrients. 2019;11 doi: 10.3390/nu11051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pervin M., Unno K., Ohishi T., Tanabe H., Miyoshi N., Nakamura Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules. 2018:23. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pervin M., Unno K., Takagaki A., Isemura M., Nakamura Y. Function of green tea catechins in the brain: epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20153630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancini E., Beglinger C., Drewe J., Zanchi D., Lang U.E., Borgwardt S. Green tea effects on cognition, mood and human brain function: a systematic review. Phytomedicine. 2017;34:26–37. doi: 10.1016/j.phymed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Unno K., Furushima D., Hamamoto S., Iguchi K., Yamada H., Morita A., Horie H., Nakamura Y. Stress-reducing function of Matcha green tea in animal experiments and clinical trials. Nutrients. 2018;10 doi: 10.3390/nu10101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J., Xu Z., Zheng W. A review of the antiviral role of green tea catechins. Molecules. 2017:22. doi: 10.3390/molecules22081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reygaert W.C. Green tea catechins: their use in treating and preventing infectious diseases. BioMed Res. Int. 2018;2018:9105261. doi: 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H.H., Liu J., Lv Y.J., Jiang Y.L., Pan J.X., Zhu Y.J., Huang M.G., Zhang S.K. Changes in intestinal microbiota of type 2 diabetes in mice in response to dietary supplementation with instant tea or Matcha. Can. J. Diabetes. 2020 February;44(1):44–52. doi: 10.1016/j.jcjd.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Barbalho S.M., Bosso H., Salzedas-Pescinini L.M., de Alvares Goulart R. Green tea: a possibility in the therapeutic approach of inflammatory bowel diseases?: green tea and inflammatory bowel diseases. Compl. Ther. Med. 2019;43:148–153. doi: 10.1016/j.ctim.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Rahman S.U., Huang Y., Zhu L., Feng S., Khan I.M., Wu J., Li Y., Wang X. Therapeutic role of green tea polyphenols in improving fertility: a review. Nutrients. 2018;10 doi: 10.3390/nu10070834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbling L., Weiss R.M., Teufelhofer O., Uhl M., Knasmueller S., Schulte-Hermann R., Berger W., Micksche M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb. J. 2005;19:807–809. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 30.Long L.H., Lan A.N., Hsuan F.T., Halliwell B. Generation of hydrogen peroxide by "antioxidant" beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic. Res. 1999;31:67–71. doi: 10.1080/10715769900300611. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.S., Quon M.J., Kim J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeta K., Nomura W., Takatsume Y., Izawa S., Inoue Y. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl. Environ. Microbiol. 2007;73:572–580. doi: 10.1128/AEM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii T., Mori T., Tanaka T., Mizuno D., Yamaji R., Kumazawa S., Nakayama T., Akagawa M. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008;45:1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Sang S., Lambert J.D., Hong J., Tian S., Lee M.J., Stark R.E., Ho C.T., Yang C.S. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- 35.Saeki K., Hayakawa S., Nakano S., Ito S., Oishi Y., Suzuki Y., Isemura M. In vitro and in silico studies of the molecular interactions of epigallocatechin-3-O-gallate (EGCG) with proteins that explain the health benefits of green tea. Molecules. 2018;23 doi: 10.3390/molecules23061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi W., Li L., Ding Y., Yang K., Chen Z., Fan X., Jiang S., Guan Y., Liu Z., Xu D., Wu L. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future Med. Chem. 2018;10:795–809. doi: 10.4155/fmc-2017-0204. [DOI] [PubMed] [Google Scholar]
- 37.DeLeon E.R., Gao Y., Huang E., Arif M., Arora N., Divietro A., Patel S., Olson K.R. A case of mistaken identity: are reactive oxygen species actually reactive sulfide species? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R549–R560. doi: 10.1152/ajpregu.00455.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson K.R. H2S and polysulfide metabolism: conventional and unconventional pathways. Biochem. Pharmacol. 2018;149:77–90. doi: 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Olson K.R. Hydrogen sulfide, reactive sulfur species and coping with reactive oxygen species. Free Radic. Biol. Med. 2019;140:74–83. doi: 10.1016/j.freeradbiomed.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Olson K.R. Reactive oxygen species or reactive sulfur species: why we should consider the latter. J. Exp. Biol. 2020;223 doi: 10.1242/jeb.196352. [DOI] [PubMed] [Google Scholar]
- 41.Olson K.R., Gao Y. Effects of inhibiting antioxidant pathways on cellular hydrogen sulfide and polysulfide metabolism. Free Radic. Biol. Med. 2019;135:1–14. doi: 10.1016/j.freeradbiomed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Olson K.R., Gao Y., Arif F., Patel S., Yuan X., Mannam V., Howard S., Batinic-Haberle I., Fukuto J., Minnion M., Feelisch M., Straub K.D. Manganese porphyrin-based SOD mimetics produce polysulfides from hydrogen sulfide. Antioxidants. 2019:8. doi: 10.3390/antiox8120639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson K.R., Gao Y., DeLeon E.R., Arif M., Arif F., Arora N., Straub K.D. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS) Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H2S) and production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biol. 2017;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson K.R., Straub K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31:60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 46.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., van Goor H., Olson K.R., Feelisch M. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020 Feb;177(4):720–733. doi: 10.1111/bph.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filipovic M.R., Zivanovic J., Alvarez B., Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018;118:1253–1337. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., Lin J., Ida T., Akaike T. Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav P.K., Martinov M., Vitvitsky V., Seravalli J., Wedmann R., Filipovic M.R., Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J. Am. Chem. Soc. 2016;138:289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kharma A., Grman M., Misak A., Dominguez-Alvarez E., Nasim M.J., Ondrias K., Chovanec M., Jacob C. Inorganic polysulfides and related reactive sulfur-selenium species from the perspective of chemistry. Molecules. 2019:24. doi: 10.3390/molecules24071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wedmann R., Bertlein S., Macinkovic I., Boltz S., Miljkovic J., Munoz L.E., Herrmann M., Filipovic M.R. Working with "H2S": facts and apparent artifacts. Nitric Oxide. 2014;41:85–96. doi: 10.1016/j.niox.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 53.May P.M., Batka D., Hefter G., Konigsberger E., Rowland D. Goodbye to S(2-) in aqueous solution. Chem. Commun. 2018;54:1980–1983. doi: 10.1039/c8cc00187a. [DOI] [PubMed] [Google Scholar]
- 54.Bibli S.I., Luck B., Zukunft S., Wittig J., Chen W., Xian M., Papapetropoulos A., Hu J., Fleming I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018;18:295–304. doi: 10.1016/j.redox.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson K.R., Briggs A., Devireddy M., Xian M., Gao Y. Are the beneficial effects of ‘antioxidant’ lipoic acid mediated through metabolism of reactive sulfur species? Free Radic. Biol. Med. 2020 January;146:139–149. doi: 10.1016/j.freeradbiomed.2019.10.410. [DOI] [PubMed] [Google Scholar]
- 56.Jennings M.L. Transport of H2S and HS(-) across the human red blood cell membrane: rapid H2S diffusion and AE1-mediated Cl(-)/HS(-) exchange. Am. J. Physiol. Cell Physiol. 2013;305:C941–C950. doi: 10.1152/ajpcell.00178.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin J., Akiyama M., Bica I., Long F.T., Henderson C.F., Goddu R.N., Suarez V., Baker B., Ida T., Shinkai Y., Nagy P., Akaike T., Fukuto J.M., Kumagai Y. The uptake and release of polysulfur cysteine species by cells: physiological and toxicological implications. Chem. Res. Toxicol. 2019;32:447–455. doi: 10.1021/acs.chemrestox.8b00340. [DOI] [PubMed] [Google Scholar]
- 58.Bogdandi V., Ida T., Sutton T.R., Bianco C., Ditroi T., Koster G., Henthorn H.A., Minnion M., Toscano J.P., van der Vliet A., Pluth M.D., Feelisch M., Fukuto J.M., Akaike T., Nagy P. Speciation of reactive sulfur species and their reactions with alkylating agents: do we have any clue about what is present inside the cell? Br. J. Pharmacol. 2019;176:646–670. doi: 10.1111/bph.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mochizuki M., Yamazaki S., Kano K., Ikeda T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta. 2002;1569:35–44. doi: 10.1016/s0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
- 60.Guo C., Liang F., Shah M.W., Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014;725:70–78. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Hourihan J.M., Kenna J.G., Hayes J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxidants Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 62.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013 May 20;18(15) doi: 10.1089/ars.2012.4645. 1906-19. [DOI] [PubMed] [Google Scholar]
- 63.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxidants Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 64.Doka E., Ida T., Dagnell M., Abiko Y., Luong N.C., Balog N., Takata T., Espinosa B., Nishimura A., Cheng Q., Funato Y., Miki H., Fukuto J.M., Prigge J.R., Schmidt E.E., Arner E.S.J., Kumagai Y., Akaike T., Nagy P. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datzmann T., Hoffmann A., McCook O., Merz T., Wachter U., Preuss J., Vettorazzi S., Calzia E., Groger M., Kohn F., Schmid A., Denoix N., Radermacher P., Wepler M. Effects of sodium thiosulfate (Na2S2O3) during resuscitation from hemorrhagic shock in swine with preexisting atherosclerosis. Pharmacol. Res. 2020;151:104536. doi: 10.1016/j.phrs.2019.104536. [DOI] [PubMed] [Google Scholar]
- 66.Howard R.M., Smith G.P. Treatment of calcinosis cutis with sodium thiosulfate therapy. J. Am. Acad. Dermatol. 2020 Jul 2 doi: 10.1016/j.jaad.2020.06.996. S0190-9622(20)32096-X. [DOI] [PubMed] [Google Scholar]
- 67.Mizuta Y., Tokuda K., Guo J., Zhang S., Narahara S., Kawano T., Murata M., Yamaura K., Hoka S., Hashizume M., Akahoshi T. Sodium thiosulfate prevents doxorubicin-induced DNA damage and apoptosis in cardiomyocytes in mice. Life Sci. 2020:118074. doi: 10.1016/j.lfs.2020.118074. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen I.T.N., Klooster A., Minnion M., Feelisch M., Verhaar M.C., van Goor H., Joles J.A. Sodium thiosulfate improves renal function and oxygenation in L-NNA-induced hypertension in rats. Kidney Int. 2020;98:366–377. doi: 10.1016/j.kint.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Sakaguchi M., Marutani E., Shin H.S., Chen W., Hanaoka K., Xian M., Ichinose F. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology. 2014;121:1248–1257. doi: 10.1097/ALN.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snijder P.M., Frenay A.R., Koning A.M., Bachtler M., Pasch A., Kwakernaak A.J., van den B.E., Bos E.M., Hillebrands J.L., Navis G., Leuvenink H.G., van G.H. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage, Nitric. Oxide. 2014;42:87–98. doi: 10.1016/j.niox.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Olson K.R., DeLeon E.R., Gao Y., Hurley K., Sadauskas V., Batz C., Stoy G.F. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 72.Liang W., Fernandes A.P., Holmgren A., Li X., Zhong L. Bacterial thioredoxin and thioredoxin reductase as mediators for epigallocatechin 3-gallate-induced antimicrobial action. FEBS J. 2016;283:446–458. doi: 10.1111/febs.13587. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Zhang H., Holmgren A., Tian W., Zhong L. Inhibitory effect of green tea extract and (-)-epigallocatechin-3-gallate on mammalian thioredoxin reductase and HeLa cell viability. Oncol. Rep. 2008;20:1479–1487. [PubMed] [Google Scholar]
- 74.Zhang H., Cao D., Cui W., Ji M., Qian X., Zhong L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (-)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010;49:2010–2018. doi: 10.1016/j.freeradbiomed.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 75.Akagawa M., Shigemitsu T., Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003;67:2632–2640. doi: 10.1271/bbb.67.2632. [DOI] [PubMed] [Google Scholar]
- 76.Bandy B., Davison A.J. Interactions between metals, ligands, and oxygen in the autoxidation of 6-hydroxydopamine: mechanisms by which metal chelation enhances inhibition by superoxide dismutase. Arch. Biochem. Biophys. 1987;259:305–315. doi: 10.1016/0003-9861(87)90497-8. [DOI] [PubMed] [Google Scholar]
- 77.Bandy B., Walter P.B., Moon J., Davison A.J. Reaction of oxygen with 6-hydroxydopamine catalyzed by Cu, Fe, Mn, and V complexes: identification of a thermodynamic window for effective metal catalysis. Arch. Biochem. Biophys. 2001;389:22–30. doi: 10.1006/abbi.2001.2285. [DOI] [PubMed] [Google Scholar]
- 78.Bousher A. Review: unidentate complexes involving borate. J. Coord. Chem. 1995;34:1–11. [Google Scholar]
- 79.Wardman P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1989;18:1637–1755. [Google Scholar]
- 80.Hatasa Y., Chikazawa M., Furuhashi M., Nakashima F., Shibata T., Kondo T., Akagawa M., Hamagami H., Tanaka H., Tachibana H., Uchida K. Oxidative deamination of serum albumins by (-)-Epigallocatechin-3-O-gallate: a potential mechanism for the formation of innate antigens by antioxidants. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bae J., Kumazoe M., Yamashita S., Tachibana H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017;7:6665. doi: 10.1038/s41598-017-06879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng X.H., Li N., Zhu H.T., Wang D., Yang C.R., Zhang Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus camellia section thea. J. Agric. Food Chem. 2019;67:5318–5349. doi: 10.1021/acs.jafc.8b05037. [DOI] [PubMed] [Google Scholar]
- 83.Koch W., Kukula-Koch W., Komsta L., Marzec Z., Szwerc W., Glowniak K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules. 2018:23. doi: 10.3390/molecules23071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin Y., Zhao J., Kim E.M., Kim K.H., Kang S., Lee H., Lee J. Comprehensive investigation of the effects of brewing conditions in sample preparation of green tea infusions. Molecules. 2019:24. doi: 10.3390/molecules24091735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marutani E., Ichinose F. Emerging pharmacological tools to control hydrogen sulfide signaling in critical illness. Intensive Care Med. Exp. 2020;8:5. doi: 10.1186/s40635-020-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hildebrandt T.M., Grieshaber M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.