Abstract
Generation of mitochondrial reactive oxygen species (ROS) is an important process in triggering cellular necrosis and tissue infarction during ischemia-reperfusion (IR) injury. Ischemia results in accumulation of the metabolite succinate. Rapid oxidation of this succinate by mitochondrial complex II (Cx-II) during reperfusion reduces the co-enzyme Q (Co-Q) pool, thereby driving electrons backward into complex-I (Cx-I), a process known as reverse electron transport (RET), which is thought to be a major source of ROS. During ischemia, enhanced glycolysis results in an acidic cellular pH at the onset of reperfusion. While the process of RsET within Cx-I is known to be enhanced by a high mitochondrial trans-membrane ΔpH, the impact of pH itself on the integrated process of Cx-II to Cx-I RET has not been fully studied. Using isolated mouse heart and liver mitochondria under conditions which mimic the onset of reperfusion (i.e., high [ADP]), we show that mitochondrial respiration (state 2 and state 3) as well as isolated Cx-II activity are impaired at acidic pH, whereas the overall generation of ROS by Cx-II to Cx-I RET was insensitive to pH. Together these data indicate that the acceleration of Cx-I RET ROS by ΔpH appears to be cancelled out by the impact of pH on the source of electrons, i.e. Cx-II. Implications for the role of Cx-II to Cx-I RET derived ROS in IR injury are discussed.
Keywords: Mitochondria, Metabolism, Acidosis, Ischemia, ROS, Complex I
Graphical abstract
Highlights
-
•
ROS from complex I (Cx-I) reverse electron transport (RET) is enhanced at acidic pH.
-
•
Mitochondrial complex II (Cx-II) activity is inhibited at acidic pH.
-
•
These effects cancel out, yielding no net pH response of Cx-II to Cx-I RET ROS.
1. Introduction
Ischemia-reperfusion (IR) injury is caused by disruption of blood flow, followed by its restoration, and is relevant to pathologies such as myocardial infarction (heart attack) and stroke [1,2]. The magnitude and duration of ischemia is a key determinant of the severity of eventual cell death, such that timely reperfusion is necessary to salvage tissue. However, paradoxically the reintroduction of oxygen by reperfusion triggers mitochondrial events including opening of the mitochondrial permeability transition (PT) pore, which initiate cell death [3].
Under physiologic conditions, pyruvate generated from glycolysis in the cytosol is shuttled into mitochondria and metabolized to acetyl-CoA to serve as a carbon source for the Krebs’ cycle. The cycle generates reducing intermediates NADH and succinate, that are consumed by complex I (Cx-I) and complex II (Cx-II) of the electron transport chain (ETC) respectively. These electrons then sequentially pass via Co-enzyme Q (Co-Q) to complex III (Cx-III), cytochrome c, complex IV (Cx-IV) and eventually O2. ETC activity pumps protons across the inner mitochondrial membrane (IMM), creating a protonmotive force (pmf), consisting of charge (ΔΨm) and chemical (ΔpH) components, and ATP synthase (Cx-V) uses this pmf to produce ATP [4].
Two major sources of acidification during ischemia are: (i) CO2 from dehydrogenases that remain active [5] and (ii) the metabolic changes that result from a lack of oxygen, with the best-known being an elevation in glycolysis to yield lactic acid [6]. The release of a proton, yielding lactate, causes a drop in cytosolic pH from ~7.4 to ~6.6 [[5], [6], [7], [8]]. In addition, the accumulation of succinate is a hallmark of ischemia [[9], [10], [11]] and is proposed to occur via two mechanisms: (i) the reversal of mitochondrial Cx-II driven by a highly reduced Co-Q pool [[11], [12], [13], [14]], and/or (ii) canonical Krebs’ cycle activity augmented by anaplerosis to α-ketoglutarate [15].
Within the first 5 min of reperfusion, accumulated succinate returns to normal levels, via a combination of its washout from tissue, and its rapid oxidation by Cx-II [15,16]. The latter process leads to a highly reduced Co-Q pool, with the downstream ETC functioning at maximum capacity, resulting in an enhanced pmf [9]. These conditions provide the driving force to push electrons backward into Cx-I, a phenomenon known as reverse electron transport (RET) (Fig. 1) [[17], [18], [19]]. The generation of reactive oxygen species (ROS) has been observed under conditions that favor RET, and such ROS generation is thought to underlie the cascade of pathologic events including PT pore opening that lead to cell-death in IR injury [20]. Several variables have been identified that regulate RET, such as the redox states of Co-Q and NAD+/NADH pools, O2 concentration, ΔpH, and pH (for clarity, herein ΔpH refers to the trans-membrane proton gradient and pH refers to the pH in a given compartment) [[21], [22], [23], [24]].
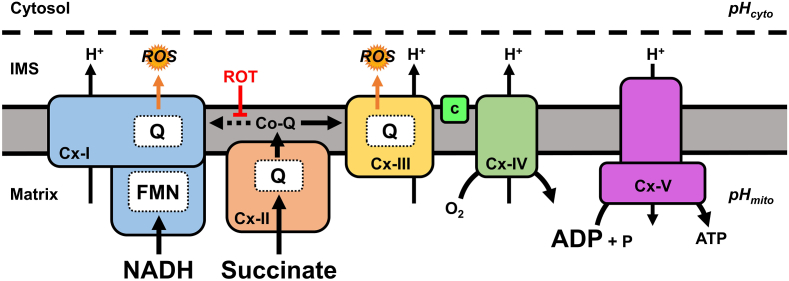
Fig. 1.
Schematic of mitochondrial ROS during reperfusion. At reperfusion, the re-introduction of O2 stimulates ETC activity and accumulated ischemic succinate is rapidly oxidized by Cx-II resulting in a highly reduced Co-Q pool. Given the metabolic conditions present at the start of reperfusion (high NADH and ADP), instead of flowing forward to Cx-III, some electrons can be driven backwards from Co-Q into Cx-I (reverse electron transport, RET, dotted line), generating ROS in a rotenone (ROT) sensitive manner. Abbreviations: pHcyto, cytosolic pH; pHmito, mitochondrial matrix pH; ETC, electron transport chain; Cx-I, complex-I; Cx-II, complex-II; Cx-III, complex-III; c, cytochrome c; Cx-IV, complex-IV; Cx-V, complex V; ROS, reactive oxygen species; IMS, intermembrane space; FMN, flavin mononucleotide; Co-Q, Co-enzyme Q; Q, Co-enzyme Q binding site.
The role of succinate oxidation by Cx-II as a driver of pathological ROS generation has been exploited for its potential as a drug target in IR injury [25,26]. We and others have demonstrated that Cx-II inhibition at the start of reperfusion is protective in animal and perfused organ models of IR injury [15,[27], [28], [29], [30]]. In addition, it is known that prolongation of ischemic tissue acidosis into the reperfusion period can improve recovery from IR injury [31,32]. While the exact protective mechanism of “acid post-conditioning” is poorly characterized, it may involve a recently described pH sensor on the PT pore [33]. Alternatively, the impact of pH on the major ROS generating sites within the ETC during reperfusion has not been extensively investigated.
RET-induced Cx-I ROS generation is thought to occur at the Co-Q binding site, and is characterized by sensitivity to Q-site inhibitors such as rotenone [21,[34], [35], [36]]. The ΔpH is thought to be a major driver of RET-induced Cx-I ROS, although the effect of collapsing ΔpH on RET-induced Cx-I ROS is somewhat controversial [21,23]. In addition, the relationship between cytosolic pH (pHcyto) and mitochondrial matrix pH (pHmito) and the independent effects of each on RET-induced Cx-I ROS are poorly understood. Importantly, many previous studies have assumed that the bulk of ROS measured under conditions of succinate supported respiration is due to Cx-I RET, without appropriate controls (i.e. subtraction of the rotenone-insensitive ROS component) [21,23].
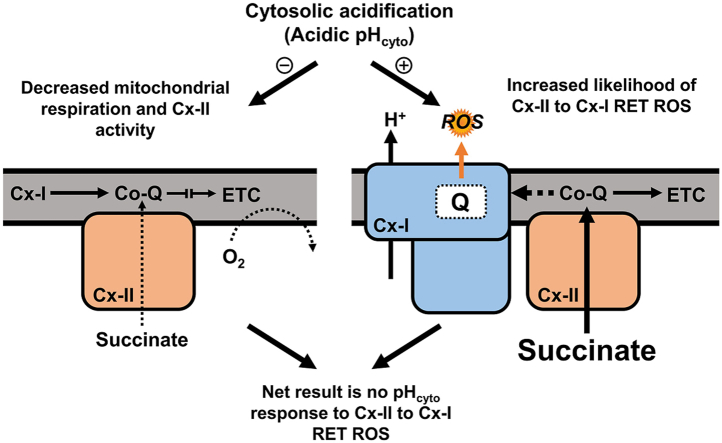
Herein, we examined the effects of pHcyto range spanning ischemic to normoxic values (pH 6.6–7.8) on RET-induced Cx-I ROS (rotenone-sensitive), also taking into consideration the effects of pHmito on the source of reduced Co-Q, i.e. Cx-II. Despite observing an inhibition of Cx-II at acidic pH, we observed no difference in the net production of RET-induced Cx-I ROS over the applied pHcyto range. We conclude that while ΔpH may indeed serve to enhance RET-induced Cx-I ROS, this is counteracted by the impact of pH on the driving force (Cx-II). These findings have important implications for the impact of pH on ROS during reperfusion injury.
2. Results
The complete original data set used for generation of the figures has been posted on the data sharing site Figshare (https://doi.org/10.6084/m9.figshare.12946931).
2.1. Acidic pH decreases mitochondrial succinate-linked respiration
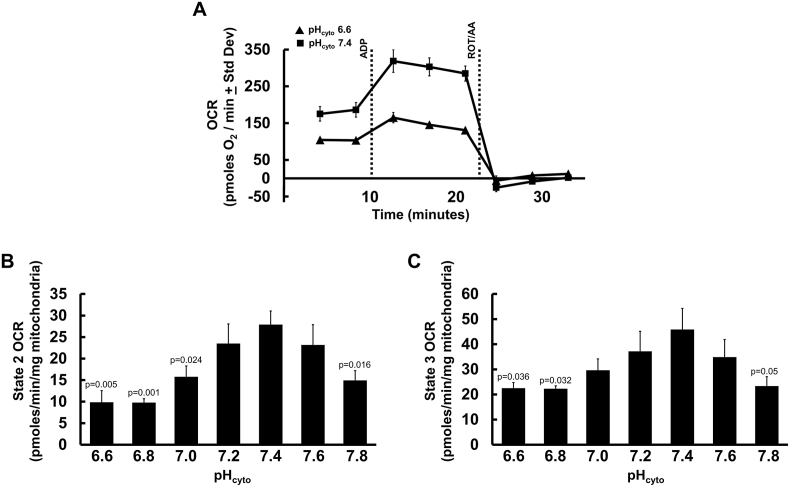
To determine the effect of pHcyto on mitochondrial respiration, we measured isolated mouse liver mitochondrial oxygen consumption rates (OCR) using a Seahorse XF96 analyzer over a pH range of 6.6–7.8. Mitochondria (5 μg protein/well) were assayed in the presence of 5 mM succinate to determine Cx-II linked respiration (state 2), followed by sequential injections of ADP (state 3) and rotenone/antimycin A (non-mitochondrial oxygen consumption). As the data in Fig. 2B and C shows, state 2 (quiescent) and state 3 (phosphorylating) respiration were lower at acidic pHcyto.
Fig. 2.
Mitochondrial respiration vs. pHcyto. (A): Representative Seahorse XF trace showing oxygen consumption rate (OCR) of mouse liver mitochondria in MRB pHcyto 6.6 (triangles) and pHcyto 7.4 (squares) sustained by succinate (state 2). ADP injection (state 3) and rotenone + antimycin A (ROT/AA) are indicated by dotted lines. Representative traces are means + Std. Dev. of one biological replicate with twelve technical replicates. (B): Oxygen consumption rates were assayed in isolated mouse liver mitochondria in succinate sustained state 2 respiration over pHcyto range 6.6–7.8. (C): Succinate + ADP sustained state 3 respiration over pHcyto range 6.6–7.8. (B and C) Data are mean OCR values relative to individual OCR values at pH 7.4 for each mitochondria preparation +SEM (N = 4) with eight-twelve technical replicates per N. One-way ANOVA with Tukey's test for multiple comparisons was performed. Student's unpaired t-test was done between all individual pHcyto values and pHcyto 7.4, values are denoted in the figure above.
2.2. Acidic pH decreases complex II activity
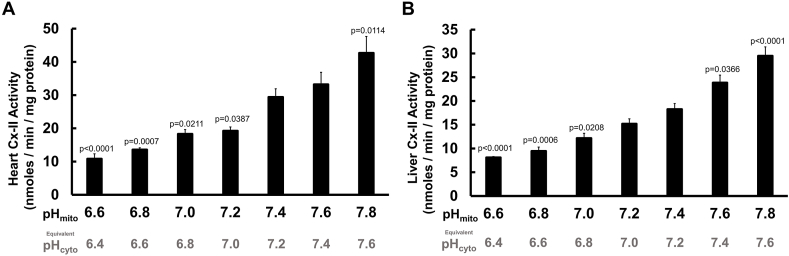
Seeking the source of the observed acidic impairment of respiration, we next determined if acidic pH could impact the activity of mitochondrial Cx-II. Generally, the ΔpH across the IMM of mitochondria ranges from 0.1 to 0.7 pH units, and is dependent on the prevailing phosphate concentration (since the mitochondrial phosphate carrier co-transports a proton) [37]. At the [Pi] used here (5 mM), we therefore assumed a ΔpH of 0.2 units, regardless of the prevailing pHcyto [21,23]. Thus, at normoxic or ischemic pHcyto values of 7.4 or 6.6, the corresponding pHmito values would be 7.6 and 6.8. Cx-II activity was assayed spectrophotometrically in freeze-thawed mouse liver or heart mitochondria over an applied pH range (i.e. pHmito) of 6.6–7.8 (equivalent to pHcyto 6.4–7.6). As shown in Fig. 3A and B, Cx-II activity was drastically reduced by acidic pH. These data suggest that under conditions of ischemic acidosis experienced during early reperfusion, Cx-II activity is low.
Fig. 3.
Isolated mitochondrial Cx-II activity vs. pH. Isolated complex-II (Cx-II) activity assays from freeze-thawed mitochondria demonstrated a pH-dependent relationship over the range pH 6.6–7.8. (A): Isolated Cx-II activity from heart mitochondria. (B): Isolated Cx-II activity from liver mitochondria. One-way ANOVA with Tukey's test for multiple comparisons was performed. Data are means +SEM. In A, N = 6 biological replicates; four technical replicates per N. In B, N = 5 biological replicates; four technical replicates per N. Statistical differences were determined between all pH values, but values denoted in the figure are in comparison to pH 7.4. For each applied pH (i.e. pHmito), the equivalent pHcyto is shown underneath, assuming ΔpH = 0.4 units.
2.3. Acidic pHcyto does not change net Cx-I RET ROS production
Incubation of mitochondria with succinate drives ROS generation both from Q site of Cx-I via RET, and from the downstream respiratory chain (likely the QO site of Cx-III). Since ROS generation from Cx-I RET can be inhibited by rotenone [21,23], the contribution of Cx-I RET to the overall ROS signal is calculated as the rotenone-sensitive component. Metabolic conditions upon tissue reperfusion include high [succinate], high [NADH], and an elevated ADP/ATP ratio favoring state 3 respiration [[38], [39], [40]]. Initial experiments utilizing succinate in the presence of pyruvate plus carnitine to generate intra-mitochondrial NADH [15,41] were unsuccessful owing to a quenching of the amplex red resorufin fluorescent signal by carnitine (data not shown). Further experiments using succinate in the presence of pyruvate plus malate for NADH production, yielded an elevation in ROS generation upon addition of rotenone, presumably due to enhancement of ROS production at the flavin site of Cx-I [42]. Thus, due to the confounding effect of NADH-generating substrates we opted to use succinate as the sole metabolic substrate, with ADP present to simulate early reperfusion.
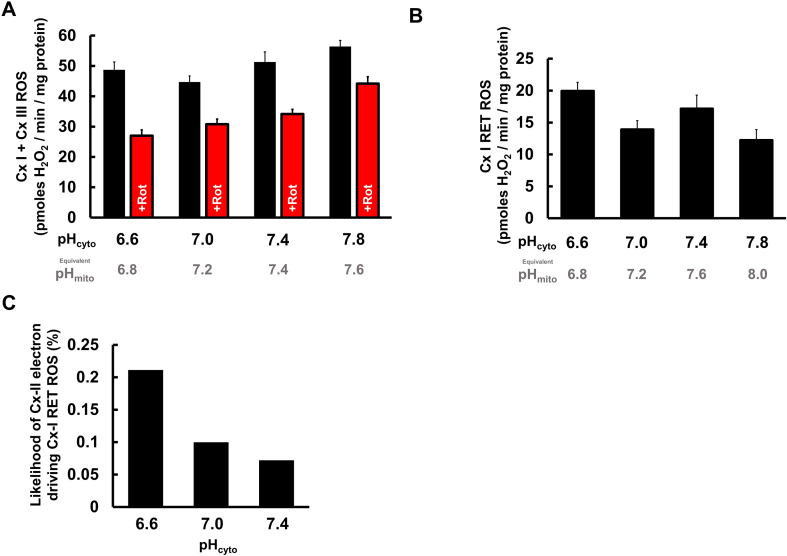
Although a high ΔpH has been proposed as sine qua non for Cx-I RET [21], Fig. 4A shows that even in the presence of ADP (state 3 respiration), ROS generation was partly rotenone-sensitive. Fig. 4B shows the calculated net rates of rotenone-sensitive ROS (i.e., Cx-I RET) across the range of pH values studied, revealing that ROS from Cx-II driven Cx-I RET is not enhanced at acidic pH.
Fig. 4.
Cx-I RET ROS generation vs. pHcyto. (A): ROS production with or without rotenone (+Rot) in isolated liver mitochondria incubated with succinate and ADP across a pH range 6.6–7.8. (B): Net difference between ROS generated in the absence and presence of rotenone from panel B across the pH range. (C): Calculated probability that an electron oxidized from succinate at Cx-II (Fig. 3B) will go backward and cause Cx-I RET ROS (Fig. 4B) across different values of pHcyto 6.6–7.4. Data in A and B are means + SEMs (N = 6) with six technical replicates per N. Data in C are calculated per Eqn. (1) (see text). For each applied pH (i.e. pHcyto), the equivalent pHmito is shown underneath, assuming ΔpH = 0.4 units.
3. Discussion
The net amount of ROS originating from any site (N) is a combination of two factors: the availability of electrons (E), and the probability the site will leak an electron onto oxygen (P).
| N = E x P | Equation 1 |
In the case of Cx-II driven Cx-I RET ROS, the availability of electrons in the Co-Q pool (E) is determined by the activity of Cx-II, and we measure the net ROS (N) directly from the rotenone-sensitive amplex red oxidation rate. So, the probability of Cx-I to donate electrons to molecular oxygen to make ROS (P) can be calculated by rearranging equation (1). As shown in Fig. 4C, acidification of pHcyto from pH 7.4 to 6.6 (equivalent pHmito 7.8 to 7.0) results in a doubling of Cx-I RET ROS probability. This is in agreement with the widely demonstrated importance of ΔpH as a determinant of RET in Cx-I.
In the context of IR injury, the findings herein are particularly relevant to the phenomenon of acidic post-conditioning [31]. Specifically, our data suggest that the ability of acidic reperfusion to improve recovery from IR is likely not due to an inhibition of mitochondrial ROS generation. While Cx-II activity was inhibited at acidic pH, the probability that Cx-I can generate ROS via RET was enhanced, such that these two phenomena cancel each other out, yielding similar ROS generation rates across the range of pH values that would be expected in ischemia/normoxia.
A number of drugs developed to protect tissues such as the heart from IR injury are known to enhance cytosolic acidosis. For example, the cardioprotective drug cariporide inhibits the Na+/H+ exchanger (NHE1), an important pathway for export of protons from the cell [43,44] Similarly, the NAD+ precursor NMN elicits cardioprotection in part via stimulation of glycolysis, which enhances lactic acidosis [45]. Furthermore, as discussed above, exogenously applied acidosis (i.e. acid reperfusion) is cardioprotective [31]. Contrasting the notion that Cx-I RET is enhanced by ΔpH, with the literature suggesting that acidic pHcyto is cardioprotective, presents a paradox – how can acid be protective if it enhances RET driven ROS? However, factoring in the pH sensitivity of the source of electrons for RET during reperfusion, i.e., Cx-II, may explain these contrasting results: An acidic pHcyto does not alter the overall Cx-II driven Cx-I RET derived ROS generation rate. Together with recent speculation that ROS may be relatively unimportant for the pathogenesis of IR injury [46], plus proposals that ROS originating from Cx-I RET can elicit beneficial signaling roles [47,48], this implies that the protective benefits of acidosis in the context of IR injury may originate elsewhere than a direct effect on ROS (e.g. effects on the PT pore) [33].
Regarding the mechanism by which acidic pH inhibits Cx-II itself, there is a possibility the protonation of succinate may render it an unsuitable substrate for the enzyme. However, succinate's pKa values of 4.2 and 5.6 are such that at a physiologic pHmito ~7.8, <1% of succinate is protonated as succinic acid, and even during ischemic acidification to pHmito ~7.0, <5% of succinate would be protonated. Thus, succinate protonation is unlikely to account for Cx-II inhibition.
A surprising finding herein was that a significant portion of ROS generation with succinate as substrate was sensitive to rotenone, despite mitochondria respiring in state 3 (ADP present). Previously, in mitochondria from skeletal muscle, heart and brain, ~90% of the succinate-supported ROS generation was reported as rotenone-sensitive [[21], [22], [23]]. However, most of those studies used mitochondria in state 2 (no ADP), which does not model the situation at the beginning of reperfusion. As such, the observation herein that ~30% of the ROS signal is rotenone-sensitive and can thus be attributed to Cx-I RET, is likely a more accurate estimate of the contribution of this process to ROS generation during early reperfusion.
Recently, it was reported that application of an extracellular acidic pH can also impair the export of succinate from the cell via the monocarboxylate transporter MCT1 [16]. It is unclear what may be the impact of the resulting succinate retention inside the cell, but it could be hypothesized that such retention would enhance IR injury due to succinate-driven ROS generation. However, the novel observation herein that acidic pH also inhibits Cx-II, may prevent retained succinate from being oxidized. Clearly, the complex dynamics of extracellular, intracellular, and mitochondrial pH in the first moments of reperfusion are poorly understood, and their further elucidation will be essential to determine the optimal manner in which succinate and Cx-II may be manipulated for therapeutic benefit in the context of IR injury.
4. Methods
4.1. Animals and reagents
Animal and experimental procedures complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals (8th edition, 2011) and were approved by the University of Rochester Committee on Animal Resources. Male and female C57BL/6J adult mice (8–12 weeks old) were housed in a pathogen-free vivarium with 12 h light-dark cycles and food and water ad libitum. Mice were administered terminal anesthesia via intra-peritoneal 2,2,2-tribromoethanol (Avertin) ~250 mg/kg.
4.2. Heart mitochondria isolation
Following anesthesia, the heart was excised and washed twice in 25 ml of ice-cold heart mitochondria isolation media (HMIM) comprising (in mM): Tris [20], EGTA [2], sucrose (300), pH 7.35. Tissue was diced using a razor blade, washed and transferred to a 50 ml conical tube containing 2 ml HMIM on ice, then homogenized using a Tekmar Tissumizer (IKA Instruments, Wilmington, NC) at 22,000 rpm for 20 s. The homogenate was centrifuged at 800 g for 5 min at 4 °C, the pellet discarded, and the supernatant transferred to a new tube and centrifuged at 10,800 g for 5 min. Pellets were washed by centrifugation twice more, and the final pellet resuspended in 60 μl HMIM. Protein content was determined using the Folin-Phenol method against a standard curve constructed using bovine serum albumin [49,50].
4.3. Liver mitochondria isolation
Following anesthesia, the liver was excised and washed in ice-cold 25 ml of liver mitochondria isolation media (LMIM) comprising (in mM): Tris [20], EGTA [2], sucrose (250), pH 7.35. The liver was chopped into 2–3 mm pieces using double scissors, washed 2–3 times and transferred into an ice-cold glass Dounce homogenizer. Tissue was first homogenized with 8–10 strokes using loose pestle A, then another 8–10 strokes using pestle B. Homogenates were centrifuged at 1000 g for 3 min at 4 °C the supernatant transferred to a new tube and spun at 10,000 g for 10 min. Pellet was resuspended in ice-cold LMIM and centrifuged at 10,000 g twice more and the final pellet resuspended in 600 μl of LMIM, transferred to a Potter-Elvehjem homogenizer and homogenized with 6–8 strokes. Protein content was determined using the Folin-Phenol method against a standard curve constructed using bovine serum albumin [49,50].
4.4. Isolated liver mitochondria respiration vs. pH
As previously described by Shiriai et al. [51] isolated liver mitochondria were loaded into a Seahorse XF96 microplate in 20 μl of mitochondria respiration buffer (MRB) pH 6.6–7.8 37 °C (0.2 pH unit intervals). MRB contained the following (in mM): KCl (120), sucrose [25], MgCl2 [5], KH2PO4 [5], EGTA [1], HEPES [10], succinate [5]. The loaded plate was centrifuged at 1000 g for 5 min at 4 °C and an additional 130 μl of MRB (pH 6.6–7.8) was added to each well. For port injections, a 1 mM HEPES MRB pH 7.0 at 37 °C was prepared, referred to as MRBinj. ADP (1.5 mM final) and rotenone/antimycin A (5 μM final) were prepared in 10x injection stocks in MRBinj. Mix and measure times were 0.5 and 3 min. Mitochondrial oxygen consumption rates (OCR) are expressed as pmols O2/min/mg mitochondrial protein. The XF assay of oxygen consumption is designed to operate across a wide pH range, since the apparatus also measures pH in the same samples, to determine extracellular acidification rate (ECAR) as a surrogate for glycolysis. As such, the respiration data were independent of pH.
4.5. Complex II activity vs. pH
A Synergy 2 Multi-Detection 96-well Microplate Reader (BioTek, Winooski, VT) was used at 37 °C for experiments assessing complex II activity over the pH range 6.6–7.8. Isolated mitochondria (heart or liver) were freeze/thawed using liquid N2 three times. Mitochondria (0.1 mg/ml) were resuspended in 100 mM KPO4 assay buffer containing the following (in mM): Coenzyme-Q2 (0.05), EDTA (0.1), dichlorophenolindophenol (DCPIP) (0.12), KCN [1], rotenone (0.01) for 10 min at 37 °C in the plate reader. Activity was measured by spectrophotometrically monitoring the absorbance change of DCPIP at 600 nm (ε = 21 mM−1 cm−1). After baseline measurements, 5 mM succinate was added for reaction initiation and data collected every 50 s for 5 min. Followed by addition of the Cx-II inhibitor 2-thenyltrifluoroacetone (TTFA, 1 mM) to determine the TTFA-sensitive rate [28]. Control experiments confirmed that buffer pH did not compromise the integrity of the assay in the readout of DCPIP reduction (data not shown).
4.6. Measurement of ROS production by RET
A Synergy 2 Multi-Detection 96-well Microplate Reader (BioTek, Winooski, VT) was used at 37 °C for experiments assessing ROS generation as result of RET over a pH range. MRB (pH 6.6–7.8) was supplemented with (in mM) Amplex Red (0.01), horse radish peroxidase (1 U/ml), superoxide dismutase (80 U/ml), ADP (0.1), succinate (2.5), ± rotenone (0.005), and incubated at 37 °C in the plate reader for 5 min. The reaction was initiated by addition of mitochondria (0.25 mg/ml), and ROS production measured as change in fluorescence from the conversion of amplex red to resorufin (ëEX 570 nm, λEM 585 nm) when reacted with H2O2. To calibrate H2O2 production a known concentration of H2O2 was added at the end of each run. ROS generation was determined with or without the Cx I inhibitor rotenone, and the net rotenone-sensitive rate was used to represent Cx-I RET ROS [52,53]. Control experiments confirmed that buffer pH did not compromise the integrity of the assay in the readout of amplex red resorufin fluorescence (data not shown).
4.7. Statistical analysis
Comparisons between groups were made unpaired Student's t-test or ANOVA with a post-hoc Tukey's test where appropriate. Statistical analysis was performed using GraphPad Prism statistical analysis software. Data are shown as means ± SEM. Numbers of biological replicates (N) and technical replicates are noted in the figure legends. Significance was set at α = 0.05.
Funding and additional information
This work was funded by a grant from the National Institutes of Health (R01-HL071158). ASM was funded by an institutional NIH training grant (T32-GM068411). CAK was funded by a post-doctoral fellowship from the American Heart Association (#19POST34380212).
Declaration of competing interest
The authors declare that they have no conflicts of interest.
ABBREVIATIONS
- ROS
Reactive oxygen species
- IR
Ischemia-reperfusion
- mPTP
Mitochondrial permeability transition pore
- ETC
Electron transport chain
- Cx-I
Complex I (NADH:ubiquinone oxidoreductase)
- Cx-II
Complex II (succinate:ubiquinone oxidoreductase, succinate dehydrogenase)
- Cx-III
Complex III
- Cx-IV
Complex IV
- Cx-V
Complex V (ATP synthase)
- Co-Q
Coenzyme Q (ubiquinone)
- RET
Reverse electron transport
- ΔpH
Trans-membrane pH gradient
- pmf
Proton motive force
- ΔΨm
Mitochondrial membrane potential
- IMM
Inner mitochondrial membrane
- IMS
Intermembrane space
- pHmito
Mitochondrial matrix pH
- pHcyto
Cytosolic pH
- OCR
Oxygen consumption rate
References
- 1.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welbourn C.R. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br. J. Surg. 1991;78:651–655. doi: 10.1002/bjs.1800780607. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi P., Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voet D. Wiley; Chichester: 2016. Fundamentals of Biochemistry. [Google Scholar]
- 5.Yan G.X., Kleber A.G. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ. Res. 1992;71:460–470. doi: 10.1161/01.res.71.2.460. [DOI] [PubMed] [Google Scholar]
- 6.Scholz W., Albus U. Na+/H+ exchange and its inhibition in cardiac ischemia and reperfusion. Basic Res. Cardiol. 1993;88:443–455. doi: 10.1007/BF00795411. [DOI] [PubMed] [Google Scholar]
- 7.Inserte J. High-fat diet improves tolerance to myocardial ischemia by delaying normalization of intracellular PH at reperfusion. J. Mol. Cell. Cardiol. 2019;133:164–173. doi: 10.1016/j.yjmcc.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Bailey I.A., Williams S.R., Radda G.K., Gadian D.G. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem. J. 1981;196:171–178. doi: 10.1042/bj1960171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouchani E.T. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochachka P.W., Dressendorfer R.H. Succinate accumulation in man during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1976;35:235–242. doi: 10.1007/BF00423282. [DOI] [PubMed] [Google Scholar]
- 11.Hochachka P.W., Owen T.G., Allen J.F., Whittow G.C. Multiple end products of anaerobiosis in diving vertebrates. Comp. Biochem. Physiol. B. 1975;50:17–22. doi: 10.1016/0305-0491(75)90292-8. [DOI] [PubMed] [Google Scholar]
- 12.Hohl C., Oestreich R., Rosen P., Wiesner R., Grieshaber M. Evidence for succinate production by reduction of fumarate during hypoxia in isolated adult rat heart cells. Arch. Biochem. Biophys. 1987;259:527–535. doi: 10.1016/0003-9861(87)90519-4. [DOI] [PubMed] [Google Scholar]
- 13.Dewan J.G., Green D.E. Coenzyme-linked reactions between dehydrogenase systems. Biochem. J. 1937;31:1074–1085. doi: 10.1042/bj0311074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanadi D.R., Fluharty A.L. On the mechanism of oxidative phosphorylation. Vii. The energy-requiring reduction of pyridine nucleotide by succinate and the energy-yielding oxidation of reduced pyridine nucleotide by fumarate. Biochemistry. 1963;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J. Accumulation of succinate in cardiac ischemia primarily occurs via canonical Krebs cycle activity. Cell Rep. 2018;23:2617–2628. doi: 10.1016/j.celrep.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prag H.A. Mechanism of succinate efflux upon reperfusion of the ischemic heart. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 18.Kussmaul L., Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Votyakova T.V., Reynolds I.J. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 20.Seidlmayer L.K. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 2015;106:237–248. doi: 10.1093/cvr/cvv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert A.J., Brand M.D. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robb E.L. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293:9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komlodi T., Geibl F.F., Sassani M., Ambrus A., Tretter L. Membrane potential and delta pH dependency of reverse electron transport-associated hydrogen peroxide production in brain and heart mitochondria. J. Bioenerg. Biomembr. 2018;50:355–365. doi: 10.1007/s10863-018-9766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selivanov V.A. The role of external and matrix pH in mitochondrial reactive oxygen species generation. J. Biol. Chem. 2008;283:29292–29300. doi: 10.1074/jbc.M801019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pell V.R., Chouchani E.T., Frezza C., Murphy M.P., Krieg T. Succinate metabolism: a new therapeutic target for myocardial reperfusion injury. Cardiovasc. Res. 2016;111:134–141. doi: 10.1093/cvr/cvw100. [DOI] [PubMed] [Google Scholar]
- 26.Kula-Alwar D., Prag H.A., Krieg T. Targeting succinate metabolism in ischemia/reperfusion injury. Circulation. 2019;140:1968–1970. doi: 10.1161/CIRCULATIONAHA.119.042791. [DOI] [PubMed] [Google Scholar]
- 27.Wojtovich A.P., Brookes P.S. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res. Cardiol. 2009;104:121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojtovich A.P., Brookes P.S. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim. Biophys. Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohlhauer M. Protection against cardiac ischemia-reperfusion injury by hypothermia and by inhibition of succinate accumulation and oxidation is additive. Basic Res. Cardiol. 2019;114:18. doi: 10.1007/s00395-019-0727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prag H.A. Ester prodrugs of malonate with enhanced intracellular delivery protect against cardiac ischemia-reperfusion injury in vivo. Cardiovasc. Drugs Ther. 2020 doi: 10.1007/s10557-020-07033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen M.V., Yang X.M., Downey J.M. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 32.Andersen A.D. The cardioprotective effect of brief acidic reperfusion after ischemia in perfused rat hearts is not mimicked by inhibition of the Na(+)/H(+) exchanger NHE1. Cell. Physiol. Biochem. 2011;28:13–24. doi: 10.1159/000331709. [DOI] [PubMed] [Google Scholar]
- 33.Antoniel M. The unique histidine in OSCP subunit of F-ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep. 2018;19:257–268. doi: 10.15252/embr.201744705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong H.S., Monternier P.A., Brand M.D. S1QELs suppress mitochondrial superoxide/hydrogen peroxide production from site IQ without inhibiting reverse electron flow through Complex I. Free Radic. Biol. Med. 2019;143:545–559. doi: 10.1016/j.freeradbiomed.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Watson M.A., Wong H.S., Brand M.D. Use of S1QELs and S3QELs to link mitochondrial sites of superoxide and hydrogen peroxide generation to physiological and pathological outcomes. Biochem. Soc. Trans. 2019;47:1461–1469. doi: 10.1042/BST20190305. [DOI] [PubMed] [Google Scholar]
- 36.Chance B., Williams G.R., Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J. Biol. Chem. 1963;238:418–431. [PubMed] [Google Scholar]
- 37.Klingenberg M., Rottenberg H. Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur. J. Biochem. 1977;73:125–130. doi: 10.1111/j.1432-1033.1977.tb11298.x. [DOI] [PubMed] [Google Scholar]
- 38.Bak M.I., Ingwall J.S. Acidosis during ischemia promotes adenosine triphosphate resynthesis in postischemic rat heart. In vivo regulation of 5'-nucleotidase. J. Clin. Invest. 1994;93:40–49. doi: 10.1172/JCI116974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennings R.B., Schaper J., Hill M.L., Steenbergen C., Jr., Reimer K.A. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ. Res. 1985;56:262–278. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- 40.Cave A.C. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation. 2000;101:2090–2096. doi: 10.1161/01.cir.101.17.2090. [DOI] [PubMed] [Google Scholar]
- 41.Muoio D.M. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabol. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryde K.R., Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slepkov E., Fliegel L. Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem. Cell. Biol. 2002;80:499–508. doi: 10.1139/o02-151. [DOI] [PubMed] [Google Scholar]
- 44.Javadov S. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc. Res. 2008;77:416–424. doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- 45.Nadtochiy S.M., Wang Y.T., Nehrke K., Munger J., Brookes P.S. Cardioprotection by nicotinamide mononucleotide (NMN): involvement of glycolysis and acidic pH. J. Mol. Cell. Cardiol. 2018;121:155–162. doi: 10.1016/j.yjmcc.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrienko T., Pasdois P., Rossbach A., Halestrap A.P. Real-time fluorescence measurements of ROS and [Ca2+] in ischemic/reperfused rat hearts: detectable increases occur only after mitochondrial pore opening and are attenuated by ischemic preconditioning. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onukwufor J.O., Berry B.J., Wojtovich A.P. Physiologic implications of reactive oxygen species production by mitochondrial complex I reverse electron transport. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicente-Gutierrez C. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metabol. 2019;1:201–211. doi: 10.1038/s42255-018-0031-6. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni C.A. ALKBH7 mediates necrosis via rewiring of glyoxal metabolism. Elife. 2020;9 doi: 10.7554/eLife.58573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 51.Acin-Perez R. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 2020;39 doi: 10.15252/embj.2019104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman D.L., Salter J.D., Brookes P.S. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 53.Tompkins A.J. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim. Biophys. Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]