Abstract
The authors present a case of acute disseminated encephalomyelitis in a COVID-19 pediatric patient with positive SARS-CoV2 markers from a nasopharyngeal swab. A previously healthy 12-year-old-girl presented with a skin rash, headache, and fever. Five days after that, she had an acute, progressive, bilateral, and symmetrical motor weakness. She evolved to respiratory failure. Magnetic resonance imaging (MRI) of the brain and cervical spine showed extensive bilateral and symmetric restricted diffusion involving the subcortical and deep white matter, a focal hyperintense T2/FLAIR lesion in the splenium of the corpus callosum with restricted diffusion, and extensive cervical myelopathy involving both white and gray matter. Follow-up examinations of the brain and spine were performed 30 days after the first MRI examination. The images of the brain demonstrated mild dilatation of the lateral ventricles and widespread widening of the cerebral sulci, complete resolution of the extensive white matter restricted diffusion, and complete resolution of the restricted diffusion in the lesion of the splenium of the corpus callosum, leaving behind a small gliotic focus. The follow-up examination of the spine demonstrated nearly complete resolution of the extensive signal changes in the spinal cord, leaving behind scattered signal changes in keeping with gliosis. She evolved with partial clinical and neurological improvement and was subsequently discharged.
Keywords: COVID-19, Brain diseases, Spinal cord diseases, ADEM, Child, Central Nervous System
Introduction
The majority of COVID-19 pediatric patients are asymptomatic [1]. In the pediatric age group, very few reports of neurological manifestations are available [2, 3]. Multiple COVID-19–related neurological manifestations have been described in adults, such as anosmia, ageusia, headache, encephalopathy, acute hemorrhagic necrotizing encephalopathy, meningitis, Guillain-Barre syndrome, stroke, myopathy, and acute disseminated encephalomyelitis (ADEM) [4–7]. Neurologic damage may occur by direct virus lesion, cytokine-related injury, and secondary hypoxia [7, 8]. We report a case of acute disseminated encephalomyelitis in a COVID-19 pediatric patient, with positive SARS-CoV2 markers from a nasopharyngeal swab.
Case presentation
A previously healthy 12-year-old girl presented with a skin rash, headache, and fever (38 °C). Headache and fever lasted 1 day, and the skin rash lasted 6 days. Five days after the onset of symptoms, she had an acute, progressive, bilateral, and symmetrical motor weakness (inability to stand, walk, and handle objects). In addition, she had tingling and numbness in the inferior limbs but no sphincter abnormalities. Up to this point, she had had no respiratory symptoms. Two days after the onset of the neurological symptoms, she developed respiratory distress (venturi mask at 50%; PCO2: 120 mmHg; PaO2 108 mmHg) that evolved to respiratory failure. She was intubated and placed on mechanical ventilation.
She was unable to follow commands. Her oculocephalic, corneal, cough, and gag reflexes were abolished but had photoreactive isochoric pupils. She also had flaccid tetraplegia, deep areflexia, and abolished abdominal cutaneous and plantar cutaneous reflexes. There was no history of cardiorespiratory arrest or visual disturbances.
White cell count was notable for leukocytosis with neutrophilia (13.87 × 103 white blood cells/μL). Red blood cells, electrolytes, urea, and creatinine were normal. Her creatine phosphokinase was 556 U/L; aspartate aminotransferase, 49 U/L; alanine aminotransferase, 104 U/L; lactate dehydrogenase, 373 U/L; C-reactive protein, 4.1 mg/dL; fibrinogen, 461 mg/dL; D-dimer, 6.470 ng/mL; and troponin, 5.5. SARS-CoV-2 real-time reverse transcriptase (rRT)-PCR from a nasopharyngeal swab, collected on the sixth day of symptoms, was positive (XGEN MASTER COVID-19—CDC China protocol—ORF1ab and N SARS-CoV-2 gene targets). CSF analysis demonstrated 18 mg/dL of protein, 74 mg/dL of glucose, no cells, and normal opening pressure. (rRT)-PCR for SARS-CoV-2, Zika virus, and dengue viruses serotypes 1 to 4 (DENV-1-4) were negative in the CSF.
Low-dose computed tomography (LDCT) of the lungs demonstrated no ground glass changes, but a right lower lobe focal atelectasis, that resolved after 3 days of respiratory therapy. LDCT of the lungs findings were not in keeping with COVID-19-related changes and interpreted as due to airway hypersecretion and mucous plug.
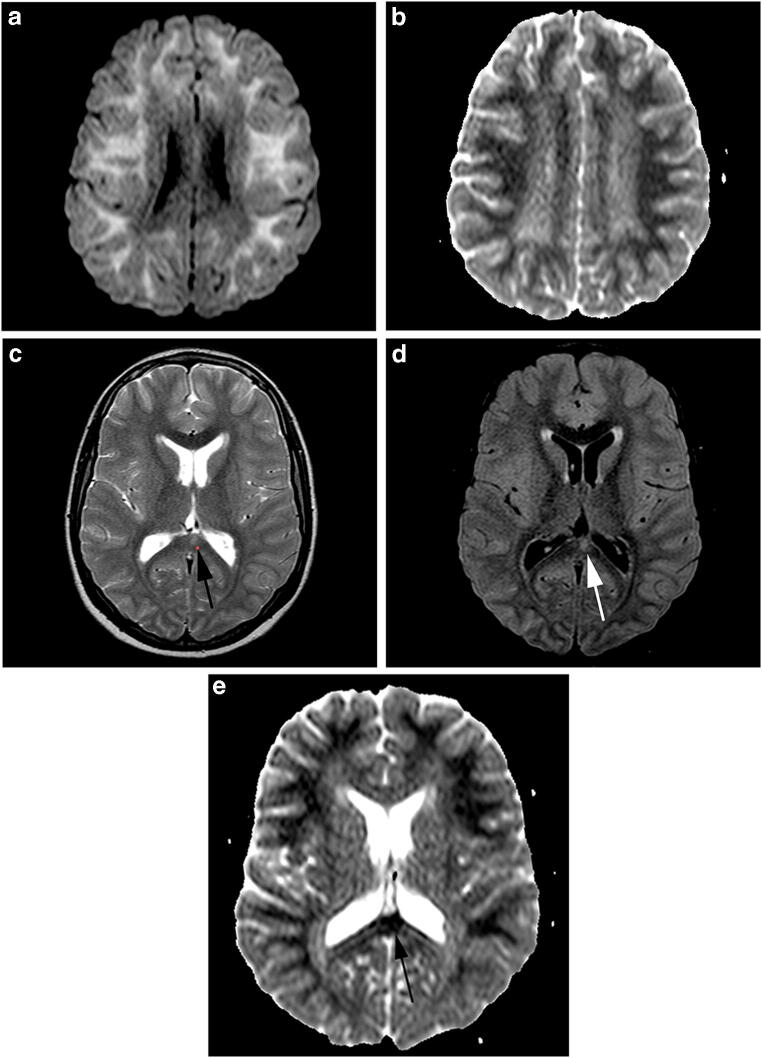
Magnetic resonance imaging (MRI) of the brain, acquired on the seventh day after the fever and on the second day after the onset of the neurological symptoms, showed extensive bilateral and symmetric restricted diffusion involving the subcortical and deep white matter (Fig. 1a and b). There was also a focal hyperintense T2/fluid-attenuated inversion recovery (FLAIR) lesion in the splenium of the corpus callosum (Fig. 1c and d) with restricted diffusion. No signs of blood–brain barrier breakdown were identified in the contrast-enhanced T1-weighted images. Susceptibility-weighted images were obtained, which did not demonstrate hemorrhagic deposits or pathological calcification in the brain parenchyma. MRI of the cervical spine showed longitudinally extensive cervical myelopathy involving both white and gray matter (Fig. 2a and b). Diffusion-weighted images were not acquired, nor gadolinium that was injected in the spine examination.
Fig. 1.
Axial diffusion (a) and apparent diffusion coefficient map images (b) showing extensive bilateral and symmetric restricted diffusion involving the subcortical and deep white matter. Axial T2 (c), fluid-attenuated inversion recovery (FLAIR) (d), and ADC map images (e) showing a focal hyperintense lesion in the splenium of the corpus callosum with restricted diffusion (arrows)
Fig. 2.
Sagittal (a) and axial T2-weighted images (b) highlighting longitudinally extensive cervical myelopathy involving both white and gray matter
She was treated clinically under the institutional protocol, including a 5-day therapy with methylprednisolone, which was repeated due to little improvement of her weakness after first pulse therapy. She remained on mechanical ventilation for 20 days. Her cognition was spared, as was her cranial nerve functions (except for mild dysphagia and dysphonia).
A follow-up examination of the brain was performed 30 days after the first MRI examination, which demonstrated mild dilatation of the lateral ventricles, widespread widening of the cerebral sulci, complete resolution of the extensive white matter restricted diffusion, and complete resolution of the restricted diffusion in the lesion of the splenium of the corpus callosum, with a residual small gliotic focus. A follow-up examination of the spine demonstrated nearly complete resolution of the extensive signal changes in the spinal cord, with residual scattered signal changes in keeping with gliosis.
Sixty-eight days after the onset of the condition, she had evolved from flaccid to spastic tetraparesis. Her strength improved from grade zero in the four limbs to grade three in the left superior limb, grade three in the lower limbs, and grade two in the right superior limb, using the Medical Research Council Scale (MRC) for muscle strength. She regained cervical control, being able to sit with support and reach objects placed nearby. Global hyperreflexia and bilateral Babinski signs were present. She had incomplete sphincter control. A second SARS-CoV-2 (rRT)-PCR from a nasopharyngeal swab was obtained 60 days after the onset of symptoms (before hospital discharge), which was negative.
Discussion
The striking neuroimaging features, in this case, are the diffuse subcortical and deep white matter–restricted diffusion, focal T2/FLAIR hyperintense lesions in the splenium of the corpus callosum, and inferior medulla, and extensive cervical myelopathy. The presence of acute and diffuse encephalomyelitis in a pediatric patient (mainly involving the cerebral white matter) following a viral infection due to SARS-CoV-2 favors the diagnosis of ADEM.
According to the consensus criteria established by the International Pediatric Multiple Sclerosis Study Group in 2013 [9], the diagnosis of pediatric ADEM requires all of the following: (1) a polyfocal, clinical central nervous system (CNS) event with a presumed inflammatory demyelinating cause; (2) an encephalopathy that cannot be explained by fever; (3) no new clinical and MRI findings emerging 3 months or more after the onset; (4) abnormal brain MRI during the acute phase. Typical MRI findings include (a) diffuse, poorly demarcated, large (> 1–2 cm) lesions involving predominantly the cerebral white matter; (b) rare white matter T1 hypointense lesions; and (c) possible deep gray matter lesions (e.g., thalamus or basal ganglia).
Five distinct patterns of cerebral involvement have been described to classify the MRI findings in children with ADEM: (1) ADEM with small lesions (less than 5 mm); (2) ADEM with large, confluent, or tumefactive lesions, with frequent extensive perilesional edema and mass effect; (3) ADEM with additional symmetrical bithalamic involvement; (4) acute hemorrhagic encephalomyelitis (AHEM), when the blood products are identified within the large T2 hyperintense demyelinating lesions; and (5) ADEM with a pseudo-leukodystrophy pattern, with a diffuse, bilateral, symmetrical, and usually nonenhancing white matter involvement [10–13]. We believe that the ADEM pattern that fits our patient is the one that courses with diffuse, bilateral, symmetrical, and non-enhancing white matter involvement (ADEM with pseudo-leukodystrophy pattern).
Well-known entities associated with involvement of the splenium of the corpus callosum include the reversible splenial lesion syndrome (RESLES) and MERS, which is a clinical-radiological entity characterized by mild encephalitis or encephalopathy associated with reversible lesion of the splenium of the corpus callosum [14]. Transient lesions in the splenium of the corpus callosum can occur in several conditions such as epilepsy, following the sudden withdrawal of antiepileptic drugs, influenza encephalitis, and other conditions such as hemolytic-uremic syndrome [15]. In addition, isolated involvement of the splenium of the corpus callosum may also occur in patients with ADEM [16]. We believe that the lesion in the splenium of the corpus callosum in our patient is demyelinating in nature (likely post-viral).
Children who have ADEM may evolve with motor or cognitive sequela. A recent study described the long-term outcome of 102 children with ADEM [17]. According to this study, 17.2% of the children had long-term motor or motor and sensory deficit and 8% had MRC grade 3 or less power in at least one limb.
Potential differential diagnosis in a child with acute leukoencephalomyelopathy includes other viral encephalitides, neuromyelitis optica (NMO), and severe hypoxic-ischemic injury. Negative rt-PCR for Zika and dengue virus speaks against a co-infection with these endemic viruses in our region and the absence of ophthalmological abnormalities and cardiorespiratory arrest make the possibility of hypoxic-ischemic injury and NMO less likely.
Our patient had neurological symptoms during the acute phase of COVID-19, which reinforces the possibility of a causal relationship between this infection and the CNS involvement. The simultaneous and monophasic brain involvement, mainly in cerebral the white matter and the spinal cord, lack of T1 hypointense lesions, and resolution of the acute MRI findings (restricted diffusion and edema) corroborates a post-infectious demyelinating process such as ADEM. This unique case report highlights the polymorphism of COVID-19 neurological manifestations and the possibility of activation of an autoimmune response against the CNS following the SARS-Cov-2 infection.
Funding
No funding was received for this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adélia Maria de Miranda Henriques-Souza, Email: adelianeuro@gmail.com.
Ana Cláudia Marques Gouveia de Melo, Email: anagouveianeuro@yahoo.com.
Bianca de Aguiar Coelho Silva Madeiro, Email: bianca.madeiro@upe.br.
Leonardo Furtado Freitas, Email: furtadoleofreitas@hotmail.com.
Pedro Augusto Sampaio Rocha-Filho, Email: pedroasampaio@gmail.com.
Fabrício Guimarães Gonçalves, Email: goncalves.neuroradio@gmail.com.
References
- 1.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirzaee SMM, Gonçalves FG, Mohammadifard M, et al (2020) Focal Cerebral arteriopathy in a COVID-19 pediatric patient. Radiology 202197 [DOI] [PMC free article] [PubMed]
- 3.Dugue R, Cay-Martínez KC, Thakur KT, Garcia JA, Chauhan LV, Williams SH, Briese T, Jain K, Foca M, McBrian DK, Bain JM, Lipkin WI, Mishra N. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Oliveira FAA, Palmeira DCC, Rocha-Filho PAS. Headache and pleocytosis in CSF associated with COVID-19: case report. Neurol Sci. 2020;41:1667. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridwell R, Long B, Gottlieb M (2020) Neurologic complications of COVID-19. Am J Emerg Med 38:1549.e3–1549.e7 [DOI] [PMC free article] [PubMed]
- 7.Sampaio Rocha-Filho PA, Voss L. Persistent headache and persistent anosmia associated with COVID-19. Headache. 2020;60:1797–1799. doi: 10.1111/head.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, for the International Pediatric Multiple Sclerosis Study Group International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 10.Sáenz-Farret M, Cansino-Torres MA, Sandoval-Rodríguez V, et al. The spectrum of acute disseminated encephalomyelitis and mild encephalopathy with reversible splenial lesion. Case Rep Neurol Med. 2019;2019:9272074. doi: 10.1155/2019/9272074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenembaum S, Chitnis T, Ness J, Hahn JS, for the International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 12.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/WNL.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 13.Kesselring J, Miller DH, Robb SA, et al. Acute disseminated encephalomyelitis. MRI findings and the distinction from multiple sclerosis. Brain. 1990;113(Pt 2):291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 14.Kontzialis M, Soares BP, Huisman TAGM. Lesions in the splenium of the corpus callosum on MRI in children: a review. J Neuroimaging. 2017;27:549–561. doi: 10.1111/jon.12455. [DOI] [PubMed] [Google Scholar]
- 15.Park SE, Choi DS, Shin HS, Baek HJ, Choi HC, Kim JE, Choi HY, Park MJ. Splenial lesions of the corpus callosum: disease spectrum and MRI findings. Korean J Radiol. 2017;18:710–721. doi: 10.3348/kjr.2017.18.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaauw J, Meiners LC. The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis. Neuroradiology. 2020;62:563–585. doi: 10.1007/s00234-019-02357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iype M, Ts A, Kunju PM, Saradakutty G, Sreedharan M, Ahamed SM. Factors related to long term motor, behavioral, and scholastic outcome in children with acute disseminated encephalomyelitis. Pediatr Neurol. 2018;89:49–57. doi: 10.1016/j.pediatrneurol.2018.08.015. [DOI] [PubMed] [Google Scholar]