Introduction
Chilblain-like lesions (CLLs) have been increasingly reported during the COVID-19 pandemic. However, their association with SARS-CoV-2 infection is currently still controversial.1 Usually, chilblains manifest as a reaction to cold and wet conditions. They are generally idiopathic and acute, although chronic forms also exist. Secondary chilblains are associated with several other disorders, among which systemic lupus erythematosus is the most common one (chilblain lupus erythematosus).2 Although infrequent, another acknowledged trigger is viral infections.3 In this case series, we describe the clinical and dermoscopic features of such unusual CLLs in young patients (age <18 years) observed at our clinic during the COVID-19 pandemic.
Methods
Fifteen patients with CLLs were recruited. All the patients were screened for SARS-CoV-2 infection with quantitative reverse transcriptase–polymerase chain reaction using nasopharyngeal and oropharyngeal swabs at the time of admission. The CLLs were diagnosed based on dermatologic, clinical, and dermoscopic examinations. Informed consent was obtained from the parents of all the patients, and the study was performed according to the ethics committee's guidelines.
The enrolled patients were divided into 2 groups according to the disease activity: group A (Fig 1) comprised patients with active disease, characterized by the presence of acral erythematous purpuric papules and macules, with possible bullous and crusty evolution or digital swelling often associated with itching and burning; group B (Fig 2) comprised patients in remission, characterized by the presence of acral blurred rosaceous erythematous maculae, sometimes scaly with postinflammatory hyperpigmentation. The following dermoscopic criteria were evaluated according to the International Society of Dermatology: (I) vessels, (II) scales, (III) follicular findings, (IV) other structures, and (V) specific clues.4
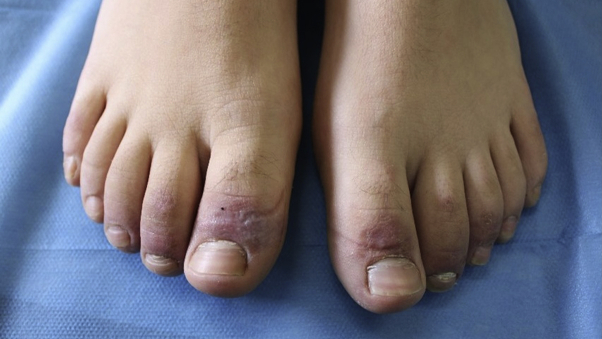
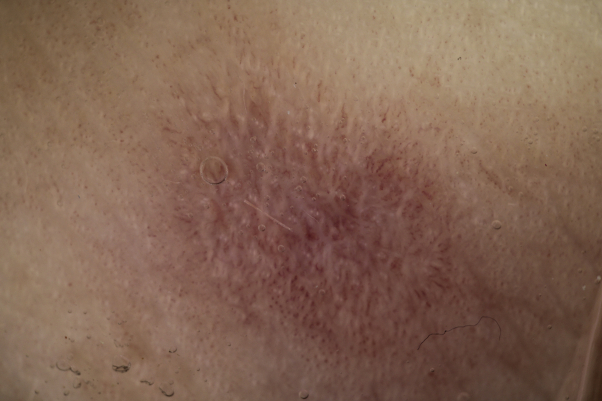
Fig 1.
CLL in the active phase of disease consisted of erythematous purpuric papules and macules, with bullous evolution with crust and digital swelling. CLL, Chilblain-like lesion.
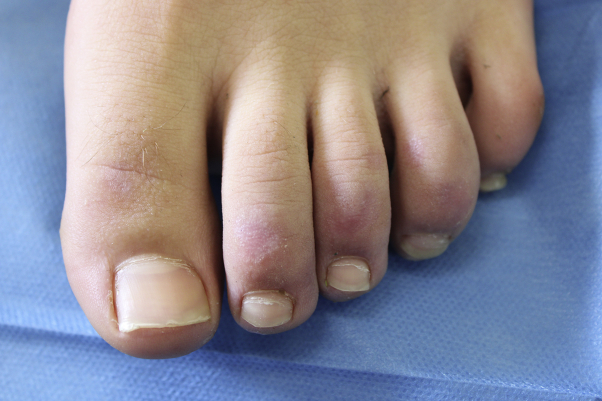
Fig 2.
CLL in the later stages appeared as blurred rosaceous erythematous maculae on the toes. CLL, Chilblain-like lesion.
Case series
We observed 15 patients. Overall, 6 (40%) were female and 9 (60%) were male. The mean age of the patients was 13 years ± 2.08 standard deviation (range: 8-17 years). Overall, there were 3 pairs of siblings. Admission to the hospital occurred, on average, on day 57 ± 43 standard deviation since the beginning of lesion manifestation. Nine (60%) patients presented with active disease (group A), and 6 (40 %) were in remission (group B). The cutaneous manifestations observed in the active phase consisted of erythematous purpuric papules and macules, with possible bullous and crusty evolution or digital swelling (Fig 1) often associated with itching and burning, and those observed during remission appeared as blurred rosaceous erythematous maculae, sometimes scaly with postinflammatory hyperpigmentation (Fig 2).
The nasopharyngeal and oropharyngeal swab specimens for examining SARS-CoV-2 infection were negative in all the patients (100%), although 3 of them had established COVID-19 contact within the family (20%). Six patients (40%) reported having had a history of COVID-19–like symptoms in the previous 4 months. Laboratory tests showed no alterations in both the groups and excluded the presence of any other autoimmune or infectious diseases. Only 1 patient in group A tested positive for anti–Epstein-Barr virus IgM.
In group A, the lesions appeared between the beginning of March and the beginning of April. Toes were affected in all cases, with sporadic involvement of the heels (22.2%, n = 2). No history of cold exposure, personal or familial autoimmunity, or recent medication intake was recorded. The dermoscopic evaluation revealed red dots (100%, n = 9), some of which appeared as dotted and comma-shaped congested vessels (77.7%, n = 7), white rosettes (88.8%, n = 8), and white streaks (66.6%, n = 6) over a pinkish-reddish background (Fig 3). The red dots or globules were mainly polymorphic; some of them were enlarged and asymmetrically distributed along the lesion, often surrounding the rosettes (66.0%, n = 6). Furthermore, the common dermoscopic features were white and yellow scales (66.6%, n = 6) often associated with yellow crusts. Focally distributed orange-yellowish structureless areas due to dermal hemosiderin deposits were also observed (66.6%, n = 6). The other dermoscopic features are shown in Table I.
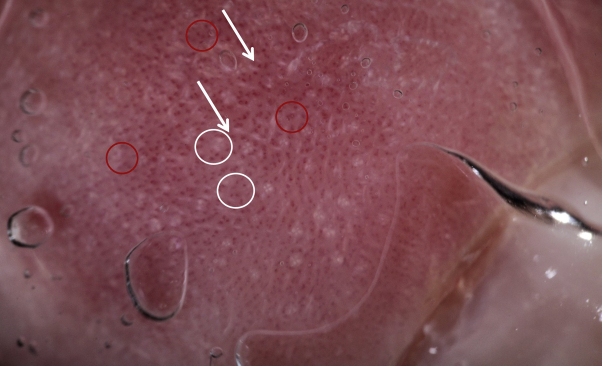
Fig 3.
The dermoscopic evaluation of the CLL in the active phase revealed (A) dotted and comma-shaped congested vessels (red circles), white rosettes (white circles), and white streaks (white arrows) and (B) white scales (blue arrows) and yellow serocrust (orange arrows). CLL, Chilblain-like lesion.
Table I.
Dermoscopic characteristics of CLLs in the active and late phase of the disease
| Dermoscopic features | Total, n (%) | Acute, n (%) | Remission, n (%) | P∗ |
|---|---|---|---|---|
| Vessels | ||||
| Linear | 0/15 (0) | 0/9 (0) | 0/6 (0) | |
| Dotted | 15/15 (100) | 9/9 (100) | 6/6 (100) | |
| Dotted enlarged | 8/15 (53.3) | 7/9 (77.7) | 1/6 (16.6) | .040 |
| Scale | ||||
| White | 8/15 (53.3) | 6/9 (66.6) | 2/6 (33.3) | |
| Follicular findings | ||||
| Follicular plugs: Rosettes | 12/15 (80) | 8/9 (88.8) | 2/6 (33.3) | |
| Follicular red dots | 7/15 (46.6) | 6/9 (66.6) | 1/6 (16.6) | |
| Perifollicular white color | 0/15 (0) | 0/9 (0) | 0/6 (0) | |
| Perifollicular pigmentation | 0/15 (0) | 0/9 (0) | 0/6 (0) | |
| Other findings | ||||
| Yellowish structureless areas | 6/15 (40) | 6/9 (66.6) | 0/6 (0) | .027 |
| White streaks | 8/15 (53.3) | 6/9 (66.6) | 2/6 (33.3) | |
| Melanin hyperpigmentation | 7/15 (46.6) | 4/9 (44.4) | 3/6 (50) | |
| Specific clue | ||||
| Wickham striae | 1/15 (6.66) | 0/9 (0) | 1/6 (16.6) |
CLLs, Chilblain-like lesions.
P < .05.
In group B, the lesions appeared between the middle of February and the end of March. Toes were affected in all cases, with sporadic involvement of the heels (66.6%, n = 4), and in 1 case (16.6%, n = 1), both hands and feet were affected. As in the patients of group A, no history of cold exposure, personal or familial autoimmunity, or recent medication intake was reported in the patients of group B. The dermoscopic evaluation revealed ubiquitous red dots (100%, n = 6), whereas only a minor proportion of patients displayed dotted and comma-shaped congested vessels (16.6%, n = 1). Rosettes were detected even though they appeared more blurred than those in group A (33.3%, n = 2), and fewer white streaks (33.3%, n = 2) over a pinkish blurred background were present (Fig 4). Moreover, a lesion on the right ankle of a patient had a dermoscopic pattern with a white crossed or annular line surrounded by red globular blood vessels at its periphery distributed in lines or rings, respectively, on an opaque erythematous background resembling “Wickham striae” (Fig 5).
Fig 4.
The dermoscopic evaluation of the CLL in the remission phase revealed red dots (red arrows) and blurred white rosettes (white circles). CLL, Chilblain-like lesion.
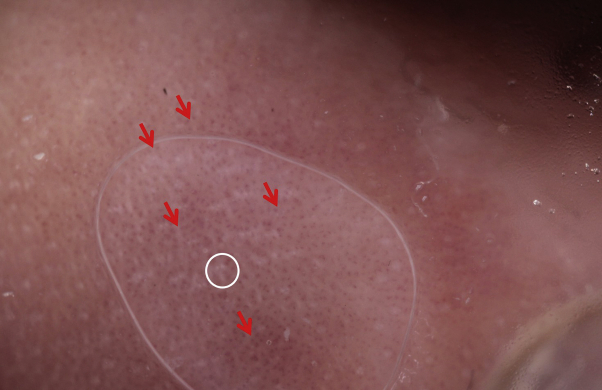
Fig 5.
The lesion on a patient's right ankle revealed in a dermoscopic pattern, with a white crossed or annular line surrounded by red globular blood vessels at its periphery distributed in lines or rings, respectively, on an opaque erythematous background resembling “Wickham striae.”
The comparative dermoscopic analysis revealed a more significant presence of the enlarged dotted vessels (77.7% vs 16.66%, P = .040) in the patients of group A compared with that in the patients of group B. Moreover, the simultaneous presence of rosettes, white streaks, and red dots was significantly more evident in group A than that in group B (66.6% vs 16.6%, P = .0440).
Discussion
Chilblains are cutaneous inflammatory lesions that commonly occur during cold and humid periods and usually resolve within a few days. However, when chilblains remain unresolved for long, they may be associated with connective tissue diseases, mostly lupus erythematosus, or a preceding viral infection.5 To date, very few studies have described the dermoscopic characteristics of CLLs.6 The main observed dermoscopic pattern of the CLLs was represented by rosettes often surrounded by enlarged dotted vessels in a patchy distribution and white scales on a pinkish-reddish background. Moreover, the simultaneous presence of the dotted and comma-shaped congested vessels, white rosettes, and white streaks is higher in the active phase of the disease than in the late phase and can, therefore, be considered a dermoscopic hallmark of disease activity.
Rosettes can be found in many skin cancers as well as inflammatory diseases and are therefore an unspecific finding.7 Rosettes probably represent the involvement of hair follicles7; white streaks reveal altered collagen in the dermis.8 Moreover, elongated, dotted blood vessels can be observed in inflamed, damaged, or traumatized skin or in overlying skin stasis, and they represent the dilatation of blood vessels with the extravasation of the red blood cells surrounding the enlarged infundibula.8
We hypothesize that such dermoscopic features are the results of compressive phenomena produced by considerable inflammation and edema in CLLs. The dermoscopic pattern of CLLs is intermediate between that of spongiotic eczema and chronic inflammatory skin diseases such as discoid lupus.8 Therefore, dermatoscopy together with a clinical examination can help in the close monitoring and follow-up of these lesions. The occurrence of such marked inflammatory lesions during the COVID-19 pandemic, in the absence of other triggering factors, suggests that the 2 events may be related. The exact pathology of chilblains secondary to SARS-CoV-2 infection is still unknown; however, the activation of interferon type 1 (IFN-1) may have a significant influence. SARS-CoV-2 infection triggers the expression of IFN-1 genes as a part of the patient's antiviral defense mechanisms. Thus, adolescent patients may develop a robust and rapid IFN-1 response toward the virus, which in turn blocks viral replication and leads to a slightly less severe disease course. However, this strong viral response can also lead to small changes in the vessels after initial infection, producing CLL that should be distinguished from ischemia and thrombosis often observed in older, infected adult patients. The pronounced IFN-1 response in adolescent patients may be advantageous, and the appearance of chilblains induced by COVID-19 infection may be related to an indolent course and favorable prognosis.9,10 However, the negative swab tests in these patients, which may be partially explained by the disappearance of the virus without symptoms after a short course, does not support this association currently. Future studies on larger samples are essential to understand the characteristics of these unusual lesions completely.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
References
- 1.Docampo-Simón A., Sánchez-Pujol M.J., Juan-Carpena G. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman P.A., Crane J.S. StatPearls Publishing; Treasure Island (FL): 2020. Pernio (Chilblains). StatPearls. [Google Scholar]
- 3.Crowson A.N., Magro C.M. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28(4):478–484. doi: 10.1016/s0046-8177(97)90038-1. [DOI] [PubMed] [Google Scholar]
- 4.Errichetti E., Zalaudek I., Kittler H. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182(2):454–467. doi: 10.1111/bjd.18125. [DOI] [PubMed] [Google Scholar]
- 5.Wang M.L., Chan M.P. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40(4):265–271. doi: 10.1097/DAD.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 6.Andina D., Noguera-Morel L., Bascuas-Arribas M. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haspeslagh M., Noë M., De Wispelaere I. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30(2):311–313. doi: 10.1111/jdv.13080. [DOI] [PubMed] [Google Scholar]
- 8.Sgouros D., Apalla Z., Ioannides D. Dermoscopy of common inflammatory disorders. Dermatol Clin. 2018;36(4):359–368. doi: 10.1016/j.det.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Kolivras A., Dehavay F., Delplace D. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V., Lind R. Chilblains in COVID-19 infection. Cureus. 2020;12(7):e9245. doi: 10.7759/cureus.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]