Abstract
Background
Yashada bhasma has been found to be very useful for the treatment of ailments like diabetes, eye disorder, urinary disorder etc. Since bhasma is a metallic preparation, so to prove its non-toxicity; modern standardization of the prepared samples is a must apart from other organoleptic tests as mentioned in the ancient text.
Objectives
The present study is aimed to synthesize bio-compatible Yashada bhasma from bio-incompatible zinc metal. Further, comparative study of their chemical and physical properties through some quality control tests, physico-chemical tests and modern tests like XRD, DLS, Zeta potential, SEM and EDAX are carried out.
Materials and methods
Yashada bhasma is prepared by a three step process i.e. Shodhan, Jarana and Marana. The inclusion of plant extracts and herbs during calcination process enhances its medicinal qualities, and reduces it to a nano size.
Results
The XRD analysis of Yashada bhasma shows hexagonal ZnO crystalline phase whereas the raw metal confirms the presence of crystallite Zinc metal. DLS shows reduction in particle size of Yashada Bhasma (339.8 nm) as compared to raw metal (2063 nm) and this reduction is further supported by SEM which shows the particle size of Yashada bhasma (324 nm) and raw metal (1-2μ). The zeta potential value confirms the stability of Yashada bhasma. EDAX revealed difference in concentration of Zinc and Oxygen in both the samples.
Conclusion
An effort has been made to characterize the preparation of Yashada bhasma using sophisticated analytical tools as a step towards standardization of the bhasma. The results help in scientifically establishing the findings in line with the principle of Ayurveda.
Keywords: Yashada bhasma, Standardization, Zeta potential, XRD, DLS, SEM
1. Introduction
Ayurveda system of medicine is almost 5000 years old and finds their mention in the Vedas. It is well known for its healing powers since a long time and it has been proved that it helps individuals gain a long and healthy life without using synthetic drugs or undergoing painful surgeries. Although it works slowly but it's is a way of finding out the root cause by deep understanding of soul and body.
Earlier, Ayurveda, the Indian system of medicine did not get promoted to the extent it should have been. It hardly gained any recognition among the countries around the globe. Also, there have been reports of toxicity related to these bhasmas in recent literature. Therefore, there is a need for an extensive research work in the field of Indian Ayurveda Shastra. It should be conducted not only by Ayurvedic researchers, but also by the experts from multidisciplinary fields for establishing the scientific basis in the practice of Ayurveda. This exploration should be exposed to the mankind with appropriate evidences relating that the Ayurveda unquestionably cures major ailments. This will help in enhancing the acceptability of our ancient system of medication all around the globe.
It should be noted here that the Chinese health practitioners have made it a point to make their method known to the entire humanity by getting their research work published worldwide [1]. This may be the reason why chinese traditional system of medicine has gained immense popularity among 3 millions.
In the recent years, there have been many findings that have shown Ayurveda to be scientifically perfect. The safety of any type of drug including ayurvedic bhasma depends upon its formulation, processing and its uses. Bhasma is a metallic preparation, so to prove its non-toxicity; many classical, physical and modern analyses are carried out. Ayurvedic bhasma is non-toxic because during its preparation process it is implied that its toxicity is abolished by reaction of different liquid media and herbal extract in Shodhan and Jarana steps, and after these two processes it is subjected to proper incineration through Marana process [2].
Yashada is a zinc-based herbo metallic preparation which is prepared from zinc metal and some herbal ingredients through purification and calcination processes that changes zinc metal into its oxide form as mentioned in the literature. To prove its authenticity, it is required to use some modern characterization techniques such as (XRD, DLS, SEM and EDAX) etc. These characterizations are authentic proofs for the bhasmas that this traditional system of medicine which uses metals as its precursor not only eliminates toxicity of metals but also induces medicinal properties making it consumable as a medicinal product for human beings and suitable for the treatment of many diseases.
2. Materials and methods
Materials and methods used in the preparation of the Yashada bhasma are based on availability, feasibility in the classical indication of Rasa Shastra, traditional value and expert opinions.
2.1. Materials used
The following section describes the major drug and associated drugs used in the preparation of Yashada bhasma.
2.1.1. Major drug
Zinc Metal was collected from the Post Graduate Department of Rasa shastra, National Institute of Ayurveda, Jaipur, Rajasthan, India.
2.1.2. Associated drugs
For samanya shodhan of Zinc: Kanji, Takra, Kulattha Kwatha, Gomutra and TilaTaila were used. Gomutra was freshly collected from the local cowshed. Takra was procured from Saras dairy, Jaipur. TilaTaila was procured from the Pharmacy and other drugs like Kanji (Sour gruel), and Kulattha Kwatha (decoction of Dolichos biflorus) were prepared freshly.
For Vishesha Shodhan: Nirgundi Patraswarasa and Haridra were used.
For Jarana: Apamarga panchangchurna was used.
For Marana: Kumariswa rasa was used.
All materials used in the preparation of Yashada bhasma are shown in Table 1.
Table 1.
Materials used in preparation of Yashada bhasma.
| S.No | Name of ingredients | Botanical name/English name | Quantity |
|---|---|---|---|
| 1 | Zinc metal | Zinc | 140 g |
| 2 | TilaTaila | Oil of Sesamum indicumlinn | 450 ml*7 = 3150 mL |
| 3 | Takra | Butter milk | 450 ml*7 = 3150 mL |
| 4 | Gomutra | Cow urine | 450 ml*7 = 3150 mL |
| 5 | Kanji | Sour gruel | 450 ml*7 = 3150 mL |
| 6 | Kulattha kwatha | Decoction of Dolichos biflorus | 450 ml*7 = 3150 ml |
| 7 | Nirgundi Patraswarasa | Vitex negundo | 2.5 kg |
| 8 | Haridra churna | Curcuma longa | 8 g |
| 9 | Apamarga panchangchurna | Achyranthes aspera | 20 g |
| 10 | Kumariswarasa | Aloe vera (Aloe barbadensis) | 30 ml*13 putas = 390 mL |
2.2. Methodology adopted
The following steps were followed for the preparation of Yashada bhasma as per classical literature given in Rasa Shastra text [2], [3], [4], [5], [6].
2.2.1. Shodhan
It is done by Dhalana (liquefying and pouring) method. This is further divided into two steps: Samanya shodhan (general purification) and Vishesha shodhan (specific purification).
Samanya shodhan of Yashada: Raw zinc (140 g) was taken in a long handled iron ladle (Loha Darvi) and heated up to melting. After complete melting, it was immediately quenched in TilaTaila, Takra, Gomutra, Kanji, and Kulattha Kwatha respectively. Every time fresh and same amount of liquid media was taken and the whole process was repeated for 7 times [3]. Vishesha shodhan of Samanya shodhita bhasma was done by using leaves of 2.5 kg of Nirgundi (Vitex negundo). Leaves of Nirgundi were grounded with water, filtered and then Haridra (Curcuma longa) was added into filtered mixture. Samanya shodhita yashada was taken, heated up to melting, and immediately quenched in the Nirgundi herbal extract and the whole process was repeated for three times [4].
During Samanya shodhan, raw yashada which was granulated became a lump after melting. Yashada became more silvery white in color after first dhalana. The blackish color was noted in the sixth and seventh dhalana and some part of Yashada changed into greyish powder. Yashada which was in the form of a lump broke up into small globules after dhalana in Taila. Yashada caught fire during sixth and seventh dhalana in Takra. Increased shining was noted during dhalana in Gomutra and the sample also became more brittle. During dhalana in kulattha kwatha, the color of yashada became dull due to a coating of kwatha over it. A characteristic smell and a rush of black fumes were observed after quenching in TilaTaila and Gomutra. As the Shodhan progressed, more and more yashada converted into blackish Powder.
During Vishesha shodhan, the characteristic smell of Nirgundi patraswarasa was observed when melted yashada was quenched in it. Haridra churna was seen precipitated after quenching and color of liquid media was also changed from bright green to dark green. Duration of melting of yashada increased in successive melting. Silvery shine samanya shodhita yashada became dull after dhalana and particles of Haridra were seen sticking to the surface.
2.2.2. Jarana
Shuddha yashada (80 g) was taken in an iron pan and was allowed to melt. A weighed quantity (20 g) of Apamarga churna was added to the molten yashada and stirred continuously with iron ladle by applying a good amount of pressure [6]. When all the metal was converted in to powder form and none of the metal remained in metallic form, the powder was collected in the center of the pan and covered with an earthen saucer and heat was increased up to maximum. Intermittently saucer was slightly lifted to check the color of the powder. When the color changed to red hot and no melted particles of free metal was observed; the heating was stopped and left for self-cooling.
During Jarana process, when the shuddha yashada was melting, the burning smell of haridra and Nirgundi was observed along with smoke. When Apamarga was added into melted yashada, it burnt and emitted smoke and in due course, the smoke ceased. No free metal was seen after the addition of the prescribed quantity of Apamarga.
2.2.3. Marana
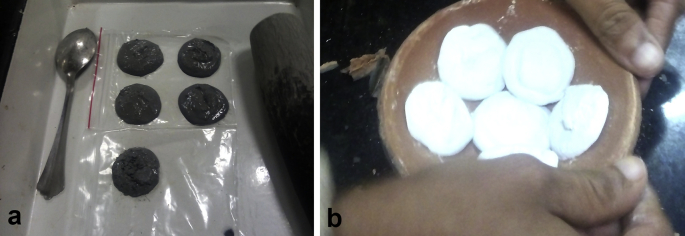
Jarita yashada (70 g) and measured amount of kumariswarasa were put together and triturated for 3 h and transferred to a plastic sheet [6] by making pellets. Prepared pellets were kept on the plastic sheet for drying (Fig. 1a). All the dried pellets were weighed properly. Pellets were arranged in one Sharava and another Sharava was kept over it. Sandhi bandhana was done with the help of mud smeared cloth and dried. The Sharavasamputa was subjected to heat in a muffle furnace. The temperature was allowed to rise up to 500 °C and was maintained for 60 min. Thereafter the furnace was switched off and allowed for self-cooling. The same procedure was repeated using fresh aloe vera juice every time until bhasma which passed the classical test was obtained. Maximum temperature was increased in successive putas.
Fig. 1.
(a) (Chakrikas) Pellets (b) Pellets after calcination.
During Marana process, after the first two puta, colors of chakrikas were greyish white and consistency was hard. The color was noticed on the outer surface of chakrikas which appeared as a coating. When the chakrikas were broken, inner surface was dull white. In the successive putas the color changed from greyish white to white and the consistency changed from hard to soft. But after 6th puta, bhasma became very soft in touch and the color of the bhasma became dull white. After seven putas, bhasma was very fine however the color was dull white (Fig. 1b). Further six more putas were given and creamish colored bhasma was obtained. In each puta, the maximum temperature was maintained for an hour; which was increased from 500 to 600 °C in successive putas.
2.3. Classical characterization of Bhasma
The classical methods of characterization called the Bhasma pariksha help in the qualitative evaluation of bhasma particles [7].
2.3.1. Varna (color)
Every bhasma has a specific color. If the color of the incinerated material complies with the classical textual reference, then it can be considered as a proper bhasma. But again the point to be noted is that the color depends on the materials used in the method of incineration.
2.3.2. Taste (Niswadu)
A pinch of bhasma has to be placed on the tongue to check its taste since metals in their natural form have a specific taste.
2.3.3. Nishchandrata (lusterless)
Presence of luster indicates that the process of bhasma preparation was not completed. So to check this, pinch of bhasma was taken and observed under bright sunlight.
2.3.4. Varitara (lightness)
This test indicates the lightness of the incinerated metal. After proper incineration, the bhasma particles become too light and they cannot break the surface tension of the stagnant water. So, they float. Clean water was taken in a glass and allowed to standstill. A pinch of yashada bhasma was sprinkled on the surface of water.
2.3.5. Unama
This is in continuation of the Varitara test where rice grains were placed gently over floating yashada bhasma.
2.3.6. Rekhapurnata (fineness)
This test indicated the fineness of the particles. A pinch of bhasma was rubbed in between thumb and index finger. If bhasma particle entered into the creases of these fingers; it indicates that the metal is incinerated properly. Finer the particle, fastest the absorption and quickest the action; is the fundamental principle of pharmacology.
2.3.7. Nirdhuma
A pinch of yashada bhasma was sprinkled on the ignited charcoal and observed for the fumes.
2.4. Physical characterization
2.4.1. Determination of total ash
About 1.1514 g of yashada bhasma (W1) was accurately weighed and grounded in a tarred silica crucible (W2). The crucible was kept in a muffle-furnace at a temperature not exceeding 450 °C- 600 °C for 4 hrs in order to get carbon-free ash. It is then cooled in a desiccator and weighed (W3). The residue was then collected on an ashless filter paper [8]. Total ash is determined by the following formula:
| (1) |
2.4.2. Water soluble ash
The total ash was boiled for 5 min with 25 mL of water. Insoluble matter was collected in an ashless filter paper. It was washed with hot water and ignited for 15 min at a temperature not exceeding 450°–600 °C. Subtract the weight (W3) of the insoluble matter from the weight of the ash; the difference in weight represents the water-soluble ash.
| (2) |
2.4.3. Acid insoluble ash
The ash was transferred in a 250 mL beaker without loss of ash and 100 mL of dil. hydrochloric acid was added. The crucible was washed with 10 mL of acid and the washings were transferred to a beaker. The beaker was heated until the liquid boils. The solution was filtered and the insoluble matter was collected on ashless filter paper. It was then dried on a hot plate and ignited at 600 °C in a muffle furnace. (Until became white ash). The residue was allowed to cool in suitable desiccators for 30 min and weighed without delay.
| (3) |
W1 = Weight of sample taken (yashada bhasma), W2 = Weight of empty crucible, W3 = (Weight of crucible + Acid insoluble Ash).
2.4.4. Determination of loss on drying (LOD)
10 g of Yashada bhasma (W1) was accurately weighed up to third decimal place (without preliminary drying) in a tarred evaporating dish + bhasma weight (W2). It was dried at 105 °C for 5 h and weighed (W3). The drying was continued and weighing was done at one hour interval until the difference between two successive weighing corresponds to not more than 0.25 percent. (Constant weight was reached when two consecutive weighing after drying for 30 min). It was then cooled for 30 min in desiccator, showed not more than 0.001 g difference.
| (4) |
2.4.5. Determination of pH
5.0 g of Yashada bhasma was taken in 100 mL of distilled water. Macerate for 15 min. Then filter through filter paper. Take the clear liquid and dip the electrode in it. Note the pH value.
2.5. Modern characterization techniques
The following section discusses physicochemical characterization of yashada bhasma using modern state-of-the-art techniques [9], [10].
2.5.1. XRD analysis
The crystallite size, structural identification, phase and purity of raw metal and yashada bhasma sample were determined by X-ray diffractometer (Bruker D8 focus) using Cu Kα radiation of wavelength λ = 0.1541 nm. The two theta value ranged from 30° to 90°.
2.5.2. DLS and zeta potential analysis
Particle size, surface charge, poly disperse index (PDI) and stability of raw metal and yashada bhasma sample were calculated by particle size and zeta potential analyzer, Nano-ZS series 633 nm laser (Malvern Instruments Limited, UK) at 25 °C. DLS is quick indicator of nano particle size distribution.
2.5.3. SEM - EDAX analysis
The nano particle morphology, particle size and elemental analysis of raw metal and Yashada bhasma sample were carried by using SEM-EDAX instrument, NOVA NANO SEM 450 (XT Microscope server, FEI Netherlands). SEM is useful for determining the three dimensional appearance and surface morphology of samples. Small portion of sample was sprinkled on to a double sided carbon tape and mounted on aluminum stubs, to get electron image for SEM and EDAX analysis.
3. Results
3.1. Classical characterization
Yashada bhasma complied with all the classical analysis methods. After complete preparation, creamish color of yashada bhasma was obtained. Its taste was found to be tasteless i.e. absence of metallic taste. There were no shining particles in the bhasma. Yashada bhasma was observed to float on the surface of water. Rice grains float over the layer of bhasma, which indicates that the process of incineration was proper. It was observed that bhasma enters the furrows of finger and no fumes were found emerging out of it.
3.2. Physical characterization
Physical parameters of yashada bhasma showed that the values of Total ash for bhasma was 99.76%, Acid insoluble ash was 46.10%, Water soluble ash was 1.66 and loss on drying was 0.01%.
3.3. Modern characterization
3.3.1. XRD analysis
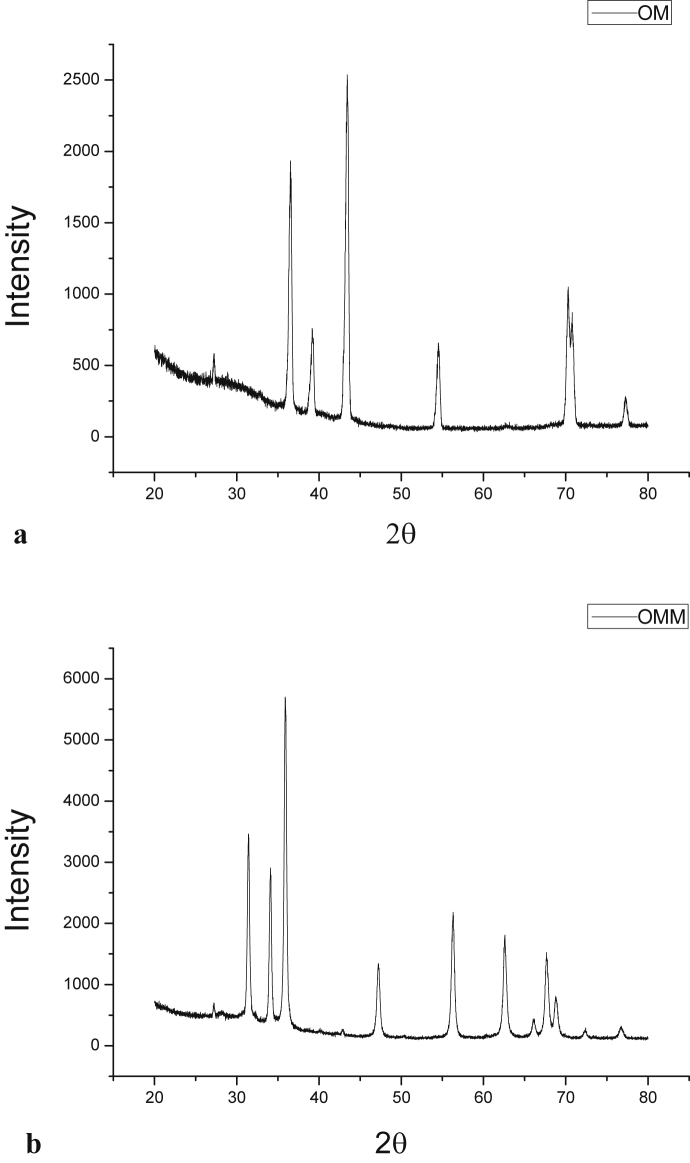
XRD data of raw metal and yashada bhasma are as shown in Fig. 2a,b (OM: raw metal, OMM: yashada bhasma) and Table 2. The XRD result of raw metal shows the presence of crystallite zinc metal, with JCPDS card 04-0831, located at 2θ = 36.54°, 39.21°, 43.44°, 54.53°, 70.28°, 70.77°, 77.28°, 82.09°, 83.7°, 86.5° and 89.9°, while the XRD analysis of yashada bhasma pattern shows hexagonal ZnO (zincite) crystalline phase, with JCPDS card 79-0208 and P63mc space group, located at 2θ = 31.42°, 34.09°, 35.91°, 47.22°, 56.28°, 62.58°, 66.09°, 67.67°, 68.80°, 72.36° and 76.71°.
Fig. 2.
a. XRD spectra of raw metal (OM). b. XRD spectra of Yashada bhasma (OMM).
Table 2.
Values of “d” and Intensity at different 2-Theta values for Raw Metal and Yashada bhasma.
| Angle |
d value |
Intensity |
Intensity % |
|---|---|---|---|
| 2-Theta° | Angstrom | Count | % |
| Raw Metal | |||
| 27.229 | 3.27243 | 170 | 7.3 |
| 36.542 | 2.45700 | 1621 | 69.6 |
| 39.211 | 2.29570 | 563 | 24.1 |
| 43.441 | 2.08143 | 2330 | 100.0 |
| 54.536 | 1.68130 | 562 | 24.1 |
| 70.286 | 1.33821 | 913 | 39.2 |
| 70.770 | 1.33023 | 698 | 29.9 |
| 77.282 | 1.23358 | 188 | 8.1 |
| Yashada Bhasma | |||
| 27.225 | 3.27300 | 179 | 3.5 |
| 31.425 | 2.84440 | 2916 | 56.2 |
| 34.095 | 2.62754 | 2412 | 46.5 |
| 35.914 | 2.49852 | 5188 | 100.0 |
| 47.221 | 1.92325 | 1145 | 22.1 |
| 56.287 | 1.63311 | 1925 | 37.1 |
| 62.585 | 1.48303 | 1552 | 29.9 |
| 66.092 | 1.41259 | 237 | 4.6 |
| 67.673 | 1.38338 | 1260 | 24.3 |
| 68.801 | 1.36342 | 596 | 11.5 |
| 72.368 | 1.30475 | 106 | 2.0 |
| 76.710 | 1.24135 | 171 | 3.3 |
No characteristic peaks from other phases of ZnO and impurities are observed, indicating high purity of the obtained ZnO in yashada bhasma sample [11].
The average crystallite size of Yashada bhasma was determined from the prominent peak of XRD pattern corresponding to 2θ = 35.91, hkl (1 0 1) reflection using the Debye–Scherrer equation (D = 0.89λ/βcosθ) [12], where D is the crystallite diameter, λ is the x-ray wavelength (0.15418 nm), β is the full width at half maximum intensity (FWHM) of the diffraction peak, and θ is the diffraction angle of the peak pattern of the of bhasma sample. The average crystallite size obtained was found as 32.79 nm.
XRD result of raw metal confirms the presence of crystallite Zinc metal and this Zinc metal showed prominent peak at d = 2.08A◦ (2θ = 43.44°), while in yashada bhasma sample this characteristic peak was at d = 2.49A◦ (2θ = 35.91°), not on d = 2.08 A◦. So, the absence of this characteristic peak on d = 2.08A◦ in yashada bhasma confirms that no crystallite zinc metal is present in yashada bhasma sample [13].
3.3.2. Particle size and zeta potential analysis
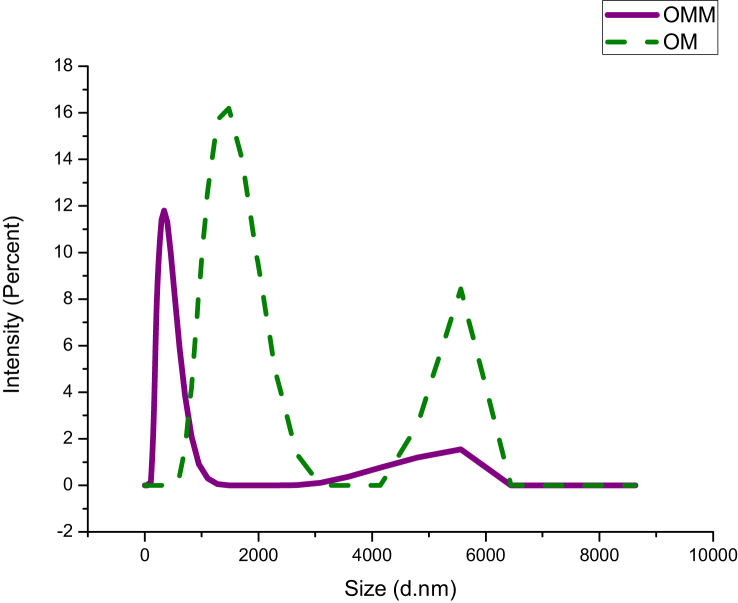
Particle size analysis of raw metal (OM: raw metal) and Yashada bhasma (OMM: Yashada bhasma) by dynamic light scattering method is as shown in Table 3 and Fig. 3. Raw metal shows mean particle diameter of 2063 nm and Yashada Bhasma had a mean particle diameter of 339.8 nm. This reveals the reduction in particle size of Yashada bhasma after processing.
Table 3.
Particle size of raw metal and Yashada bhasma.
| Size (d.nm)± sd | % Intensity | |||
|---|---|---|---|---|
| Raw Metal | ||||
| Z-Average (d.nm): | 2063 | Peak 1: | 1450 ± 427.0 | 88.9 |
| PdI: | 0.543 | Peak 2: | 5381 ± 322.2 | 11.1 |
| Intercept: | 0.658 | Peak 3: | 0.000 ± 0.000 | 0.0 |
| Yashada bhasma | ||||
| Z-Average (d.nm): | 339.8 | Peak 1: | 381.0 ± 174.2 | 95.7 |
| PdI: | 0.252 | Peak 2: | 4799 ± 735.8 | 4.0 |
| Intercept: | 0.869 | Peak 3: | 76.64 ± 11.23 | 0.3 |
Fig. 3.
Particle size of Raw Metal (OM) and Yashada bhasma (OMM).
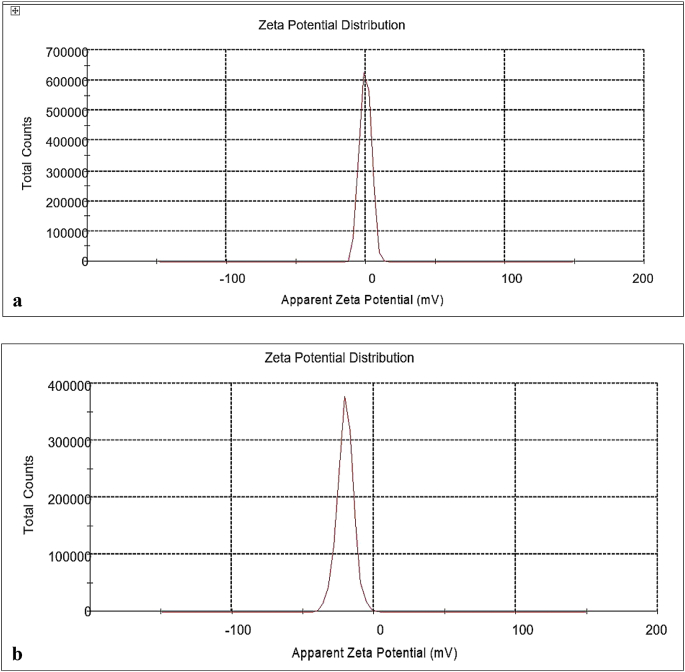
The Zeta potential of Yashada bhasma at neutral pH was found to be in standard range (−30 to +30 mV) as compared to raw metal as shown in Fig. 4a (OM: raw metal) and Fig. 4b (OMM: Yashada bhasma). Zeta potential of yashada sample has been found to be −20.1 mV, which indicates its stable condition, while raw metal had Zeta potential value of - 0.24 mV.
Fig. 4.
a. Zeta potential of raw metal (OM). b. Zeta potential of Yashada bhasma (OMM).
3.3.3. SEM analysis
Morphology and size of raw metal as well as Yashada bhasma was determined by SEM analysis and shown in Fig. 5. SEM result shows particle size range from 1 to 2 μin raw metal and that in Yashada bhasma sample, range from 137 to 793 nm. The average particle size of Yashada bhasma sample is 324 nm, which is much smaller as compared to raw metal and reveals the reduction in particle size after proper incineration [14].
Fig. 5.
SEM micrographs of raw metal (OM) and Yashada bhasma (OMM).
3.3.4. EDAX analysis
Elemental composition of raw metal and yashada sample using EDAX are shown in Table 4. EDAX study confirmed the presence of zinc and oxygen in raw metal and Yashada bhasma. In raw metal, percentage of oxygen (15%) was lesser as compared to Yashada bhasma (31%). Some other elements like phosphorus, potassium, iron and calcium were also reported to be present in yashada bhasma in low concentrations.
Table 4.
Elemental analysis by EDAX.
| Elements | Concentration (%w/w) |
|
|---|---|---|
| Raw metal | Yashada bhasma | |
| Zn | 75.14 | 65.18 |
| O | 15.02 | 30.45 |
| C | 9.38 | – |
| Si | 0.46 | – |
| P | – | 2.72 |
| K | – | 0.70 |
| Ca | – | 0.49 |
| Fe | – | 0.46 |
4. Discussion
Zinc plays a very important role in human body. Zinc has only single oxidation state i.e. Zn2+ and it is found in more than 300 enzymes, present in all six classes of enzymes characterized by the International Union of Biochemistry [15]. Zinc prevents the degradation of insulin and makes receptors suitable for the binding of insulin [16]. It plays an important role to prevent diabetes mellitus. In spite of this, it is well-known fact that direct consumption of metals is not suitable for human body as they have some toxic effect themselves. So to make them appropriate for human use, these metals are converted into an ayurvedic preparation known as bhasma. After the preparation of bhasma, characterizations are done to confirm the formation of these bhasma particles. Characterization of bhasma particles has to be done by both classical and modern methods of analysis.
In this work, Yashada bhasma has been prepared by three step process i.e. Shodhan, Jarana and Marana. During Shodhan process, for repeated heating and quenching of the zinc metal, different acidic and basic liquid media were used. The alternative melting and quenching of zinc metal in these liquid media not only deforms their external surface but also change the chemical and physical nature of metal. Thus during the shodhan process, metal may get free from impurities and change into blackish and coarse powdered form, due to partial oxidation of zinc during the melting process [17].
During Jarana process, the Shodhita sample was heated with Apamargachurna (Achyranthes aspera) in an iron pan for 5–6 h at 600 °C. The presence of potassium in the bhasma as indicated by the elemental analysis is mainly due to the presence of potassium in Achyranthes aspera [18].
During Marana process, Jarita sample was triturated with Aloe-vera juice which contains some beneficial compounds like polymannans, anthraquinoneetc [19], [20], [21]. These compounds make aloe vera juice suitable for hypoglycemic effect [15]. Both the metal Zinc and aloe vera have been found to have extensive capacity to overcome the high blood sugar effect. Therefore, it may be deduced that on the trituration of both these components together, there may be a possibility of formation of coordination bonds between the metal and organic constituents of aloe vera. This organometallic complex is subjected to calcination in muffle furnace for their physical and chemical transformation. Size of particles gets reduced during Marana process after each cycle of trituration and calcination. This reduction in size of the particles and their increased surface area makes them suitable and consumable for their easy absorption in human body.
When the process of preparing the bhasma is completed, it is the time to verify the various constituents present in it. It is essential to study the effects of the toxicity of various constituents used during the preparation of bhasma and at the same time, study the science behind their transformation in to non-toxic form, and their ability to impart therapeutic effects. It has been found that repeated incineration of metals along with plants and herbal extracts not only change its quality resulting in its non-toxicity, but makes them to have medicinal value. To prove this and to ensure its effectiveness and non-toxicity, some physico-chemical and modern tests have been conducted on yashada bhasma. Firstly, all the organoleptic tests as mentioned in the ancient scriptures were carried out. Tests like Varitara, Rekhapurnata were performed to check the lightness and fineness of bhasma as fineness and lightness will enhance the absorption and healing property of bhasma. Likewise, Nishchandrata and Nirdhuma show complete conversion of the metal into its compound forms. Physicochemical analysis i.e. total ash of the samples was determined to check proper incineration of the metal. This is an important parameter as improperly incinerated zinc has been reported to introduce deleterious effects. Accordingly, the samples under investigation showed proper incineration of zinc as evident from the total ash value (99.76%), negligible moisture content (0.01% loss on drying), acid insoluble ash (46.10%) and lower solubility in water (1.66%). The results are comparable to the reported values in the recent literature.
Modern characterization proves that this journey of the metal up to bhasma preparation has gone through extreme transformation. During the process, metal zinc reacted with different media. Some elements were added while some eliminated and radical change has been seen in the size of the bhasma by repeated incineration process.
The XRD of raw metal shows crystallite peaks of Zinc metal whereas that of Yashada bhasma sample shows hexagonal crystallite ZnO peaks in its very effective zincite form. The average crystallite size of ZnO nano particle from Scherer's formula was found to be 32.79 nm. The absence of any crystallite zinc metal peaks and presence of nano size particles is confirmed. It is observed that zinc which was present in raw metal has been converted into ZnO nanoparticles after proper incineration process and this can get easily absorbed at targeted site as it has particle size of 32 nm as particles less than 100 nm can be easily absorbed by intestine [9].
Particle size analysis of both samples shows reduction in size, as the mean particle diameter of raw metal was found to be 2063 nm and that of Yashada bhasma was found to be 340 nm. The results showing larger particle size of Yashada bhasma sample through DLS analysis as compared to XRD result suggests that, it may be due to repeated incineration process at the time of Marana process [22]and also when bhasma sample was dispersed in aqueous media, particles of bhasma colloids come together and make suspension of negatively charged hydrophobic particles. Due to this repeated incineration and the hydrophobicity; particles come closer and get clustered to a larger particle size [10]. The more negative zeta potential value of Yashada bhasma as compared to zinc metal shows the stability of Yashada bhasma sample.
It is a known fact that due to repetitive burning, ultra-structural changes occur in terms of size reduction. SEM results evaluate the morphological changes in terms of size. Particle size of raw metal was found to lie in the range of 1–2 μm whereas the particle size of proper incinerated Yashada bhasma range was found to lie in the range of 137–793 nm [14]. SEM also revealed external morphology of particles, where particles of Yashada bhasma showed granular and porous nature as compared to raw metal.
EDAX analysis shows the presence of zinc and oxygen in both the samples but their concentration is different. Higher percentage of oxygen in Yashada bhasma sample reveals that most of the zinc is converted into zinc oxide whereas in raw metal only surface of zinc metal might have converted into ZnO as evident from the low percentage of oxygen. EDAX result of both samples is in good agreement with XRD result which confirms the presence of crystallite zinc and crystallite ZnO peaks in metal and Yashada bhasma sample. Presence of some trace elements like phosphorus, potassium and calcium in the bhasma sample as compared to raw metal may be attributed to the use of some herbal juices or plant extracts during its preparation as kumariswarasahas been found to be rich in Ca, Na, and Mg. Fe might have been inserted from iron pan used during the Jarana process at high temperature.
5. Conclusion
The present study focuses on the preparation of yashada bhasma by following a proper methodology as mentioned in the shastras. An effort has been made to characterize the prepared Yashada bhasma using sophisticated analytical tools as a step towards standardization of these bhasmas. To assure the quality of the prepared bhasma, apart from classical and physical characterization techniques as mentioned in the Ayurveda texts, modern characterization techniques like XRD (X-Ray Diffraction), DLS (Dynamic light scattering), Zeta potential, SEM (Scanning electron microscopy) and EDAX (Energy-Dispersive X-ray spectroscopy) analysis of bhasma samples have been carried out. Modern characterization techniques like XRD, DLS, SEM, and EDAX reveal a significant transformation of synthesized yashada bhasma from original zinc metal. XRD reveals the conversion of zinc metal into crystallite, hexagonal ZnO phase after proper incineration process, which is in line with the elemental analysis done through EDAX analysis. DLS and SEM reveal a drastic reduction in particle size of zinc metal while transforming in to Yashada bhasma, which makes it suitable for therapeutic use. Thus, these characterization techniques give us an idea about the chemical composition and chemical form of the bhasma particles that can help us correlate the toxicity related issues and is a step towards establishing the scientific reasons behind the safety and efficacy of this ancient system of medicine.
Sources of funding
Seed money project under endowment fund (EF/2016/05-05) from Manipal University Jaipur, Jaipur, Rajasthan.
Conflicts of interest
None.
Acknowledgement
The authors are thankful to Manipal University, Jaipur for providing funds in the form of a seed money project and Prof. K. Shankar Rao and Prof. P. Suresh from National Institute of Ayurveda, Jaipur for providing the research and lab facilities related to preparation of Yashada Bhasma. The authors would also like to extend their heartfelt thanks to Prof. Brindha from Sastra University, Thanjavur and MNIT, Jaipur for providing the research facilities related to characterization of Bhasma samples.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Altern Med. 2005;2(4):465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar G., Gupta Y.K. Evidence for safety of Ayurvedic herbal, herbo-metallic and Bhasma preparations on neurobehavioral activity and oxidative stress in rats. Ayu. 2012;33(4):569–575. doi: 10.4103/0974-8520.110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayurvedic formulary of India, Part 1. 2nd revised edition. Ministry of Health & FW Government of India; New Delhi, Shodhan Prakran: 2003. [Google Scholar]
- 4.Sharma S. 11th ed. Motilal Banarsi Das publication; Varansi: 2009. Rasa Tarangini, 6/203-234, edited by Pt. Kashinath Shastri; pp. 143–145. [Google Scholar]
- 5.Acharya V.Y.T. Rasamritam. In: Joshi Damodar., editor. Chaukhamba Sanskrit Sansthan, Varanasi. 1998. [Google Scholar]
- 6.Kirtikar K.R., Basu B.D. Vol. 3. International Book Distributors; 1999. pp. 2066–2068. (Indian Medicinal plants). [Google Scholar]
- 7.Pal D., Sahu C.K., Haldar A. Bhasma: the ancient Indian nanomedicine. J Adv Pharm Technol Res. 2014;5(1):4–12. doi: 10.4103/2231-4040.126980. http://www.japtr.org/text.asp?2014/5/1/4/126980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh T.R., Gupta L.N., Kumar N. Standard manufacturing procedure of Teekshnalauha bhasma. J Ayurveda Integr Med Sci. 2016;7(2):100–108. doi: 10.7897/2277-4343.05487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kale B., Rajurkar N. Synthesis and characterization of Vanga bhasma. J Ayurveda Integr Med Sci. 2018 doi: 10.1016/j.jaim.2017.05.003. (available online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul W., Sharma C.P. Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2(1):14–22. doi: 10.4103/0974-7788.83183. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3157103/View Record in Scopus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosh Hesab Z.M., Sarfaraz M., Asadabad M.A. Preparation of ZnO nanostructures by chemical precipitation method. Synth React Inorg Metal Org Nano Metal Chem. 2011;41(7):814–819. doi: 10.1080/15533174.2011.591308. [DOI] [Google Scholar]
- 12.Taunk P.B., Das R., Bisen D.P., kumar Tamrakar R. Structural characterization and photoluminescence properties of zinc oxide nano particles synthesized by chemical route method. J Radiat Res Appl Sci. 2015;8(3):433–438. doi: 10.1016/j.jrras.2015.03.006. [DOI] [Google Scholar]
- 13.Bhowmick T.K., Suresh A.K., Kane S.G., Joshi A.C., Bellare J.R. Physicochemical characterization of an Indian traditional medicine, Jasada Bhasma: detection of nanoparticles containing non-stoichiometric zinc oxide. J Nanoparticle Res. 2009;11(3):655–664. doi: 10.1007/s11051-008-9414-zPDF]chem17.com. [DOI] [Google Scholar]
- 14.Mishra A., Mishra A.K., Tiwari O.P., Jha S. In-house preparation and characterization of an Ayurvedicbhasma: Praval bhasma. J Integr Med. 2014;12(1):52–58. doi: 10.1016/S2095-4964(14)60005-4. [DOI] [PubMed] [Google Scholar]
- 15.Crichton R.R. Metal Chelation in medicine. RSC Publishing; 2016. Metal toxicity–an introduction; pp. 1–23. [DOI] [Google Scholar]
- 16.Umrani R.D., Agrawal D.S., Paknikar K.M. Anti-diabetic activity and safety assessment of Ayurvedic medicine, Jasadabhasma (zinc ash) in rats. Indian J Exp Biol. 2013;51(10):811–822. http://nopr.niscair.res.in/bitstream/123456789/21445/1/IJEB%2051%2810%29%20811-822.pdf [PubMed] [Google Scholar]
- 17.Jagtap C.Y., Prajapati P.K., Patgiri B., Shukla V.J. Standard manufacturing procedure of TamraBhasma. J Ayu. 2012;33(4):561–568. doi: 10.4103/0974-8520.110528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiremath R., Jha C.B., Narang K.K. Vanga and its XRD. J Anc Sci Life. 2010;29(4):24–28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3336289/pdf/ASL-29-24.pdf [PMC free article] [PubMed] [Google Scholar]
- 19.Boudreau M.D., Beland F.A. An evaluation of the biological and toxicological properties of aloe Barbadensis (Miller), aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24(1):103–154. doi: 10.1080/1059050060061430310.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 20.King G.K., Yates K.M., Greenlee P.G., Pierce K.R., Ford C.R., Mcanalley B.H. The effect of Acemannan Immunostimulant in combination with surgery and radiation therapy on spontaneous canine and feline fibrosarcomas. J Am Anim Hosp Assoc. 1995;31(5):439–447. doi: 10.5326/15473317-31-5-439. [DOI] [PubMed] [Google Scholar]
- 21.Eshun K., He Q. Aloe Vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries—a review. J Crit Rev Food Sci Nutr. 2004;44(2):91–96. doi: 10.1080/10408690490424694. [DOI] [PubMed] [Google Scholar]
- 22.Wadekar M.P., Rode C.V., Bendale Y.N., Patil K.R., Gaikwad A.B., Prabhune A.A. Effect of calcination cycles on the preparation of tin oxide based traditional drug: studies on its formation and characterization. J Pharm Biomed Anal. 2006;41(4):1473–1478. doi: 10.1016/j.jpba.2006.03.032. [DOI] [PubMed] [Google Scholar]