Abstract
Background
Currently, no vaccines or modern drugs are available for dengue and chikungunya and only symptomatic relief is provided to the patients. Siddha medicine, a traditional form of indigenous medical system uses specific polyherbal formulations for the treatment of such infections with considerable success. One such polyherbal formulation for the treatment of chikungunya and dengue is Nilavembu kudineer (NVK). The mechanistic details of this drug as an antiviral for chikungunya virus (CHIKV) and dengue virus (DENV) is poorly understood.
Objectives
The current study was undertaken to study the efficacy of NVK as an antiviral formulation against CHIKV and DENV.
Materials and methods
Cytotoxicity assays (MTT) were performed to determine the role of NVK as an antiviral during chikungunya and dengue infections in the following conditions-i). post infection, ii). during active infections and iii) protective, not allowing virus infection.
Results
It was observed that NVK provides protection against CHIKV and DENV-2 during active infection as well can help to prevent virus infection in the cells and it mainly depends on the cellular availability of drugs for maximum protection against both the infections.
Conclusion
Our study establishes that extraction protocols are important to ensure maximum efficacy of NVK along with the time of addition of the drug during CHIKV and DENV infections in the cells. This study provides insights to the possible mode of action of NVK in in vitro condition during CHIKV and DENV infection.
Keywords: Nilavembu kudineer, Andrographis, Siddha, Chikungunya virus (CHIKV), Dengue virus (DENV), In vitro
Graphical abstract
Highlights
-
•
Andrographis is a major component of Nilavembu kudineer.
-
•
Andrographis is more cytotoxic when used singularly and hence is used as a polyherbal formulation.
-
•
Ethanol Extract of Nilavembu Kudineer (NVK) exhibit antiviral properties.
-
•
NVK has a prophylactic effect during chikungunya and dengue virus (CHIKV and DENV respectively) infection.
-
•
NVK exhibits antiviral activity during active CHIKV and DENV infections.
1. Introduction
Siddha system of medicine is one of the oldest branches of traditional Indian medical system and can be traced back to sangam period (500BCE-500CE) [1], [2], [3]. This system of medicine follows the holistic approach of healthcare [4], [5], [6] and is based on the concept of five proto-elements and three doshas [7], [8]. According to this system of medicine, the physiological function in the human system is mediated by three substances (Tridosham) viz. i) wind (Vatham) ii) bile (Pitham), iii) phlegm (Karpam) [8]. If these three substances function normally in the ratio 4: 2: 1 respectively [2], [8], normal health is maintained. The change in this ratio will lead to various diseases [8]. Polyherbal formulations of siddha system of medicine are used individually or in combination for maintaining the equilibrium ratio of human health. Siddha medicine maintains that the three humours predominate the humans in accordance with their nature and stage of life, and that they vary with the seasons [9]. The Siddha system of medicine is mainly concerned with the development of polyherbal formulations, which have high potency and long shelf life for their use in future. It also aims to activate the generation of cells and to maintain their longevity [10]. The resources of siddha medicine system have been categorized into three groups: plant products (mulavargam), inorganic substances (thathuvargam), and animal products (jivavargam), which are characterized by means of taste (suvai), quality (gunam), potency (veeryam), post-digestive taste (pirivu), and specific action (prabhavam) [11], [12].
According to the siddha literature, Extrinsic (External) and Intrinsic (Internal) factors account for cause of a disease [10]. Factors in the intrinsic causes include the three-humour derangement and extrinsic causes include climate, habitat, environment, trauma, poisonous bites, accidents etc [7]. The concept of epidemics is very well defined and established in Ayurveda and Siddha. Charaka (1500BC - 400AD), the great physician of Ayurveda had mentioned epidemic conditions under the head “Janapadodwamsa” [10] as Senkaraisuram [13]. As per the siddha literature the contamination and vitiation of environmental factors such as– water, air, place and season is responsible for the production of epidemics along with the disequilibrium of the doshas [8], [10] in the hosts that makes them more receptive to the diseases. Dengue and chikungunya are such entities that emerged as epidemic outbreak across the country causing discomfort and morbidity to the affected individuals. In the current times, water borne diseases, environmental diseases, epidemiological disorders and seasonal disorders due to infections can be correlated with Janapadodhwamsa Vyadhis (epidemic or endemic Diseases), a medical term well defined in the Indian traditional medical system. Ayurveda and Siddha offers several single drugs, compound herbal and herbo-mineral combinations, that may be rationally used to combat such conditions [8], [10].
Dengue and chikungunya are arboviral infections caused by a flavivirus, DENV and an alphavirus, CHIKV respectively [14]. In a country like India, dengue and chikungunya infection can occur either as mono or co-infections [14], [15]. Currently, there are no authorized drugs for these infections in modern medicine and clinicians resort to symptomatic relief to combat these infections. However, in Siddha, both these infections are effectively treated by specific course of medication that are termed as regimen. The regimen that is used during the initial days of infection in the treatment of both chikungunya fever (CF) and in dengue fever (DF) includes one formulation in common, i.e. Nilavembu Kudineer (NVK) [16]. NVK is a polyherbal concoction with Andrographis paniculata as the chief ingredient that controls all types of fever associated with body ache. This herb popularly called the Neem of ground, Bile of earth and King of bitters is native to India and Sri Lanka [17]. Other components include Vettiver (Vetiveria zizanioides), Vilamiccam ver (V. zizanioides), Cantanam (Santalum album), Peyputtal (Trichosanthes cucumerina), Koraik kilanku (Cyperus rotandus), Cukku (Zingeber officinale), Milaku (Piper nigrum) and Parpatakam (Mollugo cerviana) [16], [18]. All these plants are used traditionally in the treatment of fever, inflammation, arthralgia, arthritis, gastric ulcer, jaundice and general debility conditions, formulated with an intention to manage CF and DF. NVK controls fever in a comprehensive manner through its healing effects of temperature regulation, inflammation control and body pain relief. It acts in a way to boost immunity [16], [18]. The present study was done to investigate antiviral effects of ethanol extract of Nilavembu kudineer against CHIKV and DENV infection under in vitro conditions.
2. Materials and Methods
2.1. Cell line and maintenance
Vero (ATCC® CCL-81™) HEK-293T (ATCC® CRL-11268™) and BHK-21 (ATCC® CCL-10™) cell lines were maintained at 37 °C in DMEM media (Himedia, AL007A) supplemented with 10% FBS, antibiotics and l-glutamine. THP-1 (ATCC® TIB-202™) cell line was maintained at 37 °C in RPMI media supplemented with 10% FBS and antibiotics.
2.2. Isolation, propagation and amplification of CHIKV and DENV
CHIKV and DENV-2 were derived from sera samples obtained from viremic patients during an outbreak [19], [20]. Briefly, 20 μL of infected sera sample was inoculated in a confluent 6 wells plate of C6/36 cells for 6 days. Thereafter, 90–100% confluent T-150 flask of Vero and BHK-21 cells was used for CHIKV and DENV-2 amplification respectively, all medium but 2.5 mL was removed. 50 μL of virus stocks prepared as aforementioned (approx. MOI 0.1) was added to the flask and homogenized. The flask was incubated at 37 °C for 1 h, the volume of the flask was made to 25 mL with DMEM 2% FBS supplemented with 1% Pen/Strep and incubated until CPE was about 90% (time depending on virus). Infected media was harvested placed in a 50 mL conical tube and centrifuged at 2500 rpm for 5 min and stored at −80 °C. All procedures were carried out using sterile techniques and in a biosafety cabinet.
2.3. PEG-based virus concentration and purification method
4X PEG prepared in Tris-EDTA-NaCl (TEN) buffer (wt./volume) was prepared and added to the infected media in a way that it become 1X, the samples were vortexed for homogenization and placed at +4 °C undisturbed for 48 h. After 48 h, PEG-CHIKV mix was centrifuged at 4000 rpm for 30 min. The supernatant was discarded and pellet was resuspended in 250 μL of TEN and stored at −80 °C until further use.
2.4. Virus characterization by the plaque assay
Monolayer of Vero cells in a 96 well plate (90% confluence is better) was prepared a day prior to the experiment. Starting at dilution of 1:100 virus was double diluted for the experiment. The virus particles were incubated on the cells for 1–2 h at 37 °C for virus adsorption/penetration. Thereafter, media were removed and cells were overlaid with 150 μL of 1% CMC prepared in DMEM media supplemented with 10% FBS, 1% pen/strep and 1% l-glutamine (Overlay media). The plates were incubated at 37 °C for 48 h for CHIKV and 3–5 days for DENV-2. Post that the plates were fixed with 10% formaldehyde for 30 min, washed twice with 1X PBS and stained with 0.25% Crystal violet (prepared in 30% methanol) for 15 min. Finally, the plates were washed thrice with 1X PBS. The images of the plates were captured using Elispot reader. The number of plaques forming units (pfu) in each well was determined using the formulae as mentioned below.
2.5. Preparation of ethanol extract of Nilavembu Kudineer and Andrographis
Details of the ingredients and their percentage presence in Nilavembu Kudineer was provided in suppl. Table 1. Twenty-five grams of the powdered plants constituents were extracted with 100 mL ethanol (95% ethanol). The contents were stored at room temperature for 48 h with constant stirring at regular intervals. After the incubation period, the contents were filtered through Whatman filter paper No.1. Then filtrates were vacuum dried using rotary evaporator and concentrates were stored at 4 °C until the completion of pharmacological and biological studies.
2.6. Cell viability assay
The MTT 3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide assay (Sigma, Cat No: D4540) was performed to evaluate the cytotoxicity of ethanol extracted Nilavembu kudineer and Andrographis compound in Vero cells using previously standardized protocols [21]. Briefly, monolayers of Vero cells were grown in 96-well plate and were treated with different concentrations of the compound in 8 replicates together with negative control (media containing 0.1% DMSO). The plate was then incubated at 37 °C with 5% CO2 for 72 h before the MTT assay was performed. Three days post-treatment, MTT solution was added to the cells and incubated for 4 h at 37 °C with 5% CO2 and 150 μL of DMSO was added prior to absorbance detection at 495 nm using multiplate reader. Percentage survival of cells for the compounds was determined using Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA, 2005).
2.7. Phytochemical and physiochemical studies
2.7.1. Phytochemical analysis
Nilavembu Kudineer and Andrographis paniculata are separately taken for preliminary phytochemical analysis. The presence of different phytoconstituents were detected through prescribed methods [22].
2.7.2. Organoleptic and physicochemical evaluation
Nilavembu kudineer and Andrographis paniculata was subjected for the determination of organoleptic characters like colour, odour, taste, texture and physicochemical parameters such as total ash, acid insoluble ash, alcohol soluble extractives, loss on drying at 105 °C and were evaluated according to the standard methods described in the texts [22].
2.8. Screening of drug activity using in-vitro cell viability assays
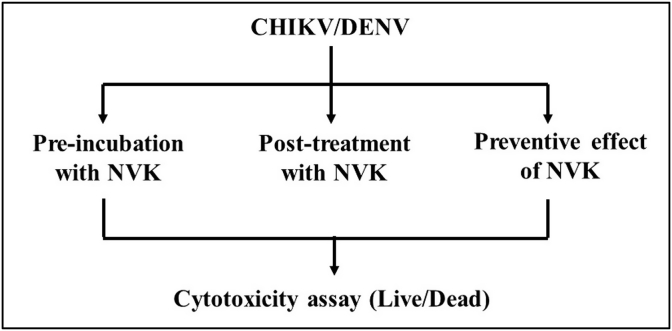
Schematic representation of the in vitro bioassays developed to evaluate antiviral activity of NVK is given in Fig. 1. In these cell viability assays, two separate sets for CHIKV and DENV-2 were used wherein Vero cells and HEK-293T cells were infected with CHIKV and Vero cells and THP-1 cells were infected with DENV-2 (MOI 1) and antiviral activity of Nilavembu kudineer was tested at indicated concentrations. Briefly, the assays have been explained in the following section.
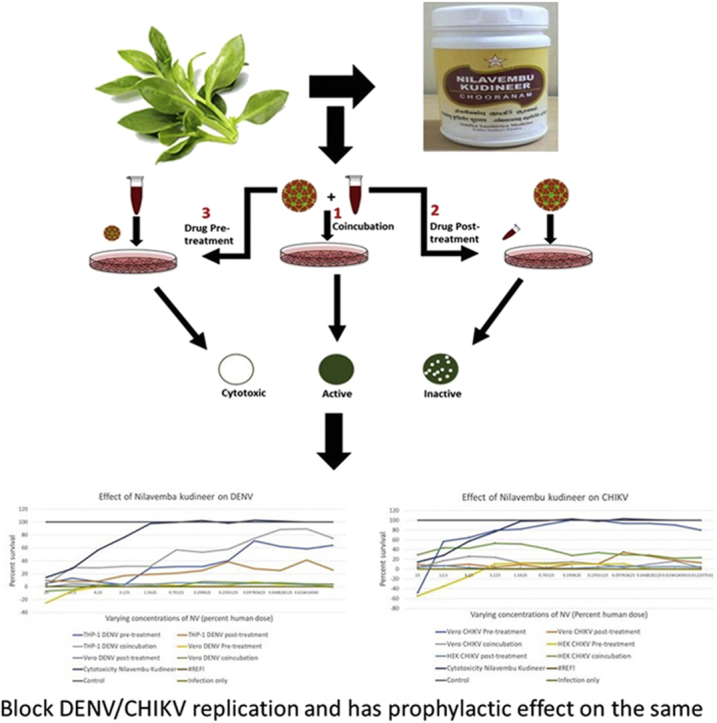
Fig. 1.
Schematic representation of the in vitro bioassays developed to evaluate antiviral activity of Nilavembu Kudineer (NVK). Three types of MTT based assays were designed to evaluate the antiviral activity were (1) Pre-incubation assay (2) Post-treatment assay and (3) Preventive assay.
2.8.1. Design of screening assays
Three bioassays were developed to identify potential DENV/CHIKV-inhibitory activity of the ethanol extract of NVK, as shown schematically in Fig. 1. The assay was performed on specific cell types i.e. Green African monkey kidney epithelial cells (Vero) and human macrophage cells (THP-1) for DENV and African green monkey kidney epithelial cells and human kidney epithelial cells (Vero and HEK-293T respectively) based on the individual replication kinetics and affinity towards that cell type. Vero cells served as a positive control.
The type-1 pre-incubation assay was designed to identify the ability of NVK to block viruses from entering susceptible cells. It was observed that NVK extract showed an IC50 value ≤ 3.125% of the human dose during the cytotoxicity assay. Based on the following observations it could be concluded that this assay had the following possible outcomes: the NVK extract could be cytotoxic (compromised monolayer), inactive (no reduction in PFU count in comparison to control infection), or active (reduced number of FU counts). However, the type-1assay may not reveal if the active NVK extracts also possessed the ability to inhibit post-entry steps in the virus life cycle.
The type-2 assay was designed to assess the capacity of the NVK extracts to inhibit viruses within the infected cell. Thus, this assay aims to study the activity of NVK post virus infection. In this respect, virus infection preceded exposure to NVK. As the polyherbal formulation must be internalized before it exerts any inhibitory effect, we decided to use higher cut-off amounts of NVK extract. Possible outcomes in the type-2 assay are as follows: once again, the extract could be cytotoxic. If not, it may be ineffective due to one of two reasons: the extract failed to enter the cells, or it was ineffective despite successful entry. The final possibility is that the extract would reduce the plaque count, indicating potential antiviral activity.
The type-3 assay was designed to monitor protective effect of NVK and for this respect, NVK treatment preceded virus infection. Although, the possible outcome of this assay was also similar to the other two assays, it was used to determine whether NVK could be used in CHIKV/DENV hyper-endemic regions to control epidemics.
2.8.2. Methodology of the assays
In these cell viability assays, specific cell types were used to study antiviral activity against CHIKV/DENV-2 infections; wherein Vero cells and HEK-293T cells were infected with CHIKV and Vero cells and THP-1 cells were infected with DENV-2. In both the cases virus was infected at MOI 1 and antiviral activity of Nilavembu kudineer was tested at indicated concentrations. Briefly, the assays have been explained in the following section.
2.8.2.1. Pre-incubation with Nilavembu Kudineer
Vero cells were seeded in 96-well plates (20,000 cells/well), a day in advance. CHIKV and DENV-2 (MOI 1) were separately pre-incubated with serial dilutions of NVK (corresponding to 25 mg/mL–12 μg/mL final concentration) in 200 μL at 37 °C for 2 h 100 μL of this pre-incubated mixture was used to infect the cells in 6-plicates in the 96-well plate. After 1 h of adsorption in the incubator (37 °C, 5% CO2), the pre-incubated media was removed, and the infected cells were supplemented with 10% DMEM complete media in case of Vero and HEK-293T cells and RMPI in case of THP-1 cells and incubated at 37 °C for 72 h. Post 72 h the cells were processed in a fashion similar to MTT as mentioned above. Briefly, MTT solution was added to the cells and incubated for 4 h at 37 °C after which 150 μL of DMSO was added prior to absorbance detection at 495 nm wavelength using multiplate reader. To assess any potential cytotoxicity, cells were exposed to the NVK formulations (in the same concentration range) in the absence of CHIKV infection. Additional control experiments, ran in parallel, included cells which were either mock-infected (negative control) or infected with CHIKV/DENV-2 in the absence of NVK. The half-maximal inhibitory concentration (IC50 value) for each concentration of NVK against CHIKV/DENV-2 was calculated, with reference to the positive control which represented 100% survival. Percentage survival of cells for the compound was determined = using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA, 2005).
2.8.2.2. Post-treatment after infection with Nilavembu Kudineer
Vero cells in 96-well plates (20,000 cells/well) were infected with CHIKV/DENV-2 (MOI 1) for 1 h. Post virus adsorption, the virus inoculum was aspirated and fed with complete medium containing NVK at concentrations as aforementioned. After 2 h of treatment, media containing NVK was aspirated and replaced with 10 % complete DMEM media in case Vero and HEK-293T cells and RPMI in case of THP-1 cells. Further, the assay was culminated in the same way as mentioned in assay-1.
2.8.2.3. Preventive effect of Nilavembu Kudineer
Vero cells in 96-well plates (20,000 cells/well) were treated with varying NVK concentrations for 2 h at 37 °C. Post treatment, NVK media was aspirated, and the cells were infected with CHIKV/DENV-2 (MOI 1) for 1 h at 37 °C. Post infection, virus media was aspirated and replaced with 10 % complete DMEM media in case Vero and HEK-293T cells and RPMI in case of THP-1 cells. Further, the assay was culminated in the same way as mentioned in assay-1.
3. Results
3.1. Virus characterization
Number of Plaque forming units of lab amplified CHIKV and DENV-2 virus was done using plaque assay as mentioned above, it was observed that PFU of CHIKV and DENV-2 was 1 × 109 PFU/mL and 1 × 108 PFU/mL respectively. Envelope gene of DENV and E1 gene of the CHIKV was sequenced. We observed that envelop of DENV belonged to the DENV-2 serotype and E1 gene of CHIKV belonged to the East/Central/South Africa (ECSA) genotype (data not shown).
3.2. Preliminary phytochemical and physio-chemical activity of Nilavembu Kudineer
The physio-chemical studies for total ash, water soluble ash, acid insoluble ash, pH and extractive nature of the powder of Nilavembu Kudineer was carried out and the results are documented in Suppl. Table 2. The colour of ethanol extract of Nilavembu Kudineer is dark brown and Andrographis is dark green. The phytochemical screening of all extract of Nilavembu Kudineer formulation was also carried out and the results showed presence of terpenoids, alkaloids, carbohydrates, tannins, steroids (Suppl. Table 3) as major components.
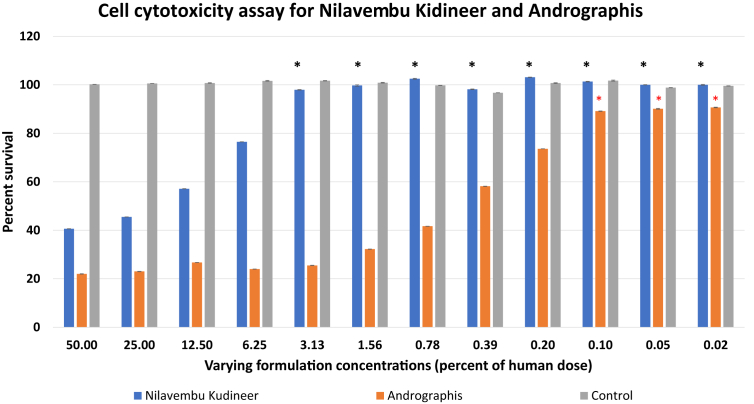
3.3. Cell cytotoxicity assay for Nilavembu kudineer and Andrographis
MTT assay was performed to evaluate cytotoxicity of the ethanol extract of NVK as well as Andrographis. It was observed that NVK was non-toxic starting 3.1% of human dose (i.e. approximately 1.8 mg/mL) onwards while Andrographis showed more of 80% death of cells at this concentration (Fig. 2). It was observed that Andrographis showed higher toxicity than NVK for all the indicated concentrations during the course for the study. Only at 12ug/mL Andrographis exhibited less than 20 % cell death as opposed to none in case of NVK. Thus, it is concluded that Andrographis was extremely cytotoxic if delivered singularly but its toxicity greatly decreased when given as a polyherbal formulation as NVK. Based on these observations, further studies were done only on NVK. Antiviral activity of NVK at 3.1 % of the human dose onwards was taken as significant (as at these concentrations, NVK showed less than 20 % cell death).
Fig. 2.
Cytotoxicity assay for NVK and Andrographis: MTT based cytotoxicity assay was performed to evaluate the cell cytotoxicity of ethanol extract of NVK and Andrographis starting at 50 % of the human dose. Andrographis was found to be more cytotoxic than NVK at varying concentrations.
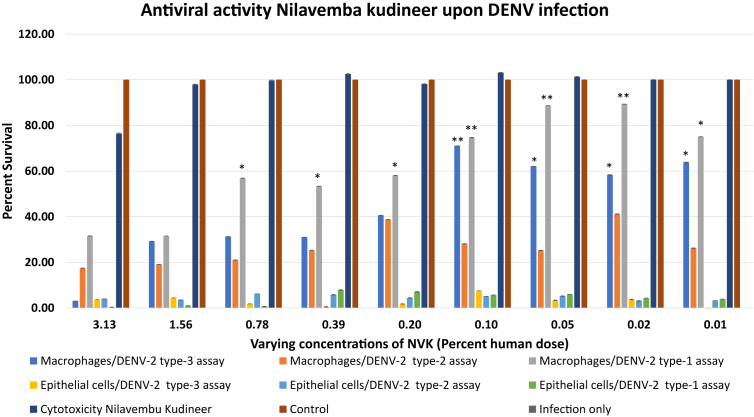
3.4. In vitro screening of Nilavembu kudineer for the treatment of DENV-2 infection
All three types of assays were performed in Vero and THP-1 cells, to determine the antiviral activity of NVK against DENV-2 virus infection (Fig. 3). It was observed that in case of human macrophage cell line (THP-1), NVK exhibited significant antiviral activity in type-1 assay from 0.78 % till 0.01% of the human dose. The formulation worked best at 0.05 % of the human dose after which the activity of the formulation decreased in a dose dependent manner (the activity reduced with the increase of the dosage) from 0.1% till 0.78 % of the human dosage. In case of type-2 assay, NVK did not exhibit any post treatment effect against DENV-2 infection. In case of type-3 assay, significant protective effect of NVK was observed at concentration of 0.1–0.10% of the human dose onwards. Maximum activity of the formulation was observed at 0.1 % of human dosage. When similar experiments were performed for epithelial cells it was observed that NVK was not unable to exhibited antiviral activity against DENV-2 infection. Briefly, it could be summarized that NVK is capable of an organ specific antiviral activity and it can be used as a potential antiviral against DENV-2 both as a preventive measure before the infection as well as an antiviral during active infection. The effectiveness of NVK on THP-1 cell lines throws light on the potential immuno-modulatory effect of NVK.
Fig. 3.
Evaluation of antiviral activity of NVK upon DENV-2 infection. Antiviral activity of NVK was evaluated based on all the three types of MTT based assays designed for the study. It was observed that upon DENV-2 infection NVK exhibited antiviral activity as prophylactic agent as well as during active infection. *denotes p-value<0.05 and **denotes p-value<0.01.
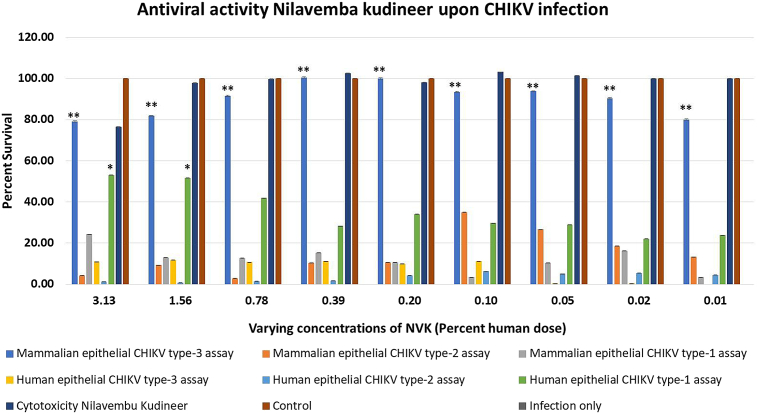
3.5. In vitro screening of Nilavembu kudineer for the treatment of CHIKV infection
All three types of assays were performed in mammalian kidney epithelial cell line i.e. Vero and human epithelial kidney cell lines i.e. HEK-293T, to determine the antiviral activity of NVK against CHIKV virus infection (Fig. 4). It was observed that in case of Vero, NVK did not show exhibit any antiviral activity for type-1 and type-2 assays whereas in case of type-3 assay NVK exhibited significant antiviral activity was observed from 0.01% till 3.13 % of the human dosage onwards. The results demonstrated the protective effect of NVK in case of Vero cells. When the similar experiments were performed in HEK-293T cells, it was observed that in case of type-1 assay NVK exhibited antiviral activity at 3.13 % and 1.56 % of the human dosage. Whereas, no antiviral activity of NVK was observed in case of type-2 and type-3 assays. Briefly, it could be summarized that NVK could be used both as a protective agent as well during active CHIKV infections. These results also demonstrated the importance of cell choice depending on cell susceptibility of the virus to perform the assays.
Fig. 4.
Evaluation of antiviral activity of NVK upon CHIKV infection. Antiviral activity of NVK was evaluated based on all the three types of MTT based assays designed for the study. It was observed that upon CHIKV infection NVK exhibited antiviral activity acts as a prophylactic agent as well as during active infection. *denotes p-value<0.05 and **denotes p-value<0.01.
In our testing assays NVK exhibited drastically different effects in monkey and human kidney epithelial cell lines based on the cell susceptibility of CHIKV on these cells.
4. Discussion
CF and DF are serious public health issues in India. While vector control is the most effective method to curb both these infections, the main concern in infection control is the lack of proper drugs or vaccines. Post a major CF outbreak in 2006, Department of AYUSH studied the effect of siddha medicines on CF patients in various places in the southern part of the Indian subcontinent and recommended specific regimen for disease management [10]. In all these regimen, it was observed that 95 % patients recruited for the study showed positive response towards the regimen. Thereafter, all the regimens were supplemented with the use of NVK both in the pyrexic and post-pyrexic phase of the disease, due to its previously known role in immunity modulation along with the other siddha herbal and minero-herbal formulations. Several previous studies have studied the impact of NVK in a variety of ailments for its antipyretic, analgesic and anti-inflammatory activities and is known to impart protection at varying dosages in different experimental models [18], [23].
Different extraction methods have shown to elicit formulation outcome in several herbal formulations [24], [25]. A main contributing factor for these differences in response lies in the inherent feature of chemical compounds in these formulations. It is known that polyherbal formulations have varying solubility in different solvents, which is determined by the structure of phenolic compounds in them that may contribute to deciding the final yield of the polyphenols from the herbal materials [26], [27]. A recent paper from our lab showed that aqueous extracts of NVK exhibited no antiviral activity upon CHIKV infection upon in vitro conditions [28], whereas the current report clearly demonstrates the antiviral activity of the ethanol extracts of NVK.
Further, we observed that the addition of additives to the active component is very important to reduce its cytotoxicity and to make it available for human consumption. Andrographis, the known active component of NVK is highly cytotoxic in in vitro conditions, which can lead to severe oral hazards when consumed alone. Similar question has been addressed in book by Norma G. Cuellar [29], wherein it has been reported that importance of herbal medicines lies in the fact that they use plant as a whole, which is an important point because if the modern concepts are used in alternative medicine and only active component is separated, then will cease to act as a herbal medicine and will plainly act as a chemical drug which can be highly toxic/hazardous to the human body and thus this approach of modern science should be avoided while working on alternative medicine system.
Lastly, this study focused on the differences observed in the antiviral activity of NVK upon CHIKV and DENV infection in organ specific cell lines. Previous studies have reported that CHIKV and DENV acts in an organ specific manner [30], [31], [32]. Role of NVK in a regime during CF and DF have been well studied by the Siddha community in the patients at various siddha centers keeping in view the major disease symptoms [10], [16]. Keeping both the points in mind, we used organ specific cell lines to study the antiviral role of NVK upon CHIKV and DENV infections. Our data clearly depicts that the time of drug treatment is important in eliciting maximum antiviral response. The ideal requisite would be to demonstrate the effects of the drug in a suitable animal model; however, lack of proper animal models to study these viruses is a hurdle to address some of the mechanistic concerns.
5. Conclusion
The present study has provided insights to the mode of action of NVK as an antiviral agent in combating CF and DF. Considering the huge public health impact of both these infections in India, information from the present study may be utilized in devising an effective disease control strategies by using NVK in prophylaxis during outbreaks.
Sources of funding
Ministry of Ayurveda, Yoga, Unani, Siddha and Homeopathy (AYUSH), Government of India, [Grant No: ND/AYU/15/019].
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2018.05.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rajantheran M., Gill S.S., Muniapan B., Silllalee K., Manimaran S. A critical analysis of siddha tradition in the context of Malaysian Hindu culture. Life Sci J. 2014;11(7) [Google Scholar]
- 2.Zysk K.G. Some reflections on siddha medicine in Tamil Nadu. Indian J History Sci. 2009;44(2):199–208. [Google Scholar]
- 3.Premachandra M.K. Paper presented at: Universitas Forum. 2011. The growing importance of traditional, alternative and complementary medicine in India. [Google Scholar]
- 4.Subbarayappa B.V. Siddha medicine: an overview. Lancet. 1997;350(9094):1841–1844. doi: 10.1016/s0140-6736(97)04223-2. [DOI] [PubMed] [Google Scholar]
- 5.Subbarayappa B.V. The roots of ancient medicine: an historical outline. J Biosci. 2001;26(2):135–143. doi: 10.1007/BF02703637. [DOI] [PubMed] [Google Scholar]
- 6.Karunamoorthi K., Jegajeevanram K., Xavier J., Vijayalakshmi J., Melita L. Tamil traditional medicinal system-siddha: an indigenous health practice in the international perspectives. Int J Genuine Trad Med. 2012;2(2):12.1–12.11. [Google Scholar]
- 7.Chaudhury R.R., Rafei U.M. WHO; Geneva: 2002. Traditional medicine in Asia. [Google Scholar]
- 8.Shukla S., Saraf S. Fundamental aspect and basic concept of siddha medicines. Sys Rev Pharm. 2011;2(1):48. [Google Scholar]
- 9.Micozzi M.S. 5 th ed. Elsevier Health Sci; 2014. Fundamentals of complementary and alternative medicine-E-book. [Google Scholar]
- 10.Lavekar G., Padhi M. 1 st ed. Central Council for Research in Ayurveda and Siddha; New Delhi: 2007. Management of chikungunya through Ayurveda and Siddha: a technical report; pp. 28–44. [Google Scholar]
- 11.Lalitha N. Protecting traditional knowledge in siddha system of medicine. J Intellect Prop Rig. 2013;18(3):272–282. [Google Scholar]
- 12.Pan S.-Y., Litscher G., Gao S.-H., Zhou S.-F., Yu Z.-L., Chen H.-Q. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathya B., Velpandian V., Ramani M., Kumar M.P. A primitive approach on review of Siddha herbs, herbo-mineral formulation exhibiting antiviral activity. Int J Pharma Bio Sci. 2014;5:138–147. [Google Scholar]
- 14.Jain J., Dubey S.K., Shrinet J., Sunil S. Dengue Chikungunya co-infection: a live-in relationship?? Biochem Biophys Res Commun. 2017;492(4):608–616. doi: 10.1016/j.bbrc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Londhey V., Agrawal S., Vaidya N., Kini S., Shastri J., Sunil S. Dengue and chikungunya virus Co-infections: the inside story. J Assoc Physicians India. 2016;64:36. [PubMed] [Google Scholar]
- 16.Parthiban P., Kumar V. Siddha medicine and clinical presentation of dengue fever at tertiary care hospital of Chennai, Tamil Nadu, India. Int J Adv Ayurveda Yoga Unani Siddha Homeopathy. 2014;3(1):209–212. [Google Scholar]
- 17.Maiti K., Mukherjee K., Murugan V., Saha B.P., Mukherjee P.K. Enhancing bioavailability and hepatoprotective activity of andrographolide from Andrographis paniculata, a well-known medicinal food, through its herbosome. J Sci Food Agric. 2010;90(1):43–51. doi: 10.1002/jsfa.3777. [DOI] [PubMed] [Google Scholar]
- 18.Anbarasu K., Manisenthil K.K., Ramachandran S. Antipyretic, anti-inflammatory and analgesic properties of nilavembu kudineer choornam: a classical preparation used in the treatment of chikungunya fever. Asian Pac J Trop Med. 2011;4(10):819–823. doi: 10.1016/S1995-7645(11)60201-0. [DOI] [PubMed] [Google Scholar]
- 19.Jain J., Nayak K., Tanwar N., Gaind R., Gupta B., Shastri J.S. Clinical, serological, and virological analysis of 572 chikungunya patients from 2010 to 2013 in India. Clin Infect Dis. 2017;65(1):133–140. doi: 10.1093/cid/cix283. [DOI] [PubMed] [Google Scholar]
- 20.Kaur N., Jain J., Kumar A., Narang M., Zakaria M.K., Marcello A. Chikungunya outbreak in Delhi, India, 2016: report on coinfection status and comorbid conditions in patients. New Microbes New Infect. 2017;20:39–42. doi: 10.1016/j.nmni.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz O., Henin Y., Marechal V., Montagnier L. A rapid and simple colorimetric test for the study of anti-HIV agents. AIDS Res Hum Retrovir. 1988;4(6):441–448. doi: 10.1089/aid.1988.4.441. [DOI] [PubMed] [Google Scholar]
- 22.Lohar D.R. 1 st ed. Depertment of AYUSH, Ministry of Health & Family Welfare, Pharmacopoeial Laboratory for Indian Medicines; 2007. Protocol for testing of ayurvedic, siddha, Unani medicines. [Google Scholar]
- 23.Rajasekaran A., Arivukkarasu R., Mathew L. A systematic comprehensive review on therapeutic potential of Andrographis paniculata (Burm. f.) Wall. ex Nees. J Pharmacogn Phytochem. 2016;5(5):189. [Google Scholar]
- 24.Dhanani T., Shah S., Gajbhiye N., Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem. 2013;10:S1193–S1199. [Google Scholar]
- 25.Złotek U., Mikulska S., Nagajek M., Świeca M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J Biol Sci. 2016;23(5):628–633. doi: 10.1016/j.sjbs.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rababah T.M., Banat F., Rababah A., Ereifej K., Yang W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J Food Sci. 2010;75(7):C626–C632. doi: 10.1111/j.1750-3841.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrini N., Salvatore S., Valtuena S., Bedogni G., Porrini M., Pala V. Development and validation of a food frequency questionnaire for the assessment of dietary total antioxidant capacity. J Nutr. 2007;137(1):93–98. doi: 10.1093/jn/137.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Jain J., Pai S., Sunil S. Standardization of in vitro assays to evaluate the activity of polyherbal siddha formulations against chikungunya virus infection. Virus Dis. 2017;29(1):32–39. doi: 10.1007/s13337-018-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuellar N.G. Conversations in complementary and alternative medicine: insights and perspectives from leading practitioners. J Altern Complement Med. 2007;13(1):179–180. [Google Scholar]
- 30.Jain J., Narayanan V., Chaturvedi S., Pai S., Sunil S. In vivo evaluation of Withania somnifera based Indian traditional formulation (Amukkara Choornam), against chikungunya virus induced morbidity and arthralgia. J Evid Based Compl Altern Med. 2017;26(23) doi: 10.1177/2156587218757661. 2156587218757661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz O., Albert M.L. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 32.Guabiraba R., Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141(2):143–156. doi: 10.1111/imm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.