Abstract
Background
Viscum articulatum Burm. (Family: Loranthaceae) is commonly known as mistletoe. In ayurveda, the plant parts are used in “Kapha”, “Vata”, diseases of the blood, ulcer, and epilepsy. The plant parts are also used in urinary tract infection and wound infection. The plant contains five triterpenoids such as α-amyrin, lupeol, betulin, betulinic acid and oleanolic acid, exhibiting several pharmacological activities including antimicrobial, anti-HIV, antitumor, antiviral activity.
Objective
To ensure the content of uniformity of oleanolic acid, a RP-HPLC method has been developed for estimation of oleanolic acid in V. articulatum aerial part.
Material and methods
The RP-HPLC method was carried out in reverse phase C18 column, using methanol and water as mobile phase in the ratio of 95:5 (v/v), at the flow rate of 1 mL/min. The pH of aqueous phase was adjusted 3.2 with 1% (v/v) glacial acetic acid. The λmax was set at 210 nm.
Results
The retention time of oleanolic acid was found at 21.5 ± 0.05 min. The linearity of the response was found to be 10–800 μg/mL. The coefficient of determinants of oleanolic acid was found to be (r2) 0.995 and equation Y = 19462X + 16,172. The LOD and LOQ were found to be for oleanolic acid (1.96% w/w) 0.197 ± 0.63 and 0.623 ± 0.87 μg/mL, respectively. The developed method was accurate, specific, precise and reproducible.
Conclusion
This RP-HPLC may be useful for quantitative estimation of the chemical constituents present in the plant extract as well as the quality assessment of the herbal product.
Keywords: RP-HPLC, Viscum articulatum Burm, Oleanolic acid, Ayurveda, Method validation
Highlights
-
•
An ethnomedicinal plant – Viscum articulatum used for analysis.
-
•
Method validation of oleanolic acid through RP-HPLC.
-
•
Quality control of Ayurvedic medicinal Plant.
1. Introduction
Viscum articulatum Burm. (Family: Loranthaceae) is an ethnomedicinal plant, commonly known as mistletoe and it is distributed widely in India, Bangladesh and south west of China [1], [2]. V. articulatum is a parasitic herbs; branches are hanging or move in a twisted and convoluted fashion. They are yellowish-green with 3–4 cm long and 3–5 mm wide. Flowers are subtended by two coalescent bracteoles and originating at the nodes. Fruits are sub orbicular and whitish in color with 4 mm in diameter [3]. V. articulatum is traditionally used in Chinese medicine for the treatment of hemorrhage, pleurisy, gout, heart disease, arthritis and hypertension [4]. In ayurveda, the different plant parts of V. articulatum are used in “Kapha”, “Vata”, diseases of the blood, ulcer, epilepsy and biliousness [5], [6]. As per Ayurveda, Stem paste and decoction is given to cure cuts, wounds, bone fracture, ulcers and blood diseases, epilepsy and sprain. The stem part of the plant is also used for fractures, joint pain and skin diseases [7], [8]. The plant parts are also used in urinary tract infection, low back pain, dysentery, uterine bleeding and to treat weakness [1]. The plant contains five triterpenoids such as α-amyrin, lupeol, betulin, betulinic acid and oleanolic acid [9]. The plant also contains two flavanones, (2S)-pinocembrin 7-O-[b -d-apiosyl (1–2)]-b –Dglucoside, and (2S)-pinocembrin 7-O-[cinnamoyl (1–5)-b -d-apiosyl (1–2)]-b -D-glucoside [10]. The leaves and stems contain flavanone glycosides, visartisides A-B, visartisides D-F [11]. RP-HPLC analysis showed the presence of oleanolic acid in this plant extract [12]. RP-HPLC standardization of some medicinal plant used in Ayurveda including Acromas calamus [13], Glycyrrhiza glabra [14], Murraya koenigii [15], Swertia chirata [16], Carissa spinarum [17] has been reported from our laboratory. The marker profiling and standardization are very importance for finding phytoconstituents present in medicinal plants [18]. The quality related safety issue and quantification of phytoconstituents has major concern in herbal formulation [19]. In this context, the HPLC is one of the most convenient and comprehensive analytical technique for separating individual components in plant extracts. The HPLC technique has potential importance for authentication, fingerprinting, quantification, quality control of herbal products [20]. The oleanolic acid has several pharmacological activities including antimicrobial, anti-HIV, antitumor, antiviral activity. A thorough literature survey revealed that there is no report available for estimation of oleanolic acid present in V. articulatum. In this context, the aim of the present study is to evaluate of oleanolic acid in V. articulatum by using RP-HPLC method.
2. Materials and methods
2.1. Collection and extraction of plant
V. articulatum was collected from the South Bengal region in the month of December, 2014. The collected plant material was authenticated and a voucher specimen number (SNPS-JU/2014/1467) has been kept in the School of Natural Product Studies (SNPS), Jadavpur University, Kolkata, India for future reference. The plant material was dried under shade and pulverized by using a mechanical grinder to make a coarse powder. Then 940 gm powder was soaked with 95% methanol at room temperature (25 °C) for successive extraction. The whole extract was collected, filtered and the solvent was evaporated to dryness under reduced pressure and temperature (45 °C) by using Eyela Rotary Evaporator (Japan). The yield of the aerial part of V. articulatum methanol extract was found to be 11.35% w/w. Dried extract was stored at 4 °C for further use.
2.2. Chemicals and reagents
Acetonitrile (HPLC grade), water (Milli-Q) and glacial acetic acid (HPLC grade) were used. All the chemicals and reagents are analytical grade and were purchased from Merck Ltd. (Mumbai, India). Standard oleanolic acid was purchased from Sigma Aldrich (St. Louis, MO, USA).
2.3. RP-HPLC instrumentation
The HPLC system (Waters, Milford, MA, USA) used for the analysis consisted of a 600 controller pump, a multiple-wavelength UV-Visible detector equipped with an in-line degasser AF 2489 and a rheodyne 7725i injector equipped with a 20 μl loop. Quantitative estimation was performed with Empower 2 software programs using the external calibration method. A Milli-Q academic water purification system (Bedford, MA, USA) equipped with 0.22 μm Millipak express filter and Eyela (Tokyo, Japan) rotary vacuum evaporate were used. Membrane filters of 0.45 μm pore size (Millipore) were used for filtration of the mobile phase and Whatman's syringe filters (NYL 0.45 μm) were used for the filtration of the sample.
2.4. Preparation of standard and sample solutions
A standard solution of oleanolic acid was prepared in methanol (1 mg/mL). Calibration samples were prepared in the range of 10–800 μg/mL. The sample solution also was prepared by dissolving 10 mg of extract in 1 mL methanol. Both the standard and sample solutions were filtered through Whatman NYL 0.45 μm syringe filter. The responses were measured as peak areas vs concentration.
2.5. Preparation of mobile phase
Mobile phase was prepared by using methanol and water in the ratio of 95: 5 (v/v). The pH of water was adjusted 3.2 with 1% (v/v) glacial acetic acid and then both solvent was filtered through 0.45 mm Millipore membrane filter followed by ultra-sonication to de-gas the solvent.
2.6. Method validation
The method was validated for linearity, specificity, limit of detection (LOD), limit of quantification (LOQ), accuracy and precision according to ICH guidelines [21].
2.6.1. Linearity
The linearity range of oleanolic acid was analyzed (n = 6) of the standard solutions containing of oleanolic 10–800 μg/mL, in the optimized chromatographic conditions. The calibration curve was made by plotting the main peak area in Y-axis vs the concentration in X-axis and linearity was determined by the linear regression analysis.
2.6.2. Specificity
The specificity of the method was determined by comparing the retention time of the standard and test samples. This mainly ensures the identity and the purity of the analyte and to minimize the error of the result.
2.6.3. Limit of detection (LOD) and limit of quantification (LOQ)
The LOD and LOQ were calculated based on the ICH guideline by determining the SD of the response (σ) and the slope of the linear equation (S). The LOD and LOQ were calculated by the following equation LOD = 3.3 σ/S, LOQ = 10 σ/S.
2.6.4. Accuracy and precision
Intra-day and inter-day assay accuracy and precision for each analyte were determined at Low quality control (LQC), Medium quality control (MQC) and High quality control (HQC). Both data were assessed by comparing the data within one run (n = 6). Accuracy of the method was determined by standard addition technique and expressed in terms of % RSD. The precision of the method was analyzed by performing intra-day and inter-day variation, assessed by injecting six replicates at three different concentrations of the reference compounds. The values were represented as % RSD.
2.6.5. Robustness
Robustness study was performed by changing different mobile phase composition, flow rate and detection of wave length to determine their influence on the retention time.
Statistical analysis was performed using the Graph Pad Prism Version 5.0 and results are represented as the mean ± % RSD.
3. Results
3.1. Optimization of chromatographic conditions
The chromatographic separation was determined through reversed-phase C18 column (Waters Spherisorb 5 mm ODS2, 250 mm × 4.6 mm, 5 μm particle size) at optimum temperature (25 °C). The mobile phase consisted of acetonitrile: water (1% glacial acetic acid) in the ratio of 95:5 (v/v) at 1.0 ml/min flow rate. The λmax was set at 210 nm.
3.2. Linearity
The calibration range of oleanolic acid was found to be 10–800 μg/mL. The coefficient of determinants (r2) of oleanolic acid was found to be 0.995 with linear equation Y = 19462X + 16,172.
3.3. Specificity
By the specificity test, the well-shaped peak indicated that other constituent present in the V. articulatum aerial part does not interfere with the main peak of oleanolic acid.
3.4. Limit of detection (LOD) and limit of quantification (LOQ)
The LOD and LOQ of oleanolic acid were found to be 0.197 ± 0.63 and 0.623 ± 0.87 μg/mL respectively.
3.5. Accuracy
The high recovery values for oleanolic acid (99.49–99.91%) indicated the accuracy of the method (Table 1).
Table 1.
Recovery studies for determination of oleanolic acid in Viscum articulatum extract.
| Biomarker | Amount added | Sample concentration (μg/mL) | Theoretical concentration (μg/mL) | Actual concentration (μg/mL) | Percentage recovery |
|---|---|---|---|---|---|
| Oleanolic acid | 100 | 1238.5 | 1338.5 | 1331.7 | 99.49 |
| 400 | 1238.5 | 1638.5 | 1634.2 | 99.73 | |
| 800 | 1238.5 | 2038.5 | 2036.8 | 99.91 |
3.6. Precision
The % RSD of intra-day and inter-day precision was found to be <2%, which confirms high repeatability of the method (Table 2).
Table 2.
Intra-day and inter-day precision of oleanolic acid by using HPLC method.
| Oeanolic acid | |||||||
|---|---|---|---|---|---|---|---|
| Intra-day (n = 6) |
Inter-day (n = 6) |
||||||
| RT (min) |
Response (AU) |
RT (min) |
Response (AU) |
||||
| Mean | % RSD | Mean | % RSD | Mean | % RSD | Mean | % RSD |
| 21.54 | 0.45 | 24,032.33 | 1.74 | 21.53 | 0.70 | 22,802.00 | 1.98 |
| 21.51 | 1.02 | 25,407.67 | 1.03 | 21.57 | 0.97 | 23,876.33 | 1.69 |
| 21.52 | 1.11 | 28,228.00 | 1.86 | 21.53 | 1.06 | 27,192.00 | 1.52 |
3.7. Robustness
The robustness was evaluated by analyzing (n = 6) the standard solution of oleanolic acid (400 μg/mL) under the small changes (±2) in the optimum conditions such as column temperature, flow rate, detection of wavelength and pH. But no significant changes were observed in the retention time, peak area and recovery study.
3.8. Content of oleanolic acid in V. articulatum
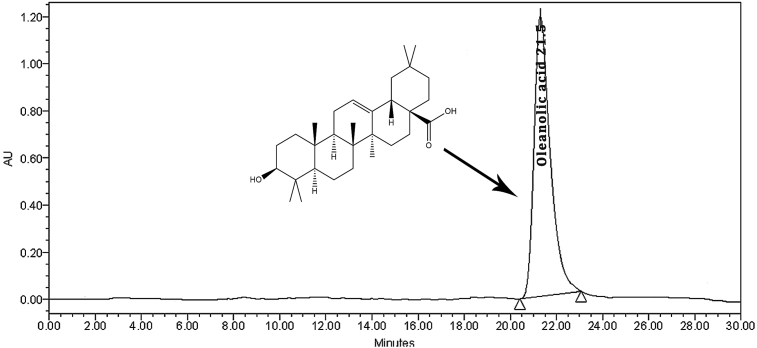
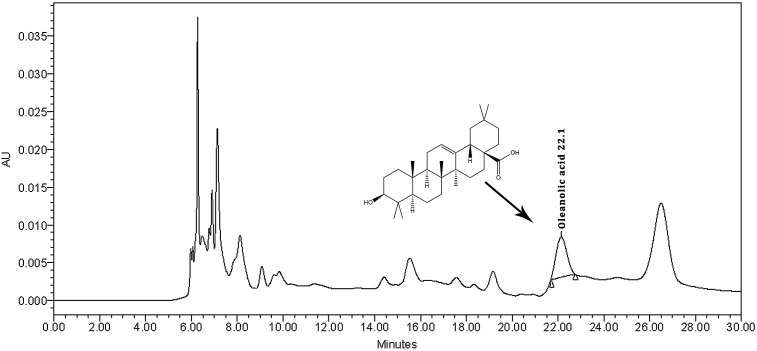
The mean retention time of oleanolic acid was observed 21.5 ± 0.05 min by comparing between standard (Fig. 1) and extract chromatograms (Fig. 2). The content of oleanolic acid in V. articulatum aerial part methanol extract was 1.96% (w/w).
Fig. 1.
RP-HPLC chromatogram of oleanolic acid standard.
Fig. 2.
RP-HPLC chromatogram of Viscum articulatum extract.
4. Discussion
Major challenges for Quality of Herbal medicine may vary depending on harvest seasons, plant origins, drying processes, contamination of heavy metals, microbial contains and other related factors. Thus, it is utmost essential to determine most of the phytochemicals of medicinal plant products in order to ensure the reliability and repeatability of pharmacological research to ensure the quality of the medicinal plant products [22]. Chemical marker plays an important role to ensure the quality of medicinal plants and their products. The limited evidence of chemical marker remains a major problem for the quality control of herbal medicines. Keeping view, our present study dealt with the RP-HPLC method validation of aerial part of V. articulatum plant to ensure the content of active phytomolecule as well as reproducibility of the developed method [23]. The developed method was validated to quantify the amount of oleanolic acid in the aerial part of V. articulatum. This method was also found accurate, specific, precise, robust and reproducible with a narrow linearity range. The amount of oleanolic acid found in V. articulatum aerial part is 1.96% (w/w). The presence of oleanolic acid was found significant amount in V. articulatum.
5. Conclusion
This HPLC may be useful for quantitative estimation of the chemical constituents present in the plant extract as well as quality assessment of the herbal product.
Sources of funding
Tata Innovation Fellowship programme (D.O. No. BT/HRD/35/01/04/2014), Dept. of Biotechnology, New Delhi.
Conflicts of interest
None.
Acknowledgements
The authors are thankful to the Department of Biotechnology, Government of India, New Delhi, India for financial support.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Mukherjee P.K., Rai S., Bhattacharya S., Wahile A., Saha B.P. Marker analysis of polyherbal formulation, triphala-a well-known Indian traditional medicine. Indian J Tradit Know. 2008;7:379–383. [Google Scholar]
- 2.Harwansh R.K., Mukherjee K., Bhadra S., Kar A., Bahadur S., Mitra A. Cytochrome P450 inhibitory potential and RP-HPLC standardization of trikatu - a rasayana from indian ayurveda. J Ethnopharmacol. 2014;153:674–681. doi: 10.1016/j.jep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi S., Ray S., Mondal A.K., Verma N.K. Rare ethno medicinal plants of south West Bengal, India with their different medicinal uses: needs conservation. Int J Life Sc Bt Pharm Res. 2013;2(2):114–122. [Google Scholar]
- 4.Li Y., Zhao Y.L., Huang N., Zheng Y.T., Yang Y.P., Li X.L. Two New phenolic glycosides from Viscum articulatum. Molecules. 2008;13:2500–2508. doi: 10.3390/molecules13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indian Biodiversity Portal (IBP) 2012. Viscum articulatum Burm. [Google Scholar]
- 6.Bachhav S.S., Bhutada M.S., Patil S.D., Baser B., Chaudhari K.B. Effect of Viscum articulatum Burm. (Loranthaceae) in Nw-nitro-L-arginine methyl ester induced hypertension and renal dysfunction. J Ethnopharmacol. 2012;142:467–473. doi: 10.1016/j.jep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Shanavaskhan A.E., Sivadasan M., Alfarhan Ahmed H., Thomas J. Ethnomedicinal plantsfrom Paderu division of Visakhapat-nam district, AP, India. IJTK. 2012;11(2) [Google Scholar]
- 8.Patel B.P., Singh P.K. Viscum articulatum Burm. f.: a review on its phytochemistry, pharmacology and traditional uses. J Pharm Pharmacol. 2017 doi: 10.1111/jphp.12837. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarani D.K., kumbhojkar M.S. Traditional use of family Loranthaceae from Western Maharastra, India. Anc Sci Life. 2002;21(3):178. [PMC free article] [PubMed] [Google Scholar]
- 10.Kirtikar K., Basu B.D. 1987. Indian medicinal plants. Dehradun, India. [Google Scholar]
- 11.Nag M., Mukherjee P.K., Biswas R., Chanda J., Kar A. Evaluation of antimicrobial potential of some indian ayurvedic medicinal plants. Pharmacogn J. 2016;8(6):525–533. [Google Scholar]
- 12.Jadhav N., Patil C.R., Chaudhari K.B., Wagh J.P., Surana S.J., Jadhav R.B. Diuretic and natriuretic activity of two mistletoe species in rats. Pharmacogn Mag. 2010 doi: 10.4103/0974-8490.60576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandit S., Mukherjee P.K., Ponnusankar S., Venkatesh M., Srikanth N. Metabolism mediated interaction of α-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 2011;82:369–374. doi: 10.1016/j.fitote.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Pandit S., Bandyopadhyay A., Ponnusankar S., Ota S., Mukherjee P.K. Exploring the possible metabolism mediated interaction of Glycyrrhiza glabra extract with CYP3A4 and CYP2D6. Phytother Res. 2011;25:1429–1434. doi: 10.1002/ptr.3426. [DOI] [PubMed] [Google Scholar]
- 15.Pandit S., Kumar M., Ponnusankar S., Pal B.C., Mukherjee P.K. RP-HPLC-DAD for simulataneous estimation of Mahanine and Mahanambine in Murraya koenigii. Biomed Chromatogr. 2011;25:959–962. doi: 10.1002/bmc.1561. [DOI] [PubMed] [Google Scholar]
- 16.Ahmmed Sk M., Mukherjee P.K., Bahadur S., Harwansh R.K., Kar A., Bandyopadhyay A. CYP450 mediated inhibition potential of Swertia chirata: an herb from Indian traditional medicine. J Ethnopharmacol. 2016;178:34–39. doi: 10.1016/j.jep.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Chanda J., Mukherjee Pulok K., Harwansh R., Bhadra S., Chaudhary S.K., Choudhury S. RP-HPLC simultaneous estimation of betulinic acid and ursolic acid in Carissa spinarum. Nat Prod Res. 2014 doi: 10.1080/14786419.2014.953496. [DOI] [PubMed] [Google Scholar]
- 18.Yann-Lii Leu, Shih-Ming Kuo, Tsong-Long Hwang, Shau-Ting Chiu. The inhibition of superoxide anion generation by neutrophils from Viscum articulactum. Chem Pharm Bull. 2004;52(7):858–860. doi: 10.1248/cpb.52.858. [DOI] [PubMed] [Google Scholar]
- 19.Kuo Y.J., Yang Y.C., Zhang L.J., Wu M.D., Kuo L.M.Y., Kuo Y.C. Flavanone and 1-phenylpropane glycosides and glycosidic acyl esters from Viscum articulatum. Nat Prod Res. 2010;73:109–114. doi: 10.1021/np9004294. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee P.K. 1st ed. Business Horizons; New Delhi: 2002. Quality control on herbal drugs. [Google Scholar]
- 21.ICH Q2 (R1) 2005. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, validation of analytical procedures: text and methodology. [Google Scholar]
- 22.Mukherjee P.K., Wahile A. Perspectives of safety for natural health products. In: Sharma R.K., Arora R., editors. Herbal drugsda twenty first century perspectives. Jaypee Brothers. Medicinal Publishers Ltd; New Delhi: 2006. [Google Scholar]
- 23.Mukherjee P.K., Bahadur S., Chaudhary S.K., Kar A., Mukherjee K. In: Quality related safety issue-evidence-based validation of herbal medicine farm to pharma in evidence based validation of herbal medicine. Mukherjee P.K., editor. Elsevier Science; Netherlands, USA: 2015. pp. 1–28. [Google Scholar]