Abstract
Background
Laghu Sutashekhara Rasa (LSR) is a herbo mineral formulation containing Shuddha Gairika (Fe2O3) and Shunthi (Zingiber officinale Roxb.) with the levigation of Nagawalli Swarasa (fresh juice of Piper betel Linn.) prepared as per the reference of Rasatarangini Parishistha. This is an important formulation in Ayurveda therapeutics, but its shelf life is not evaluated till date. The Govt. of India Gazette specifies the shelf life of various Ayurvedic medicines. However, there is a need to revalidate the shelf life of individual formulations by following parameters prevalent in respective scenario.
Objectives
To evaluate shelf life of Laghu Sutashekhara Rasa.
Materials and methods
Laghu Sutashekhara Rasa was prepared in the Pharmacy, Gujarat Ayurved University, Jamnagar following classical guidelines. The samples were subjected to accelerated stability study maintaining temperature and humidity 40 ± 2 °C and 75 ± 5% respectively. Relevant analytical parameters were analyzed at an interval of 0, 1, 3 and 6 months to check the degradation levels in the formulation.
Result
Product was free from microbial contamination and heavy metals were within the prescribed limits. There were insignificant changes in physico-chemical profiles at different intervals of analysis. On extrapolation of the observations, the shelf life of Rasayoga was found to be 2 years and 8 months.
Conclusion
The shelf life of Laghu Sutashekhara Rasa was found to be less than the given standards in official gazettes of Govt. of India. This decreased shelf life may be because of the predominantly (approximately 70%) herbal component present in the formulation.
Keywords: Accelerated stability, Laghu Sutashekhara Rasa, Saviryata avadhi, Shelf life
1. Introduction
Shelf-life of a drug product is defined as the time at which the average drug characteristic (e.g., potency) remains within an approved specification after manufacture [1]. Shelf life depends on the degradation mechanism of a specific product. It can be influenced by factors like:-exposure to light, heat, moisture, transmission of gases, mechanical stresses, contamination by micro-organisms etc. [2] General belief that Ayurvedic medicines do not have any expiry date and their shelf life is infinite is not true. In Ayurveda, Shelf life is known under the term Saviryata Avadhi i.e. indicative of specific time period during which the Virya (potency) of the drug remain above certain threshold provided that it is stored in mentioned conditions. The concept of Virya explained in ancient Ayurvedic literature is very clear and it denotes the main property which is solely responsible for all the therapeutic actions of the drug. Although this concept was exclusively described in text such as Sharangadhara Samhita for different Ayurvedic dosage forms. Acc. to Sharangadharacharya, generally medicinal recipes lose their potency after one year of their preparation; Churnas (powders) after two months, Gutikas (pills) and Lehyas (confections) after one year; Ghritas and Taila (Ghee, oils) after four months; recipes which are digested easily and quickly become poor in action after one year; while Asavas (fermented liquors) and Dhatus (metal and mineral recipes) become more potent as they become old [3]. Rule 161 (B) of Drugs and Cosmetics Act, 1940 and rules has made it mandatory to print the manufacture and expiry date of all Ayurvedic, Siddha and Unani (ASU) drugs from April 1, 2010 onwards and specified as-“Under no circumstances, consumers should buy these drugs after their expiry date”. Though, shelf life of different categories of Ayurveda formulations has been mentioned in the Gazettes of Govt. of India; there is a need to evaluate shelf life of individual formulations [4]. However, no stability profiles of this formulation are available till date. Considering this, an attempt has been made to evaluate shelf life of Laghu Sutashekhara Rasa [5] (LSR) with the help of modern analytical techniques.
2. Materials and methods
2.1. Collection of raw materials
LSR is a herbo-mineral formulation consisting of powdered Shuddha Gairika and Shunthi in the ratio of 2:1 respectively and levigated with Nagawalli Swarasa. Gairika and Shunthi were procured from the Pharmacy, Gujarat Ayurved University, Jamnagar and were authenticated in the Pharmacognosy Laboratory, Institute for Post Graduate Teaching and Research in Ayurveda, Jamnagar.
2.2. Pharmaceutical preparation of formulation
LSR was prepared out of Shuddha Gairika (Fe2O3) and Shunthi (Zingiber officinale Roxb.) with the levigation of Nagawalli Swarasa (fresh juice of Piper betel Linn.) and converted into tablets. For Shodhana of Gairika, it was roasted with 1/4th part of cow ghee till the appearance of chief desire characteristics Betel leaves were washed with water, chopped into small pieces, grinded in grinder machine and juice was expressed out. This juice was used as the levigating media. The finished product thus obtained was converted into tablets through wet granulation method [6] by adding 1% gum acacia and 1% sugar as binding agents.
2.3. Shelf life evaluation
2.3.1. Sample quantity and packing
Samples were supplied in four transparent plastic bottles with transparent screw cap. Each bottle contains 100 g of LSR. Voucher specimen number of the ingredient is R-2179.
2.4. Storage conditions
Accelerated stability study was conducted as per ICH Guidelines Q1A (R2) [7]. Temperature was maintained at 40 °C ± 2 °C while relative humidity was maintained at 75% ± 5%.
2.4.1. Frequency of withdrawal
The products were withdrawn from the container and analyzed initially, and at a gap of 1, 3 and 6 months.
2.4.2. Parameters
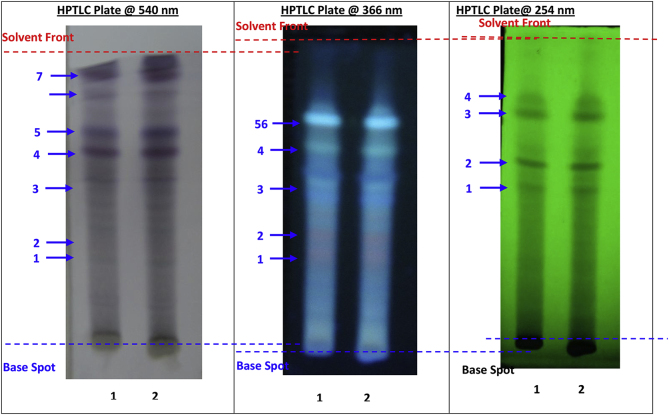
Basic analytical parameters including Average weight of tablets mg, Tablet hardness kg/cm [2], Loss on drying %w/w [8], Water soluble extractive %w/w [9] and Total ash contents %w/w [10] were evaluated at intervals specified earlier. Test for microbial contamination was done initially and at the end of 6 months of storage by following standard guidelines [11]. Chromatographic profiles (HPTLC) were evaluated under 254, 366 and 540 nm initially and after six months of storage. Analysis for Heavy metals using AAS was carried out initially [12].
3. Results
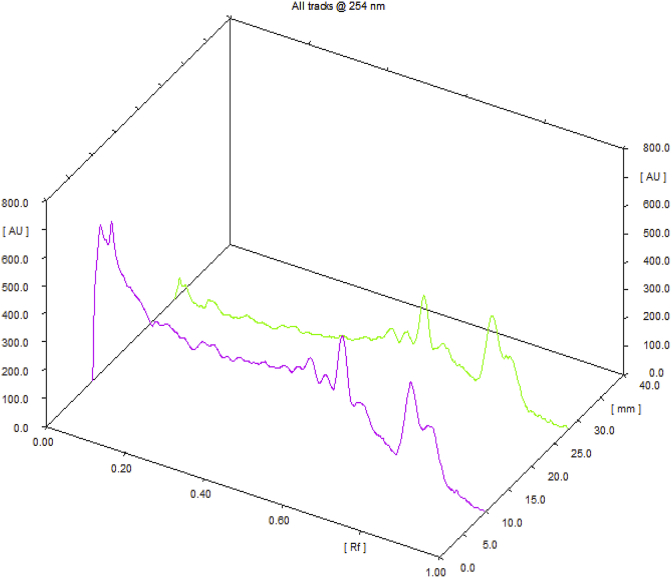
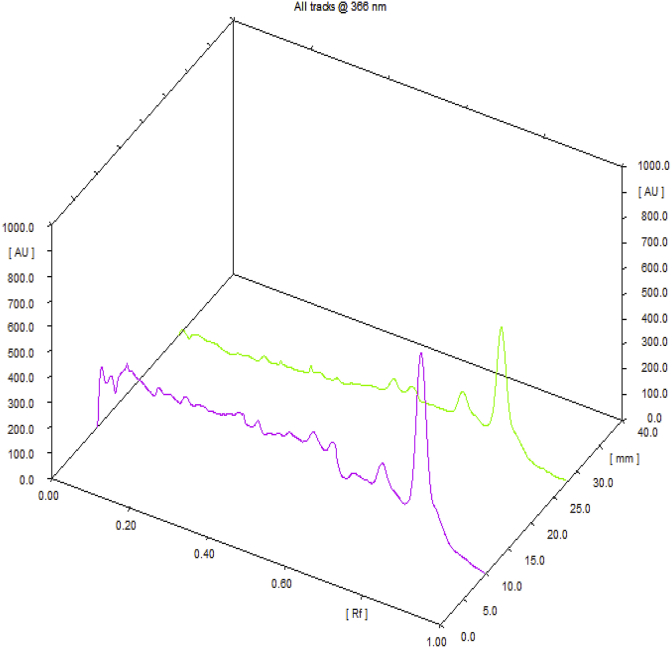
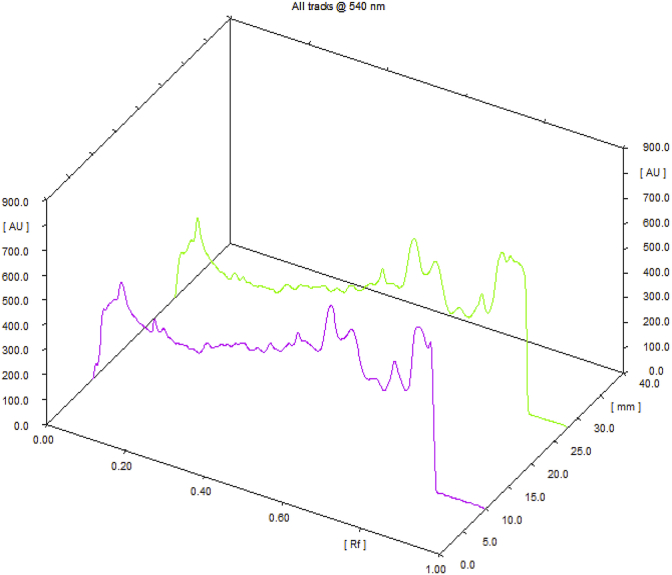
Observations of Physico-chemical analysis of LSR at initial, 1, 3, 6-month interval are shown in Table 1. Water Soluble Extractive value was observed 6.62 at initial stage, 6.03 at one month interval, 5.22 at three months interval and 3.73 at six months interval. Microbial growth was found below prescribed limits [13] initially and after 6th month [Table 2]. Heavy metals (Mercury, Cadmium, Arsenic, Lead) were also found to be within the prescribed limits [14]. HPTLC showed 4 spots at 254 nm with Rf values 5 spots at 366 nm and 7 spots at 540 nm (Fig. 1, Fig. 2, Fig. 3, Fig. 4). Rf values recorded were similar in both samples initially and at the end of 6th month. Based on the physico-chemical values, intercept and slope were calculated followed by expected time for 10% degradation for individual parameters. 10% degradation was set as the acceptable point to extrapolate the accelerated stability data. Real time aging factor 5 and 3.3 was used for extrapolation of shelf life for climatic Zone I & II countries and climatic Zone III & IV countries respectively. India comes under climatic zone III & IV. Number of months when 10% degradation was occurred was calculated using following formula:
| Months when 10% degradation occurs = [0 month assay value − {[0 month assay value × 10/100}] − Intercept Slope |
Table 1.
Physico chemical parameters of LSR.
| Physico chemical parameters | ||||
|---|---|---|---|---|
| Parameters | 0 month (Initial) | 1st month | 3rd month | 6th month |
| Average weight of tablets | 501 mg | 501 mg | 501 mg | 501 mg |
| Tablet hardness | 2 kg/cm2 | 2 kg/cm2 | 2 kg/cm2 | 2 kg/cm2 |
| Loss on drying (%w/w) | 2.70 | 2.63 | 2.63 | 2.50 |
| Water soluble extractive (%w/w) | 6.62 | 6.03 | 5.22 | 3.73 |
| Total Ash (%w/w) | 59.73 | 60.58 | 59.96 | 61.90 |
Table 2.
Total microbial growth in LSR.
| Organism | 0 month (Initial) | “6” months | Permissible Limits |
|---|---|---|---|
| Total plate count (cfu/g) | <10 cfu/g | <10 cfu/g | 105/g |
| Total fungal count (cfu/g) | <10 cfu/g | <10 cfu/g | 103/g |
| E. coli | Ab | Ab | Absent |
| Pseudomonas aeruginosa | Ab | Ab | Absent |
| Staphylococcus aureus | Ab | Ab | Absent |
| Salmonella Spp | Ab | Ab | Absent |
cfu: Colony Forming Units, Ab: Absent, g: Grams.
Fig. 1.
Comparative HPTLC Plate @ 540 nm, 366 nm and @254 nm.
Fig. 2.
3D overlay chromatogram @ 256 nm.
Fig. 3.
3D overlay chromatogram @ 366 nm.
Fig. 4.
4D overlay chromatogram @ 540 nm.
For present study, On extrapolation of these values; mean months for 10% degradation was found 9.89 and the shelf life of LSR was found to be 2 years and 8 months [Tables 3 and 4].
Table 3.
Intercept and slope of LSR for different parameters.
| Parameters | Intercept | Slope |
|---|---|---|
| Loss on Drying | 2.69 | 0.03 |
| Water Soluble Extract | 6.58 | 0.472 |
| Ash Value | 59.78 | 0.306 |
Table 4.
Approximate period (in month) for 10% degradation of LSR.
| Parameters | Initial (0 month) | 10% Degradation | Approximate Months required for 10% degradation |
|---|---|---|---|
| Loss on Drying | 2.70 | 2.43 | 8.66 |
| Water soluble extract | 6.62 | 5.958 | 1.32 |
| Ash Value | 59.73 | 53.757 | 19.68 |
| Mean Months | 9.886667 | ||
4. Discussion
Saviryata Avadhi is the time limit by which the drug reduces its original potency up to some extent and can be utilized for therapeutic purposes until it retains its fragrance, color, and taste etc. [15] The purpose of shelf life testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of variety of environmental factors such as temperature, humidity, and light, to establish a retest period for drug substance or a shelf life for drug products. Insignificant differences were observed in basic physico-chemical profiles in the drugs at different stages of analysis except Water soluble extractive value. The moisture content was found to be decreasing gradually with storage. Moisture is one of the main parameters that determine the shelf life of a product, and is the main causative factor in product deterioration. Moisture in a product is sufficient to activate different enzymes, which slowly decompose the product resulting in its degradation [16]. Water soluble extractive value plays an important role in evaluation of crude drugs. Less extractive value indicates addition of exhausted material, adulteration or incorrect processing during drying or storage or formulating. A high ash value is indicative of contamination, substitution, adulteration or carelessness in preparing the drug or drug combinations for marketing [17]. In present study, decreasing value of Water soluble extract and increasing value of Ash is indicates less efficacy of formulation after a period. Microbial count and Heavy metals were within the prescribed limits indicating safety and quality of the product.
A few studies reported shelf life of Rasayana Churna [18], Vasavaleha [19], and Kamsaharitaki Avaleha [20], Shirishashwagandhadi Avaleha [21], Hridya Yoga Churna [22], Shirishavaleha [23] but for LSR, the same is not available. Findings of earlier studies make substantiate to results of LSR except Shirishashwagandhadi Avaleha that is found more stable. The changes observed in the physico-chemical parameters at regular intervals were analyzed to evaluate the shelf life of LSR that is found to be 2 years and 8 months. Sharangadharacharya opines that, the Rasa preparations retain therapeutic potency for long time, while general shelf life mentioned in the official gazettes for Gutika/tablet containing Rasa/Uparasa/Bhasma along with Kasthaushadhi is five years and for Gutika/tablet containing only Kasthaushadhi is two years. As LSR contains Shunthi in bulk amounts and also levigated with Nagawalli Swarasa i.e. herbal fraction in its composition; possibly the shelf life came down to 2 years and 8 months.
5. Conclusion
Microbial count and heavy metals were within permissible limits in sample indicating its standards and safety for therapeutic utilization. Shelf-life of LSR is found to be 2 years and 8 months using the analytical parameters i.e. Loss on drying, Water soluble extractive value, Ash value. This observation is specific to LSR. No study has been carried out on shelf life of herbo-mineral Rasayoga till date. Studies involving many more Rasayoga with Kasthaushadhi needed to substantiate the observations of the current study.
Sources of funding
IPGT&RA, Gujarat Ayurved University, Jamnagar, India.
Conflicts of interest
None.
Acknowledgments
Authors are sincerely thankful to the management of Vasu Healthcare Pvt. Ltd., for the state of the art testing facility to carry out this work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Shao J., Chung S. Chow University of Wisconsin and Stat Plus, Inc Statistica Sinica; 2001. Drug shelf-life estimation; pp. 737–745. [Google Scholar]
- 2.Azanha A.B., Faria J.A.F. Use of mathematical models for estimating the shelf-life of cornflakes in flexible packaging. Packag Technol Sci. 2005;Chapter 18(4):161–222. [Google Scholar]
- 3.Sharangadhara Samhita . Chaukhamba Orientalia; Varanasi: 2009. Sharangadhara, jivanprada Hindi commentary, poorva khanda, prathama adhyaya (1:51) p. 12. [Google Scholar]
- 4.Anonymous . Ministry of Health and Family Welfare, Dept. of AYUSH; notification New Delhi: 2005. Drugs and Cosmetics (Amendment) rules. [Google Scholar]
- 5.Tarangini Rasa, Sadananda Sharma. 11th ed. Motilal Banarasi Das publication; India: 2004. Rasa, tarangini parishista; p. 771. [Google Scholar]
- 6.Leon Lachman, Liberman HerbertA., Kanig Joseph L. 3rd ed. Varghese publishing house; India: 1999. “Tablets” the theory and practice of industrial pharmacy; pp. 320–321. [Google Scholar]
- 7.Anonymous . February, 2003. ICH harmonised tripartite guideline. Stability testing of new drug substances and products – Q1A (R2) [Google Scholar]
- 8.Anonymous . 1st ed. vol. I. Ministry of Health and Family Welfare; Part II; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-2, (2.2.10),141. [Google Scholar]
- 9.Anonymous . 1st ed. vol. I. Ministry of Health and Family Welfare; Part II; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-2, (2.2.8),141. [Google Scholar]
- 10.Anonymous . 1st ed. vol. I. Ministry of Health and Family Welfare; Part II; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-2, (2.2.3),140. [Google Scholar]
- 11.Anonymous . 1st ed. vol. I. Ministry of Health and Family Welfare; Part II; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-2, (2.4)163. [Google Scholar]
- 12.Anonymous . 1st ed. vol. I. Ministry of Health and Family Welfare; Part II; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-2, (2.3.3)153. [Google Scholar]
- 13.Anonymous . 1st ed. vol. IX. Ministry of Health and Family Welfare; Part I; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-3, (3.1.1)153. [Google Scholar]
- 14.Anonymous . 1st ed. vol. IX. Ministry of Health and Family Welfare; Part I; Govt. of India: 2007. (The ayurvedic pharmacopoeia of India). Appendix-3, (3.1.1)153. [Google Scholar]
- 15.Acharya Y.T., editor. vol. 6:16. Chaukhambha Surbharti Prakashana; Varanasi: 2011. p. 704. (Caraka Samhita of Agnivesha, (Siddhi Sthana)). [Google Scholar]
- 16.Sharma R., Amin H., Shukla V.J., Kartar D., Galib R., Prajapati P.K. Quality control evaluation of Guduchi Satva (solid aqueous extract of Tinospora cordifolia (Willd.) Miers): an herbal formulation. Int J Green Pharm. 2013;7(3):258–263. [Google Scholar]
- 17.Singh Chandel Harinarayan, Pathak A.K., Mukul Tailang. Standardization of some herbal antidiabetic drugs in polyherbal formulation. Pharmacogn Res. 2011 Jan-Mar;3(1):49–56. doi: 10.4103/0974-8490.79116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma P., Galib, Patgiri B., Prajapati P.K. Shelf-life evaluation of Rasayana Churna: a preliminary study. Ayu. 2014;35:184–186. doi: 10.4103/0974-8520.146247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ankit P., Galib, Patgiri B.J., Prajapati P.K. Shelf-life evaluation of Vasavaleha and its granules – a preliminary study. Sri Lanka J Indigen Med. 2014;4(1):242–245. [Google Scholar]
- 20.Khemuka N., Galib R., Patgiri B.J., Prajapati P.K. Shelf-life evaluation of Kamsaharitaki avaleha and its granules: a preliminary study. Ancient Sci Life. 2015;35(2):96–100. doi: 10.4103/0257-7941.171670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave Parth P., Vaghela D.B., Galib, Jadav Hasmukh R. Shelf life assessment of Shirishaashwagandhadi avaleha – a preliminary assessment. J Med Pharmaceut Allied Sci. 2016:302–313. [Google Scholar]
- 22.Unnikrishnan Vidhya, Nishteshwar Karra, Patel Bhupesh R. Shelf life evaluation of Hridya Yoga Churna. Phcog J. 2016;8(3):234–238. [Google Scholar]
- 23.Kaur H., Galib R., Prajapati P.K. Shelf life evaluation of Shirishavaleha: a preliminary study. BLDE Univ J Health Sci. 2016;1:120–124. [Google Scholar]