Abstract
Background
Baby corn silk extract can be used as an antioxidant dietary supplement. However, insufficient data exists for this extract to guarantee its quality, efficacy and safety.
Objective
To determine phytochemical constituents, contents of phenolics and flavonoids, antioxidant activities, heavy metal concentrations, and microbial contamination of baby corn silk extracts.
Material and methods
Baby corn silks including Pacific 271 and Zeba SG 17 hybrids were individually extracted with 40% v/v ethanol and distilled water to obtain Pacific 271 ethanol extract (PE), Zeba SG 17 ethanol extract (ZE), Pacific 271 aqueous extract (PA), and Zeba SG 17 aqueous extract (ZA). The analysis of phytochemical constituents was carried out using phytochemical tests, TLC screening, UV-visible, FTIR, and 1H NMR experiments. The contents of phenolics and flavonoids were determined using the modified Folin-Ciocalteu and aluminium chloride colorimetric procedures, respectively. Antioxidant activities were investigated using DPPH and FRAP assays. The concentrations of heavy metals were analyzed by ICP-MS. Microbial enumeration tests were carried out according to the United States Pharmacopeia (USP) 41.
Results
PE and ZE were composed of flavonoids, tannins, terpenoids, and steroids while PA and ZA contained flavonoids and tannins. PE and ZE exhibited higher total phenolic and flavonoid contents and significantly stronger antioxidant activities than PA and ZA. All extracts conformed to the microbiological and heavy metal requirements according to Association of South East Asian Nations (ASEAN) guidelines.
Conclusion
PE and ZE were considered appropriate to use as natural extracts of phenolics and flavonoids with antioxidant activities and safety.
Keywords: Corn silk, Antioxidant, Flavonoid, Contamination
Highlights
-
•
Baby corn silk including Pacific 271 and Zeba SG 17 hybrids at the silking stage were separately extracted with 40% v/v ethanol and distilled water to obtain Pacific 271 ethanol extract (PE), Zeba SG 17 ethanol extract (ZE), Pacific 271 aqueous extract (PA), and Zeba SG 17 aqueous extract (ZA).
-
•
PE and ZE were composed of flavonoids, tannins, terpenoids, and steroids while PA and ZA contained flavonoids and tannins. Additionally, antioxidants, flavone glycosides, and unsaturated and aromatic compounds were also found in all extract.
-
•
PE and ZE exhibited higher total phenolic and flavonoid contents and significantly stronger antioxidant activities than PA and ZA.
-
•
40% v/v Ethanol is suitable for extraction of phenolic compounds and flavonoids with antioxidant activities from baby corn silk.
1. Introduction
Corn silk, the stigma and style from the flower of maize (Zea mays Linn.), is a biological by-product from cultivation of corn and has been widely reported to exhibit various pharmacological activities such as anti-inflammatory, anti-depressant, anti-hyperlipidemic, anti-diabetic, anti-fatigue, and antioxidant activities as well as neuroprotective, diuretic, and kaluretic effects [1]. Corn silk is the origin of several bioactive materials including steroids, alkaloids, saponins, carotenoids, anthocyanins, and other phenolics with helpful effects on physical health such as reducing blood pressure, lowering blood glucose, decreasing inflammation, and promoting relaxation [1,2]. The content of phenolic compounds, particularly flavonoids and anthocyanins in corn silk was in good correlation with their biological activities [3,4]. Corn silk is also composed of lipids, carbohydrates, proteins, vitamins, minerals, and volatiles oils [5]. In order to promote their safe use, the toxicity study in Wistar rats suspected that corn silk was harmless because of the absence of histopathological and adverse effects [6].
The antioxidant properties of plant extracts were attributed to the contents of phenolics and flavonoids. Furthermore, antioxidant extractions were affected by the polarity of used solvents [7]. Heavy metals and pathogens were the most common contaminants found in plant extracts. These harmful contaminants may derive from the environment in which the plants are cultivated, storage of plant materials, and extraction conditions [8]. The production of commercial baby corn with the use of fertile baby corn hybrids including Pacific 271 and Zeba SG 17 needs detasseling [9]. Baby corn silk, unfertilized female flowers, may have valuable substances, which can be employed as antioxidant dietary supplements. The information regarding the consequences of extracting solvents and antioxidant capacities of baby corn silk is still lacking. Furthermore, heavy metal and microbial contamination is related to the quality control of baby corn silk extracts. Therefore, the goal of this research was to compare phytochemical constituents, contents of phenolics and flavonoids, antioxidant activities, heavy metal concentrations, and microbial contamination of baby corn silk extracts obtained from Pacific 271 and Zeba SG 17 hybrids.
2. Materials and methods
2.1. Chemicals
Ultrapure water was generated by GenPure equipment (TKA Wasseraufbereitungssysteme GmbH, Germany). Analytical grade solvents including ethanol, methanol, chloroform, acetic anhydride, glacial acetic acid, hydrochloric acid, nitric acid, and sulfuric acid and phytochemical reagents including bismuth sub-nitrate, bromine solution, ferric chloride, gelatin solution, iodine, magnesium ribbons, potassium iodide, p-anisaldehyde, Dragendorff's reagent, and iodine were acquired from Sigma–Aldrich Chemical Corporation (St. Louis, MO, USA). Potassium bromide (FTIR grade), Folin-Ciocalteu, l-ascorbic acid, and ICP multi-element standard solution XIII were supplied by Thermo Fisher Scientific (Massachusetts, USA), Loba Chemie (Mumbai, India), Chem-Supply Pty Ltd (Gillman, South Australia), and Agilent Technologies (Santa Clara, USA), respectively. Dimethyl sulfoxide-d6, gallic acid, sodium carbonate, aluminium chloride, and rutin hydrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), acetate buffer, and ferric chloride hexahydrate were obtained from Sigma–Aldrich Chemical Corporation (St. Louis, MO, USA). General purpose nutrient media, selective media, and silica gel 60 F254 aluminium sheets were bought from Merck KGaA (Darmstadt, Germany).
2.2. Baby corn silk and extraction
Baby corn silk samples were collected from Pacific 271 and Zeba SG 17 hybrids that were cultivated through organic farming at the Siam Ostrich Farm in Song Phi Nong District, Suphan Buri, Thailand. The samples were identified, authenticated, and deposited at the herbarium of Department of Pharmacognocy, Faculty of Pharmacy, Silpakorn University, Thailand. The colors of silk were yellow and red (Fig. 1). The silk was harvested 7 days (silking stage) after their emergence. Fresh silk was cleaned with tap water, drenched with distilled water, and dried in an oven at 45 °C until constant weight. The dried silk was mashed into a fine powder (particle size < 0.149 mm) and extracted individually using 40% v/v ethanol and distilled water. Each dried sample (300 g) was macerated three times with 40% v/v ethanol (1.5 L) for 24 h at room temperature and digested with distilled water (1.5 L) for 1 h at 45 °C. The extract solutions were filtered separately through a filter paper (Whatman No. 1) and then the solvents were evaporated using a rotary evaporator (R-100, Buchi, Japan) at 40 °C. The water residue in each extract was eliminated using a freeze dryer (Model 6112974, Labconco, USA). Appearances of extracts including Pacific 271 ethanol extract (PE), Zeba SG 17 ethanol extract (ZE), Pacific 271 aqueous extract (PA), and Zeba SG 17 aqueous extract (ZA) were of dark brown mass, as shown in Fig. 2. The percent yield was expressed as the mass of extract obtained per 100 g of dried baby corn silks. All extracts were stored at −20 °C before analysis.
Fig. 1.
Characteristics of corn silk hybrids Zeba SG 17 (A) and Pacific 271 (B).
Fig. 2.
Appearances of Pacific 271 ethanol extract (PE), Zeba SG 17 ethanol extract (ZE), Pacific 271 aqueous extract (PA), and Zeba SG 17 aqueous extract (ZA).
2.3. Qualitative analysis
2.3.1. Phytochemical investigation
Each of the extracts was exposed to phytochemical analysis for identification of chemical components using procedures as described previously [[10], [11], [12], [13], [14], [15]]. All tests were done in triplicate.
2.3.1.1. Tests for flavonoids
Cyanidin's test (Shinoda's test): The methanolic solution of the extract when treated with magnesium ribbons and concentrated hydrochloric acid gave the magenta color of flavonoid solutions.
Ferric chloride test: The extract was reacted with some drops of 10% w/v ferric chloride solution. The resulting blackish green color designates the existence of flavonoids.
2.3.1.2. Tests for tannins
Gelatin solution: 5% w/v Gelatin solution and 10% w/v sodium chloride solution were poured into the solution of extract. If white precipitates are obtained, tannins are present.
Ferric chloride test: 10% w/v Ferric chloride solution was added to the extract solution. A green or brownish green color indicates the presence of tannins.
Bromine water test: The bromine solution was added to the extract solution. If yellow precipitates are observed, tannins are present.
2.3.1.3. Tests for steroids and terpenoids
Liebermann–Burchard test: The extract was treated with acetic anhydride and chloroform followed by concentrated sulfuric acid, and shaken well. Appearance of green and reddish brown colors indicates the presence of steroids and terpenoids, respectively.
Salkowski test: Each extract solution was added to chloroform, and then concentrated sulfuric acid was carefully poured into a mixture. The development of reddish purple and reddish brown at the interface confirmed the presence of steroids and terpenoids, respectively.
2.3.1.4. Tests for alkaloids
Dragendroff's test: The extract solution was treated with Dragendorff's reagent (bismuth potassium iodide) and the development of orange red precipitates indicates the existence of alkaloids.
Wagner's test: The Wagner's reagent (iodine in potassium iodide) was added to the extract solution. The emergence of reddish brown precipitates indicates the existence of alkaloids.
2.3.2. Thin layer chromatography (TLC) investigation
TLC analysis of extracts was with a stationary phase of TLC silica gel 60 F254 aluminium sheets, a migratory distance of 8 cm. Five microliters of each extract solution (20 mg/mL) was applied on a TLC plate. Mobile phase systems were chloroform: methanol: water: glacial acetic acid (6:5:1:1, v/v/v/v) and chloroform: methanol: glacial acetic acid (7:3:1, v/v/v). The TLC chromatograms were detected under visible light and short-wave UV light at 254 nm (Camag UV cabinet, USA). The spots on the plates were then exposed to different visualizing reagents such as Dragendorff's reagent, 10% v/v sulfuric acid - anisaldehyde, DPPH, and iodine vapor [16]. The detected spots were recorded according to their retardation factor (Rf) values.
2.4. Compound characterization
UV-visible spectrophotometric analysis was conducted on extracts dissolved in ultrapure water using UV-visible spectrophotometer (Model U-2990, Hitachi, Japan) with a 10 mm path length cell at room temperature. The extracts were examined under UV and visible light in the wavelength ranging from 250 to 800 nm. The functional groups and chemical structures of all extracts were elucidated using Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR) at 400 MHz and Fourier-Transform Infrared Spectroscopy (FTIR) in the region 4000-400 cm−1. For 1H NMR experiment, approximately 15 mg of dried extract was solubilized in 5 mL of dimethyl sulfoxide-d6 with an internal standard (tetramethylsilane, TMS) and then it was transferred to the NMR tube. 1H NMR spectra were obtained at 25 °C using a Bruker AVANCE 400 NMR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). In order to prepare the translucent pellet, approximately 5 mg of the dried extract was blended with 100 mg of dried potassium bromide and then compressed using a hydraulic press. FTIR spectra were recorded on a FTIR spectrometer (Thermo Electron Scientific Instruments Corporation, Madison, WI, USA).
2.5. Quantitative analysis
2.5.1. Total phenolic contents
The contents of phenolics in all extracts were quantified in line with the modified Folin-Ciocalteu method [15]. Briefly, 1 mg/mL extract solution (0.5 mL) was combined thoroughly with 10% v/v Folin-Ciocalteu reagent (3 mL) for 5 min, and then added 7.5% w/v sodium carbonate solution (2.5 mL). All solutions were kept at 25 °C in the dark for 60 min and absorbance measurements were carried out at 765 nm using a U-2990 UV-visible spectrophotometer (Hitachi, Japan). From the calibration curve of gallic acid solutions at concentrations of 20, 30, 50, 60, 80, 100 μg/mL, the contents of phenolics were calculated as mg of gallic acid equivalents per g of dried extract (mg GAE/g dried extract). All extracts were performed in triplicate.
2.5.2. Total flavonoid contents
The contents of flavonoids in all extracts were evaluated by the modified aluminium chloride colorimetric procedure [17] and rutin hydrate was employed as a calibration standard. In brief, 1 mg/mL extract solution (0.5 mL) was combined with methanol (2.5 mL) and then 0.01 M aluminium chloride solution (3.0 mL) was added. After incubation at 25 °C for 10 min, the absorbance of reaction solution was read at 400 nm with a U-2990 UV-visible spectrophotometer (Hitachi, Japan). The content of flavonoids was established using the standard curve of rutin at concentrations of 20, 30, 50, 60, 80, 100 mg/mL in methanol and expressed as mg of rutin hydrate equivalents per g of extract in dry mass (mg RE/g dried extract). Triplicate analyses were performed on each extract.
2.6. Antioxidant activity measurements
2.6.1. DPPH assay
The DPPH free radical scavenging activities of extracts were measured as described earlier [15]. Initially, 1 mg/mL extract solution (2.5 mL) was mixed with 0.2 mM DPPH in methanol (3.5 mL) and then the reaction solution was kept in the dark at room temperature for 30 min. Absorbance of solution was recorded at 517 nm using a U-2990 UV-visible spectrophotometer (Hitachi, Japan). The standard curve was prepared by plotting the absorbance against the concentration of l-ascorbic acid in methanol (3, 6, 9, 12, 15, 18, 21, and 24 μg/mL). The results have been expressed as EC50 (μg/mL). Each extract was done in triplicate.
2.6.2. FRAP assay
Ferric reducing antioxidant power (FRAP) was assayed in line with our previous report [15] with minor modifications. The FRAP solution was composed of 0.3 M acetate buffer (pH 3.5), 0.1 M TPTZ in 0.5 M hydrochloric acid, and 0.2 M ferric chloride hexahydrate in ultrapure water. The FRAP solution (2 mL) was incubated with 1 mg/mL extract solution (2 mL) in the dark at 37 °C for 30 min. The absorbance was read at 593 nm using a U-2990 UV-visible spectrophotometer (Hitachi, Japan) against the reagent blank. Regression linear equation of calibration curve of each extract was used to estimate EC50 value. l-Ascorbic acid was used as a standard and each extract analysis was done in triplicate.
2.7. Determination of heavy metal concentrations
The concentrations of As, Cd, Hg, and Pb in four extracts were assessed by acid digestion and inductively coupled plasma - mass spectrometry (ICP-MS) [15]. Each extract (1 g) put in a Teflon vessel was completely digested with 65% v/v nitric acid solution (7 mL) using a microwave digester Model ETHOS ONE (Milestone Corporation, Sorisole, Italy) for 15 min at 1500 W of power. The obtained solutions were scrutinized by 7500ce ICP-MS instrument (Agilent Technologies, Santa Clara, USA) operated with radio frequency power 1500 W, carrier gas flow rate 1.09–1.20 L/min, and auxiliary gas flow rate 0.89 L/min [18]. Each extract was established in triplicate. The standard curves of each metal were constructed using 6 concentrations of an ICP multi-element standard solution XIII in 5% v/v nitric acid solution.
2.8. Microbial limit test
All extracts were stored separately in tightly closed containers at 4 ± 1 °C with 75 ± 2% RH (relative humidity) for 6 months. The microbiological examination of extracts comprised of total aerobic microbial count (TAMC) and total yeast and mold count (TYMC) as well as the tests for bile-tolerant gram negative bacteria, Salmonella spp., and Escherichia coli were done according to the United States Pharmacopeia (USP) 41 [19]. Tryptic soy agar at 32.5 ± 2.5 °C for 3–5 days and Sabouraud dextrose agar at 23.7 ± 1.0 °C for 5–7 days were used for determination of TAMC and TYMC, respectively. Bile-tolerant gram negative bacteria were cultivated in Tryptic soy broth (TSB) at 23.7 ± 1.0 °C for 2–5 h and Enterobacteria enrichment broth-Mossel (at 32.5 ± 2.5 °C for 24–48 h) was employed as a selective enrichment medium, with subculture executed on Violet red bile dextrose agar at 32.5 ± 2.5 °C for 18–24 h. Salmonella spp. was determined with TSB at 32.5 ± 2.5 °C for 18–24 h. The samples were selectively enriched in Rappaport-Vassiliadis broth and Tetrathionate bile brilliant green broth, followed by discriminating isolation on Xylose-lysine-desoxycholate agar, Brilliant green agar, and Bismuth sulfite agar. The survival of E. coli inoculated into TSB at 32.5 ± 2.5 °C for 18–24 h was done. The performance of MacConkey broth (at 43.0 ± 1.0 °C for 24–48 h) and MacConkey agar (at 32.5 ± 2.5 °C for 18–72 h) in supporting colony development of incubated colonies was verified.
2.9. Statistical analysis
Data is expressed as mean ± standard deviation (SD) of at least three independent experiments. Slopes and intercepts of standard curves were calculated by linear regression. Correlation coefficient (R2) values were obtained from MS Office Excel 2010 software. One-way analysis of variance (ANOVA) in SPSS v 16.0 was used to analyze the differences among total phenolic contents, total flavonoid contents, and EC50 of various extracts with least significant difference (LSD) p < 0.05 as a level of significance.
3. Results and discussion
3.1. Chemical constituents
3.1.1. Phytochemical tests
The phytochemicals of 40% v/v ethanol and distilled water extracts from baby corn silk cultivated in Thailand are presented in Table 1. Flavonoids, tannins, terpenoids, and steroids were found in PE and ZE, while only flavonoids and tannins were detected in PA and ZA. In this study, alkaloids were absent in all extracts whereas the ethanol extract from mature corn silk cultivated in India have been reported to contain alkaloids [20]. The results showed that the differences in phytochemical constituents of corn silk might depend on various factors such as baby corn hybrids, maturity stage, cultivation region, and solvent polarity.
Table 1.
Qualitative analysis of the phytochemicals of baby corn silk extracts.
| Phytochemical constituents | Pacific 271 hybrid |
Zeba SG 17 hybrid |
||
|---|---|---|---|---|
| Ethanol extract (PE) | Aqueous extract (PA) | Ethanol extract (ZE) | Aqueous extract (ZA) | |
| Flavonoids | + | + | + | + |
| Tannins | + | + | + | + |
| Terpenoids | + | – | + | – |
| Steroids | + | – | + | – |
| Alkaloids | – | – | – | – |
+ = Present; – = Absent.
3.1.2. TLC screening
The separated phytochemicals using TLC can be detected by several methods such as physical (quenching of compounds under UV light) and chemical (colorimetric reaction between compounds and visualizing reagent) methods [21]. The visualizing reagents can detect and distinguish many separated compounds on TLC plates. After development, alkaloids, sugars, antioxidants, and unsaturated and aromatic compounds on chromatographic plates were visualized using Dragendorff, 10% v/v sulfuric acid - p-anisaldehyde, DPPH, and iodine vapor, respectively. The results indicate the presence of glycosides, antioxidants, and unsaturated and aromatic compounds in all extracts because the spots of these compounds on TLC plates produced greenish gray, yellow, and brown colors, respectively [16,21]. Furthermore, antioxidants and unsaturated and aromatic compounds also exhibited quenching spots under short wavelength UV light (254 nm) on fluorescent TLC plates. However, all extracts produced negative results with Dragendorff's reagent indicating absence of alkaloids.
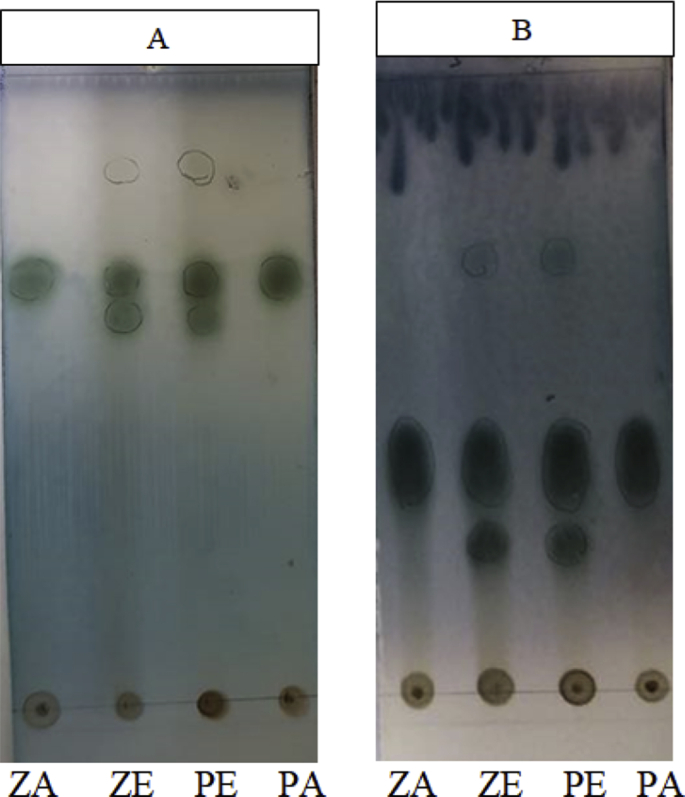
A mixture of 10% v/v sulfuric acid - p-anisaldehyde was sprayed onto the TLC plate and then the plate was heated at 110 °C for a few minutes. The relationship of the similarities of the spots and the Rf values found for analysis of extracts are presented in Table 2 and Fig. 3. All extracts showed greenish gray spots at Rf = 0.66 (PA and ZA) and Rf = 0.60, 0.66, 0.85 (PE and ZE) using the mobile phase chloroform: methanol: water: glacial acetic acid (6:5:1:1, v/v/v/v) and Rf = 0.40 (PA and ZA) and Rf = 0.26, 0.40, 0.63 (PE and ZE) using the mobile phase chloroform: methanol: glacial acetic acid (7:3:1, v/v/v).
Table 2.
Rf values for each component obtained from baby corn silk extracts.
| Code of extracts |
Rf values |
|||||
|---|---|---|---|---|---|---|
| chloroform: methanol: water: glacial acetic acid (6:5:1:1, v/v/v/v)a | chloroform: methanol: glacial acetic acid (7:3:1, v/v/v)a | |||||
| PE | 0.60 | 0.66 | 0.85 | 0.26 | 0.40 | 0.63 |
| PA | 0.66 | 0.40 | ||||
| ZE | 0.60 | 0.66 | 0.85 | 0.26 | 0.40 | 0.63 |
| ZA | 0.66 | 0.40 | ||||
Pacific 271 ethanol extract (PE), Pacific 271 aqueous extract (PA), Zeba SG 17 ethanol extract (ZE), Zeba SG 17 aqueous extract (ZA).
The separated spots were detected as dark spots under UV 254 nm and visualized as greenish gray spots after spraying with 10% v/v sulfuric acid - p-anisaldehyde spraying reagent.
Fig. 3.
TLC chromatograms of Zeba SG 17 aqueous extract (ZA), Zeba SG 17 ethanol extract (ZE), Pacific 271 ethanol extract (PE), and Pacific 271 aqueous extract (PA); Visualizing reagent: 10% v/v sulfuric acid - p-anisaldehyde; Developing solvents: chloroform: methanol: water: glacial acetic acid (6:5:1:1, v/v/v/v) (A) and chloroform: methanol: glacial acetic acid (7:3:1, v/v/v) (B).
In conclusion, PE and ZE contained similar phytochemicals including flavonoids, tannins, terpenoids, and steroids, while PA and ZA were composed of almost identical compounds such as flavonoids and tannins. Furthermore, all extracts were composed of glycosides, antioxidants, and unsaturated and aromatic compounds. According to previous research, baby corn silk extracts were plenty of phytochemicals [22]. The chemical structures and antioxidant activities of these phytochemicals would be examined in the next section.
3.1.3. Compound characterization
After investigating phytochemical tests and TLC screening, PE and ZE contain more phytochemicals than PA and ZA. Therefore, the characterization of compounds using UV-visible, FTIR, and 1H NMR was carried out only on PE and ZE. The absorption peaks appeared between 200 and 400 nm indicate the existence of auxochromes or unsaturated functional groups and the UV absorbance at particular wavelength is a characteristic of flavonoid type. Absorption spectra in the UV region of PE and ZE solutions in ultrapure water exhibited λmax at 266, 335 and 272, 341 nm with the absorption 2.01, 1.25 and 2.70, 1.60, respectively. Consistent with a previous report [23,24], these spectra confirm the presence of organic chromophores such as aromatic ring and conjugated system and may presume existence of flavone glycosides within PE and ZE. Nevertheless, the elucidation of phytochemical components using UV-visible spectra is restricted by complications in the assignment of absorption peaks to any particular components in the extract. The results obtained from the UV-visible spectrophotometric method must therefore be supplemented with FTIR and 1H NMR techniques to elucidate structural compounds in the extracts.
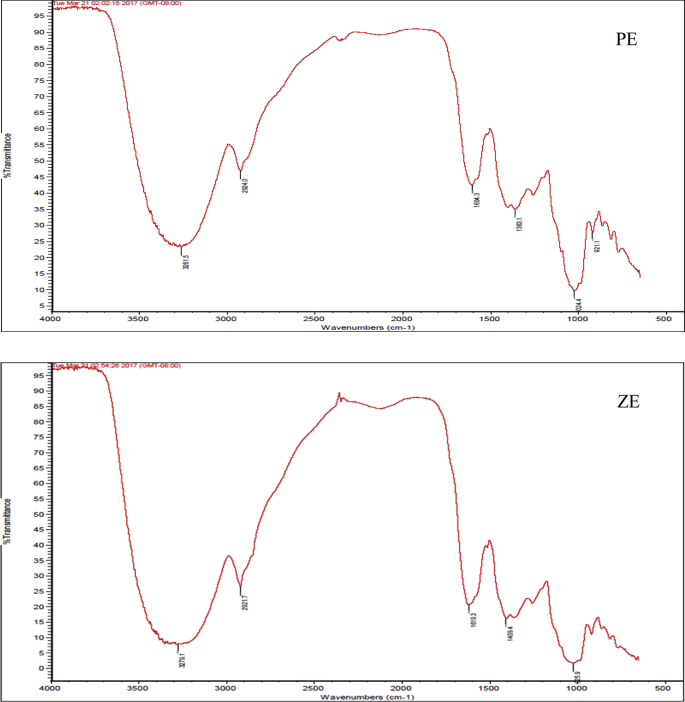
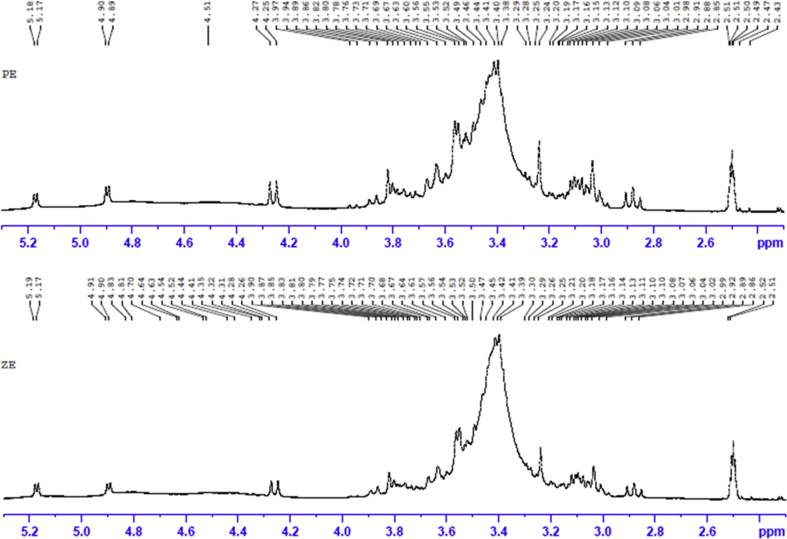
As shown in Fig. 4, the FTIR spectra of PE and ZE showed broad bands (3279.1 and 3261.5 cm−1) in harmony with hydroxyl groups and medium bands (2921.7 and 2924.0 cm−1) and pairs of ring stretch absorption bands (1619.3, 1409.4 and 1604.3, 1363.1 cm−1) in line with aromatic rings. These results suggest that compounds in PE and ZE were flavonoid derivatives. As shown in Fig. 5, PE and ZE showed almost similar 1H NMR spectra. The singlet signal at δ 3.82 should be designated to a methoxy substituent at the 3′-position in the aromatic B-ring of flavonoid derivatives. Additionally, aromatic proton signals at δ 6.58–6.60 in B-ring were observed. The anomeric protons originating from the sugar moiety appeared at δ 5.17 (1H, d) and δ 4.90 (1H, d), which were ascribed to H-1″ and H-1‴. The proton signals at δ 2.8–3.8 were assigned to a sugar moiety according to the ppm values found in the previous report [24].
Fig. 4.
FTIR spectra of Pacific 271 ethanol extract (PE) and Zeba SG 17 ethanol extract (ZE).
Fig. 5.
1H NMR spectra of Pacific 271 ethanol extract (PE) and Zeba SG 17 ethanol extract (ZE).
The results obtained from UV-visible, FTIR, and 1H NMR of PE and ZE provide similar spectra determining the presence of flavone glycosides. According to the literatures [23,24], the major constituent of PE and ZE should be either maysin or methoxyluteolin.
3.2. Percent yields, total phenolic contents, and total flavonoid contents
3.2.1. Yields
PE and PA of Pacific 271 hybrid and ZE and ZA of Zeba SG 17 hybrid were obtained through the extraction with 40% v/v ethanol, and distilled water, respectively. As shown in Table 3, ZE had the highest yield ((32.11 ± 0.82%), followed by PE (31.98 ± 0.79%) and PA (29.67 ± 0.50%), while ZA had the lowest yield (25.98 ± 0.48%). This highlights that 40% v/v ethanol is efficient in extracting phytochemical constituents from baby corn silk more than distilled water. In addition, this result implies that most of phytochemicals in all extracts are polar compounds such as phenolics and glycosides.
Table 3.
Percent yields, total phenolic contents, total flavonoid contents, and antioxidant activities of baby corn silk extracts.
| Code of extracts | Percent yield (%) | Total phenolic contents (mg GAE/g extract) | Total flavonoid contents (mg RE/g extract) | DPPH EC50 (μg/mL) | FRAP EC50 (μg/mL) |
|---|---|---|---|---|---|
| PE | 31.98 ± 0.79 | 44.58 ± 0.75b | 22.46 ± 0.48b | 117.08 ± 0.38b | 58.16 ± 1.01b |
| PA | 29.67 ± 0.50 | 33.57 ± 0.49a | 12.59 ± 0.35a | 292.89 ± 3.10e | 139.54 ± 1.49d |
| ZE | 32.11 ± 0.82 | 49.95 ± 0.40c | 21.07 ± 0.52b | 132.09 ± 0.84c | 64.28 ± 1.24c |
| ZA | 25.98 ± 0.48 | 33.06 ± 0.45a | 11.25 ± 0.31a | 270.92 ± 2.24d | 135.25 ± 1.32d |
| ascorbic acid | n/a | n/a | n/a | 14.60 ± 0.05a | 25.12 ± 0.24a |
Pacific 271 ethanol extract (PE), Pacific 271 aqueous extract (PA), Zeba SG 17 ethanol extract (ZE), Zeba SG 17 aqueous extract (ZA); n/a: not applicable; Each value is represented as mean ± SD (n = 3); Means with different letters (a–e) within the same column differed significantly (p < 0.05).
3.2.2. Total phenolic contents
The contents of phenolics in extracts were quantified using a linear standard curve of gallic acid (y = 0.0085x + 0.0273; R2 = 0.9997). The total phenolic contents of 40% v/v ethanol extracts (ZE; 49.95 ± 0.40 mg GAE/g dried extract and PE; 44.58 ± 0.75 mg GAE/g dried extract) were found to be significantly higher than those of aqueous extracts (PA; 33.57 ± 0.49 mg GAE/g dried extract and ZA; 33.06 ± 0.45 mg GAE/g dried extract) as shown in Table 3. PE and ZE therefore contained higher phenolic compounds which dissolved more in 40% v/v ethanol compared to distilled water. However, total phenolic contents of all baby corn silk extracts (from 33.06 ± 0.45 to 49.95 ± 0.40 mg GAE/g dried extract) were noticeably higher than the quantities reported in the studies carried on 80% v/v methanol extracts from ten varieties of corn silk cultivated in Thailand [25] and 95% v/v ethanol extract from mature corn silk gathered in China [3] which ranged from 38.7 up to 189.1 μg GAE/g dried extract. The results revealed that the difference of hybrid and maturity stage of corn silks and solvent polarity influences on the total phenolic content.
3.2.3. Total flavonoid contents
The contents of flavonoids in extracts were calculated from regression equation of calibration curve (y = 4.3265x + 0.0182, R2 = 0.9993). As seen from Table 3, the higher total flavonoid contents were found in 40% v/v ethanol extracts with amounts of 22.46 ± 0.48 mg RE/g dried extract (PE) and 21.07 ± 0.52 mg RE/g dried extract (ZE), which are significantly different to the values for aqueous extracts including 12.59 ± 0.35 mg RE/g dried extract (PA) and 11.25 ± 0.31 mg RE/g dried extract (ZA). The difference of total flavonoid contents tends to believe that 40% v/v ethanol is suitable for extraction of flavonoids. Furthermore, total flavonoid contents of all baby corn silk extracts (from 11.25 ± 0.31 to 22.46 ± 0.48 mg RE/g dried extract) were considerably higher than the contents reported in previous studies performed on 80% v/v methanol extracts from Thai corn silk at three maturity stages [25] and 95% v/v ethanol extract from mature corn silk gathered in China [3] which ranged from 17.9 to 119.6 μg RE/g dried extract. The data revealed that total flavonoid contents were affected by hybrid and growth stage of corn silk and ethanol strength.
3.3. Antioxidant activities
3.3.1. DPPH assay
The EC50 values of scavenging DPPH radicals for four extracts are shown in Table 3. Lower value of EC50 indicates higher free radical scavenging activity. The study found that 40% v/v ethanol extracts (PE and ZE) with EC50 values of 117.08 ± 0.38 and 132.09 ± 0.84 μg/mL, respectively, exhibited stronger DPPH radical scavenging activities than their aqueous extracts (PA and ZA) with EC50 values of 292.89 ± 3.10 and 270.92 ± 2.24 μg/mL, respectively. These results suggest that the active free radical scavenging compounds are better extracted in 40% v/v ethanol.
PE had the lowest EC50 of DPPH scavenging activity (117.08 ± 0.38 μg/mL) while ascorbic acid exhibited DPPH scavenging activity with EC50 value of 14.60 ± 0.05 μg/mL 40% v/v Ethanol extracts (PE and ZE) had the EC50 values of 117.08 ± 0.38 and 132.09 ± 0.84 μg/mL, respectively, which were classified as medium antioxidants. Meanwhile, aqueous extracts (PA and ZA) showed the EC50 values of 292.89 ± 3.10 and 270.92 ± 2.24 μg/mL, respectively, which were categorized as weak antioxidants. Our findings are in agreement with a former study, which showed that 95% v/v ethanol extract of mature corn silk exhibited medium antioxidant activity with the EC50 value of 163.45 ± 6.34 μg/mL [3]. The experimental results indicate that the aqueous ethanol solution would be more efficient to extract antioxidants from corn silk.
3.3.2. FRAP assay
The lower EC50 means had the higher ferric ion reducing antioxidant power. As shown in Table 3, PE gave the highest reducing power with EC50 value of 58.16 ± 1.01 μg/mL while ascorbic acid exhibited reducing power activity with EC50 value of 25.12 ± 0.24 μg/mL. The reducing power activities of 40% v/v ethanol extracts (PE and ZE) with EC50 values of 58.16 ± 1.01 and 64.28 ± 1.24 μg/mL, respectively, were significantly higher than aqueous extracts (PA and ZA) with EC50 values of 139.54 ± 1.49 and 135.25 ± 1.32 μg/mL, respectively (Table 3). The results proved that 40% v/v ethanol was an efficient solvent for extracting antioxidants from baby corn silk. Furthermore, the aqueous ethanol solution has been known as a suitable solvent for the extraction of antioxidants from corn silk [25].
3.4. Heavy metal concentrations
The present study attempts to analyze the levels of As, Cd, Hg, and Pb in baby corn silk extracts using ICP-MS. As shown in Table 4, the trace heavy metal concentrations in all analyzed extracts were ranged from 0.215 ± 0.019 to 0.351 ± 0.022 mg/kg for As, 0.004 ± 0.015 to 0.007 ± 0.010 mg/kg for Cd, 0.112 ± 0.015 to 0.152 ± 0.020 mg/kg for Hg, and 1.256 ± 0.105 to 1.965 ± 0.125 mg/kg for Pb. The results were compared with authorized limits of these metals in Association of South East Asian Nations (ASEAN) guidelines [26] and the concentrations of all these heavy metals were found to be within permissible limits (Table 4). These findings indicate that all baby corn silk extracts are safe from the toxicity of heavy metals.
Table 4.
Heavy metal concentrations of baby corn silk extracts.
| Code of extracts | Heavy metal concentrations (mg/kg) |
|||
|---|---|---|---|---|
| As | Cd | Hg | Pb | |
| PE | 0.324 ± 0.021 | 0.007 ± 0.010 | 0.112 ± 0.015 | 1.256 ± 0.105 |
| PA | 0.215 ± 0.019 | 0.006 ± 0.020 | 0.152 ± 0.020 | 1.965 ± 0.125 |
| ZE | 0.351 ± 0.022 | 0.005 ± 0.012 | 0.138 ± 0.018 | 1.358 ± 0.109 |
| ZA | 0.303 ± 0.028 | 0.004 ± 0.015 | 0.142 ± 0.011 | 1.769 ± 0.110 |
| Permissible limitsa | 5.0 | 0.3 | 0.5 | 10.0 |
Pacific 271 ethanol extract (PE), Pacific 271 aqueous extract (PA), Zeba SG 17 ethanol extract (ZE), Zeba SG 17 aqueous extract (ZA); each value is represented as mean ± SD (n = 3).
Permissible limits set by ASEAN guidelines [26].
3.5. Microbial contamination
Insufficient drying of baby corn silk extracts leads to increased levels of microbial contamination. Moreover, storage conditions and packing materials for these extracts should be appropriated in order to avert microbial growth [27]. Therefore, all extracts were stored individually in tightly closed glass containers at 4 ± 1 °C with 75 ± 2% RH for 6 months. ASEAN guidelines have been issued in microbial limits and absence of some specified microorganisms in traditional medicines and health supplements [26]. TAMC and TYMC expressed as colony-forming units per gram of sample (CFU/g), the acceptance criteria for oral administration of herbal extracts based upon TAMC and TYMC were not more than 5 × 105 and 5 × 104 CFU/g, respectively. In addition, the number of bile-tolerant gram negative bacteria was not more than 104 CFU/g, and none of the specified pathogens including E. coli and Salmonella spp. were observed in 1 g and 25 g of extract, respectively. These limitations are used as indices of microbiological quality. The results showed that TAMC, TYMC, and specified microorganisms were zero for all freshly prepared extracts. After storage for 6 months, all stored extracts were contaminated with lower than 10 CFU/g in tests for TAMC, TYMC, and bile-tolerant gram negative bacteria. However, E. coli and Salmonella spp. were not detected in any stored extracts. It could be concluded that all observed extracts complied with the microbiological requirements.
4. Conclusion
Percent yields ranging from 25.98 to 32.11% were observed in 40% v/v ethanol extracts (ZE and PE) and aqueous extracts (PA and ZA) of baby corn silk from Pacific 271 and Zeba SG 17 hybrids. ZE had the highest percent yield followed by PE and PA whereas ZA had the lowest percent yield. The results of phytochemical test, TLC screening, UV-visible, FTIR, and 1H NMR confirmed that PE and ZE consisted of resembled phytochemical constituents including flavonoids, tannins, terpenoids, and steroids, while PA and ZA contained the same components such as flavonoids and tannins. Additionally, antioxidants, flavone glycosides, and unsaturated and aromatic compounds were found in all extracts. The contents of phenolic compounds and flavonoids of 40% v/v ethanol extracts were higher than those of aqueous extracts. Furthermore, In DPPH and FRAP assays, the antioxidant activities of 40% v/v ethanol extracts were stronger than those of aqueous extracts. Therefore, it could be assumed that the high antioxidant activities displayed by 40% v/v ethanol extracts were associated with the large contents of phenolic compounds and flavonoids present in these extracts. From the results, 40% v/v ethanol was more efficient than distilled water in extracting several phytochemicals especially flavonoids and phenolic compounds with antioxidant properties. In this study, the hybrid and maturity stage of corn silks and solvent polarity affect percent yields, phytochemical constituents, contents of phenolic compounds and flavonoids, and antioxidant activities. According to our results, baby corn silks at the silking stage of Pacific 271 and Zeba SG 17 hybrids are suitable for use as sources of flavonoids and phenolic compounds exhibiting antioxidant activities and 40% v/v ethanol is a good solvent for extracting these compounds. The data from ICP-MS and microbial limit tests were compared with prescribed limits of heavy metals and microbial contamination in ASEAN guidelines and the results of all extracts were found to be within safe limits. In summary, 40% v/v ethanol extracts (ZE and PE) of baby corn silk from Pacific 271 and Zeba SG 17 hybrids could be novel sources of natural antioxidants and were safe for further development of dietary supplements and functional foods.
Sources of funding
This research has 2 Research Funders: Research and Creative Fund, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, Thailand (Grant numbers: RGG 009/2562), Siam Ostrich Farm, Song Phi Nong, Suphan Buri, Thailand.
Conflicts of interest
None.
Acknowledgements
The authors thank Siam Ostrich Farm and Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, Thailand for providing baby corn silk and research facilities.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Hasanudin K., Hashim P., Mustafa S. Corn silk (Stigma Maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012;17:9697–9715. doi: 10.3390/molecules17089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maksimović Z., Malenčić Đ., Kovačević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005;96:873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Wang C., Wang Z., Zhang C., Lu S., Liu J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011;126:261–269. [Google Scholar]
- 4.Maksimovic Z.A., Kovačević N. Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia. 2003;74:144–147. doi: 10.1016/s0367-326x(02)00311-8. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimzadeh M.A., Pourmorad F., Hafe S. Antioxidant activities of Iranian corn silk. Turk J Biol. 2008;32:43–49. [Google Scholar]
- 6.Wang C., Zhang T., Liu J., Lu S., Zhang C., Wang E. Subchronic toxicity study of corn silk with rats. J Ethnopharmacol. 2011;137:36–43. doi: 10.1016/j.jep.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Boeing J.S., Barizão É., Silva B.C., Montanher P.F., Almeida V.C., Visentainer J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J. 2014;8:48. doi: 10.1186/s13065-014-0048-1. http://doi:10.1186/s13065-014-0048-1 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosalec I., Cvek J., Tomić S. Contaminants of medicinal herbs and herbal products. Arh Hig Rada Toksikol. 2009;60:485–501. doi: 10.2478/10004-1254-60-2009-2005. [DOI] [PubMed] [Google Scholar]
- 9.Aekatasanawan C. In: Baby corn. Specialty corns. Hallauer A.R., editor. CRC Press; Boca Raton: 2001. pp. 275–292. [Google Scholar]
- 10.Edeoga H.O., Okwu D.E., Mbaebie B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotech. 2005;4:685–688. [Google Scholar]
- 11.Shabbir M., Khan M.R., Saeed N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation, and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement Altern Med. 2013;13:143. doi: 10.1186/1472-6882-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pati P.K., Sharma M., Salar R.K., Sharma A., Gupta A.P., Singh B. Studies on leaf spot disease of Withania somnifera and its impact on secondary metabolites. Indian J Microbiol. 2008;48:432–437. doi: 10.1007/s12088-008-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal E., Salim K.A., Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. JKSUS. 2015;27:224–232. [Google Scholar]
- 14.Chandra S., Khan S., Avula B., Lata H., Yang M.H., ElSohly M.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. J Evid Based Complementary Altern Med. 2014;2014:253875. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krongrawa W., Limmatvapirat S., Pongnimitprasert N., Meetam P., Limmatvapirat C. Formulation and evaluation of gels containing coconut kernel extract for topical application. AJPS. 2018;13:415–424. doi: 10.1016/j.ajps.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner H., Bladt S., editors. Plant drug analysis: a thin layer chromatography atlas. 2 nd ed. Springer-Verlag; Berlin: 1995. pp. 53–54. [Google Scholar]
- 17.Sembiring E.N., Elya B., Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J. 2018;10:123–127. [Google Scholar]
- 18.Limmatvapirat C., Limmatvapirat S., Charoenteeraboon J., Wessapan C., Kumsum A., Jenwithayaamornwech S. Comparison of eleven heavy metals in Moringa oleifera lam. products. Indian J Pharm Sci. 2015;77:485–490. doi: 10.4103/0250-474x.164782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The United States Pharmacopeial Convention . The United States Pharmacopeia, 41st rev., and the national formulary. 36th ed. The United States Pharmacopeial Convention; Rockville (MD): 2018. <61> Microbiological examination of nonsterile products: microbial enumeration tests. [Google Scholar]
- 20.Milind P., Isha D. Zea maize: a modern craze. IRJP. 2013;4:39–43. [Google Scholar]
- 21.Sherma J., Fried B., editors. Handbook of thin-layer chromatography. 3 rd ed. Marcel Dekker; New York: 2003. pp. 1126–1187. [Google Scholar]
- 22.Bhaigyabati T., Kirithika T., Ramya J., Usha K. Phytochemical constituents and antioxidant activity of various extracts of corn silk (Zea mays. L) RJPBCS. 2011;2(4):986–993. [Google Scholar]
- 23.Ren S., Qiao Q., Ding X. Antioxidative activity of five flavones glycosides from corn silk (Stigma maydis) Czech J Food Sci. 2013;31:148–155. [Google Scholar]
- 24.Blunder M., Orthaber A., Bauer R., Bucar F., Kunert O. Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chem. 2017;218:600–609. doi: 10.1016/j.foodchem.2016.09.077. [DOI] [PubMed] [Google Scholar]
- 25.Sarepoua E., Tangwongchai R., Suriharn B., Lertrat K. Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chem. 2015;169:424–429. doi: 10.1016/j.foodchem.2014.07.136. [DOI] [PubMed] [Google Scholar]
- 26.The 24th ASEAN traditional medicines and health supplements scientific committee meeting (ATSC). Association of South East Asian Nations (ASEAN) guidelines on limits of contaminants for traditional medicines and health supplements. 2015. https://www.hsa.gov.sg/content/dam/HSA/HPRG/Complementary_Health_Products/Annexes/ANNEX%20III%20ASEAN%20GL%20on%20Limits%20of%20Contaminations%20TMHS%20V1.0(13Nov14).pdf URL. [Google Scholar]
- 27.Tripathy V., Basak B.B., Varghese T.S., Saha A. Residues and contaminants in medicinal herbs-A review. Phytochem Lett. 2015;14:67–78. [Google Scholar]