Abstract
Background
Dental caries is a biofilm-related infectious disease with a multifactorial etiology, over five billion inhabitants have affected worldwide due to this disease.
Objective
Antimicrobial efficacy of a mixed herbal powder extract (MHPE) against cariogenic microorganisms was investigated.
Materials and methods
MIC, MBC, kinetics of killing, biofilm disruption and anticaries effect of MHPE were determined. For biofilm disruption, biofilms of Streptococcus mutans, Lactobacillus casei, Actinomyces viscosus and Candida albicans were treated with MHPE for 30 min and attached cells were quantified after staining. For live/dead staining biofilm assay, S. mutans biofilm treated with MHPE for 1min, 5min and 1 h was examined with confocal laser scanning system after live/dead staining. Efficacy was experimented by structural quality using Scanning Electron Microscope (SEM). Anticaries effect was determined by formation of caries-like lesion in continuous flow biofilm model.
Results
MHPE exhibited inhibition zones ranging from 12.5 to 24.0 mm. The highest inhibition zone was recorded at concentration of 50 μg/ml. MIC for S. mutans was between 12.23 and 36.7 μg/ml, while the MBC values ranged from 36.7 to 110.65 μg/ml. Inhibitory concentration of MHPE was three fold higher than CHLX. Significant reduction of cell count (49–95%) was observed with increasing time and higher concentration. Percentage biofilm reduction compare with negative control was 96.9% (A. viscosus), 94% (C. albicans), 99.8% (L. casei) and 91.7% (S. mutans). For MHPE-treated biofilm, live/dead staining demonstrated significant (p < 0.05) higher in deceased red fluorescence areas in all kinetics points from 53.6% (1min) to 85% (1h). SEM confirmed the damage in the outer layers of S. mutans. MHPE has components with effective antibacterial activity against caries-inducing microorganisms.
Conclusion
The anti-adherence and anti-biofilm effect as well as the faster killing activity suggests that MHPE formula has effective antibacterial activity and could be a useful source of anti-cariogenic agents in near future.
Keywords: Plant extract, Dental caries, Plaque biofilm, Anticaries agents, Artificial mouth assay
1. Introduction
Dental caries is a biofilm-related preventable infectious disease with a multifactorial etiology [1]. About five billion people have experienced dental caries worldwide [2], thus it constitutes a main source of global disease and financial burden [3], [4], [5]. Despite the great progress in caries control, a skewed distribution of caries has been observed in many developed countries [5], [6] thus posing a great need to search for novel preventive strategies/therapies. Considerable efforts in the past decades have identified multiple caries risk factors, among which are cariogenic bacteria and oral hygiene [7]. It makes sense, therefore, that one of the strategies for caries control and prevention involves reduction and/or elimination of bacteria accumulation as plaque biofilm on tooth surfaces. At the moment, commercially available mouthrinses and dentifrices contain antimicrobial agents that are effective in decreasing dental plaque, such as povidone iodine, chlorhexidine and cetylpyridinium chloride, triclosan and zinc citrate. However, besides side effects such as teeth staining, development of antimicrobial resistant strains is a growing concern against these products. Although chlorhexidine was found to be effective in decreasing salivary mutans streptococci, it is ineffective as a caries-preventive agent [8], [9]. These drawbacks necessitates further research and development on natural antimicrobial agents targeting specific oral pathogens while being safe for the host [10].
Natural antibacterial substances are presently attracting attention as useful antimicrobials to be incorporated into oral health care products. Extracts of tea tree oil, peppermint, manuka honey and green tea have been incorporated into oral care products for antimicrobial therapy [8]. The dried powders of A. arabica (bark), Terminalia chebula (fruits), Terminalia bellerica (fruits) and Emblica officinalis (fruits) have the potential to cure oral diseases [10] and theses four plant powders have been used in traditional tooth powder formulas in India for more than 100 years (unpublished data). The objective of the present study was to investigate the antimicrobial effect and mode of action of a tooth powder containing more than one plant material.
2. Materials and method
2.1. Collection of plant materials
Bark of Acacia a rabica (wattles), fruits of Terminalia Chebula, T. bellerica and E. officinalis (Triphala) procured from authorized Indian herbal medicinal stores, Cuddalore, Tamil Nadu, India. All herbs were ground to coarse powders and then the powders were mixed together in accordance with the traditional tooth powder formulation (A. arabica (70%), T. chebula (10%), T. bellerica (10%), E. officinalis (10%)).
2.2. Preparation of plant extracts
The mixed herbal powder extract (MHPE) was prepared as follows. Methanol was poured into 50 g of the tooth powder at the ratio of 10:1 (v/w), and the mixture was flushed with nitrogen and kept under orbital shaking incubator at room temperature for 24 h under dark condition. Likewise, second and third extractions were produced from the powder with chloroform/methanol mixture (1:1 (v/v)) and chloroform-only respectively. These three different extracts, representing lower polar, polar, and non-polar components, were then pooled together and evaporated under reduced pressure using rotary flash evaporator. All the resulting extracts were lyophilized and stored in refrigerator (−4 °C) and extracts were dissolved in 1% DMSO for further use (1% DMSO was maintained as negative control in all the experiments as 1% DMSO did not show any activity).
2.3. Agar diffusion method
Aliquots of standardized cell suspension (200 μl, 107 CFU/ml) of Streptococcus mutans, Lactobacillus casei, Actinomyces viscosus and Candida albicans were spread on Brain Heart Infusion agar (BHI) (20 ml per plate). Wells were punched in the agar plate and each well was filled with 100 μl of different concentrations (1, 10, 25 and 50 μg/ml) of MHPE. Chlorohexidine digluconate 0.12% (v/v) was used as positive control. The lowest concentration of MHPE that showed a zone of inhibition around the well was recorded as the minimum inhibitory concentration (MIC).
2.4. MIC and MBC on S. mutans
MHPE was serially diluted with S. mutans cell suspension (107 CFU/ml) and was incubated at 37 °C overnight. The lowest concentration showing no growth was recorded as the MIC. For minimum bactericidal concentration (MBC) testing, aliquots was plated onto Brain Heart Infusion (BHI) agar and incubated overnight at 37 °C. The lowest concentration showing 99.9% inhibition was recorded as the MBC. Chlorohexidine digluconate 0.12% (v/v) was used as positive control [11].
2.5. Kinetics of killing on S. mutans
Overnight bacterial cultures of S. mutans (107 CFU/ml) were added to 1, 10, 50 and 100 μg/ml of the MHPE in micro titer plates (200 μl inoculum added in 1.8 ml of medium). Same concentrations of CHLX were used as positive control. Following addition of the bacterial culture, 1 ml sample was retrieved from each plate at 1, 5, 15, 30 and 60 min to assess microbial viability by measuring the absorbance at 490 nm [11], [12].
2.6. Adherence tests with S. mutans
Overnight bacterial cultures of S. mutans (107 CFU/ml) were added to different concentration (1, 10, 50 and 100 μg/ml) of MHPE with BHI broth and 2% sucrose supplementation in 5 ml glass test tubes. Same concentrations of CHLX were used as positive control. Tubes were incubated aerobically at 37 °C (to simulate conditions in the mouth) for 24 h inclined at 30° angle. Attached bacteria were fixed with 5 ml of methanol per tube, for 15 min. The tubes were then emptied and air dried. Each tube was then stained for 5 min with 5 ml of 1% (v/v) crystal violet. Excess stain was rinsed off by tap water. The tubes were air dried and the dye bound to the adherent cells was removed with 5 ml of 33% (v/v) glacial acetic acid per tube. The absorbance of the resulting solutions was read at 595 nm [11], [12].
2.7. Biofilm studies
Biofilm formation in plastic micro plates was performed as previously described by Stepanovic et al. [13]. A 20 μl portion of an overnight broth culture of S. mutans, L. casei, A. viscosus and C. albicans (107 CFU/ml) was added to each well of a 24-well plate which was incubated aerobically, with mild agitation at 70 rpm, for 72 h at 37 °C. Every 12 h, the medium containing suspended bacterial cells were removed and an equal volume of fresh medium was added (BHI broth and 2% sucrose supplementation). Negative controls were obtained by incubating the micro plates with media without inoculum. All the experiments were performed thrice. After removing the supernatant media, the biofilm were treated with 10, 50, 100 and 150 μg/ml of MHPE, for 30 min, at room temperature and without agitation. Same concentrations of CHLX were used as positive control. Following treatment, the extract was removed and the wells were gently washed twice with sterilized distilled water. Quantification of viable cells in biofilm formed in plastic microplates was performed as previously described by Stepanovic et al. [13].
2.8. Live and dead staining (S. mutans viability test)
For the biofilm viability test, S. mutans was grown in 4-well Lab-Tek chamber slides with cover slide (Nalge Nunc International, Naperville, IL) for 24 h with BHI broth and 2% sucrose supplementation. The grown biofilm was treated with MBC concentration of MHPE (110.65 μg/ml) for 1 min, 5 min and 1 h. CHLX 0.12% (v/v) was used as positive control. Following treatment, the biofilm was stained with L 7012 LIVE/DEAD® BacLight™ bacterial viability kit from Molecular Probes Inc. (Eugene, OR) as described by Neu and Lawrence [14] The samples were immediately examined via an Olympus FV1000 confocal system on an IX81 microscope (Olympus Life Science, Center Valley, PA) at excitation wavelengths of 488 and 543 nm. Image analysis was conducted as described by Al-Ahmad et al. [15]. In order to quantify green (live) and red (dead) areas of the biofilms, a maximal projection of each image stack was built using the program LSM Image Browser (Zeiss, Oberkochen, Germany). Using the image analysis program Image J1.42q (Wayne Rasband National Institute of health, USA), the red and green projections were converted into merged black and white (B/W) images. In order to determine the area covered by all cells, live or dead, B/W intensity thresholds were manually set for each of the measured biofilm areas. The resulting green and red ratios were analyzed for their statistical significance.
2.9. Effect of MHPE on S. mutans morphology
Scanning electron microscope (SEM) images were carried out on biofilm formed overnight in 6-well plates with glass slides at the bottom of each well. Prior the SEM imaging the biofilm was treated with MBC concentration of MHPE (110.65 μg/ml) for 30 min at room temperature. The MHPE containing medium was removed and the wells were gently washed twice with sterilized distilled water. Samples were placed in a fixative (4% formaldehyde [vol/vol], 1% glutaraldehyde [vol/vol] in PBS) until ready for scanning. The samples were rinsed in 0.1 M phosphate buffer (2 times, 3 min each) and then placed in 1% Zetterquist's osmium for 30 min. The samples were subsequently dehydrated in a series of ethanol washes (70% for 10 min, 95% for 10 min, and 100% for 20 min), treated (2 times, 5 min each) with hexamethyldisilizane (Polysciences Inc., Warrington, PA), and finally air dried in a desiccator. The specimens were coated using a DESK IV Model Sputter Coater (Denton Vacuum, Moorestown, NJ) with a gold-palladium (40%/60%) target. After processing, samples were observed with a LEO 435VP Variable Pressure Digital Scanning Electron Microscope (LEO Electron Microscopy Ltd., Cambridge, England) in high vacuum mode at 15 kV [16].
2.10. Inhibition of tooth surface demineralization (caries formation)
The ability of the MHPE to prevent demineralization of human tooth tissue by the acid produced by bacteria in dental plaque biofilm was investigated using a continuous flow biofilm model acting as Artificial Mouth. This artificial mouth system has been described and used in our previous study [17]. Briefly, the system was composed of multiple chambers containing cylindrical clear acrylic rods with grooves in which human teeth were embedded. Three chambers, the three experimental groups (32 tooth blocks/group), were used in this study. The simulated oral fluid (SOF) used in this system is Bacto™ Todd Hewitt broth (Fisher Scientific, Pittsburgh, PA) and this was continuously circulated to simulate saliva. Continuous circulation through the chambers at individually controlled flow rates (2 ml/min) via a digital programmable pump was maintained from a reservoir. A complete circulatory system was established by a return-flow line from the chamber back into the reservoir. The reservoir content was changed daily. 10% sucrose was circulated every 6 h for 6 min to simulate meals. All fluids flowed uniformly as a thin film over the tooth surfaces. The entire assembly was housed inside a reach-in CO2 incubator maintained at 5% CO2 and at a constant physiological temperature of 37 °C. The completely assembled system with reservoirs and contents were sterilized using ethylene oxide gas prior to experiment. Caries development on the experimental blocks was initiated by inoculation of the chambers by 1 h circulation of mixed S. mutans, A. viscosus, L. casei and candida albicans culture in Todd Hewitt broth (broth to inoculum ratio 10:1) through the chambers. Experimental groups A was treated with MBC concentration of MHPE (110.65 μg/ml), group B treated with Chlorhexidine mouth rinse and group C treated with sterile water once daily for 1 min on each occasion. The experiment lasted for 14 days, and the tooth samples were harvested and processed for demineralization assessment using caries measurement device, DIAGNO dent (KaVo Dental Inc., Charlotte, NC). Briefly DIAGNOdent caries detecting tool have 655 nm diode lasers, the laser allows for detection of non cavitated, occlusal pit and fissure tooth decay [18]. DIAGNOdent works based on the mineral structure and fluorescence of teeth, as the incident laser light is disseminated into the site, two-way hand piece optics allows the unit to simultaneously quantify the reflected laser light energy [19]. Three repeated measurements have been taken one sample for the caries measurement.
2.11. Statistical analysis
The software used was SPSS Version 15 with level of significance prechosen at α = 0.05. The presence of normal distribution was evaluated to perform parametric analysis. Data were analyzed by ANOVA followed by Tukey was performed to observe differences among the experimental groups in all tests performed.
3. Results
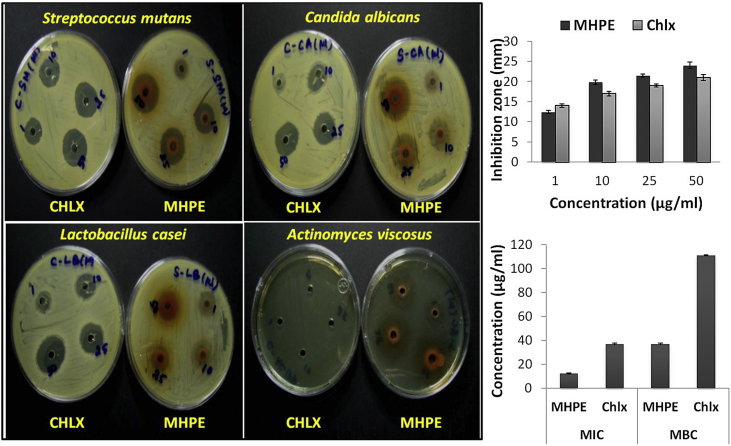
Fig. 1 showed that when the antimicrobial activity of MHPE against S. mutans, L. casei, A. viscosus and Candida albicans was quantitatively assessed by inhibition zone, MHPE was effective with the inhibition zones ranging from 12.5 to 24.0 mm. The highest inhibition zone was recorded at concentration of 50 μg/ml. MIC for S. mutans was between 12.23 and 36.7 μg/ml, while the MBC values ranged from 36.7 to 110.65 μg/ml. Inhibitory concentration of MHPE was three fold higher than CHLX.
Fig. 1.
Mixed herbal powder extract (MHPE) inhibition zone at varied concentration (1,10, 25 and 50 μg/ml) in BHI agar with Streptococcus mutans graphs of inhibition zone measurements, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).
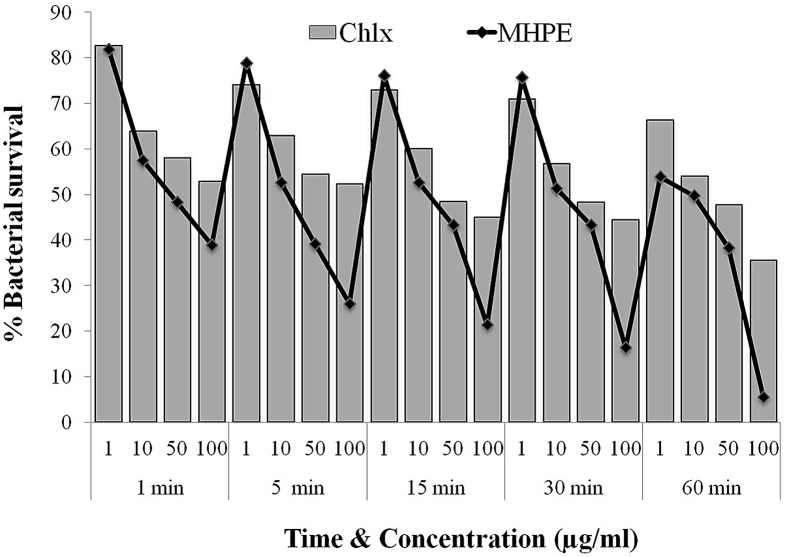
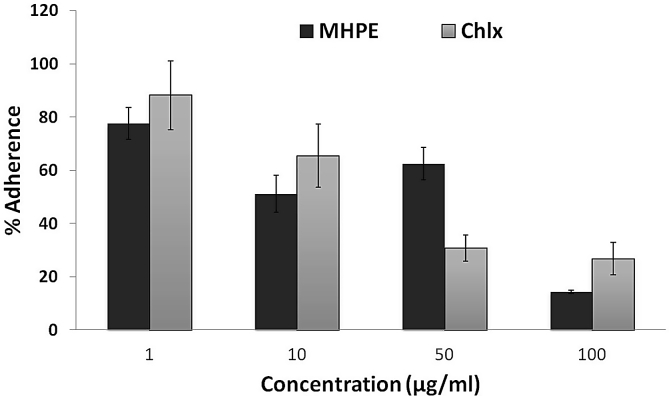
Fig. 2 showed the killing curves of the MHPE against planktonic S. mutans. It was observed that MHPE had faster and more powerful bactericidal activity than CHLX at different concentrations. Reduction of cell count was observed with increasing time and highest concentration. Significantly (P > 0.05) higher killing of S. mutans was observed with MHPE (49–94.73%) at 60 min when compared with CHLX (36.56–65.93%). In Fig. 3, there was a significantly (P < 0.05) more inhibition of the adherence of S. mutans by MHPE (up to 85.64%) at the 100 μg/ml concentration when compared with CHLX (up to 73.21%).
Fig. 2.
Time kinetics of mixed herbal powder extract (MHPE) and chlorhexidine digluconate (CHLX) on S. mutans.
Fig. 3.
Effect of the mixed herbal powder extract (MHPE) on the adherence of S. mutans at different concentration (1, 10, 50 and 100 μg/ml).
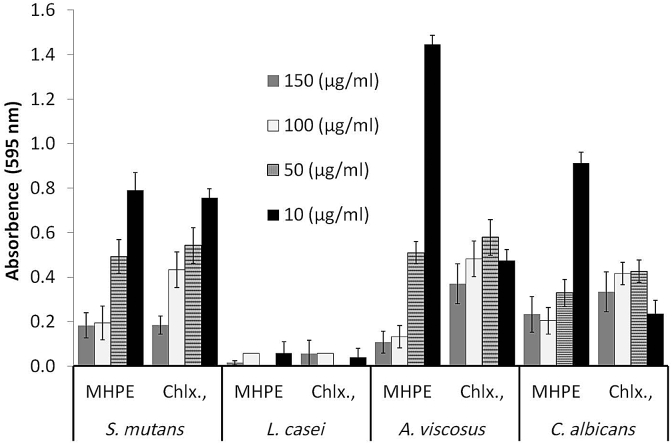
Regarding the biofilm disruption and removal, MHPE exhibited highest activity against L. casei biofilm compared to that of the other three microorganisms (Fig. 4). Percentage biofilm reductions by MHPE when compared with negative control (without treatment) were 96.9% (A. viscosus), 94.1% (C. albicans), 99.8% (L. casei) and 91.7% (S. mutans). In assessing the ability of MHPE to remove S. mutans biofilm, 30 min exposure gave maximum biofilm reduction. Biofilm removal increased significantly (P < 0.05) with increasing MHPE concentration with highest activity observed at the highest concentration tested (150 μg/ml).
Fig. 4.
Crystal violet staining to assess the effect of the mixed herbal powder extract (MHPE) on biofilm of S. mutans, L. casei, A. viscosus and C. albicans. CHLX = chlorhexidine digluconate.
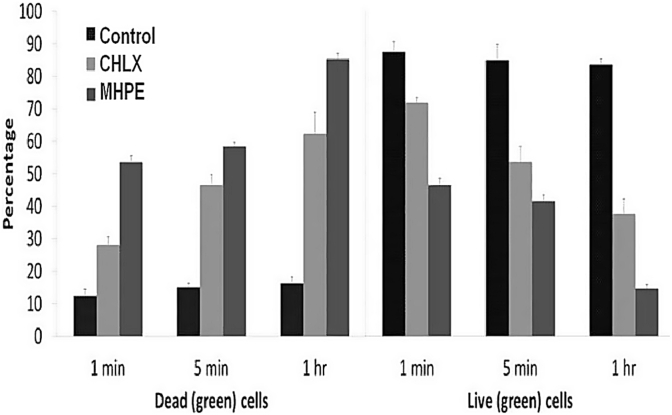
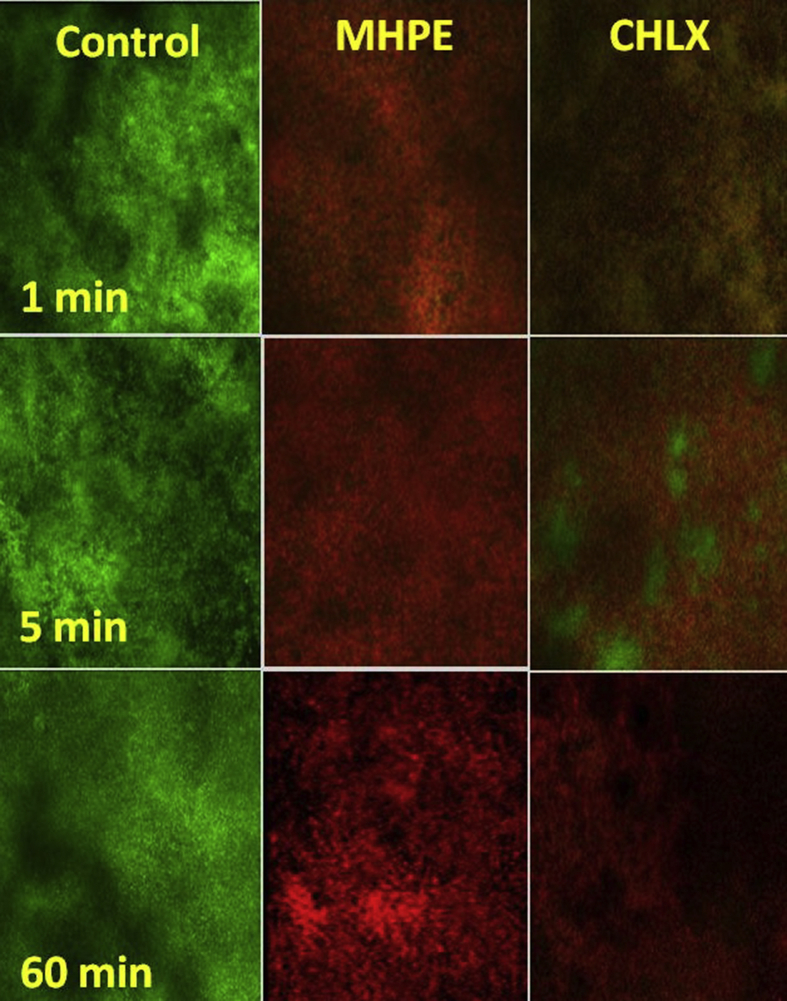
Confocal microscope examination of biofilm stained with LIVE/DEAD bacterial viability kit demonstrated MHPE to be effective against S. mutans biofilm (Fig. 5 and 6). In the control groups, most of the biofilm stained “live” (green), whereas in the treatment (DF/NE) groups cells throughout the structure stained “dead” (red). Syto 9 and propidium iodide are the two fluorescent nucleic acid stains present in LIVE/DEAD stain. SYTO 9 is used to enumerate “live” cells as it can pass through cells under every condition. Propidium iodide is used to enumerate “dead” cells since it is a highly charged molecule and is unable to pass through cells having a strong electrochemical gradient across the membrane. The images reflect different green and red fluorescence intensities. The increasing the dead cell area with increased time, up to 53.6% in 1 min and 85% in 1 h, MHPE increased the dead cell area significantly (p < 0.05) higher than CHLX and negative control (without treatment). With 28% dead cell area in 1 min, chlorhexidine exhibited approximately one half of the MHPE activities.
Fig. 5.
Live/dead staining to assess the effect of the mixed herbal powder extract (MHPE) on Streptococcus mutans (Control- No treatment; CHLX - Chlorhexidine digluconate).
Fig. 6.
Confocal Laser Scanning micrographs of Streptococcus mutans biofilms (Control - no treatment; MHPE-treated with plants tooth powder extract; CHLX-treated with Chlorhexidine digluconate at 1, 5 min and 1 h. Each micrograph represents 3 optical sections-green, red and combined green and red from two channel images. Red = dead cells; Green = live cells.
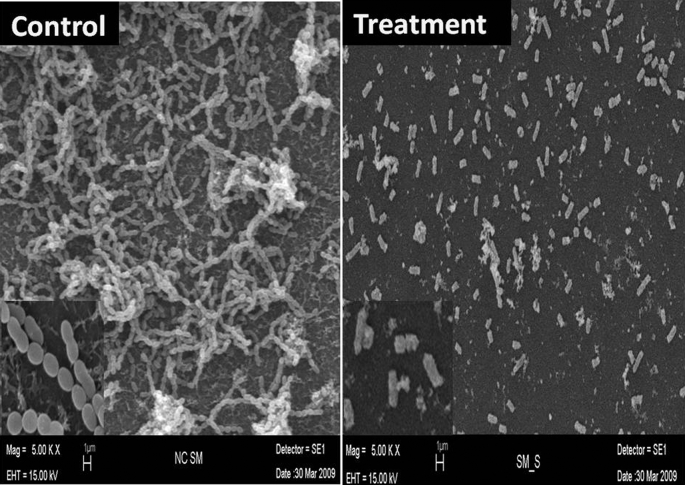
Investigating the possible morphological changes in S. mutans caused by MHPE treatment using the SEM, significant morphological changes were observed in the cells treated with MHPE (Fig. 7) when compared with intact cells from the negative control. The cell surface was remarkably disintegrated. Irregular boundaries were observed, margins of cell walls were unclear and complete collapse of biofilm was observed.
Fig. 7.
Scanning electron micrographs of control (no treatment) and MHPE-treated S. mutans biofilm.
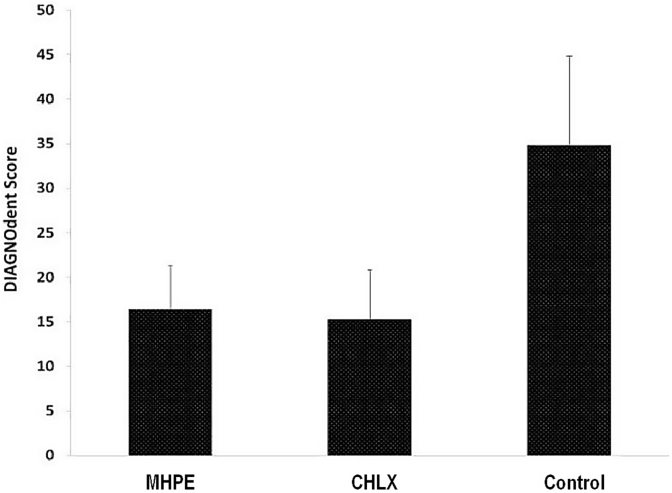
Fig. 8 showed the level of tooth enamel demineralization caused by acid produced by plaque microorganisms when treated with MHPE, CHLX or nothing. There was a statistically significant (P < 0.001) high demineralization in control (untreated) group was observed, when compared with MHPE or CHLX groups. There was no significant difference in the level of demineralization observed among MHPE and CHLX groups.
Fig. 8.
Tooth demineralization as measured by DIAGNOdent caries detection and measurement device. (Control- No treatment; CHLX - Chlorhexidine digluconate; MHPE- Mixed herbal powder extract).
4. Discussion
The dried powders A. arabica (bark), T. Chebula (fruits), T. bellerica (fruits) and E. officinalis (fruits) plants have been used in traditional tooth powder formulation in India for more than 100 years (unpublished). Already many reports are available for the validation of numerous of potential benefits of these four plant material including anti-cariogenic properties [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] and these plant materials possess abundance of bioactive compound like quercetin, luteolin, saponins, anthraquinones, amino acids, fatty acids, various carbohydrates and polyphenols such as chebulinic acid which can exhibit the antimicrobial activities [34], [35]. The above four plant materials used in this study having the potential to protect users against oral diseases, but this potential and the possible mechanism has not been investigated. The present study investigated the potential antimicrobial action of an extract from a tooth powder derived from mixture of the plant materials named above. The main objective was to determine its potential to prevent the development of dental caries through inhibition of the growth and biofilm formation by most common cariogenic microorganisms, S. mutans, L. casei, A. viscosus and C. albicans. The results of the present study demonstrated that the mixed herbal powder extracts (MHPE) has a higher antimicrobial activities against these microorganisms than the most potent oral antimicrobial, chlorhexidine gluconate. This was evident from the observed growth inhibition zone caused by the MHPE, which increased with increasing concentrations, with highest zone recorded at concentration of 100 μg/ml. Inhibitory concentration of MHPE was three fold higher than chlorhexidine digluconate (CHLX). These strong activities of MHPE can be attributed to the polar and non-polar extract component of the MHPE into which more organic compounds were leached.
Time kinetic assays showed that MHPE had faster and more powerful bactericidal activity than CHLX at different concentrations and reduction of cell count was observed with increasing time and higher concentration. MHPE demonstrated a higher level of inhibition toward S. mutans. The time period tested in this study can be achieved in vivo by incorporating the MHPE active compound as ingredient in agents like chewing gum, mouthwash or toothpaste that have prolong clearance intraorally. Glass adherence assay used in the present study is an effective model for assessing sucrose dependent bacterial adhesion [36], [37], [38]. Previous studies used this model to isolate from Psidium guajava, guaijaverin that inhibited the adherence of S. mutans to glass surface.
The membrane disruption mechanism observed when the possible mechanism of antimicrobial action of MHPE was investigated using SEM, has been reported in other studies with several phytochemicals including plant lectins [39], [40], [41] and the tea polyphenols [42] Catechins act by damaging the bacterial membranes and disrupting the function of cell surface proteins [42], [43] and the alkaloids and terpenoids has ability to intercalate with DNA and membrane disruption [44] Curcuma xanthorrhiza extract (Xan) exerts its activity by mediating morphological changes in S. mutans [45].
One of the reported strategies for preventing dental caries has been the control of plaque biofilms [46]. However, bacteria in biofilms display lower susceptibility to antimicrobial agents compared with planktonic bacteria [47]. However, in the present study, MHPE demonstrated the ability to disrupt biofilm, with higher activity against the biofilms formed by L. casei compare other three organisms, while highest biofilm reduction was observed at 150 μg/ml concentration.
The antimicrobial actions of MHPE demonstrated in the present study strongly suggest that MHPE shows significant anticariogenic effect compare to untreated groups. This was investigated by applying MHPE as a mouthrinse in an artificial mouth during the development of cariogenic dental plaque on human tooth surfaces. There was a statistically higher demineralization in control (untreated) group when compared with MHPE or CHLX groups but MHPE with CHLX showing less difference between the groups. However MHPE can provide cure from side effects such as teeth staining, development of antimicrobial resistant caused by the usage CHLX. The bacterial plaque was aimed to produce acid from sucrose metabolism to cause demineralization of the tooth tissue thus producing dental caries. The significant higher demineralization produced in the control group in this study further confirmed the fact that the active component in MHPE can prevent caries formation through its antibiofilm and antimicrobial action if incorporated into an oral healthcare product.
5. Conclusion
In conclusion, mixed herbal powder extract (MHPE) showed remarkable inhibitory effects on the growth of cariogenic microorganism at very low concentration when compare to CHLX. Plants extracts effectively inhibited sucrose mediated adherence of S. mutans and cariogenic organisms biofilm formation. Dead area 85% showed higher activity of MHPE and damage to cell membranes of S. mutans by extract was demonstrated using scanning electron microscopy (SEM). The anti-adherence and anti-biofilm effect as well as the faster killing activity suggests that MHPE formula has effective antibacterial activity and highlights that this material could be a useful source of anti-cariogenic agents in near future. We are currently testing extracts from individual plants and separating components with chromatography.
Sources of funding
AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy, Sanction Order No. Z. 28015/209/2015-HPC) and DST Science and Engineering Research Board (SERB) [Grant number- SERB/LS-267/2014].
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Lukacs J.R., Largaespada L.L. Explaining sex differences in dental caries prevalence: saliva, hormones, and "life-history" etiologies. Am J Hum Biol. 2006;18(4):540–555. doi: 10.1002/ajhb.20530. [DOI] [PubMed] [Google Scholar]
- 3.CDC . 2006. Fact sheet: surveillance for dental caries, dental sealants, tooth retention, edentulismand enamel fluorosis - United States, 1988–1994 and 1999-2002. [PubMed] [Google Scholar]
- 4.Chandra R.K. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(3):73–76. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- 5.Kaste L.M., Selwitz R.H., Oldakowski R.J., Brunelle J.A., Winn D.M., Brown L.J. Coronal caries in the primary and permanent dentition of children and adolescents 1-17 years of age: United States, 1988-1991. J Dent Res. 1996;75:631–641. doi: 10.1177/002203459607502S03. [DOI] [PubMed] [Google Scholar]
- 6.Pitts N.B., Chestnutt I.G., Evans D., White D., Chadwick B., Steele J.G. The dentinal caries experience of children in the United Kingdom 2003. Br Dent J. 2006;200(6):313–320. doi: 10.1038/sj.bdj.4813377. [DOI] [PubMed] [Google Scholar]
- 7.Cai L., Wu C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod. 1996;59:987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- 8.Almas K. The antimicrobial effects of seven different types of Asian chewing sticks. Odonto Stomatol Trop. 2001;24(96):17–20. [PubMed] [Google Scholar]
- 9.Autio-Gold J. The role of chlorhexidine in caries prevention. Operat Dent. 2008;33(6):710–716. doi: 10.2341/08-3. [DOI] [PubMed] [Google Scholar]
- 10.Biradar Y.S., Jagatap S., Khandelwal K.R., Singhania S.S. Exploring of antimicrobial activity of triphala mashi-an ayurvedic formulation. Evid Based Complement Alternat Med. 2008;5(1):107–113. doi: 10.1093/ecam/nem002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karthikeyan R., Amaechi B.T., Rawls H.R., Lee V.A. Antimicrobial activity of nanoemulsion on cariogenic Streptococcus mutans. Arch Oral Biol. 2011;56:437–445. doi: 10.1016/j.archoralbio.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramalingam K., Amaechi B.T., Rawls H.R., Lee V.A. Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch Oral Biol. 2012;57:15–22. doi: 10.1016/j.archoralbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepanovic S., Cirkovic I., Ranin L., Svabic-Vlahovic M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 14.Neu T.R., Lawrence J.R. Development and structure of microbial stream biofilms as studied by confocal laser scanning microscopy. FEMS Microbiol Ecol. 1997;24:11–25. [Google Scholar]
- 15.Al-Adham I.S.I., Al-Hmoud N.D., Khalil E., Kierans M., Collier P.J. Microemulsions are highly effective anti-biofilm agents. Appl Microbiol. 2003;36:97–100. doi: 10.1046/j.1472-765x.2003.01266.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramage G., Saville S.P., Wickes B.L., López-Ribot J.L. Inhibition of Candida albicans biofilm formation by farnesol a Quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee V.A., Karthikeyan R., Rawls H.R., Amaechi B.T. Anticariogenic effect of a cetylpyridinum chloride containing nanoemulsion. J Dent. 2010;38:742–749. doi: 10.1016/j.jdent.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lussi A. Clinical performance of the laser fluorescence system DIAGNOdent for detection of occlusal caries (in German) Acta Med Dent Helv. 2000;5:15–19. [Google Scholar]
- 19.Lussi A. Validity of diagnostic and treatment decisions of fissure caries. Caries Res. 1991;25:296–303. doi: 10.1159/000261380. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasa Rao Dola, Penmatsa Tanuja, Kranthi Kumar Alapati, Narendra Reddy M., Sai Gautam Nalam, Nalam Radhika Gautam Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: an invitro study. J Pharm BioAllied Sci. 2014;6(1):S140–S145. doi: 10.4103/0975-7406.137426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tandon S., Gupta K., Rao S., Malagi K.J. Effect of Triphala mouthwash on the caries status. Int J Ayurveda Res. 2010;1:93–99. doi: 10.4103/0974-7788.64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinagesh J., Pushpanjali K. Assessment of antibacterial efficacy of triphala against mutans streptococci: a randomised control trial. Oral Health Prev Dent. 2011;9:387–393. [PubMed] [Google Scholar]
- 23.Srinagesh J., Krishnappa P., Somanna S.N. Antibacterial efficacy of triphala against oral streptococci: an in vivo study. Indian J Dent Res. 2012;23:696. doi: 10.4103/0970-9290.107423. [DOI] [PubMed] [Google Scholar]
- 24.Shanbhag V.K. Triphala in prevention of dental caries and as an antimicrobial in oral cavity—a review. Infect Disord Drug Targets. 2015;15:89–97. doi: 10.2174/1871526515666150513105009. [DOI] [PubMed] [Google Scholar]
- 25.Pradeep A.R., Suke D.K., Martande S.S., Singh S.P., Nagpal K., Naik S.B. Triphala, a new herbal mouthwash for the treatment of gingivitis: a randomized controlled clinical trial. J Periodontol. 2016;87:1352–1359. doi: 10.1902/jop.2016.130406. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharjee R., Nekkanti S., Kumar N.G., Kapuria K., Acharya S., Pentapati K.C. Efficacy of triphala mouth rinse (aqueous extracts) on dental plaque and gingivitis in children. J Investig Clin Dent. 2015;6:206–210. doi: 10.1111/jicd.12094. [DOI] [PubMed] [Google Scholar]
- 27.Naiktari R.S., Gaonkar P., Gurav A.N., Khiste S.V. A randomized clinical trial to evaluate and compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. J Periodontal Implant Sci. 2014;44:134–140. doi: 10.5051/jpis.2014.44.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakouie S., Eskandarinezhad M., Gasemi N., Milani A.S., Samiei M., Golizadeh S. An invitro comparison of the antibacterial efficacy of triphala with different concentrations of sodium hypochlorite. Iran Endod J. 2014;9:287–289. [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhakar J., Senthilkumar M., Priya M.S., Mahalakshmi K., Sehgal P.K., Sukumaran V.G. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: an invitro study. J Endod. 2010;36:83–86. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal V., Kapoor S., Agrawal I. Critical review on eliminating endodontic dental infections using herbal products. J Diet Suppl. 2017;14:229–240. doi: 10.1080/19390211.2016.1207004. [DOI] [PubMed] [Google Scholar]
- 31.Thomas S., Asokan S., John B., Priya G., Kumar S. Comparison of antimicrobial efficacy of diode laser, triphala, and sodium hypochlorite in primary root canals: a randomized controlled trial. Int J Clin Pediatr Dent. 2017;10:14–17. doi: 10.5005/jp-journals-10005-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxena D., Saha S.G., Saha M.K., Dubey S., Khatri M. An invitro evaluation of antimicrobial activity of five herbal extracts and comparison of their activity with 2.5% sodium hypochlorite against Enterococcus faecalis. Indian J Dent Res. 2015;26:524–527. doi: 10.4103/0970-9290.172080. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar J., Balagopal S., Priya M.S., Selvi S., Senthilkumar M. Evaluation of antimicrobial efficacy of Triphala (an Indian Ayurvedic herbal formulation) and 0.2% chlorhexidine against Streptococcus mutans biofilm formed on tooth substrate: an invitro study. Indian J Dent Res. 2014;25:475–479. doi: 10.4103/0970-9290.142539. [DOI] [PubMed] [Google Scholar]
- 34.Belapurkar P., Goyal P., Tiwari-Barua P. Immunomodulatory effects of triphala and its individual constituents: a review. Indian J Pharm Sci. 2014;76:467–475. [PMC free article] [PubMed] [Google Scholar]
- 35.Olennikov D.N., Kashchenko N.I., Chirikova N.K. Invitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of Padma Hepaten formulation. Nutrients. 2015;7:8456–8477. doi: 10.3390/nu7105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo H., Pearson S.K., Scott-Anne K., Abranches J., Cury J.A., Rosalen P.L. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol. 2002;17:337–343. doi: 10.1034/j.1399-302x.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- 37.Carter K., Landini G., Malmseys A.D. Plaque removal characteristics of electric toothbrushes using an in vitro plaque model. J Clin Periodontol. 2001;28:1045–1049. doi: 10.1034/j.1600-051x.2001.281109.x. [DOI] [PubMed] [Google Scholar]
- 38.Nostro A., Cannatelli M.A., Crisafi A.D., Musolino A.D., Procopio F., Alonzo V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbiol. 2004;38:423–427. doi: 10.1111/j.1472-765X.2004.01509.x. [DOI] [PubMed] [Google Scholar]
- 39.Staat R.H., Doyle R.J., Langley S.D., Studdick R.P. Modification of in vitro adherence of Streptococcus mutans by plant lectins. Adv Exp Med Biol. 1978;107:639–647. doi: 10.1007/978-1-4684-3369-2_72. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons R.J., Dankers I. Lectin-like con-stituents of foods which react with components of serum, saliva and Streptococcus mutans. Appl Environ Microbiol. 1981;41:880–888. doi: 10.1128/aem.41.4.880-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu-Yuan C.D., Chen C.Y., Wu R.T. Gallo-tannins inhibits growth, water-insoluble glucan synthesis and aggregation of mutans streptococci. J Dent Res. 1988;67:51–55. doi: 10.1177/00220345880670011001. [DOI] [PubMed] [Google Scholar]
- 42.Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochin Biophys Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton-Miller J.M.T. Anti-cariogenic effects of tea (Camellia sinensis) J Med Microbiol. 2001;50:299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 44.Cowan M.M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J.E., Kim H.E., Hwang J.K., Lee H.J., Kwon H.K., Kim B.I. Antibacterial characteristics of Curcuma xanthorrhiza extract on Streptococcus mutans. Biofilm J Microbiol. 2008;46:228–232. doi: 10.1007/s12275-007-0167-7. [DOI] [PubMed] [Google Scholar]
- 46.Baehni P.C., Takeuchi Y. Anti-plaque agents in the prevention of biofilm associated oral diseases. Oral Dis. 2003;9(1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 47.Marsh P.D. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]