Abstract
Background
Bhasmas are traditional Ayurvedic medicines prepared from minerals and metals by tedious process which removes toxic properties of metals and minerals and enhances medicinal properties. We have synthesized abhraka bhasma by two traditional methods and were analyzed during each stage of preparation.
Objective
The present study deals with the synthesis and characterization of abhraka bhasma by two different methods, method-1 is commonly used for treating anemia and tropical sprue and method-2 for treating chronic cough. Hence attempts have been made to see the physico-chemical differences between these methods by using different analytical techniques.
Material and methods
Different steps in preparation of abhraka bhasma includes shodhana (purification), dhanyabhraka, marana (incineration) and amritikarana. The prepared bhasma were analyzed by classical ayurvedic tests and modern analytical techniques like XRD, FTIR, Raman spectroscopy, SEM, TEM, EDX, BET, DLS and TGA/DTA.
Results
The morphological characterization revealed that as-prepared abhraka bhasma products were nano crystalline in nature. Elemental analysis confirms presence of various elements along with carbon suggesting bhasma as herbo-mineral compound. The XRD studies revealed the presence of KMg3(Si3Al)O10(OH)2 in bhasmas.
Conclusion
Characterization study revealed presence of various bonds of different functional groups and formation of nano particles. Abhraka bhasma prepared by method-2 has more proportion of nano particles than that prepared by method-1 and hence will be more effective in its use.
Keywords: Abhraka bhasma, Elemental analysis, XRD, SEM
1. Introduction
Ayurveda is the divine knowledge in which the holistic ancient Indian system of medicines is recited. Ayurvedic medicines are prepared from plants, animals and metals/minerals origin [1]. Rasashastra is a branch of Ayurveda in which detailed knowledge of metals and minerals have been explained. However, due to requirement of higher dosage, non-palatability and less shelf-life, the herbal medicines have their limitations. To overcome this, Bhasmas are the best alternatives as they can be prepared from the natural minerals and metals along with herbs by the process of Bhasmikarana in which toxic compounds are converted into non-toxic and bio-acceptable form [2], [3]. Moreover, they can be easily acceptable, palatable, fast acting and effective in small dosages and have long shelf life without loosing their potency. Use of bhasmas in clinical practice is also preferred by physicians. Although, bhasmas are superior to herbal products, improperly prepared bhasmas can exhibit potentially severe, toxic and adverse effects on the body, e.g. heavy metal poisoning and sometimes may lead to death. In metal based drugs, the use of plant extracts eradicate the toxic effects of metals [4], [5]. Therefore, in ancient Rasashastra texts, more emphasis is given on the preparation of bhasma. For instance, preparation of particular bhasma from a single metal or mineral have been shown with different routes to enhance their therapeutic utility and ultimately medicinal values [6]. By changing the method of preparation, bhasmas can be obtained in different forms, colors and with different physiochemical properties [7]. These properties were used to be tested in Ayurveda by variety of classical tests [8, ch 8/ 27-30] to ensure essential quality of bhasma such as fineness, lusterlessness, ability to float on fluid etc [9] to check the efficacy of bhasmikarana process to form biological nanocrystals in bhasma [7]. The effectiveness of bhasma depends on its nano-sized crystalline nature, homogeneous chemical composition and biological activity [7]. However, in ancient times no proper instrumentation facilities were available for such characterization. Modern analytical techniques can now be used to throw a light on the hidden properties and composition of these compounds which are prepared using ancient knowledge.
In the previous papers from our laboratory, we have reported the work on synthesis and characterization of vanga bhasma [10], naga bhasma [11], mandur bhasma, yashad bhasma and rajat bhasma. In the present work, we have selectively prepared abhraka bhasma by two different methods as explained in Rasashastra texts [8, Ch 2/16-17, 21: 12, Ch 10/46-48, 32, 70-71]. with slight modification. In classical text, three times Gajaputa is prescribed. However we have used electric muffle furnace which gives uniform heating and the sample was incinerated till proper bhasma was formed (confirmed by classical and modern analytical techniques). In method-2 instead of latex of Calotropis procera, juice of C. procera (Arka patra swarasa) was used. It was particularly chosen, as it is an important ayurvedic medicine commonly used in day to day treatments to cure several diseases such as in asthma, pulmonary tuberculosis, diabetes mellitus etc [8, ch / 2; 10, 51; 12, ch 10/72-73]. If not prepared properly, the abhraka bhasma shows side effects like diabetes, anorexia, loss of appetite, intestinal obstruction, intestinal perforation and may lead to death [8, Ch 2/13; 12; 12, Ch 11/7-9]. In Ayurveda, abhraka bhasmas prepared by different methods are used for treating different diseases. Abhraka bhasma prepared by method-1 is commonly used for treating anemia and tropical sprue and that by method-2 is commonly used for treating chronic cough. The synthesis of abhraka bhasma mainly consists of shodhana (purification of abhraka), dhanyabhraka (insertion of abhraka in sour gruel with rice husk), marana (incineration) and amritikarana (heating with cow ghee) [12, Ch 10/71] During all these processes, the impurities in the mica get removed reducing the toxicity with increased medicinal value. Thus, the product at each step is needed to be examined. Herein, we report the preparation of abhraka bhasma by two preparation routes along with the physicochemical characterization of the products obtained after each major processing step. For this purpose XRD, EDAX, FTIR, Raman, TG/DTG, SEM, TEM, BET and DLS techniques were used. The as-prepared bhasma products were also compared with two commercial products.
2. Materials and methods
The traditional process was used for the preparation of abhraka bhasma. The abhraka bhasma was prepared using 2 different traditional methods as shown in Fig. 1. The prepared bhasmas were also compared with two commercial bhasma samples. The material and process used for the preparation is given below.
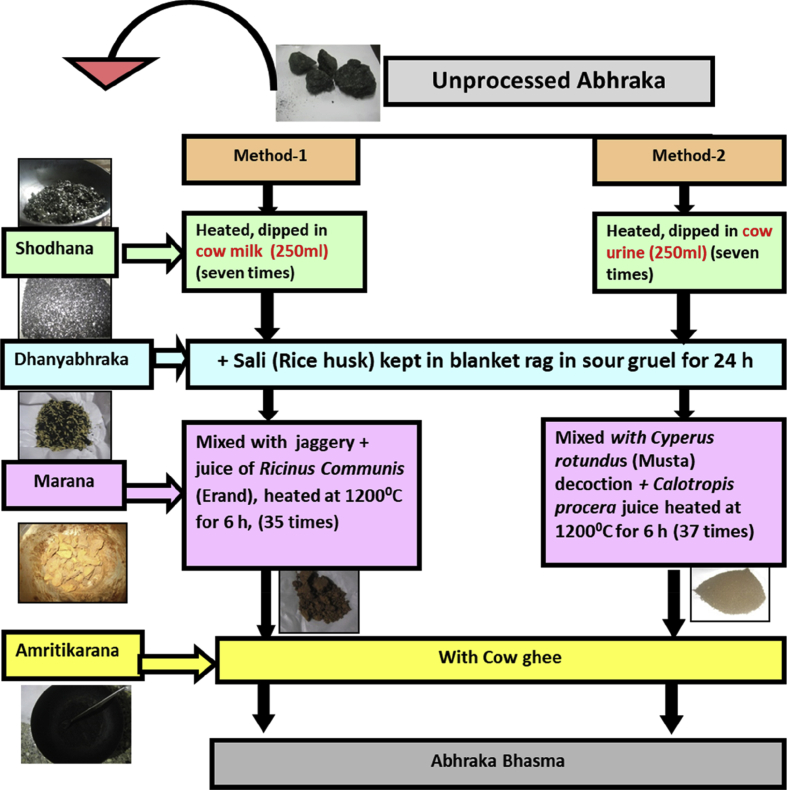
Fig. 1.
Flow-sheet for the preparation of abhraka bhasma by two different methods with actual photographs of products.
2.1. Materials
Authenticated raw materials were used for synthesis of bhasma. Abhraka (Mica-Biotite) was procured from Goradia Gandhi Aushadhalaya, Mumbai. Sesame oil procured from D.G. Pardeshi and son's laboratory, Pune. Turmeric powder, rice husk (Sali), jaggery, Ficus religosa Linn. powders were purchased from local ayurvedic medical shop. Fresh cow milk and cow's urine were brought from local cow shed. Sour gruel, decoction of horse gram and buttermilk were prepared as per references of Ayurvedic texts [13], [14], [15]. Juices of Ricinus communis Linn.(Eranda patra swarasa) and Calotropis procera Aiton R.B. (Arka patra swarasa) were prepared by crushing of the leaves of these plants with water in a mixer-grinder [12, Ch 11/7-9] Two commercial abhraka bhasmas were procured from local market.
2.2. Preparation procedures of bhasma
Abhraka bhasma was prepared by two different methods: Method-1 and Method-2. General preparation of bhasma involves four major stages viz. shodhana, dhanyabhraka, marana and amritikarana. The first step in both the methods was shodhana. In this process, the pieces of mica [(166.19 g (method-1), 153.46 g (method-2)] were heated till they became red hot and dipped in cow milk (250 mL) and cow urine (250 mL) respectively and then allowed to cool. This procedure was repeated 7 times [8, Ch 2/16-17] After shodhana process dhanyabhraka process was carried out for both the methods. In this process purified mica was powdered and 1/4th part of rice husk [(54.9 g (method-1), 38 g (method-2)] was put in blanket-rag and tied firmly and then it was kept in sour gruel for a day. Thereafter it was rubbed with hands so that mica entirely seeps into the sour gruel. The mica was collected from sour gruel, washed and dried under sunshine [8, Ch 2/21]. In marana process, the dhanyabhraka product was treated with jaggery and juice of R. communis Linn. Leaves (100 mL) (method-1) [12, Ch 10/46-48] and decoction of C. rotundus Linn. (100 mL) with juice of C. procera Aiton R.B. (50 mL) (method-2) [12, Ch 10/32]. Finally the mixtures were incinerated at 1200 °C for 6 h and the process was repeated 35 and 37 times for method-1 and method-2 respectively. As incineration process is to be repeated until proper bhasma is formed, each method required different steps. Amritikarana process was carried on incinerated product in both the methods [12, Ch 10/70-71]. During this process bhasma after marana process [49.75 g (method-1), 58.18 g (method-2)] was kept in iron pan and was roasted with equal quantity of cow ghee [(50 mL (method-1), 60 mL (method-2)] for 2–3 h till it got dry. After Amritikarana, yield of abhraka bhasma by method-1 and method-2 was found to be 51% and 63% respectively.
2.3. Classical ayurvedic tests of bhasma
The as-prepared abhraka bhasmas were first analyzed by traditional methods described in classical ayurvedic texts. These are Varitara – (ability of bhasma to float on water), Unnama – (a grain floats on a film of sprinkled bhasma on water), Rekhapurnatava – (particle should occupy the furrows of the finger), Susukshma - (reduced particle size), Niswadatvam or Gata Rasatvam – (tasteless), Nishchandratvam – (lusterless), Apunarbhavatva (non-reformation or non-reproduction of bhasma to original form). Niruttha: in this process in which Abhrak bhasma was treated with silver at high temperature in furnace. The weight of silver should remain unchanged [12, Ch 2/53-57].
2.4. Physicochemical characterization
The as-prepared Bhasmas at each intermediate stage (i.e. Shodhana, Dhanyabhraka and Marana) was characterized by using sophisticated instruments. X-ray diffractometer SHIMADZU AA -7000, equipped with photo scintillation detector, between 2θ ranges 10–80°, rate of scanning was kept at 5°/min was used for XRD studies. The FTIR analysis was carried out using FTIR Model, SHIMADZU 8400. The spectra were recorded between 4000 and 400 cm−1. Raman spectroscopy was done using RENISHAW – Invia Raman Microscope in the region 2000-100 cm−1 at wavelength λ = 532 nm. Thermal analysis (TGA-DTA) was carried out using METTLER TOLEDO –DSC821 instrument. The heating rate was 10 °C/min over the range of 25°-1000 °C in air atmosphere. Brauner Emmet Teller method (BET) was used to calculate surface area using SURFER Thermo Scientific instrument. Scanning electron microscope (SEM) Model: NOVANANO SEM-450 equipped with Energy dispersive X-rays analysis (EDAX) was used to study the morphology and elemental analysis. TEM was carried out using PHILIPS model - CM 200 with accelerating voltage 20–200 kv and resolution 0.24 nm.
3. Results
The as-prepared Abhraka bhasmas were analyzed by traditional methods described in classical ayurvedic texts [12, Ch 2/53-57] and by modern analytical techniques.
All the classical ayurvedic tests were found to be positive for the prepared bhasmas indicating formation of proper bhasma and are shown in Table 1.
Table 1.
Analysis of synthesized abhraka bhasma by classical ayurvedic tests.
| Test | Observation | Results |
||
|---|---|---|---|---|
| Method- 1 | Method- 2 | |||
| 1 | Varitara 
|
Abhraka bhasma floated on stagnant water. | +ve | +ve |
| 2 | Unnama 
|
The rice grain remained as it is on the layer of floated bhasma. | +ve | +ve |
| 3 | Rekhapurnata 
|
The bhasma filled the minute furrows of the finger tips | +ve | +ve |
| 4 | Nirchandrata | No luster was observed in sunlight. | +ve | +ve |
| 5 | Slakshnata | The Abhraka bhasma was soft and smooth to touch. | +ve | +ve |
| 6 | Niruttha | Weight of the silver remained same. No increase was observed. | +ve | +ve |
Characterization of Abhraka bhasma was carried out using XRD, FTIR, TGA/DTA, SEM, EDX, DLS and BET techniques.
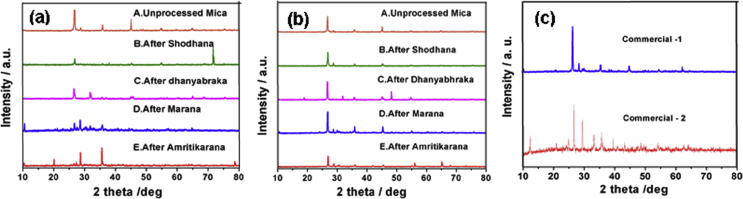
The powdered XRD patterns of as-prepared abhraka bhasmas and two commercial abhraka bhasmas are displayed in Fig. 2. The crystallite sizes calculated from the Scherrer equation for all the products are recorded in Table 2.
Fig. 2.
XRD pattern of abhraka bhasma prepared by two different methods (a) Method-1 (b) Method-2 at each stage of processing and (c) two commercial bhasma.
Table 2.
Mean Crystallite size data for as-prepared abhraka bhasma products after each stage.
| After Stage | Crystalline size/nm |
|
|---|---|---|
| Method-1 | Method-2 | |
| Shodhana | 114 | 119 |
| Dhanyabraka | 47 | 106 |
| Marana | 62 | 54 |
| Amritikarana | 85 | 80 |
| Commercial sample 1 | 95 | |
| Commercial sample 2 | 45 | |
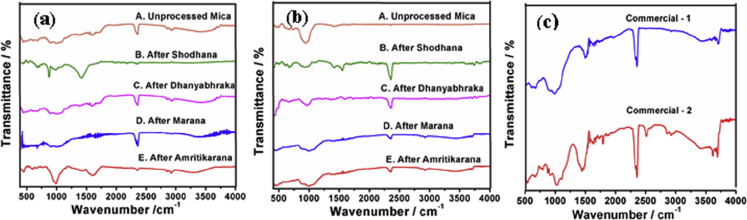
The FTIR spectrum of Abhraka bhasma was studied in the region of 4000 to 400 cm−1 and is shown in Fig. 3.
Fig. 3.
FTIR spectrum of abhraka bhasma prepared by two different methods (a) Method-1 (b) Method-2 at each stage of processing and (c) two commercial bhasma.
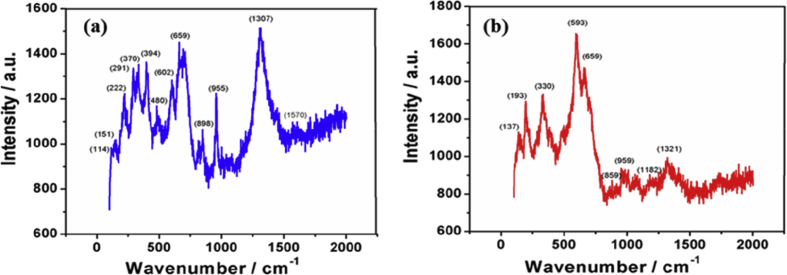
The Raman spectrum in region of 2000-100 cm−1 of abhraka bhasma is shown in Fig. 4 and the pertinent peak values and respective functional groups of as prepared abhraka bhasma are tabulated in Supplementary Table S1.
Fig. 4.
Raman Spectrum of abhraka bhasma prepared by two different methods (a) Method-1 (b) Method-2 after Marana process.
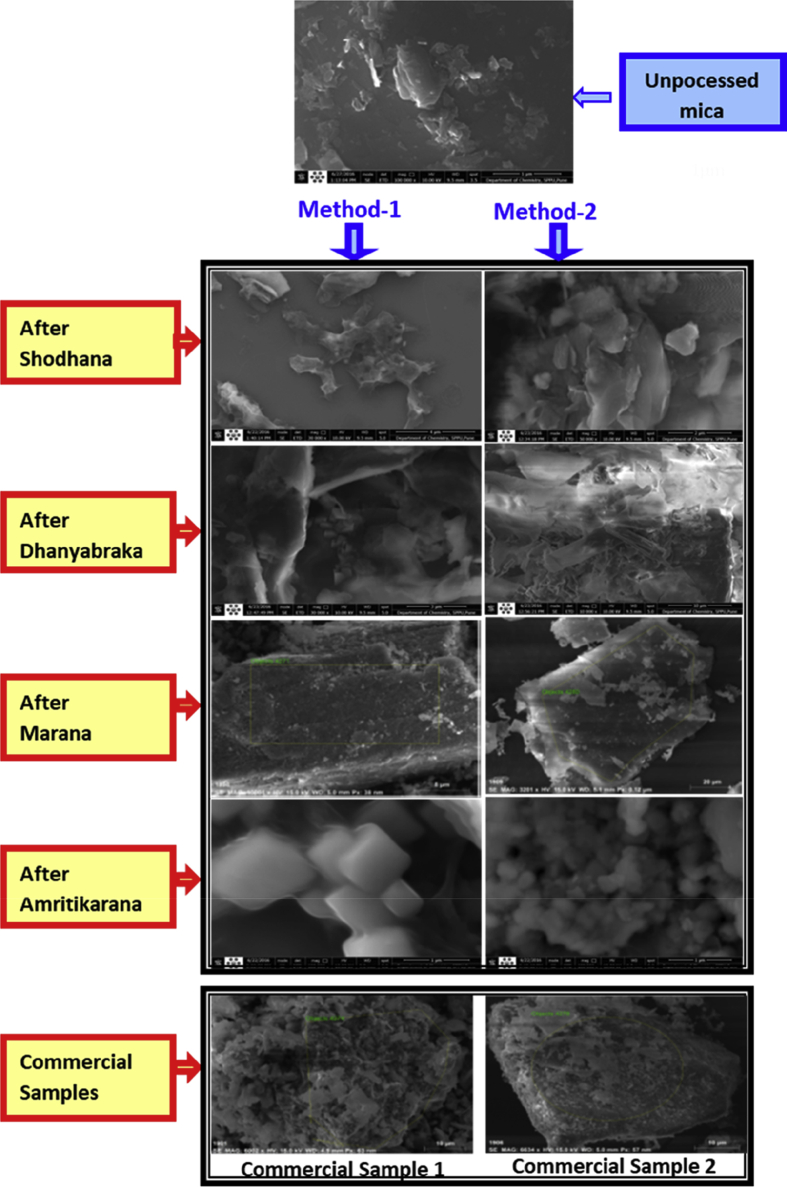
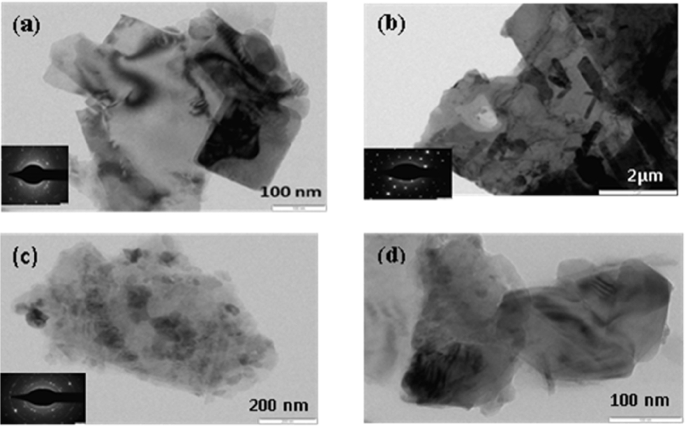
SEM and TEM images of abhraka bhasma are shown in Fig. 5 and Fig. 6 respectively. It is to be noted that magnification of SEM images is 1,00,000× for unprocessed mica, after shodhana (method-1) and after amritikarana (method-1 and method-2) images; 50,000× for dhanyabhraka (method-1) and after shodhana (method-2) images; 10,000× for after marana (method-1) and after dhanyabhraka (method-2) images; 6,000× for commercial-1 image; 3,000× for after marana (method-2) and commercial-2 images. Magnification of TEM images is 17,000× for of Fig. 6(a) and (d) images; 8,000× for Fig. 6(b) image and 500× for Fig. 6(c) image.
Fig. 5.
SEM Micrographs of abhraka bhasma after each stage of preparation and two commercial abhraka bhasma.
Fig. 6.
TEM photographs of abhraka bhasma after marana process prepared by two different methods (a) Method-1 (b) Method-2 (c) & (d) two commercial bhasma respectively.
The elemental composition of abhraka bhasmas obtained from EDX analysis is presented in Supplementary Table S2.
The results obtained after BET analysis are presented in Table 3 and that obtained after DLS analysis are shown in Supplementary Fig. S1 and Table 4.
Table 3.
BET surface area analysis results of as-prepared products after marana and commercial samples.
| Serial No | Sample name | Surface area/m2 g−1 | Pore volume/ncc g−1 | Maximum Pore size/Å | Average Pore size/Å |
|---|---|---|---|---|---|
| 1 | Method-1 | 5.05 | 1.16 | 8.063 | 7.15 |
| 2 | Method-2 | 50.11 | 11.52 | 8.33 | 7.06 |
| 3 | Commercial 1 | 4.13 | 0.95 | 8.42 | 6.86 |
| 4 | Commercial 2 | 9.35 | 2.15 | 8.52 | 7.01 |
Table 4.
DLS parameters of abhraka bhasma.
| Bhasma method | Effective Diameter/nm | Polydispersity | Diffusion coefficient/10−9 cm2s−1 |
|---|---|---|---|
| Method-1 | 902.54 | 0.270 | 5.437 |
| Method-2 | 548.94 | 0.091 | 8.940 |
| Commercial1 | 531.86 | 0.092 | 9.227 |
| Commercial2 | 333.09 | 0.178 | 1.473 |
The TG/DTG graphs of the bhasma are shown in Supplementary Fig. S2.
4. Discussion
4.1. Characterization of abhraka bhasma by XRD
All the XRD patterns (Fig. 2) exhibited major crystalline peaks pertaining to mica and matches well with monoclinic structure of KMg3(Si3Al)O10(OH)2 [16] (JCPDS card no.10-0495). The major diffraction peaks were observed at ∼ 2θ values, 8.89, 26.62, 28.39, 35.72, 45.08, and 54.88° and can be corresponded to (001) (003) (112) (004) (005) (135) planes, respectively. The XRD patterns are also matching very well with commercial samples. However, the peaks in the as-prepared products are comparatively broadened as compared with commercial products suggesting the nano-crystalline nature of the material.
An examination of Table 2 reveals that the crystallite size goes on decreasing with the process such as shodhana, dhanyabhraka and marana. This can be attributed to the decomposition of impurities and other associated organic moieties and ultimately the formation of nanosized bhasma with reduced particle size. After amritikarana, there is again little increase in the crystallite size due to formation of complex with organic moieties from cow ghee. The intensity of peaks in XRD pattern becomes more and more sharpened from successive stages i.e. from shodhana process to amritikarana process in both the methods.
4.2. Characterization of abhraka bhasma by Fourier Transform Infrared (FTIR) technique
The FTIR spectrum of abhraka bhasma was studied in the region of 4000 to 400 cm−1 (Fig. 3). The major band in the region of 2700 cm−1–3700 cm−1 can be attributed to the hydroxyl group (O-H). The O-H band with wavenumber 3654, 3594 and 3562 are due to the Mg2Al-OH stretching, Mg2Fe3+-OH stretching and MgFe3+-OH stretching respectively [17]. The presence of hydroxyl group (O-H) may be because of hydrophilic nature of bhasmas and moisture. The O-H band at this region gradually becomes more and more prominent in successive stages i.e. from shodhana to amritikarana. The O-H band becomes more prominent after amritikarana process using cow ghee. This trend was observed in both the methods. In original compound (unprocessed mica) this band is absent.
Strong sharp band in the region of 2250 cm−1–2500 cm−1 and prominent band at 2330 cm−1 present in both the bhasmas which represents O=C=O stretching bond indicating carbon dioxide and carbonate group. Intensity of this band goes on increasing from shodhana to marana stages which can be seen in method-1 which indicates incorporation of carbon during incineration process and also gradual formation of bhasma. In method-2; O=C=O stretching band gradually decreases from shodhana to marana stage. After amritikarana the intensity of this band decreases in both bhasmas which is suggestive of removal of carbon. Amritikarana is the process used to remove extra ash from compound. In original compound (unprocessed mica) this band is absent. Sharp band at the region of 1000 cm−1-1250 cm−1, represents Si-O stretching (Wavenumber 1063 and 1007) and S=O (sulfate) group. Intensity of this band goes on decreasing from shodhana to marana stages which is seen in both the methods which indicates removal of silicon and sulfur from bhasma compound. In amritikarana, intensity of this band decreases further. Strong band at the region of 470 cm−1–750 cm−1 indicates presence of Si =O (Silicate) group and wavenumber 712, wavenumber 681 attributed to Mg2-OH deformation and wavenumber 461 represent Si-O-Si deformation [17]. Strong sharp band in the region of 500 cm−1–690 cm−1 shows presence of (C-Br) halo compound.
4.3. Characterization of abhraka bhasma by Raman spectroscopy
The FTIR results were further supported by Raman spectroscopy in region 2000-100 cm−1 (Fig. 4, Supplementary Table S1). The peaks in the region 100-210 cm−1 are due to lattice vibrations. The peak near 197 cm−1 may be attributed to Al-OH translation [18], [19]. The strong peaks in the region of 150–250 cm−1 represents metallic oxides such as Fe-O, Mg- O and K-O. These peaks are also assigned to Al-OH, O-Si-O and O-Al-O. The peak in the region 291 cm−1 represents Se-Se bond. The sharp peaks in the region 480–800 cm−1 are attributed to halo compounds like C-I, C-Cl and C-Br and are consistent with FTIR results. Peaks in the region 552–682 cm−1 and strong peak at 1307 cm−1 may be assigned as Si-O-Si bond. Peak in the region 425–550 cm−1 and at 659 cm−1 can be attributed to S-S and C-S bonds respectively. This shows presence of sulfur in abhraka bhasma. The peaks in region 800–900 cm−1 are attributed to C-O-O bond and are consistent with FTIR spectrum.
4.4. Characterization of abhraka bhasma by Scanning Electron Microscopy (SEM)
An examination of SEM images (Fig. 5) shows that initially the particles of mica are in micron size with multilayered structure. After shodhana process, there was reduction in particle size and also the crystallinity was found to be increased (observed from increased white matter in the figures after shodhana process). After dhanyabhraka process, there was increase in the fibrous strands in the products which can be seen in Figure. This was due to treatment with sour gruel (Kanji). After marana process, the fibrous structures disappeared and agglomerated clumps of finite particles were observed. The agglomeration can be because of heat treatment of about ∼1200 °C during marana process. Along with these agglomerated clumps, very fine particles were also observed located on the surface of the clumps. After amritikarana, these clumps get deagglomerated and the particles separate out with increase in the grain size. The shape of the particles was also found to be changed after amritikarana. Depending on method of preparation the morphological variation was observed. In case of method 1 the square-type particles were observed whereas in case of method 2 spherical and rod-like particles were observed respectively. The particle size and morphologies were found to be differing with change in the preparation route because of different processing conditions, calcinations temperatures and cycles. After amritikarana, the edges of particles become smoother.
4.5. Characterization of abhraka bhasma by Transmission Electron Microscopy (TEM)
More close observation of particles sizes and morphologies was performed by TEM analysis. TEM photographs (Fig. 6) exhibits the particles of different sizes and shapes ranging from 50 nm to 1 μm with change in the method of preparation. All the as-prepared products show agglomerated structures, however the agglomerates are soft and clear boundaries of individual particles can be seen from the figures. In case of products obtained from method 1 and 2 rectangular plate like structures are observed with width and length ∼70 and 100 nm respectively. In case of product obtained from method 2 these rectangular plates are more elongated and tending to form nano-rod like structures. Occurrence of few nano-rods can also be seen in the photograph. Moreover at some points these particles have started a faceted growth. In case of commercial samples irregular shaped particles were observed. Beside few faceted structures were also located. The size of the particles is in the range of 100–200 nm. Thus, overall morphologies of as-prepared products are more porous and nanosized.
The SAED pattern of as-prepared bhasma products are represented as inset along with respective TEM photograph. It can be seen that all the products shows polycrystalline nature, among these products obtained from method-2 shows well defined crystalline SAED pattern.
4.6. Characterization of abhraka bhasma by Energy Dispersive X-ray spectroscopy (EDX)
An examination of Suppleementary Table S2 confirms abhraka (mica) as biotite with presence of elements Si, Al, Mg, Fe K, Na, Ca at major level and Mn, Cr, Li, Ti, Ba, Rb, Cs at minor levels. It is observed that the concentration of silicon was found to be increased after shodhana process which decreases after marana process. This trend was observed in both the methods. After amritikarana, the concentration of Si was again found to be increased. However in case of aluminium, there is a slight increase after shodhana stage which was found to be decreased after amritikarana stage. Al is a toxic element, the decrease in its concentration suggests the detoxification. Concentration of iron and magnesium was found to be decreased after shodhana process which further increases after marana process; this trend was seen in bhasmas prepared by both the methods. Nevertheless, after amritikarana, concentration of Fe was found to be increased, whereas concentration of Mg decreased. These elements such as Mg, Fe and Ca are useful elements in digestion and assimilation of bhasma. Also the presence of carbon along with these elements suggests the formation of organo-metallic compound. In case of all bhasma samples the percentage of carbon was found to be increased after amritikarana process which is the indication of formation of metal complex with the organic moieties present in the cow ghee. The presence of carbon was further confirmed by TG/DTG analysis. Commercial bhasma samples show similar results.
4.7. Characterization of abhraka bhasma by Brauner Emmet Teller (BET) method
BET analysis (Table 3) reveals that the surface area of all the as-prepared products was found to be higher (∼5–50 m2 g−1) than the commercial samples. The product obtained from method 2 showed highest surface area along with higher porosity.
4.8. Characterization of abhraka bhasma using Dynamic Light Scattering (DLS)
An examination of Supplementary Fig. S1 reveals bimodal distribution of particles with ∼50% of particles are in nanorange (50–500 nm) in method-1 and remaining particles are in micron range. The micron size nature of particles can be attributed to agglomeration of fused structure. Abhraka bhasma prepared using method-2 shows homogeneous distribution of particles with nearly 90% of particles falling in the region of 50–500 nm. Commercial-1 and commercial-2 shows similar pattern like Abhraka bhasma prepared using method-2. Particle size in bhasma prepared by method-2 appears to be smaller than method-1.
An examination of Table 4 shows that the effective diameter of bhasmas by method-1 and method-2 ranges between 500 and 1000 nm. Polydispersity was found to be least in bhasma prepared by method-2.
4.9. Characterization of abhraka bhasma by Thermogravimetric Analysis (TGA)/Differential Thermal Analysis (DTA)
An examination of Supplementary Fig. S2 shows minute weight loss (0.2–0.3%) at ∼100 °C presumably due to adsorbed moisture on the surface of abhraka bhasma. Thereafter, there was no change in weight up to 800 °C confirming the perfect formation of bhasma. Between 800 °C–900 °C there was a gradual weight loss of ∼6% which can be attributed to the decomposition of organic moieties. Similar pattern was seen in commercial-2 sample (Supplementary Fig. S1). In case of method-2, TGA graph shows gradual increase in weight which can be attributed to adsorption of oxygen which requires further investigation. The similar pattern is seen in commercial-1 sample.
5. Conclusion
Prepared Abhra ka bhasma cleared physical as well as physico-chemical tests mentioned in Ayurvedic literature. EDX spectrum shows presence of elements Si, Al, Mg, Fe K, Na, Ca at major level and also traces of Mn, Cr, Li, Ti, Ba, Rb, Cs at minor levels. All the XRD patterns exhibited major crystalline peaks pertaining to mica and matches well with monoclinic KMg3(Si3Al)O10(OH)2. XRD pattern shows highly crystalline nature of bhasma. FTIR and Raman study reveals presence of various bonds of different functional groups which indicates organo-metallic nature of bhasma. SEM/TEM study reveals crystalline nature of bhasma of different size and shapes ranging from 50 nm to 1 μm and formation of nano particles. BET study shows surface area of bhasma ranging from ∼ 5–50 m2g-1. TGA analysis shows weight loss due to moisture and decomposition of organic moieties. DLS curve shows particle size distribution in nano sized region (50–500 nm) [50% (method-1) and 90% (method-2)].
Characterization study revealed that abhraka bhasma prepared by method-2 has more proportion of nano particles than that prepared by method -1 and hence will be more effective in its use.
Sources of funding
Board of College and University Development (BCUD), Savitribai Phule Pune University (Ref. No. Fin/2015-2016/42 dated 09/04/2015).
Conflicts of interest
None.
Acknowledgement
Authors are thankful to Board of College and University Development (BCUD), Savitribai Phule Pune University, Pune for financial assistance.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2018.11.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Agnivesha, editor. Charaka Samhita: Chapter 1, Verse 69. 2nd ed. Varanasi: Chaukhamba sanskrita sansthana; 2000 p. 12.
- 2.Tripathi Y.B., Sharma G.M.K., Singh V.P., Sinha R.K., Singh D. X-rays diffraction and microscopic analysis of tamra bhasma: an Ayurvedic metallic preparation. Indian J Tradit Knowl. 2003;2:107–117. [Google Scholar]
- 3.Wadekar M.P., Patel R.K. Preparation and characterization of a copper based Indian traditional drug: tamra bhasma. J Pharmaceut Biomed Anal. 2005;39(2):951–955. doi: 10.1016/j.jpba.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Joaquin P. An investigation on the activity pattern of alchemical transmutations. J Sci Explor. 2002;16:593–6023. [Google Scholar]
- 5.Das B., Achintya M., Jayaram H. Management of madhumeha (diabetes mellitus) with current evidence and intervention with ayurvedic rasausadhies. Indian J Tradit knowl. 2011;10(4):624–628. [Google Scholar]
- 6.Rajput D., Mesram P., Patgiri B.J. Critical review on pharmaceutical prospects of nagabhasma (incinerated lead) Int. J. Pharmaceut Biol Arch. 2014;5(5):46–53. [Google Scholar]
- 7.Rasheed A., Marri A., Madhu Naik M. Standardization of bhasma-importance and prospects. J Pharm Res. 2011;4(6):1931–1933. [Google Scholar]
- 8.Tripathi I.D., editor. Hindi commentary of Rasaratnasammuchhaya. Chaukhamba Sanskrit Sansthan; Varanasi: 2006. [Google Scholar]
- 9.Kokate C.K., Purohit A.P., Gokhale S.B. Analytical pharmacognosy. Pharmacognosy. 2005:1–99. 30th ed. [Google Scholar]
- 10.Kale B., Rajurkar N. Synthesis and characterization of vanga bhasma. J Ayurveda Integr Med. 2017:1–9. doi: 10.1016/j.jaim.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantak S., Rajurkar N. Synthesis and characterization of naga (lead) bhasma. J Appl chem. 2017;6(2):291–298. [Google Scholar]
- 12.Shastri K.N., editor. Rasatarangini, Hindi commentary. 11th ed. Motilal Banarasi Das; Delhi: 2009. [Google Scholar]
- 13.Kulkarni D.A., editor. Hindi commentary of Rasaratnasammuchhaya. Meharchand Laxamandas publication; New Delhi: 1982. p. 217. [Google Scholar]
- 14.Sharangadhara, editor. Sharangadhara samhita, adhamala, dipika commentary, madhyam khanda. 6th ed. Chaukhamba orientalia; 2/1, Varanasi: 2005. [Google Scholar]
- 15.Shastri AD, editor. Hindi commentary of Sushruta samhita, Sootrashtana: Chapter 45, Verse 85. 8th ed. Varanasi: Chaukhamba Sanskrit Sansthan.
- 16.Luo Z., Yang J., Ma H., Ma X. Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia. Hydrometallurgy. 2015;157:188–193. [Google Scholar]
- 17.Janek M., Lorenc D., Chorvat D., Bugar I. Terahertz time-domain spectroscopy of selected layered silicates. Clay Clay Miner. 2009;57(4):416–424. [Google Scholar]
- 18.Singha M., Singh L. Vibrational spectroscopic study of muscovite and biotite layered phyllosilicates. Indian J Pure Appl Phys. 2016;54:116–122. [Google Scholar]
- 19.Tlili A., Smith D.C., Beny J.M., Boyer H. A Raman microprobe study of natural micas. Mineral Mag. 1989;53:165–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.