Abstract
Background
In view of the gravity of the problem of antimicrobial resistance among pathogenic bacteria against conventional bactericidal agents, investigation on alternative approaches to combat bacterial infections is warranted.
Objective
Current study aimed at investigating anti-infective potential of a polyherbal ayurvedic formulation namely panchvalkal against three different pathogenic bacteria.
Materials and methods
The panchvalkal formulation available as Pentaphyte P5® was tested for its possible in vitro quorum-modulatory potential against Chromobacterium violaceum, Serratia marcescens, and Staphylococcus aureus through broth dilution assay. Invivo efficacy was demonstrated employing Caenorhabditis elegans as the model host for test pathogens.
Results
This formulation was found to exert quorum-modulatory effect on C. violaceum, S. marcescens, and S. aureus at 250–750 μg/ml. Besides altering production of the quorum sensing-regulated pigments in these bacteria, the test formulation also had in vitro effect on antibiotic susceptibility, catalase activity and haemolytic potential of the pathogens. Invivo assay confirmed the protective effect of this panchvalkal formulation on C. elegans, when challenged with the pathogenic bacteria. Repeated exposure of S. aureus to panchvalkal did not induce resistance in this bacterium.
Conclusion
To the best of our awareness, this the first report on quorum-modulatory potential of panchvalkal formulation, validating the anti-infective potential and moderate prebiotic property of this polyherbal preparation.
Keywords: Quorum sensing, Antimicrobial resistance (AMR), Panchvalkal, Polyherbal, Staphylococcus aureus, Antibiotic susceptibility
1. Introduction
Antimicrobial resistance (AMR) is one of the forefront health challenges staring at the mankind in the current times. Modern antibiotic molecules have helped control the infectious diseases to a great extent, however relying solely on these conventional microbicidal antibiotics is not feasible owing to the pathogen's capacity to evolve to a resistant phenotype [1]. Search for novel alternatives to the conventional antimicrobial therapy is actively being pursued by researchers, and natural products seem to offer a bright ray of hope [2]. In the ancient medicinal texts, there are descriptions of many plant extracts for treatment of microbial infections [3], [4]. Plant products prescribed in ancient literature may be effective, but for their wider acceptance in the modern world, their efficacy has to be demonstrated through appropriate experiments, and also their mode of action needs to be elucidated [5], [6]. All effective antimicrobial formulations may not exert microbicidal effect; they may be exerting their efficacy by targeting virulence of the pathogens without necessarily affecting their growth heavily.
A peep into Ayurved (ancient system of Indian traditional medicine) reveals that a Panchvalkal formulation (PF) containing the barks of different Ficus species has been prescribed as a therapy for maintaining the health of female reproductive system. This formulation has also been mentioned to possess burn and wound-healing activity [7]. Panchavalkala Kwatha has been indicated in ayurvedic texts like Chakradutta, Bhaishajyaratnavali and Yogratnakar to be useful in management of mukharog e.g. tonsillitis. Panchvalkal has been mentioned to be relevant with respect to kapharaktaharadoshkarma [8]. Sushrut and Vaghbatachary mention panchvalkal for its utility in treatment of mukhadushika (one of the skin disease under the Kshudra roga). According to Bhavprakash nighantu, panchavalakal may be used for the treatment of sthaulya (obesity), in combination with gomutra (cow urine) and puran madhu (honey) [9]. Charak Samhita mentions following five plants as the ingredients of PF: Ficus bengalensis, F. religiosa, F. racemosa, F. lacor, and F. hispida. Later Bhavprakash nighantu replaced F. hispida with Albizia lebbeck. This modified PF has been studied in past for its toxicity, phytopharmacological aspects, and clinical efficacy in the patients suffering from chronic osteomyelitis [10]. We undertook to investigate interaction of this PF with three different pathogenic bacteria viz. Chromobacterium violaceum, Serratia marcescens, and S. aureus. C. violaceum is being considered as an emergent pathogen; its infection in humans is reported to be fatal [11], [12]. This bacterium is known to be resistant to penicillins and cephalosporins [13], [14]. S. marcescens has also been considered as an emerging opportunistic pathogen causing infections of respiratory tract, urinary tract, meningitis, septicaemia, pneumonia, and wound infections [15]. It has been shown to be responsible for frequent nosocomial outbreaks in infants and neonates [16]. S. aureus is a major cause of nosocomial infections globally. S. aureus infections are often difficult to treat owing to the large population heterogeneity, phenotypic switching, intra-strain diversity, and hypermutability [17]. Pigment production in all these three bacteria is quorum sensing (QS)-regulated. This study assayed the PF for its potential QS-modulatory (QSM) activity against these bacteria, since QS circuit is currently being viewed as a legitimate target for novel anti-virulence agents. QS is an intercellular communication process using acyl homoserine lactone (AHL) or auto-inducing peptidesas signal molecules, which regulates expression of a large number of genes including those coding for virulence [18], [19].
2. Materials and methods
2.1. Test organisms
C. violaceum (MTCC 2656), S. marcescens (MTCC 97), S. aureus (MTCC 737), and Lactobacillus plantarum (MTCC 2621) were procured from MTCC (Microbial Type Culture Collection, Chandigarh), whereas Bifidobacterium bifidum (NCDC 255) was procured from NCDC (National Collection of Dairy Cultures), NDRI (National Dairy Research Institute, Karnal). C. violaceum and S. marcescens were grown in nutrient broth, whereas S. aureus was grown in Tryptone Yeast Extract (TYE) broth (ISP HiVeg™ Medium No. 1, HiMedia, Mumbai) with additional 0.3%w/v yeast extract. L. plantarum was grown in Lactobacillus MRS medium (HiMedia, Mumbai), and B. bifidum was grown on MRS with 0.05% Cysteine. Incubation temperature for C. violaceum, S. aureus, L. plantarum, and B. bifidum was 37°C, and for S. marcescens, it was 28°C. Incubation time for C. violaceum, S. marcescens, L. plantarum, and B. bifidum was kept 22–24 h, and for S. aureus, it was 46–48 h. Antibiotic susceptibility profile of the bacterial strains used in this study was generated using the antibiotic discs-Dodeca Universal – I, Dodeca G - III - Plus and Icosa Universal -2 (HiMedia, Mumbai). C. violaceum and S. marcescens were found to be resistant to cefadroxil (30 μg), ampicillin (10 μg), cloxacillin (1 μg), penicillin (10 μg). S. marcescens showed resistance against vancomycin (30 μg), whereas S. aureus was found to be sensitive against all the tested antibiotics.
2.2. Test formulation
Capsules of Panchvalkal extract (Pentaphyte P5®) containing mixtures of bark extracts of F. bengalensis, F. religiosa, F. racemosa, F. lacor, and A. lebbeck, were procured from Dr. Palep's Medical Research Foundation Pvt. Ltd., Mumbai. Powder contained inside the capsules was dissolved in DMSO (Merck, Mumbai) for bioassay.
2.3. Broth dilution assay
Assessment of QS-regulated pigment production by test pathogens in presence or absence of the test formulation, was done using broth dilution assay [20]. Organism was challenged with different concentrations (250–1000 μg/ml) of Panchvalkal extract. Nutrient broth or TYE was used as a growth medium. Inoculum standardized to 0.5 McFarland turbidity standard was added at 10%v/v, followed by incubation at appropriate temp for each organism. Appropriate vehicle control containing DMSO was also included in the experiment, along with abiotic control (containing extract and growth medium, but no inoculum). Catechin (50 μg/ml; Sigma Aldrich, USA) was used as positive control.
2.3.1. Measurement of bacterial growth and pigment production
At the end of the incubation, bacterial growth was quantified at 764 nm [21]. This was followed by pigment extraction and quantification, as per the method described below for each of the pigment. Purity of each of the extracted pigment was confirmed by running a UV–vis scan (Agilent Cary 60 UV–visible spectrophotometer). Appearance of single major peak (at the λmax reported in literature) was taken as indication of purity.
2.3.2. Violacein extraction
One ml of the culture broth was centrifuged (Eppendorf 5417 R) at 15,300 g for 10 min at room temperature, and the resulting supernatant was discarded. The remaining cell pellet was resuspended into 1 ml of DMSO, and vortexed, followed by centrifugation at 15,300 g for 10 min. The purple coloured violacein was extracted in the supernatant; OD was measured at 585 nm. Violacein unit was calculated as OD585/OD764. This parameter was calculated to nullify the effect of change in cell density on pigment production [22].
2.3.3. Prodigiosin extraction
One ml of the culture broth was centrifuged at 10,600 g for 10 min. Centrifugation was carried out at 4°C, as prodigiosin is a temperature-sensitive compound. The resulting supernatant was discarded. Remaining cell pellet was resuspended in 1 ml of acidified methanol (4 ml of HCl into 96 ml of methanol; Merck), followed by incubation in dark at room temperature for 30 min. This was followed by centrifugation at 10,600 g for 10 min at 4°C. Prodigiosin was obtained in the resulting supernatant [23]; OD was taken at 535 nm. Prodigiosin unit was calculated as OD535/OD764.
2.3.4. Staphyloxanthin extraction
One ml of the S. aureus culture broth was centrifuged at 15,300 g for 10 min at room temperature. The resulting pellet was suspended in equal volume of methanol, and was kept in water bath (55°C) for 5 min. This was followed by centrifugation at 15,300 g for 10 min, and absorbance of the supernatant containing staphyloxanthin was measured at 450 nm [24]. Staphyloxanthin unit was calculated as OD450/OD764.
2.4. AHL extraction
OD of overnight grown culture of C. violaceum was standardized to 1.00 at 764 nm. It was centrifuged at 5000g for 5 min. Cell free supernatant was filter sterilized using 0.45 μm filter (Axiva, Haryana), and was mixed with equal volume of acidified ethyl acetate [0.1% formic acid (Merck) in Ethyl acetate (Merck)]. Ethyl acetate layer was collected, and evaporated at 55°C, followed by the reconstitution of the dried crystals in 100 μl phosphate buffer saline (pH 6.8) [25]. Identity of thus extracted AHL was confirmed by thin-layer chromatography (TLC). Rf value of purified AHL, while performing TLC [Methanol (60): Water (40); TLC Silica gel 60 F254plates; Merck] [26] was found to be 0.70, near to that (0.68) reported for N-hexonyl homoserine lactone (C6-HSL) [27].
2.5. Antibiotic susceptibility test
After in vitro assessment of QSM property of the test formulation, effect of this PF on antibiotic susceptibility of each of the test pathogen was investigated. For this purpose, the most effective quorum modulatory concentration of the PF against a particular bacterium was used. This investigation was done in two different ways. In one set of experiments, we challenged the test pathogen with PF and antibiotic simultaneously, wherein susceptibility of test pathogens to sub-MIC concentration of different antibiotics in absence and presence of test formulation was assessed through broth dilution assay. In another set of experiments the bacterial cells pre-treated with PF were subsequently challenged with antibiotic.
2.6. Haemolysisassay
OD of overnight grown culture was standardized to 1.00 at 764 nm. Cell free supernatant was prepared by centrifugation at 15,300 g for 10 min. 10 μl of human blood was incubated with this cell free supernatant for 2 h at 37°C, followed by centrifugation at 800 g for 15 min. 1% Triton X-100 (CDH, New Delhi) was used as a positive control. Phosphate buffer saline was used as a negative control. OD of the supernatant was read at 540 nm, to quantify the amount of haemoglobin released [28].
2.7. Catalase assay
OD of the culture was adjusted to 1.00 at 764 nm. 400 μl of phosphate buffer was added into a 2 ml vial followed by 400 μl H2O2. To this 200 μl of the bacterial culture was added, and the mixture was incubated for 10 min. Then 10 μM of sodium azide was added to stop the reaction [29] followed by centrifugation at 12,000 rpm for 10 min. OD of the supernatant was measured at 240 nm to quantify remaining H2O2 [30], with phosphate buffer as blank.
2.8. In vivo assay
In vivo efficacy of the test formulation at the concentration(s) found effective during in vitro screen was evaluated using the nematode worm C. elegans as the model host. This worm was maintained on Nematode Growing Medium (NGM; 3 g/l Nacl, 2.5 g/l peptone, 1 M Cacl2, 1 M MgSO4, 5 mg/ml cholesterol, 1 M phosphate buffer of pH 6, 17 gl agar–agar) with E. coli OP50 as the feed [31]. Worm population to be used for the in vivo assay was kept on NGM plates not seeded with E. coli OP50 for three days.
Test bacterium was incubated with the PF for 24 h. Following incubation, OD of the culture suspension was equalized to that of the DMSO control. 100 μl of this bacterial suspension was mixed with 900 μl of the M9 buffer containing 10 worms (L3-L4 stage). This experiment was performed in 24-well (sterile, non-treated) polystyrene plates (HiMedia), and incubation was carried out at 22°C. Number of live vs. lead worms was counted everyday till five days by putting the plate (with lid) under light microscope (4×). Standard antibiotic and catechin treated bacterial suspension were used as positive control. Straight worms were considered to be dead. On last day of the experiment, when plates could be opened, their death was also confirmed by touching them with a straight wire, wherein no movement was taken as confirmation of death.
2.9. Statistical analysis
All the experiments were performed in triplicate, and measurements are reported as mean ± standard deviation (SD). Statistical significance of the data was evaluated by applying t-test using Microsoft Excel®. p values ≤ 0.05 were considered to be statistically significant.
3. Results
3.1. C. violaceum
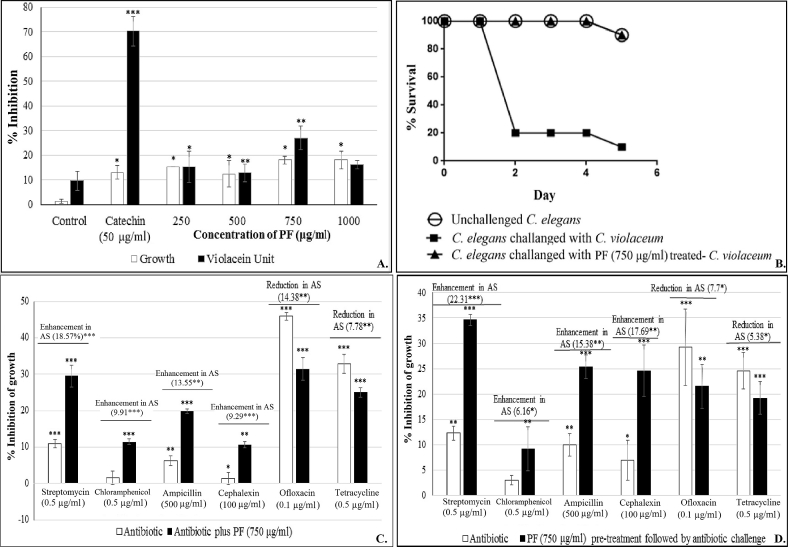
For in vitro studies, C. violaceum was challenged with the PF at 250–1000 μg/ml. PF exerted in vitro quorum inhibitory effect till 750 μg/ml, but interestingly not at 1000 μg/ml (Fig. 1A), whereas growth, though not heavily, was affected at all the test concentrations. Effect of this herbal preparation on growth of C. violaceum did not increase with increase in concentration. All the growth inhibition values at different test concentrations ranging from 10.52 to 18.18% did not differ statistically significantly. PF exerted QS inhibitory (QSI) effect at 250–750 μg/ml, with 750 μg/ml being the most effective concentration, causing a reduction of 27.02% in QS-regulated violacein production.
Fig. 1.
Effect of panchvalkal formulation on C. violaceum (*p < 0.05, **p < 0.01, ***p < 0.001; AS: Antibiotic susceptibility; QS: Quorum sensing; PF: Panchvalkal formulation) 1 (A). Effect of PF on growth and QS regulated violacein production in C. violaceum: Bacterial growth was measured as OD764; OD of violacein was measured at 585 nm, and violacein unit was calculated as the ratio OD585/OD764 (an indication of violacein production per unit of growth) ‘Control’ bar in this figure is the ‘vehicle control’ representing the % change values in comparison to the ‘growth control’ i.e. tube containing only growth medium plus organism, but no DMSO. 1 (B). Kaplan–Meier survival curve showing the protective effect of PF (750 μg/ml) on C. elegans, when challenged with C. violaceum: Catechin (50 μg/ml) and ampicillin (500 μg/ml) employed as positive controls conferred 100% protection. DMSO present in the ‘vehicle control’ at 0.5% v/v did not affect virulence of the bacterium towards C. elegans. 1 (C). C. violaceum challenged with PF and antibiotic together. 1 (D). C. violaceum challenged with antibiotic following pre-treatment with PF (750 g/ml).
Once we found 750 μg/ml PF to be the most effective quorum inhibitory concentration against C. violaceum, we tried to know whether this PF acts as a signal supply inhibitor or signal response inhibitor. This was checked by supplementing the QS-inhibited C. violaceum culture tube with extracted AHL. The QSI effect of PF was not found to be reversed upon addition of AHL (data not shown), indicating the PF to be acting as a signal response inhibitor.
Next we investigated whether the PF affects the bacterial traits other than pigment production i.e. catalase activity, haemolytic activity, and antibiotic susceptibility. Catalase activity of C. violaceum culture exposed to PF (750 μg/ml) was enhanced to a minor (2.46%) but significant extent (Table 1), which can be taken as an indication of mild activation of the stress response machinery of the bacterium when challenged with PF. Haemolytic ability, which is an important virulence trait [32], of this bacterium suffered a decrease of 7.01% under the influence of PF (Table 1).
Table 1.
Effect of Panchvalkal formulation on catalase and haemolytic activity of test bacteria.
| Organism | Concentration of extract (μg/ml) | % change in catalase activity | % change in haemolysis |
|---|---|---|---|
| C. violaceum | 750 | 2.46∗∗ ± 0.61 | −7.01∗ ± 2.37 |
| S. marcescens | 250 | 1.44∗ ± 1.17 | −3.95∗∗∗ ± 1.65 |
| 500 | 2.06∗∗∗ ± 0.37 | −5.42∗∗ ± 2.36 | |
| 750 | 2.13∗∗∗ ± 0.42 | −5.15∗∗∗ ± 0.15 | |
| 1000 | 2.21∗∗∗ ± 0.71 | −4.65∗∗ ± 0.03 | |
| S. aureus | 250 | 1.82∗∗∗ ± 0.97 | −4.95∗ ± 1.60 |
| 500 | 2.09∗∗ ± 0.76 | −4.42∗ ± 0.86 | |
| 750 | 3.59∗∗ ± 2.66 | −4.56∗∗ ± 0.20 | |
| 1000 | 4.22∗∗∗ ± 1.89 | −5.03∗ ± 1.43 |
*p < 0.05, **p < 0.01, ***p < 0.001; ‘−’ sign indicates a decrease over control; DMSO in ‘vehicle control’ tube had no effect on catalase and haemolytic activity of any of the three bacteria.
To investigate whether the susceptibility of C. violaceum to different antibiotics is altered under the influence of the QSI formulation, we challenged C. violaceum with sub-MIC concentration of six antibiotics belonging to five different classes, in presence as well as absence of PF at its most effective QSI concentration (Fig. 1C). Additionally we also tested the effect of antibiotics on C. violaceum culture pre-treated with PF i.e. the QS-inhibited culture (Fig. 1D). The PF caused an enhancement in the susceptibility of C. violaceum to four of the test antibiotics. However, it also caused a reduction in its susceptibility to ofloxacin and tetracycline. These results suggest that though simultaneous use of QS inhibitors and conventional antibiotics may be a good idea, selection of the antibiotic should be done carefully.
After confirming the in vitro efficacy of PF on multiple traits (pigment production, catalase activity, haemolysis, and antibiotic susceptibility) of C. violaceum, we assayed the PF for its in vivo efficacy using the nematode worm C. elegans as the model host. The worms were challenged with bacteria previously grown in broth containing (as well as that not containing) 750 μg/ml PF, and their survival was monitored up to five days. Appropriate controls containing the worms challenged with bacteria grown in broth containing 0.5% v/v DMSO or ampicillin (500 μg/ml) or catechin (50 μg/ml) were also included in the experiment. Bacteria previously treated with the PF could kill only 10% of worm population, as against 90% killed by the bacteria not exposed to the PF (Fig. 1B). Onset of death in worm population was also delayed by 2 days, when challenged with PF-treated bacteria.
3.2. S. marcescens
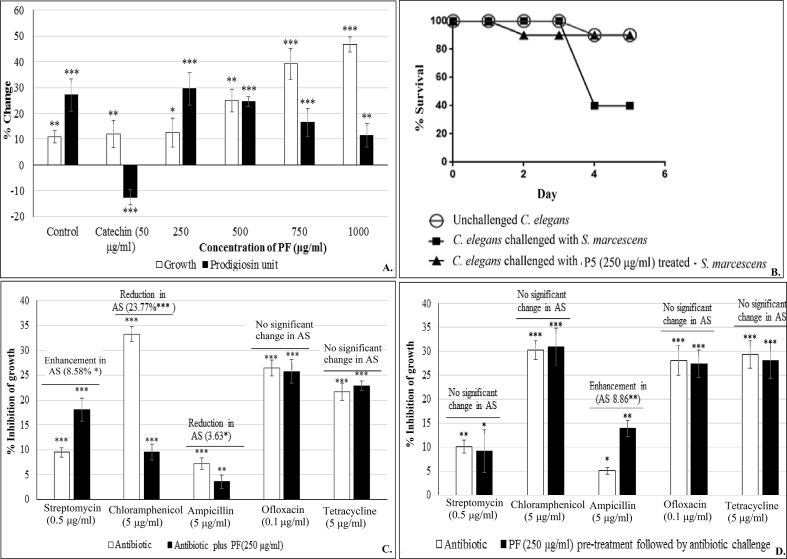
For in vitro studies, S. marcescens was challenged with the test formulation at 250–1000 μg/ml. Effect of this PF on S. marcescens growth was stimulatory from 250 μg/ml onwards (Fig. 2A). Though the effect on prodigiosin production was also stimulatory, magnitude of this effect decreased with increase in concentration. As production of the red pigment prodigiosin in S. marcescens is under control of QS [33], the PF can be said to have QSM effect on this bacterium. Catechin used as the positive control exerted stimulatory effect on S. marcescens growth, and inhibitory effect on prodigiosin production. As an ideal QS modulator is expected to have minimal or no effect on bacterial growth, we used the PF at 250 μg/ml for further experiments with S. marcescens, for the fact that among all evaluated concentrations, this was the concentration exerting lowest effect on bacterial growth, while affecting pigment production maximally.
Fig. 2.
Effect of panchvalkal formulation on S. marcescens (*p < 0.05, **p < 0.01, ***p < 0.001; AS: Antibiotic susceptibility; QS: Quorum sensing; PF: Panchvalkal formulation) 2 (A)Effect of PF on growth and QS regulated prodigiosin production in S. marcescens: Bacterial growth was measured as OD764; OD of prodigiosin was measured at 535 nm, and prodigiosin unit was calculated as the ratio OD535/OD764 (an indication of prodigiosin production per unit of growth). ‘Control’ bar in this figure is the ‘vehicle control’ representing the % change values in comparison to the ‘growth control’ i.e. tube containing only growth medium plus organism, but no DMSO. 2 (B). Kaplan–Meier survival curve showing the protective effect of PF (250 μg/ml) on C. elegans, when challenged with S. marcescens: Catechin (50 μg/ml) and Ofloxacin (0.1 μg/ml) employed as positive controls conferred 100% and 80% protection respectively. DMSO present in the ‘vehicle control’ at 0.5%v/v did not affect virulence of the bacterium towards C. elegans. 2 (C). S. marcescens challenged with PF and antibiotic together. 2 (D). S. marcescens challenged with antibiotic following pre-treatment with PF (250 μg/ml).
PF at 250 μg/ml caused a minor (1.44%) but statistically significant enhancement in catalase activity, and reduction in the haemolytic activity (by ∼4%) of the S. marcescens culture (Table 1). It was also able to alter the susceptibility of this bacterium to three different antibiotics (streptomycin, chloramphenicol, and ampicillin), when tried in combination with these antibiotics (Fig. 2C). Susceptibility of this bacterium against chloramphenicol was reduced by nearly 24% in presence of PF; however the S. marcescens culture pre-treated with PF did not experienced any alteration in its susceptibility to chloramphenicol (Fig. 2D). Antibacterial efficacy of ampicillin in combination with PF was reduced marginally (∼4%), but interestingly PF-pre-treated S. marcescens became bit more (∼9%) susceptible to ampicillin. This might be due to some sort of interaction between ampicillin and the PF, when present together.
In vivo assay demonstrated the ability of PF to confer survival benefit on C. elegans, when challenged with S. marcescens (Fig. 2B). S. marcescens receiving no pre-treatment with PF could kill 60% of worms by 5th day, whereas that treated with PF could kill only 10%. In this case, the survival benefit conferred by PF was equivalent to that conferred by ofloxacin (0.1 μg/ml) used as positive control, as the difference in number of surviving worms for these two cases was statistically not significant.
3.3. S. aureus
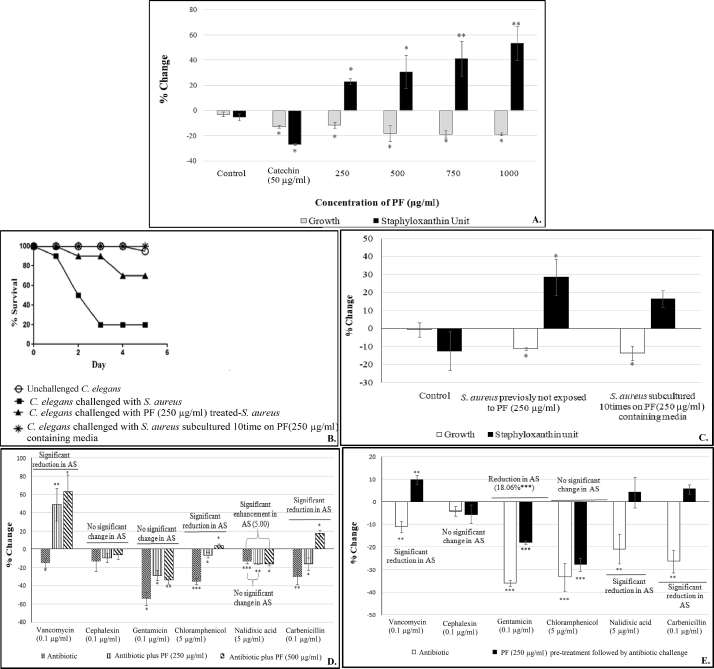
The PF had a negative effect on the growth of S. aureus at all the four test concentrations (Fig. 3A), whereas production of the carotenoid staphyloxanthin was enhanced in a dose-dependent fashion. Staphyloxanthin production in S. aureus has been known to be regulated by QS [34], and hence the PF can be said to have QSM effect on S. aureus. Catalase and haemolytic activity of this bacterium were respectively affected upward and downward, to a marginal extent under the influence of PF (Table 1). Increased catalase activity as well as higher staphyloxanthin production in presence of the PF can be taken as the indication of the bacterium experiencing higher oxidative stress when challenged with this formulation. Carotenoid pigments quench toxic singlet oxygen. They are potent antioxidants making them an important survival factor for detoxifying reactive oxygen species (ROS) [35]. It can be said that the PF used in this study is forcing S. aureus to over-activate its antioxidant machinery, by creating oxidative stress for this pathogen.
Fig. 3.
Effect of panchvalkal formulation on S. aureus (*p < 0.05, **p < 0.01, ***p < 0.001; AS: Antibiotic susceptibility; QS: Quorum sensing; PF: Panchvalkal formulation) 3(A). Effect of PF on growth and QS regulated staphyloxanthin production in S. aureus: Bacterial growth was measured as OD764; OD of staphyloxanthin was measured at 450 nm, and staphyloxanthin unit was calculated as the ratio OD450/OD764 (an indication of staphyloxanthin production per unit of growth); ‘Control’ bar in this figure is the ‘vehicle control’ representing the % change values in comparison to the ‘growth control’ i.e. tube containing only growth medium plus organism, but no DMSO. 3(B). Kaplan–Meier survival curve showing the protective effect of PF on C. elegans, when challenged with S. aureus: Catechin (50 μg/ml) and Gentamicin (0.1 μg/ml) employed as positive controls conferred 100% and 80% protection respectively. DMSO present in the ‘vehicle control’ at 0.5%v/v did not affect virulence of the bacterium towards C. elegans. 3(C). Effect of PF on S. aureus growth remained unaltered even after repeated exposure to PF, but that on staphyloxanthin was reversed. 3(D). S. aureus challenged with PF and antibiotic together. 3(E). S. aureus challenged with antibiotic following pre-treatment with PF (250 μg/ml).
When PF was tried in combination with different antibiotics against S. aureus, this bacterium exhibited reduced susceptibility to vancomycin, chloramphenicol, and carbenicillin; and higher susceptibility to nalidixic acid (Fig. 3D). PF-pre-treatment resulted in reduced susceptibility towards four of the antibiotics (Fig. 3E).
In vivo experiments confirmed the anti-infective efficacy of the PF, wherein it offered survival benefit to the nematode worm challenged with S. aureus. Interestingly the lower concentration (250 μg/ml) of the PF offered 10% better (p < 0.001) survival benefit to the worms, than the higher concentration (500 μg/ml) (Fig. 3B).
Among the three pathogenic bacteria employed in this study, S. aureus is the most dangerous human pathogen. Hence we conducted an additional study with this bacterium to investigate whether repeated exposure of S. aureus to PF can induce resistance in this bacterium. For this study, S. aureus was subcultured on PF (250 μg/ml) containing agar plates. Culture obtained after such 10 subculturings, was again assayed in vitro (Fig. 3C) and in vivo (Fig. 3B). Though the enhancement in staphyloxanthin production observed with ‘control’ S. aureus culture (i.e. not previously exposed to PF) was not observed with the PF-pre-exposed culture, the in vivo efficacy remained unaltered, suggesting that developing resistance against such polyherbal formulations may not be easy for the pathogenic bacteria.
3.4. Effect on probiotic strains
An ideal antimicrobial/anti-infective formulation should be selectively effective against pathogenic strains, with no or minimal effect on the human microbiota. We tested the effect of PF on two probiotic strains i.e. L. plantarum and B. bifidum (Table 2). PF had (statistically) similar growth promoting effect on latter at all the concentrations tested. It also promoted growth of L. plantarum at 750 μg/ml. Hence it can be said to possess moderate prebiotic and bifidogenic properties.
Table 2.
Effect of PF on L. plantarum and B. bifidum.
| Organism | Concentration of PF (μg/ml) | % Change compared to control |
|---|---|---|
| L. plantarum | 250 | 1.36 ± 1.12 |
| 500 | 2.97 ± 2.73 | |
| 750 | 8.21∗± 3.19 | |
| B. bifidum | 250 | 18.4* ± 4.76 |
| 500 | 20∗± 4.26 | |
| 750 | 13.84∗± 2.72 |
Bacterial growth was measured as OD660; *p < 0.05.
4. Discussion
This study has demonstrated the PF to be an effective quorum modulator against gram-positive (S. aureus) and gram-negative (C. violaceum and S. marcescens) bacteria at 250–750 μg/ml. Its spectrum of quorum modulatory action can be said to be broad against C. violaceum, it seemed to be acting as a signal-response inhibitor, however it is possible that it being a polyherbal formulation may contain multiple quorum modulatory compounds, some of which may act as signal-supply inhibitor too. Though modern medicine relies heavily on identification of a single active ingredient to be developed as a therapeutic molecule, ancient traditional medicines have almost always relied on crude extracts from one or multiple plants. Concept of polyherbalism to achieve greater therapeutic efficacy has been mentioned in Ayurved too [36]. Whether individual extracts of the bark of constituent plants of PF can exert any meaningful activity remains to be investigated. In context of AMR, the infectious microbes may find it difficult to develop resistance against a multi-component polyherbal formulation, than against a single antibiotic molecule, because in a poly-component formulation there are likely to be multiple compounds exerting antimicrobial action targeting more than one component of bacterial cellular machinery. This may be a reason why the ancient medicinal practises has relied on polyherbal formulations like PF or Ya-Sa-Marn-Phlae (YSMP) for treatment of infections [37].
All the three test bacteria used in this study are involved in a variety of human infections including wound infections. Successful treatment of a wound involves proper healing as well as preventing infections till healing is not complete. PF seems to be capable of enhancing wound healing as well as preventing infection. Wound healing potential of a Pañcavalkala formulation in a post-fistulectomy wound was reported by Meena et al. [38]. Bhat et al. reported that PF facilitates wound-healing by reducing microbial load of the wound [39]. However, one of the ingredient plant species mentioned in these studies was different from the PF used in our study. PF has been reported to be useful in treatment of burn wounds and vaginal infections [40], [41].
In the present study, antibiotic susceptibility of the test bacteria was found to alter (increase or decrease) either when antibiotics were used in combination of PF, or against bacteria pre-treated with PF. Pre-treatment with PF could enhance the susceptibility of both S. maracescens and C. violaceum to ampicillin. Against these two bacteria, streptomycin exerted better efficacy, when used simultaneously with PF. Chloramphenicol exerted better effect against S. aureus and C. violaceum, but inferior effect against S. marcescens, when used in combination with PF. Direction of such modulating effect may be determined by nature of antibiotic applied. In combination with ciprofloxacin and ampicillin, Samoilova et al. found certain extracts to provide protective effect, whereas with kanamycin the bactericidal action was enhanced [42]. Mechanisms through which plant products may alter antibiotic susceptibility in bacteria may possibly involve antioxidant, iron-chelating, or prooxidant properties of plant metabolites like polyphenols. Polyphenols were indicated to be involved in conferring protection on E. coli against antibiotic toxicity [43].
One of the most famous plant compounds, curcumin was shown to reduce the antimicrobial activity of ciprofloxacin against S. typhimurium and S. typhi [44]. Combined effect of plant extracts and antibiotics may originate from perturbation of cell membrane and cell wall by the plant extract, resulting in altered influx of antibiotics into the bacterial cells [45]. Hence the lesson is that though combination therapy involving simultaneous use of plant extracts and conventional antibiotics may be a logical idea, this needs to be practised with caution. Indiscriminate co-administration of antibiotics with herbal formulations may turn out to be therapeutically wasteful or counter-productive, putting the patient at risk, if right combinations are not selected [46].
Though different types of biological activities have been reported in various Ficus species [47], [48], [49], to the best of our awareness, this study is the first report on QSM potential of PF. Interestingly in this study, in vitro efficacy (in terms of % alteration of QS-regulated pigment production) at any particular concentration of PF was found to be lower than its in vivo efficacy (in terms of survival benefit conferred on C. elegans). This suggests that while screening any test product for its possible anti-infective potential, relatively lower in vitro efficacy should not be considered a discouraging factor, as for a quorum-modulator it may not be necessary to inhibit QS to the fullest extent, for being therapeutically useful. Present study is a good demonstration of use of modern scientific approach for validation of traditional therapeutic formulations. More detailed insights can be developed regarding the mode of action of such plant formulations by investigating their effect on bacterial transcriptome and/or proteome profile.
Sources of funding
None disclosed.
Conflicts of interest
None.
Acknowledgement
Authors thank NERF (Nirma Education and Research Foundation) for financial and infrastructural support. PP, CJ, VK thank Dr. H. S. Palep for suggesting ‘panchvalkal’ as a test formulation, and for making the Pentaphyte P5®capsules available for study, and appreciate his zeal for scientific validation of ‘Ayurved’. Biology division, Sophia College for Women, Mumbai is thanked for providing training to CJ on C. elegans handling.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.O'Neill J. 2016. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. Welcome Trust, HM Government.https://amr-review.org/sites/default/files/160518_Final/paper_with/cover.pdf Available at: [Google Scholar]
- 2.Tillotson G.S., Theriault N. New and alternative approaches to tackling antibiotic resistance. F1000 Prime Rep. 2013;5:51. doi: 10.12703/p5-51. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva N., Junior A.F. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 2010;16(3):402–413. [Google Scholar]
- 4.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(4):303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katiyar C., Kanjilal S., Gupta A., Katiyar S. Drug discovery from plant sources: an integrated approach. Ayu. 2012;33(1):10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahlou M. The Success of natural products in drug discovery. Pharmacol Pharm. 2013;4(3):17–31. [Google Scholar]
- 7.Vaidya A.D.B. Reverse pharmacological correlates of ayurvedic drug actions. Indian J Pharmacol. 2006;38(5):311–315. [Google Scholar]
- 8.Ahuja D.K., Shakya J.K., Thakur B.S., Sharma S.K., Vandana Clinical study on Yavaksharadi vati and Panchvalkal kwath in the management of Tundikeriw.s.r. to tonsillitis. J Ayurveda Holist Med. 2014;2(6):23–31. [Google Scholar]
- 9.Gajarmal A.A., Shende M.B., Chothe D.S. A clinical evaluation of Panchavalkala - a review article. Unique J Ayur Herbal Med. 2014;2(4):6–9. [Google Scholar]
- 10.Palep H., Kothari V., Patil S. Quorum sensing inhibition: a new antimicrobial mechanism of Panchavalkal, an ayurvedic formulation. Bombay Hosp J. 2016;58(2):198–204. [Google Scholar]
- 11.Swain B., Otta S., Sahu K., Panda K., Rout S. Urinary tract infection by Chromobacterium violaceum. J Clin Diagn Res. 2014;8(8):01–02. doi: 10.7860/JCDR/2014/9230.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottieau E., Mukendi D., Kalo J.R., Mpanya A., Lutumba P., Barbe B. Fatal Chromobacterium violaceum bacteraemia in rural bandundu, democratic republic of the Congo. New Microbes New Infect. 2015;3:21–23. doi: 10.1016/j.nmni.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fantinatti-Garboggini F., de Almeida R., Portillo V.A., Barbosa T.A.P., Trevilato P.B., Neto C.E.R. Drug resistance in Chromobacterium violaceum. Genet Mol Res. 2004;3(1):134–147. [PubMed] [Google Scholar]
- 14.Umadevi S., Kumar S., Stephen S., Joseph N.M. Chromobacterium violaceum: a potential nosocomial pathogen. Am J Infect Control. 2013;41(4):386–388. doi: 10.1016/j.ajic.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Annapoorani A., Jabbar A.K.K.A., Musthafa S.K.S., Pandian S.K., Ravi A.V. Inhibition of quorum sensing mediated virulence factors production in urinary pathogen Serratia marcescens PS1 by marine sponges. Indian J Microbiol. 2012;52(2):160–166. doi: 10.1007/s12088-012-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajput A., Shah U., Chauhan B., Shah P. Serratia—an emerging pathogen in hospital environment. Gujarat Med J. 2009;64:70–71. [Google Scholar]
- 17.Costa A.R., Batistao D.W.F., Ribas R.M., Sousa A., Pereira M.O., Botelho C.N. Staphylococcus aureus virulence factors and disease. In: Méndez-Vilas A., editor. Microbial pathogens and strategies for combating them: science, technology and education. 2013. pp. 702–710. Formatex. [Google Scholar]
- 18.Stevensa A.S., Schusterb M., Rumbaughc K.P. Working together for the common good: cell-cell communication in bacteria. J Bacteriol. 2012;194(9):2131–2141. doi: 10.1128/JB.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm A., Vikstrom E. Quorum sensing communication between bacteria and human cells: signals, targets, and functions. Front Plant Sci. 2014;5:309. doi: 10.3389/fpls.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhari V., Gosai H., Raval S., Kothari V. Effect of certain natural products and organic solvents on quorum sensing in Chromobacterium violaceum. Asian Pac J Trop Med. 2014;7:S204–S211. doi: 10.1016/S1995-7645(14)60233-9. [DOI] [PubMed] [Google Scholar]
- 21.Joshi C., Kothari V., Patel P. Importance of selecting appropriate wavelength, while quantifying growth and production of quorum sensing regulated pigments in bacteria. Recent Pat Biotechnol. 2016;10(2):145–152. doi: 10.2174/1872208310666160414102848. [DOI] [PubMed] [Google Scholar]
- 22.Choo J.H., Rukayadi Y., Hwang J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 23.Pradeep B.V., Pradeep F.S., Angayarkanni J., Palaniswamy M. Optimization and production of prodigiosin from Serratia marcescens MBB05 using various natural substrates. Asian J Pharm Clinic Res. 2013;6(1):34–41. [Google Scholar]
- 24.Song Y., Liu C., Lin F.Y., No J.H., Hensler M., Liu Y. Inhibition of Staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: In vitro, in vivo, and crystallographic results. J Med Chem. 2009;52(13):3869–3880. doi: 10.1021/jm9001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C.Y., Krishnan T., Wang H., Chen Y., Yin W.F., Chong Y.M. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci Rep. 2014;4:7245. doi: 10.1038/srep07245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClean K.H., Winson M.K., Fish L., Taylor A., Chhabra S.R., Camara M. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol. 1997;143(12):3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 27.Shaw P.D., Ping G., Daly S.L., Cha C., Cronan J.E., RinehartKL Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neun B.W., Ilinskaya A.N., Dobrovolskaia M.A. Nanotechnology Characterization Laboratory; Frederick, MD: 2015. Analysis of hemolytic properties of nanoparticles. NCL method ITA-1 Version 1.2. [Google Scholar]
- 29.Iwase T., Tajima A., Sugimoto S., Okuda K., Hironaka I., Kamata Y. A simple assay for measuring catalase activity: a visual approach. Sci Rep. 2013;3:3081. doi: 10.1038/srep03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2009;5(1):51–56. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eng S.A., Nathan S. Curcumin rescues Caenorhabditis elegans from a Burkholderia pseudomallei infection. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00290. Article 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goebel W., Chakraborty T., Kreft J. Bacterial hemolysins as virulence factors. Antonie Leeuwenhoek. 1998;54(5):453–463. doi: 10.1007/BF00461864. [DOI] [PubMed] [Google Scholar]
- 33.Khanafari A., Assadi M.M., Fakhr F.A. Review of prodigiosin, pigmentation in Serratia marcescens. Online J Biol Sci. 2006;6(1):1–13. [Google Scholar]
- 34.Koch G., Yepes A., Forstner K.U., Wermser C., Stengel S.T., Modamio J. Evolution of resistance to a Last-Resort antibiotic in Staphylococcus aureus via bacterial competition. Cell. 2014;158(5):1060–1071. doi: 10.1016/j.cell.2014.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaupp R., Ledala N., Somerville G.A. Staphylococcal response to oxidative stress. Front Cell Infect Microbiol. 2012;2:33. doi: 10.3389/fcimb.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Pharmacogn Rev. 2014;8(16):73–80. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chusri S., Tongrod S., Saising J., Mordmuang A., Limsuwan S., Sanpinit S. Antibacterial and anti-biofilm effects of a polyherbal formula and its constituents against coagulase-negative and -positive staphylococci isolated from bovine mastitis. J Appl Anim Res. 2017;45(1):364–372. [Google Scholar]
- 38.Meena R., Dudhamal T., Gupta S.K., Mahanta V. Wound healing potential of Pañcavalkala formulations in a postfistulectomy wound. Anc Sci Life. 2015;35(2):118–121. doi: 10.4103/0257-7941.171673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhat K.S., Vishwesh B.N., Sahu M., Shukla V.K. A clinical study on the efficacy of Panchavalkala cream in Vrana Shodhana W.S.R to its action on microbial load and wound infection. Ayu. 2014;35(2):135–140. doi: 10.4103/0974-8520.146216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi J., Rege V., Bhat R., Vaidya R. Cervical cytology, vaginal pH and colposcopy as adjuncts to clinical evaluation of Panchavalkal, an Ayurvedic preparation, in leucorrhoea. J Cytol. 2004;21:33–38. [Google Scholar]
- 41.Raut A.A., Chorghade M.S., Vaidya A.D.B. Elsevier; 2017. Reverse pharmacology. Innovative approaches in drug discovery. [DOI] [Google Scholar]
- 42.Samoilova Z., Smirnovaa G., Muzykaa N., Oktyabrskya O. Medicinal plant extracts variously modulate susceptibility of Escherichia coli to different antibiotics. Microbiol Res. 2014;169:307–313. doi: 10.1016/j.micres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Smirnova G., Samoilova Z., Muzyka N., Oktyabrsky O. Influence of plant polyphenols and medicinal plant extracts on antibiotic susceptibility of Escherichia coli. J Appl Microbiol. 2012;113:192–199. doi: 10.1111/j.1365-2672.2012.05322.x. [DOI] [PubMed] [Google Scholar]
- 44.Marathe S.A., Kumar R., Ajitkumar P., Nagaraja V., Chakravortty D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella Typhimurium and SalmonellaTyphi. J Antimicrob Chemother. 2013;68:139–152. doi: 10.1093/jac/dks375. [DOI] [PubMed] [Google Scholar]
- 45.Sibanda T., Okoh A.I. The challenges of overcoming antibiotic resistance: plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr J Biotechnol. 2007;6(25):2886–2896. [Google Scholar]
- 46.Eze E.A., Oruche N.E., Eze C.N. Interaction of the extracts of three medicinal plants with antibiotics against some antibiotic resistant bacteria. Sci Res Essays. 2013;8(28):1360–1367. [Google Scholar]
- 47.Khan K.Y., Khan M.J., Ahmad M., Hussain I., Mazari P., Fazal H. Hypoglycemic potential of genus Ficus L.: a review of ten years of plant based medicine used to cure diabetes (2000-2010) J Appl Pharm Sci. 2011;01(06):223–227. [Google Scholar]
- 48.Salem M.Z.M., Salem A.Z.M., Camacho L.M., Ali H.M. Antimicrobial activities and phytochemical composition of extracts of Ficus species: an over view. Afr J Microbiol Res. 2013;7(33):4207–4219. [Google Scholar]
- 49.Bhalerao S.A., Sharma A.S. Ethenomedicinal, phytochemical and pharmacological profile of Ficus religiosa Roxb. Int J Curr Microbiol App Sci. 2014;3(11):528–538. [Google Scholar]