Abstract
Purpose
Currently, there are about 15 ongoing clinical studies on low dose radiation therapy for Coronavirus Disease 2019 pneumonia. One of the underlying assumptions is that irradiation of 0.5 to 1.5 Gy is effective at ameliorating viral pneumonia. We aimed to reanalyze all available experimental radiobiologic data to assess evidence for such amelioration.
Methods and Materials
With standard statistical survival models, and based on a systematic literature review, we reanalyzed 13 radiobiologic animal data sets published in 1937 to 1973 in which animals (guinea pigs/dogs/cats/rats/mice) received radiation before or after bacterial or viral inoculation, and assessing various health endpoints (mortality/pneumonia morbidity). In most data sets absorbed doses did not exceed 7 Gy.
Results
For 6 studies evaluating postinoculation radiation exposure (more relevant to low dose radiation therapy for Coronavirus Disease 2019 pneumonia) the results are heterogeneous, with one study showing a significant increase (P < .001) and another showing a significant decrease (P < .001) in mortality associated with radiation exposure. Among the remaining 4 studies, mortality risk was nonsignificantly increased in 2 studies and nonsignificantly decreased in 2 others (P > .05). For preinoculation exposure the results are also heterogeneous, with 6 (of 8) data sets showing a significant increase (P < .01) in mortality risk associated with radiation exposure and the other 2 showing a significant decrease (P < .05) in mortality or pneumonitis morbidity risk.
Conclusions
These data do not provide support for reductions in morbidity or mortality associated with postinfection radiation exposure. For preinfection radiation exposure the inconsistency of direction of effect is difficult to interpret. One must be cautious about adducing evidence from such published reports of old animal data sets.
Introduction
Low dose radiation therapy (LDRT) for Coronavirus Disease 2019 (COVID-19) pneumonia was proposed in early April 2020.1 , 2 At least 15 clinical studies are currently ongoing in 9 countries.3 The rationale for clinical benefit, in other words the effectiveness of irradiation at the level of 0.5 to 1.5 Gy in treating viral pneumonia, largely relies on early human case studies or animal studies mostly obtained in the preantibiotic era, when a number of attempts were made to treat various noncancer diseases with ionizing radiation, including virally or bacterially associated pneumonia. An influential article underlying a number of proposals made for use of LDRT to treat COVID-19 pneumonia1 , 2 was Calabrese and Dhawan4 who reviewed 17 articles describing various relatively small case series, describing outcomes from LDRT with x-rays for pneumonia. Their sampling frameworks are unknown, and therefore they are subject to ascertainment bias and are largely uninterpretable. Calabrese and Dhawan4 also identified 4 radiobiologic animal studies of postinoculation LDRT, all from experiments done in the 1940s, namely Fried et al5 using a guinea pig model, Lieberman et al6 using a canine model, Baylin et al7 using a cat model, and Dubin et al8 using a murine model, the first 2 of these for bacterially induced pneumonia, and the last 2 for virally induced pneumonia. However, Calabrese and Dhawan4 did not consider 4 other radiobiologic studies relating to postinoculation RT, or results of 8 others relating to preinoculation RT and did not attempt any statistical reanalysis of these old data.
The aim of the present article is to look at the totality of published radiobiologic data relating to radiation exposure before or after inoculation with a viral or bacterial agent likely to result in pneumonia. Owing to the age of the data, there are shortcomings in the original statistical analysis that was performed; indeed in all but a few cases,9 , 10 there was no formal statistical analysis in the original reports. It is the purpose of this article to report reanalysis of the data abstracted from the original publications so far as that is achievable, using standard statistical survival models to assess modification of pneumonia morbidity or mortality risk by radiation exposure before or after inoculation.
Methods and Materials
We aimed to capture all radiobiologic data sets relating to moderate or LDRT whether given before or after viral or bacterial inoculation leading to pneumonia. We searched literature by means of a PubMed search (using terms [["radiation" OR "radiotherapy"] AND "pneumonia" AND "viral" AND "animal"] OR [["radiation" OR "radiotherapy"] AND "pneumonia" AND "bacterial" AND "animal"]) conducted on August 8, 2020, which returned 184 articles. We also searched for citations of the articles of Fried et al,5 Lieberman et al,6 Baylin et al,7 Dubin et al,8 and an authoritative contemporary review (in 1951) by Taliaferro and Taliaferro11 on the same date. We did not restrict by date or language of the publication. We selected from these searches all relevant articles with information on radiobiologic animal experiments in which there was any type of ionizing radiation exposure with determination of mortality or morbidity from bacterially or virally induced pneumonia. The data sets used are listed in Table 1 . It should be noted that the data sets we used include 3 of the 4 cited by Calabrese and Dhawan,4 but did not include the article of Fried et al,5 which we judged did not contain any quantitatively useful information. In Appendix E1, we provide details of the process used to abstract data from the publications that we identified as being potentially informative. The data were abstracted independently 3 times by M.P.L., W.Z., and R.v.D. We convert the given free-in-air dose in legacy units radiation absorbed dose (rad), roentgen (R), or rep in all studies to absorbed dose in gray (Gy) via the scaling 1 R/rep = 0.00877 Gy; 1 rad = 0.01 Gy.12
Table 1.
Radiobiologic animal data used for reanalysis of effects of low dose radiation therapy on bacterially or virally induced pneumonia
| Author | Animal strain | Infective agent(s) | Endpoint(s) analyzed | Mean (range) cumulative dose (Gy) | No. of fractions | No. of animals | Statistical model used for reanalysis |
|---|---|---|---|---|---|---|---|
| Radiation exposure after inoculation | |||||||
| Fried14∗ | Guinea pig | Staphylococcus aureus haemolyticus | Improvement in pneumonia morbidity | 0.357 (0-0.833) | 1 | 7 | Exact logistic |
| Lieberman et al6∗ | Dogs | Type I + III Pneumococcus | Mortality | 1.513 (0.0-4.096) | 1-3 | 45 | Linear + loglinear Cox |
| Baylin et al7∗ | Cats | Feline virus (Baker) | Degrees of pneumonia | 0.598 (0.0-1.754) | 1-2 | 22 | Loglinear logistic, exact logistic + ordinal |
| Tanner and McConchie23∗ | CFW mice | Theilers FA mouse encephalitis virus | Mortality | 3.114 (0.0-5.262) | 6 | 196 | Linear binomial logistic |
| Bond et al24∗ | Sprague-Dawley rats | Endemic coccobacillus | Mortality | 3.912 (0.0-8.770) | 1 | 1059 | Loglinear binomial logistic |
| Radiation exposure before and after inoculation | |||||||
| Dubin et al8∗ | White mice | Swine influenza virus | Mortality | 0.366 (0.0-1.754) | 1-3 | 252 | Linear + loglinear Cox |
| Radiation exposure before inoculation | |||||||
| Beutler and Gezon25 | Germantown white mice | PR8 strain type A influenza virus (mouse adapted, egg-adapted) | Mortality and morbidity | 1.682 (0-5.262) | 1 | 1635 | Linear + loglinear binomial logistic |
| Hale and Stoner26 | Swiss mice | Type III Pneumococcus | Mortality | 2.443 (0-5.701) | 1 | 140 | Linear + loglinear binomial logistic |
| Hale and Stoner27 | Swiss mice | Influenza type A, Trichinella spiralis, type III Pneumococcus | Mortality | 3.112 (0.0-6.139) | 1 | 658 | Linear binomial logistic |
| Quilligan et al18 | C57BL male mice | PR8 strain type A influenza virus | Mortality | 6.331 (0.0-14.471) | 15 | 30-34 | Linear binomial logistic |
| Berlin9 | CF-1 adult albino male mice | CAM A-prime strain influenza virus | Morbidity + mortality | 1.594 (0.0-3.070) | 1 | 362 | Linear binomial logistic |
| Berlin and Cochran10 | CF-1 adult albino male mice | PR8 strain type A influenza virus | Morbidity + mortality | 1.797 (0.0-4.385) | 1 | 660 | Linear binomial logistic |
| Lundgren et al28 | Female C57BL/6J mice | Type A0 influenza virus | Mortality | 88.763 (0.0-190.0) | Continuous | 364 | Linear binomial logistic |
NA, not available.
Data sets with information on radiation exposure after inoculation.
Statistical methods
Details of the statistical models fitted are given in Table 1, and some additional details on adjustments used are also given in the summary Table 2 . Mortality and morbidity risks in the radiobiologic cohorts of Lieberman et al6 and Dubin et al8 were assessed using a Cox proportional hazards model,13 with time after radiation exposure, if that followed the inoculation, or time after bacterial or viral inoculation, if that followed the radiation exposure, as timescale, in which the relative risk (= hazard ratio) of death for animal i at time a after radiation/inoculation was given by a linear model in dose:
| (1) |
Table 2.
Summary of modifying effects of preinoculation or postinoculation radiation exposure on bacterially or virally induced pneumonia in reanalyzed radiobiologic data
| Author | Infective agent(s) | Endpoint(s) analyzed | Mean (range) cumulative dose (Gy) | Statistical model used for reanalysis | Other notes on regression | Excess relative risk/Gy, excess odds ratio/Gy (+95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Radiation exposure after inoculation | |||||||
| Fried14∗ | Staphylococcus aureus haemolyticus | Improvement in pneumonia morbidity | 0.357 (0-0.833) | Exact logistic | Only animals receiving inoculation | 2.42† (−0.46‡ to +∞‡) | .075 |
| Lieberman et al6∗ | Type I + III Pneumococcus | Mortality | 1.513 (0.0-4.096) | Linear Cox | Model stratified by 3 groups | −0.23 (−0.24 to −0.16) | <.001 |
| Baylin et al7∗ | Feline virus (Baker) | Degrees of pneumonia | 0.598 (0.0-1.754) | Ordinal logistic | 0.55 (−0.62 to 1.76) | .358 | |
| Tanner and McConchie23∗ | Theilers FA mouse encephalitis virus | Mortality | 3.114 (0.0-5.262) | Linear binomial logistic | Adjusted for ln[virus concentration] | 0.08 (−0.10 to 0.59) | .525 |
| Bond et al24∗ | Endemic coccobacillus | Mortality | 3.912 (0.0-8.770) | Loglinear logistic | Model adjusted for likelihood of infection | 0.85 (0.75 to 0.95) | <.001 |
| Dubin et al8∗ | Swine influenza virus | Mortality | 0.366 (0.0-1.754) | Linear Cox | Model stratified by 3 experiments | −0.13 (−0.35 to 0.27) | .451 |
| Radiation exposure before inoculation | |||||||
| Dubin et al8 | Swine influenza virus | Mortality | 0.366 (0.0-1.754) | Linear Cox | Model stratified by 3 experiments | −0.62 (−0.90 to −0.09) | .029 |
| Beutler and Gezon25 | PR8 strain type A influenza virus (mouse adapted) | Mortality | 1.682 (0-5.262) | Linear binomial logistic | Adjusted for virus dilution | 0.23 (0.08 to 0.43) | <.001 |
| Hale and Stoner26 | Type III Pneumococcus | Mortality | 2.443 (0-5.701) | Linear binomial logistic | Only animals with type III Pneumococcus administered | 1.40 (0.39 to 5.47) | <.001 |
| Hale and Stoner27 | Influenza type A, type III Pneumococcus | Mortality | 3.112 (0.0-6.139) | Linear binomial logistic | Adjusted for challenge infection type, fitted to experiments with influenza and Pneumococcus challenge infections only | 1.71 (0.97 to 3.02) | <.001 |
| Quilligan et al18 | PR8 strain type A influenza virus | Mortality | 6.331 (0.0-14.471) | Linear binomial logistic | 8 mice in 1st control group assumed | 4.24 (0.49 to 96.97) | <.001 |
| Berlin9 | CAM A−prime strain influenza virus | Pneumonitis morbidity | 1.594 (0.0-3.070) | Linear binomial logistic | −0.24 (−0.28 to −0.17) | <.001 | |
| Berlin and Cochran10 | PR8 strain type A influenza virus | Mortality | 1.797 (0.0-4.385) | Linear binomial logistic | Analysis of influenza mortality adjusting for mode of administration of virus | 0.25 (0.05 to 0.57) | .009 |
| Lundgren et al28 | Type A0 influenza virus | Mortality | 88.763 (0.0-190.0) | Linear binomial logistic | 0.007 (0.002 to 0.017) | .002 | |
Data sets with information on radiation exposure after inoculation.
Median unbiased estimator.
Exact 95% confidence interval (CI).
or alternatively using a log-linear model in dose:
| (2) |
where D i is the total dose (in Gy), α is the excess relative risk coefficient per unit dose (Gy). For most of the other data sets a linear logistic model is fitted to the data (generally on number of animals that died in each group):
| (3) |
In some cases, the more standard loglinear logistic model is fitted to the data (generally on number of animals that died in each group):
| (4) |
For the data of Fried,14 numbering only 7 animals and using as outcome improvement in pneumonia in relation to unirradiated controls, an exact logistic model was used,15 as nonexact methods did not converge. It is well known that the excess odds ratio (EOR) approximates to the excess relative risk.16 All confidence intervals (CIs) and 2-sided P values are profile-partial-likelihood based.17 In the murine data set of Dubin et al8 in various subgroups risks were assessed in relation to radiation dose administered after inoculation or dose before inoculation. In the murine data set of Quilligan et al18 preinoculation dose was given to all animals; there is ambiguity in Quilligan et al18 as to whether radiation exposure may also have been given postinoculation. There is some uncertainty associated with the number of mice in the first of the control groups in this data set, and a range is used, spanning the plausible range of 6 to 10 mice, with 8 as the central estimate (Table 2). The model was stratified by the 3 experiments reported in the data of Dubin et al8 and by the 3 groups used by Lieberman et al.6 Tables E2 and E3 and Figures 1 and 2 show the risks in relation to dose for these 2 data sets. In various other data sets adjustment was made for various other covariates, as detailed in Tables E3 to E14. In the fits to the pneumonia intensity data of Baylin et al7 we used either loglinear logistic regression (as described above) comparing each pneumonia intensity group and those with greater intensity versus every group with reduced intensity. Due to the small number of animals19 we also used exact logistic methods15; we also used ordinal regression with log-linear link,20 fitting to all the ordered intensity groups. In fits of the days of infection data of Baylin et al7 we used a linear regression model, estimating the CIs via the bias-corrected advanced method.21 All models were fitted via Epicure,22 R,19 or LogXact.15 Two-sided levels of statistical significance are reported in all cases, with a conventional threshold for type I error of 2-sided P < .05 used to assign statistical significance. All statistical analyses were independently performed by M.P.L. and W.Z. to check for concordance. All data sets and analysis files are available in online supporting information (Appendix E2).
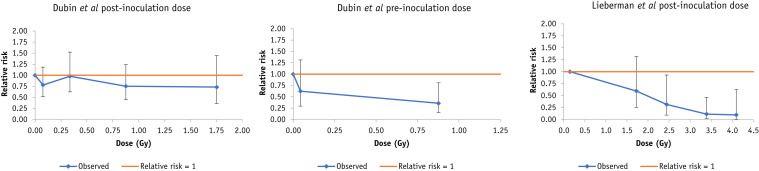
Fig. 1.
Dose response for mortality (+95% confidence interval) in the murine data of Dubin et al8 and in the canine data of Lieberman et al.6 Breakpoints for Dubin et al are at 0.01, 0.2, 0.5, and 1 Gy post inoculation dose, 0.01, 0.1 Gy preinoculation dose, for Lieberman et al at 1, 2, 3, and 4 Gy.
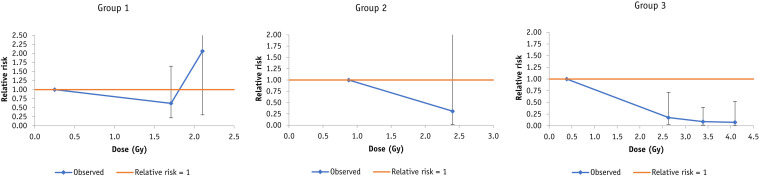
Fig. 2.
Dose response for mortality (+95% confidence interval) after radiation exposure postinoculation in the canine data of Lieberman et al,6 by study group. Breakpoints are at 1, 2 Gy [group 1], 2 Gy [group 2], 2, 3, and 4 Gy [group 3].
Results
We present results of analyses of risk in relation to whether radiation exposure occurred after inoculation or before inoculation. The results are given in summary form in Table 2, which also provides summary details of the models used and assumptions made in fitting, and in more detail in Appendix E3 and Tables E1 to E14.
Irradiation after inoculation
Table 2 (and Table E1) shows that there are weak indications (0.05 < P < .1) of decreased risk of pneumonia with postinoculation dose in the data set of Fried,14 whether for all guinea pigs or restricting to the 6 guinea pigs receiving Staphylococcus aureus inoculation. There is a highly significant decreasing trend (P < .001) of mortality with postinoculation dose in the data set of Lieberman et al6 with EOR per Gy = −0.23 (95% CI, −0.24 to −0.16; Table 2, Table E2), as also shown by Figure 1. However, Table E2 shows that this is largely driven by a single group, group 3, as also shown by Figure 2. The 3 groups in the Lieberman et al study6 were treated with slightly different x-ray energies, 80 kVp, 135 kVp, and 200 kVp, respectively, and mean doses also slightly differed, 0.947 Gy, 1.639 Gy, and 2.027 Gy, respectively (Table E2).
There are few indications of trend of degree of pneumonia infection with dose in the feline data of Baylin et al,7 whether using logistic, exact logistic or ordinal models (Table 2, Table E3). However, Table E4 indicates that there is a significant decreasing trend of days of acute infection with dose in this data set, with days of infection/Gy changing by −2.56 (95% CI, −4.59 to −0.33; P = .015), that is, duration of infection decreasing with dose, as also shown by Figure E1.
There is a nonsignificant positive trend with postinoculation dose (P > .4) in the murine data of Tanner and McConchie23 (Table 2, Table E5, Fig. 3 ); results did not appreciably vary with the type of model used (linear logistic, log-linear logistic) or whether or not adjustment was made for the virus concentration (Table E5).
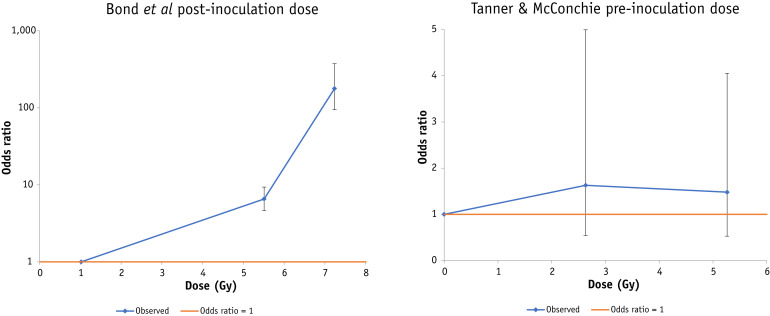
Fig. 3.
Mortality (+95% confidence interval) in the Sprague-Dawley rat data of Bond et al24 (radiation exposure after endemic coccobacillus infection) and in the mouse data of Tanner and McConchie23 (radiation exposure before mouse encephalitis virus infection). Breakpoints for Bond et al are at 5, 7 Gy, and for Tanner and McConchie at 2.5, 4.5 Gy.
There is a highly significant increasing trend (P < .001) of mortality risk with dose after endemic coccobacillus infection in the rat data of Bond et al,24 whether adjusting for likelihood of infection or not (Table 2, Table E6), with EOR per Gy = 0.85 (95% CI, 0.75 to 0.95), as also shown by Figure 3.
Radiation administration before and after inoculation
There is no significant trend (P > .4) with postinoculation dose in the data of Dubin et al,8 whether using linear or log-linear models, which is confirmed also by Figure 1 (Table E7). However, there is a borderline significant decreasing trend (P = .029) of mortality with preinoculation dose in this data set, with EOR/Gy = −0.62 (95% CI, −0.90 to −0.09; Table 2, Table E7) again confirmed by Figure 1.
Irradiation before inoculation
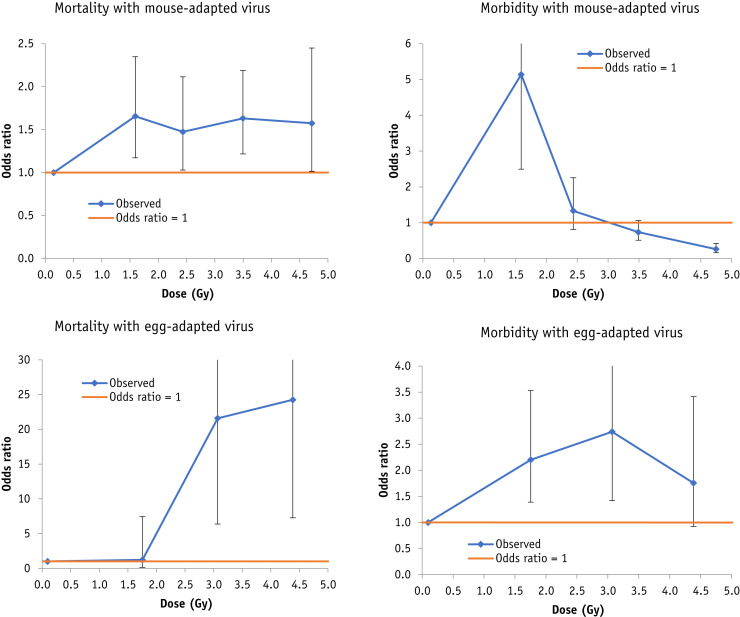
In the murine data of Beutler and Gezon25 there are highly significant (all P < .005) increasing trends of mortality with postinoculation dose, whether in relation to mouse-adapted or egg-adapted PR8 influenza A virus and irrespective of the type of statistical model (linear logistic, loglinear logistic) used; for example with a linear logistic model the EOR per Gy is 0.23 (95% CI, 0.08 to 0.43; Table 2, Table E8), also shown in Figure 4 . The morbidity trends exhibit more heterogeneity. For the mouse-adapted virus the trends are generally negative. For example, with a linear logistic model, the EOR per Gy is −0.19 (95% CI, −0.19 to −0.18; Table E8). However, for the egg-adapted virus, the trends are generally positive with dose, and, for example, with a linear logistic model the EOR per Gy is 1.02 (95% CI, 0.39 to 2.17; Table E8), as also shown in Figure 4.
Fig. 4.
Mortality and morbidity risks (+95% confidence interval) in Germantown white mice associated with preinoculation radiation exposure to mouse-adapted or egg-adapted PR8 influenza A virus in the data of Beutler and Gezon.25 Breakpoints are at 1, 2, 3, and 4 Gy (mouse-adapted virus, mortality + morbidity), 1.5, 3, and 4 Gy (egg-adapted virus, mortality + morbidity).
In the Swiss mice data of Hale and Stoner,26 there is no overall trend (P > .2) of mortality with radiation dose given before inoculation with type III pneumococcus. However, if attention is restricted to the animals that received inoculation there is a highly significant increasing trend with dose (P < .001), and the EOR per Gy is 1.40 (95% CI, 0.39 to 5.47; Table 2, Table E9). The same researchers went on to study a wider range of infective agents in the same strain of mice, and observed a generally highly significant (P < .005) increase in mortality risk associated with radiation before inoculation with influenza virus, pneumococcus type III bacterial infection or Trichinella spiralis larval infection,27 whether adjusted or not for type of first immunizing infection, and, for example, without such adjustment and excluding the Trichinella spiralis challenge infections the EOR/Gy = 1.71 (95% CI, 0.97 to 3.02; Table E10, Table 2).
There is a highly significant (P < .001) increase in mortality risk in the C57BL mouse data of Quilligan et al18 associated with postinfluenza-inoculation radiation dose with EOR/Gy, ranging from 3.73 (95% CI, 0.42 to 85.85) to 4.75 (95% CI, 0.56 to 108.10) depending on how many mice are assumed to be in the first control group (Table E11, Table 2).
Pneumonitis morbidity and mortality were significantly decreased (P < .001) after 3.5 Gy whole body air-dose exposure in adult male albino CF-1 mice in the data of Berlin,9 so that for pneumonitis morbidity, the EOR/Gy = −0.24 (95% CI, −0.28 to −0.17), and for pneumonitis mortality, the EOR/Gy = −0.21 (95% CI, −0.26 to −0.14; Table E12, Table 2). In contrast, Table 2 (see also Table E13) and Figure E2 show reanalysis of slightly later data of Berlin and Cochran,10 which exhibits slightly heterogeneous results, with one set of experiments (given in Table III of Berlin and Cochran10), indicating a significant increase (P ≤ .02) in influenza mortality, whether or not adjusted for mode of administration of virus, but a different experimental set (reported in Table II of the article) showing no significant effect (P > .1) of radiation exposure on influenza morbidity or mortality. These experiments use a similar murine system, also given 3.5 Gy whole body air-dose exposure, as in the earlier paper of Berlin.9 Lundgren et al28 used a novel type of radiation exposure, aerosolized 144CeO2, which delivers localized β dose to the lungs of C57BL/6J mice. There was a small but highly significant increase in mortality risk associated with radiation exposure, with EOR/Gy = 0.007 (95% CI, 0.002 to 0.017, P = .002; Table 2, Table E14).
Discussion
We have reanalyzed 13 radiobiologic animal data sets, dating from the late 1930s to the early 1970s, in which bacterial or viral agents were administered to induce pneumonia in animals that were also exposed to varying fractionated doses of radiation before or after inoculation. The statistical analysis in the original articles was limited; indeed, in all but 2 cases9 , 10 there was no formal statistical analysis in the publications. We therefore judged it necessary to statistically reanalyze the data from the original publications with standard statistical models.
For the 6 studies that evaluated postinoculation radiation exposure (which is more relevant to LDRT for COVID-19 pneumonia) the results are heterogeneous, with the study of Bond et al24 showing a significant increase (P < .001) in mortality associated with radiation exposure, and another, that of Lieberman et al,6 showing a significant decrease (P < .001) in mortality associated with radiation exposure. Among the remaining 4 studies, mortality risk was nonsignificantly increased in 2 studies, those of Baylin et al7 and Tanner and McConchie23 (P = .358, P = .469, respectively), and nonsignificantly decreased in 2 others, those of Fried14 and Dubin et al8 (P = .075, P = .451, respectively). Risks were only elevated in the third of the 3 groups studied by Lieberman et al6; the groups were treated with slightly different x-ray energies, and mean doses also slightly differed (see Results and Table E2). It is possible that these variations in mean dose and radiation energy may have some bearing on the differences observed (Table E2). For preinoculation exposure the results are also heterogeneous, with 6 (of 8) data sets showing significant increase in mortality risk associated with radiation exposure, namely those of Beutler and Gezon,25 Hale and Stoner,26 Hale and Stoner,27 Berlin and Cochran,10 Quilligan et al,18 and Lundgren et al28 (P < .001, P < .001, P < .001, P = .009, P < .001, P = .002, respectively), and the other 2, those of Dubin et al8 and Berlin et al,9 showing a significant decrease in mortality (and in 1 case pneumonitis morbidity) risk (P = .029, P < .001, respectively; Table 2). There was no clear systematic explanation for the heterogeneity in direction of effects, but reasons could include the different model systems (guinea pigs, dogs, cats, rats, mice) being used, also possibly due to the various types of challenging infection, which included both bacterial agents (Pneumococcus types I, III, Staphylococcus aureus haemolyticus, coccobacillus) and viral ones (swine influenza, feline, Thylers mouse encephalitis, influenza type A, CAM A-prime influenza, influenza A/PR8, and influenza A0; Table 1). It is possible that the range of doses used, and the variable degree of fractionation used may also be factors, although there does not appear to be an obvious pattern linking these to the direction or strength of effect, as shown by Tables 1 and 2. More recently, Hasegawa et al29 showed that irradiation before influenza vaccination and a succeeding lethal challenge influenza inoculation exacerbates mortality from influenza, but that vaccination before irradiation confers protection against a subsequent challenge influenza inoculation. Dadachova et al30 reported that targeted radionuclide immunotherapy induces Streptococcus pneumoniae killing in vivo, thereby alleviating bacterial pneumonia. As can be inferred, both these publications29 , 30 address somewhat different questions to those of this article.
Calabrese and Dhawan4 reviewed 3 of the studies we consider here,6, 7, 8 and a fourth,5 which we judged did not contain any quantitatively useful information, and stated that “these studies constitute the entire set of animal model studies assessing the capacity of x-rays to affect pneumonia-induced clinical symptoms and mortality. Each study demonstrated some measure of support for the hypothesis that x-ray treatment could reduce the effects of the pneumonia induced by bacteria or viruses.” Manifestly this is not the entirety of the literature relating to postinoculation radiation exposure (Tables 1 and 2), and a review of our results (Table 2) demonstrates that there is little evidence overall of reduction of morbidity or mortality with increasing radiation dose. Calabrese and Dhawan4 also reviewed 17 articles (all published before 1945), describing 15 or 16 mostly relatively small case series (the uncertainty reflecting whether or not an otherwise unpublished case series mentioned by a discussant in an article of Powell et al31 was included) and concluded that “x-ray therapy was successful in decreasing the mortality rate in untreated patients from about 30 percent to 5 to 10 percent.” However, as noted in the Introduction, without a well-defined sampling framework, such data are likely subject to ascertainment bias and are effectively uninterpretable. The radiation doses used in some of the case series are also unknown. Similar conclusions have been arrived at by others in discussing this article.32 , 33
The major strength of the present analysis is that we use standard statistical models to assess the totality of published radiobiologic data on LDRT given either before or after virally or bacterially induced pneumonia. Moreover, unlike the review of Calabrese and Dhawan4 we have undertaken a systematic review of the literature. The data we used was independently abstracted from the published reports by 3 of the authors. A significant weakness is the heterogeneity in the study designs, both the animal model systems and the infective agents used, alluded to above. There is some uncertainty as to precisely what experimental procedures were followed in some of these old data sets and one cannot be sure that the experimenters in these studies were blinded to the exposure status of the animals. This is exemplified by the study of Lieberman et al,6 where in the third group, most recovered animals received irradiation at 3 days after bacterial inoculation, which exceeded the average lifetime of 2.1 days in control (infected but not irradiated) animals: this would suggest an experimental selection bias. As noted in the Methods there is ambiguity in Quilligan et al18 as to whether radiation exposure may have been given postinoculation as well as preinoculation. It is also not always clear what the disease endpoints were, as for example in the data of Bond et al.24 Finally, about a third (4 of 13) of the radiobiologic data sets we analyzed here dealt exclusively with bacterial pneumonia, which is less relevant to discussion of LDRT for COVID-19 pneumonia, due to a significant difference in the pathogenesis of bacterial and viral pneumonia. Reliant as we were on electronic publication databases (in particular PubMed, ISI Thompson) it is possible that our literature search could have missed some relevant older data sets, given the incompleteness in coverage of publications 80 or more years previously.
Altogether, the early radiobiologic data we have reviewed does not suggest that there are strong variations in mortality or morbidity after radiation exposure after bacterial or viral inoculation. In particular, the heterogeneity in the results of our statistical analysis suggest that these early data sets do not serve as strong supportive evidence that LDRT of infected individuals reduces mortality. Although there are stronger indications of modifications of risk by radiation exposure before inoculation, the inconsistency of direction of effect makes this body of data difficult to interpret and has little relevance to LDRT for COVID-19 pneumonia.
Rödel et al34 reviewed some of the human epidemiologic and radiobiologic literature on LDRT. Although acknowledging limitations in understanding the possible mechanism, Rödel et al34 suggested that LDRT may stimulate antiviral immunity via the modulating effects of type I interferons in the early stages of SARS-CoV-2 infection. Rödel et al34 concluded that LDRT with a single dose of 0.5 Gy to the lungs warranted clinical investigation, while acknowledging the need for strict monitoring and disease phase-adapted treatment based on lung function tests and clinical markers (eg, IL-6 and D-dimer in serum). Schaue and McBride35 echoed some of the concerns of Rödel et al34 on the importance of correctly timing the use of LDRT in treatment of SARS-CoV-2, but were much more cautious, and suggested that, for example, it was unlikely that LDRT would effectively counter the virally induced cytokine storm that is a feature of the more severe forms of infection. Schaue and McBride35 and even more forcefully Kirsch et al36 suggested that the known deleterious adverse late health effects of 0.5 to 1.5 Gy administered to the lungs via increased risk of cancer and circulatory disease37 38 must be weighed against the uncertain therapeutic benefits of LDRT. Kirsch et al concluded that “based on the available data, the potential risks of such LDRT trials outweigh the potential benefits” and recommended that “further preclinical work is needed to demonstrate efficacy of radiation therapy to provide scientific justification for a clinical trial in patients with COVID-19.”36
Collectively, these animal data do not provide clear support for reductions in morbidity or mortality associated with postinfection radiation exposure. For preinfection radiation exposure, the inconsistency of direction of effect makes this body of data difficult to interpret. Nevertheless, one must be cautious about adducing evidence from the published reports of these old animal data sets.
Acknowledgments
The authors gratefully acknowledge the detailed and helpful comments of the referee.
Footnotes
Disclosures: Authors declare no conflicts of interest.
This work was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
All research data used in the paper are given online in Appendix E2.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.09.052.
Supplementary Materials
References
- 1.Kirkby C., Mackenzie M. Is low dose radiation therapy a potential treatment for COVID-19 pneumonia? Radiother Oncol. 2020;147:221. doi: 10.1016/j.radonc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghadimi-Moghadam A., Haghani M., Bevelacqua J.J., et al. COVID-19 tragic pandemic: Concerns over unintentional “directed accelerated evolution” of novel coronavirus (SARS-Cov-2) and introducing a modified treatment method for ARDS. J Biomed Phys Engineering. 2020;10:241–246. doi: 10.31661/jbpe.v0i0.2003-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ClinicalTrials.gov. 18 studies found for Covid-19 radiation. https://clinicaltrials.Gov/ct2/results?Cond=covid-19+radiation&term=&cntry=&state=&city=&dist=2020 Available at: Accessed September 24, 2020.
- 4.Calabrese E.J., Dhawan G. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale J Biol Med. 2013;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- 5.Fried C. The roentgen treatment of experimental pneumonia in the guinea-pig. Radiology. 1941;37:197–202. [Google Scholar]
- 6.Lieberman L.M., Hodes P.J., Leopold S.S. Roentgen therapy of experimental lobar pneumonia in dogs. Am J Med Sciences. 1941;291:92–100. [Google Scholar]
- 7.Baylin G.J., Dubin I.N., Gobbel W.G., Jr. The effect of roentgen therapy on experimental virus pneumonia. I. On feline virus pneumonia. Am J Roentgenol Radium Ther. 1946;55:473–477. [PubMed] [Google Scholar]
- 8.Dubin I.N., Baylin G.J., Gobble W.G., Jr. The effect of roentgen therapy on experimental virus pneumonia. II. On pneumonia produced in white mice by swine influenza virus. Am J Roentgenol Radium Ther. 1946;55:478–481. [PubMed] [Google Scholar]
- 9.Berlin B.S. Sparing effect of x-rays for mice inoculated intranasally with egg-adapted influenza virus, CAM strain. Proc Soc Experimental Biol Med. 1964;117:864–869. doi: 10.3181/00379727-117-29720. [DOI] [PubMed] [Google Scholar]
- 10.Berlin B.S., Cochran K.W. Delay of fatal pneumonia in x-irradiated mice inoculated with mouse-adapted influenza virus, PR8 strain. Radiat Res. 1967;31:343–351. [PubMed] [Google Scholar]
- 11.Taliaferro W.H., Taliaferro L.G. Effect of x-rays on immunity: a review. J Immunol. 1951;66:181–212. [PubMed] [Google Scholar]
- 12.Wikipedia Roentgen unit. https://en.Wikipedia.Org/wiki/roentgen_(unit) Available at: Accessed September 24, 2020.
- 13.Cox D.R. Regression models and life-tables. J Royal Statist Soc Series B. 1972;34:187–220. [Google Scholar]
- 14.Fried C. Die artefizielle Pneumonie und ihre Bestrahlung. Experimentalbeitrag zur Frage der Wirkung der Röntgenstrahlen auf Entzündungsgewebe [Irradiation in artificial pneumonia: Experimental contribution to the question of the effect of X-rays on inflammatory tissue] Strahlentherapie. 1937;58:430–448. [in German] [Google Scholar]
- 15.LogXact 11 version 11.0.0. Cytel, Inc; Cambridge, MA 02139: 2015. [Google Scholar]
- 16.Breslow N.E., Day N.E. Statistical methods in cancer research. Volume II: The design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 17.McCullagh P., Nelder J.A. 2nd edition. Chapman and Hall/CRC; Boca Raton, FL: 1989. Generalized Linear Models; pp. 1–526. [Google Scholar]
- 18.Quilligan J.J., Jr., Boche R.D., Carruthers E.J., et al. Continuous cobalt-60 irradiation and immunity to influenza virus. J Immunol. 1963;90:506–511. [PubMed] [Google Scholar]
- 19.R Project version 3.6.1. R: A language and environment for statistical computing. Available at: https://www.R-project.org. Vienna, Austria: R Foundation for Statistical Computing; 2019. Accessed July 28, 2020.
- 20.McCullagh P. Regression models for ordinal data. J Royal Statist Soc Series B. 1980;42:109–142. [Google Scholar]
- 21.Efron B. Better bootstrap confidence intervals. J Am Statist Assoc. 1987;82:171–185. [Google Scholar]
- 22.Epicure version 2.0.1.0. 55 Metcalfe, K1P 6L5. Risk Sciences International; Canada: 2015. [Google Scholar]
- 23.Tanner W.A., McConchie J.E. Effect of roentgen therapy on mouse encephalitis. Radiology. 1949;53:101–103. doi: 10.1148/53.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Bond V.P., Shechmeister I.L., Swift M.N., et al. The effects of X irradiation on a naturally occurring endemic infection. J Infect Di.s. 1952;91:26–32. doi: 10.1093/infdis/91.1.26. [DOI] [PubMed] [Google Scholar]
- 25.Beutler E., Gezon H.M. The effect of total body X irradiation on the susceptibility of mice to influenza A virus infection. J Immunol. 1952;68:227–242. [PubMed] [Google Scholar]
- 26.Hale W.M., Stoner R.D. The effect of cobalt-60 gamma radiation on passive immunity. Yale J Biol Med. 1953;25:326–333. [PMC free article] [PubMed] [Google Scholar]
- 27.Hale W.M., Stoner R.D. Effects of ionizing radiation on immunity. Radiat Res. 1954;1:459–469. [PubMed] [Google Scholar]
- 28.Lundgren D.L., Sanchez A., Thomas R.L., et al. Effects of inhaled 144CeO2 on influenza virus infection in mice. Proc Soc Experimental Biol Med. 1973;144:238–244. doi: 10.3181/00379727-144-37564. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa H., Kadowaki S., Takahashi H., et al. Protection against influenza virus infection by nasal vaccination in advance of sublethal irradiation. Vaccine. 2000;18:2560–2565. doi: 10.1016/s0264-410x(99)00553-8. [DOI] [PubMed] [Google Scholar]
- 30.Dadachova E., Burns T., Bryan R.A., et al. Feasibility of radioimmunotherapy of experimental pneumococcal infection. Antimicrob Agents Chemother. 2004;48:1624–1629. doi: 10.1128/AAC.48.5.1624-1629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell E.V. Radiation therapy of lobar pneumonia. Texas State J Med. 1936;32:237–240. [Google Scholar]
- 32.Salomaa S., Cardis E., Bouffler S.D., et al. Low dose radiation therapy for COVID-19 pneumonia: is there any supportive evidence? Int J Radiat Biol. 2020;96:1224–1227. doi: 10.1080/09553002.2020.1762020. [DOI] [PubMed] [Google Scholar]
- 33.Salomaa S., Bouffler S.D., Atkinson M.J., et al. Is there any supportive evidence for low dose radiotherapy for COVID-19 pneumonia? Int J Radiat Biol. 2020;96:1228–1235. doi: 10.1080/09553002.2020.1786609. [DOI] [PubMed] [Google Scholar]
- 34.Rödel F., Arenas M., Ott O.J., et al. Low-dose radiation therapy for COVID-19 pneumopathy: what is the evidence? Strahlenther Onkol. 2020;196:679–682. doi: 10.1007/s00066-020-01635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaue D., McBride W.H. Flying by the seat of our pants: is low dose radiation therapy for COVID-19 an option? Int J Radiat Biol. 2020;96:1219–1223. doi: 10.1080/09553002.2020.1767314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirsch D.G., Diehn M., Cucinotta F.A., et al. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother Oncol. 2020;147:217–220. doi: 10.1016/j.radonc.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) United Nations; New York: 2008. UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. [Google Scholar]
- 38.Little M.P., Azizova T.V., Bazyka D., et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.