Abstract
Objective
To evaluate the nutritional risk and therapy in severe and critical patients with COVID-19.
Methods
A total of 523 patients enrolled from four hospitals in Wuhan, China. The inclusion time was from January 2, 2020 to February 15. Clinical characteristics and laboratory values were obtained from electronic medical records, nursing records, and related examinations.
Results
Of these patients, 211 (40.3%) were admitted to the ICU and 115 deaths (22.0%). Patients admitted to the ICU had lower BMI and plasma protein levels. The median Nutrition risk in critically ill (NUTRIC) score of 211 patients in the ICU was 5 (4, 6) and Nutritional Risk Screening (NRS) score was 5 (3, 6). The ratio of parenteral nutrition (PN) therapy in non-survivors was greater than that in survivors, and the time to start nutrition therapy was later than that in survivors. The NUTRIC score can independently predict the risk of death in the hospital (OR = 1.197, 95%CI: 1.091–1.445, p = 0.006) and high NRS score patients have a higher risk of poor outcome in the ICU (OR = 1.880, 95%CI: 1.151–3.070, p = 0.012). After adjusted age and sex, for each standard deviation increase in BMI, the risk of in-hospital death was reduced by 13% (HR = 0.871, 95%CI: 0.795–0.955, p = 0.003), and the risk of ICU transfer was reduced by 7% (HR = 0.932, 95%CI:0.885–0.981, p = 0.007). The in-hospital survival time of patients with albumin level ≤35 g/L was significantly decreased (15.9 d, 95% CI: 13.7–16.3, vs 24.2 d, 95% CI: 22.3–29.7, p < 0.001).
Conclusion
Severe and critical patients with COVID-19 have a high risk of malnutrition. Low BMI and protein levels were significantly associated with adverse events. Early nutritional risk screening and therapy for patients with COVID-19 are necessary.
Keywords: Nutritional risk, COVID-19, NUTRIC score, NRS score
1. Introduction
The novel coronavirus outbreak in Wuhan, China has resulted in a pandemic, leading to >1,000,000 infections and nearly 600,000 deaths [1]. The new coronavirus can affect many organs by binding to the angiotensin-converting enzyme 2 receptor [[2], [3], [4]], although the lungs are the most affected. Current epidemiological evidence shows that the critical illness incidence rate in patients with the 2019 novel coronavirus disease (COVID-19) infection is 5% [5], and that mortality rate in intensive care unit (ICU) patients is approximately 45–60% [6,7]. Presently, due to the absence of effective antiviral drugs and vaccines, the treatment of every severe patient is a significant challenge in clinical practice.
Wu et al. have shown that of 201 patients with COVID-19 pneumonia, 84 eventually developed adult respiratory distress syndrome (ARDS) [8]. Approximately 6–8% of patients with COVID-19 pneumonia need to be admitted to the ICU for intensive care [9]. Sang et al. have demonstrated that the mortality rate in ICU patients with COVID-19 was 60% and 40% in patients receiving invasive ventilator treatment [6]. Patients admitted to the ICU often had more organ dysfunction conditions and chronic basic diseases. A cytokine storm is manifested by uncontrolled production of inflammatory cytokines which are significantly higher in ICU patients than non-ICU patients hospitalized with COVID-19 [10]. These are significantly higher in ICU than in non-ICU patients hospitalized with COVID-19 [10]. Manjili et al. have described COVID-19 as an acute inflammatory disease [11]. Therapeutic strategies for the management of severe symptoms have been focused on the control of viremia and/or inflammation [11].
Systemic inflammatory response and organ dysfunction in critical patients can lead to energy intake and utilization disorders. The incidence of malnutrition in the ICU is 38–78% [12] and is independently related to poor prognosis. Wu et al. have also found that patients who developed ARDS had lower levels of low-density lipoprotein cholesterol (LDL-c), albumin (ALB), and prealbumin (PA) [8]. However, specific nutritional risk screening tools have not been widely used in clinical practice to identify COVID-19 patients with a higher malnutrition risk. The application of nutritional risk screening tools is an important part of nutritional assessment of severely ill COVID-19 patients and the first step in nutritional support therapy. Nutritional status appears to be a relevant factor influencing the outcome in COVID-19 patients. However, not much information has emerged so far on the impact of early nutritional support in pre-ICU COVID-19 patients [13]. The National Health Commission of the People's Republic of China and the National Administration of Traditional Chinese Medicine recommend implementing “strengthened supportive care to ensure sufficient energy intake” [14].
The Nutritional Risk Screening (NRS) 2002 score is a recommended tool for nutritional risk screening [15]. While established based on data from general patients, it has since been validated in ICU patients [16,17]. The Nutrition Risk in the Critically Ill (NUTRIC) score, while developed for ICU patients by Canadian researchers [18], does not contain nutritional data, and is calculated retrospectively based on severity scores. The American Society for parenteral and enteral nutrition (ASPEN) recommends to use both scores [19], while the European Society for Clinical nutrition (ESPEN), recommends only the NRS score [16]. However, the clinical evidence for the association between nutritional risk assessment tools and clinical outcomes in patients with COVID-19 is limited. Therefore, the present retrospective study was designed to analyze the risk of malnutrition in severe and critical COVID-19 patients. The main objective was to evaluate nutritional metabolism in COVID-19 patients upon admission and prognostic value of the NUTRIC and NRS score in COVID-19 patients in the ICU.
2. Methods
This is a multicenter retrospective observational study. The retrospective analysis was carried out at four designated hospitals for COVID-19, including two critical and two general designated hospitals. Severely and critically ill patients were defined according to the guidelines of National Health Commission of the People's Republic of China [20]. Severely ill patients were included in the study if they met any of the following criteria: 1) respiratory distress and respiratory rate was ≥30 times/min; 2) oxygen saturation in a resting state was ≤93%; and 3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) was ≤300 mm Hg. Critically ill patients were included if they met any of the following criteria: 1) respiratory failure and need for mechanical ventilation; 2) shock; and 3) other organ failure requiring ICU monitoring. Positive results for real-time polymerase chain reaction testing of respiratory or blood samples were defined as confirmed cases [21]. The inclusion time was from January 2, 2020 to February 15, 2020 for discharged and dead patients. The severity of COVID-19 patients was determined using the analysis of electronic medical records, nursing records, and related examinations. All data review was performed by experienced ICU doctors. A total of 523 patients participated in the study, including 377 severe and 146 critically ill patients. Of these, 211 patients were admitted to the ICU, with hospital death and transfer to ICU considered as the end events. Due to the speed of COVID-19 spread and the risk of infection, exemption from written informed consent was obtained. This study complied with the Declaration of Helsinki and was approved by the ethics committee of Hubei Provincial Hospital of Traditional Chinese Medicine (HBZY2020-C14-01).
2.1. Data collection
Data for age, gender, history of chronic diseases (hypertension, coronary heart disease, and diabetes), vital signs, laboratory values, hospitalization time, chest imaging characteristics, and prognosis for COVID-19 patients were collected. In-hospital death or survival were defined as the prognosis in this study. In addition, the Glasgow Coma Scale, sequential organ failure assessment (SOFA), acute physiology and chronic health assessment II scores (APACHE II), PaO2 and lactate concentration, PaO2/FiO2, NRS score, and other indicators for subsequent analysis data were collected from the hospital's electronic medical and nursing records. Except for the 43 patients who were transferred to the ICU with endotracheal intubation and were unable to provide the NRS score, the remaining patients completed the NRS score evaluation on the first day of admission to the ICU. The nutritional risk for each patient was assessed upon ICU admission using the modified NUTRIC (m NUTRIC) score. This score (0–9 points) was calculated based on the NUTRIC score by eliminating IL-6 values. It consisted of five variables: age, APACHE II score upon admission, SOFA score upon admission, number of comorbidities, and pre-ICU hospital length of stay [22]. A score of >5 indicated that a patient had a high nutritional risk.
2.2. Statistical analysis
Demographic and medical data meeting normal distribution requirements were represented as mean ± SD. Data with a skewed distribution were presented as median (IQR). Categorical variables were described as frequency rates and percentages. The differences between survivors and non-survivors were assessed using two-sample t test or Mann–Whitney test depending on normal or skewed distribution of data for continuous variables and Chi-squared test for categorical variables. Logistic regression was used to analyze the association between nutritional and metabolic factors with a death risk in the hospital or other clinical adverse outcomes. Survival curves for body mass index (BMI) and ALB groups were estimated according to the Kaplan–Meier method and compared using the log-rank test. We used multivariate Cox regression to examine the relationships between the mNUTRIC score and BMI with end points. All statistical analyses were performed using the SPSS 22.0 software (SPSS Inc, Chicago, Illinois) and P < 0.05 was considered to be statistically significant.
3. Results
In this study, the average age of 523 patients was 54.2 ± 15.9 years, of which 250 (47.9%) were men. A total of 115 (22.0%) deaths occurred in the course of the study. Among the 523 patients, 211 were admitted to the ICU for intensive care. Patients in the ICU were older and predominantly male. They also had chronic diseases, higher fasting blood glucose (FBG) levels, and markers of renal function and inflammatory factors. Among the indicators reflecting nutritional status, patients admitted to the ICU had lower BMI and high-density lipoprotein cholesterol and plasma protein levels (Table 1 ). Of the 211 patients in the ICU, 95 (45.0%) eventually died. Similarly, patients who died in the ICU were older, had more chronic diseases, higher basal blood pressure, higher FBG levels, and worse renal and cardiac function indexes (Table 1). They also had lower plasma protein levels, absolute lymphocyte counts, and LDL-c levels. Although there was no difference in mean BMI between patients who died in the ICU and those who survived (22.1 ± 2.7 vs 21.6 ± 2.9, p = 0.208), the proportion of patients who died with BMI ≤20.5 was higher (Table 1).
Table 1.
Baseline characteristics of patients with severe COVID-19 pneumonia.
| Non-ICU(n = 312) | ICU(n = 211) | p value | ICU Survivors (n = 116) | ICU Non-survivors (n = 95) | p value | All patients, n = 523 | Normal range | |
|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||||
| Age (years) | 48.9 ± 15.1 | 62.1 ± 13.4 | <0.001 | 56.3 ± 12.1 | 69.2 ± 11.4 | <0.001 | 54.2 ± 15.9 | NA |
| Male n, (%) | 131 (42.1%) | 119 (56.4%) | 0.001 | 63 (54.3%) | 56 (58.9%) | 0.296 | 250 (47.9%) | NA |
| BMI | 23.5 ± 2.9 | 21.7 ± 2.7 | <0.001 <0.001 |
21.6 ± 2.9 | 22.1 ± 2.7 | 0.208 0.003 |
22.8 ± 2.9 | 18.5–24.9 |
| ≤20.5 | 70 (22.5%) | 99 (46.9%) | 44 (37.9%) | 55 (57.9%) | ||||
| >20.5 | 241 (77.5%) | 112 (53.1%) | 72 (62.1%) | 40 (42.1%) | ||||
| Admission SBP (mmHg) | 124.2 ± 15.4 | 126.8 ± 17.7 | 0.135 | 122.7 ± 15.2 | 132.2 ± 19.4 | <0.001 | 125.6 ± 16.7 | <140 |
| Admission DBP (mmHg) | 78.0 ± 10.1 | 78.4 ± 11.3 | 0.717 | 78.1 ± 10.9 | 78.9 ± 11.8 | 0.687 | 78.2 ± 10.8 | <90 |
| Hospitalization days | 9.0 (7,11) | 14.0 (11,17) | <0.001 | 13 (11,16) | 15 (11,21) | 0.042 | 10.0 (8,14) | NA |
| Comorbidities | ||||||||
| Coronary artery disease n, (%) | 24 (7.7%) | 14 (6.7%) | 0.009 | 4 (3.5%) | 10 (10.5%) | 0.04 | 38 (7.3%) | NA |
| Hypertension n, (%) | 65 (20.9%) | 65 (31.1%) | <0.001 | 25 (21.9%) | 40 (42.1%) | 0.001 | 130 (25.0%) | NA |
| Diabetes mellitus n, (%) | 50 (16.1%) | 44 (20.9%) | 0.101 | 20 (17.2%) | 24 (25.3%) | 0.105 | 94 (18.0%) | NA |
| Biochemical | ||||||||
| ALT (U/L) | 35.2 ± 38.2 | 41.1 ± 40.7 | 0.095 | 39.2 ± 35.0 | 43.6 ± 46.8 | 0.443 | 37.6 ± 39.2 | 9–50 |
| AST (U/L) | 31.2 ± 38.1 | 42.6 ± 27.2 | <0.001 | 35.4 ± 20.0 | 51.3 ± 32.0 | <0.001 | 35.9 ± 34.5 | 15–40 |
| Total bilirubin (ummol/L) | 15.7 ± 8.5 | 13.9 ± 6.9 | 0.011 | 14.0 ± 5.3 | 13.8 ± 8.4 | 0.910 | 15.0 ± 8.0 | 0–26 |
| TC (mmol/L) | 3.97 ± 0.85 | 3.83 ± 0.84 | 0.086 | 3.92 ± 0.81 | 3.71 ± 0.88 | 0.101 | 3.92 ± 0.85 | <5.2 |
| TG (mmol/L) | 1.50 ± 0.89 | 1.53 ± 0.71 | 0.699 | 1.54 ± 0.75 | 1.51 ± 0.64 | 0.790 | 1.51 ± 0.82 | <1.7 |
| LDL-c (mmol/L) | 2.21 ± 0.65 | 2.17 ± 0.66 | 0.496 | 2.28 ± 0.66 | 2.01 ± 0.63 | 0.007 | 2.19 ± 0.65 | <3.4 |
| HDL-c (mmol/L) | 1.06 ± 0.29 | 0.98 ± 0.28 | 0.004 | 1.00 ± 0.26 | 0.95 ± 0.30 | 0.180 | 1.03 ± 0.29 | 1.16–1.55 |
| Total protein (g/L) | 68.0 (64,72) | 62.0 (55,68) | <0.001 | 65.0 (61,72) | 55.0 (46,62) | <0.001 | 66.0 (62,72) | 65–85 |
| Albumin (g/L) | 41 (38,43) | 36 (33,40) | <0.001 <0.001 |
39 (36,41) | 33 (29,36) | <0.001 <0.001 |
39 (37,43) | 40–55 |
| ≤35 g/L | 24 (7.7%) | 88 (41.7%) | 23 (19.8%) | 65 (68.4%) | ||||
| >35 g/L | 286 (92.3%) | 123 (58.3%) | 93 (80.2%) | 30 (31.6%) | ||||
| Globulin (g/L) | 27.0 (26,29) | 25.0 (22,27) | <0.001 | 26.0 (24,30) | 22.0 (18,25) | <0.001 | 26 (22,29) | 20–40 |
| Prealbumin (mg/L) | 182.0 (134,233) | 142.0 (107,191) | <0.001 | 164.0 (124,208) | 122.0 (94,160) | <0.001 | 164 (120,214) | 180–350 |
| BUN(mmol/L) | 4.87 ± 3.63 | 6.68 ± 4.59 | <0.001 | 5.20 ± 2.68 | 8.60 ± 5.72 | <0.001 | 5.61 ± 4.14 | 3.1–8.0 |
| Cr (ummol/L) | 66.6 ± 30.7 | 83.2 ± 57.1 | <0.001 | 73.9 ± 43.4 | 95.4 ± 69.6 | <0.001 | 73.3 ± 44.1 | 57–111 |
| UA (ummol/L) | 264.9 ± 82.2 | 231.1 ± 95.4 | <0.001 | 232.2 ± 90.1 | 229.6 ± 101.8 | 0.845 | 251.2 ± 89.2 | 155–428 |
| FBG (mmol/L) | 6.36 ± 2.99 | 7.62 ± 3.53 | <0.001 | 6.70 ± 2.58 | 8.83 ± 4.21 | <0.001 | 6.87 ± 3.28 | 3.9–6.1 |
| NT-proBNP(pg/ml) | 69 (29,203) | 67 (32,210) | 0.681 | 53 (16,112) | 114 (52,284) | 0.028 | 69.0 (30.0,185.6) | 0–125.2 |
| cTnI (ng/ml) | 0.037 ± 0.278 | 0.105 ± 0.374 | 0.091 | 0.078 ± 0.362 | 0.199 ± 0.426 | 0.041 | 0.078 ± 0.341 | 0–0.06 |
| Hematologic | ||||||||
| WBC, × 109/L | 5.2 (4.0,7.0) | 6.5 (4.6,8.9) | 0.001 | 5.8 (4.0,7.7) | 7.6 (5.3,10.6) | <0.001 | 5.55 (4.10,7.69) | 3.5–9.5 |
| Hb (g/L) | 128.4 ± 20.6 | 136.1 ± 21.2 | <0.001 | 137.0 ± 21.6 | 135.0 ± 20.6 | 0.496 | 131.5 ± 21.2 | 115–175 |
| GRAN,% | 70.0 ± 13.9 | 79.3 ± 12.0 | 0.001 | 73.0 ± 11.7 | 84.4 ± 9.7 | <0.001 | 73.7 ± 13.9 | 40–75 |
| ALC, × 109/L | 1.13 (0.76,1.60) | 0.83 (0.57,1.17) | 0.001 | 1.01 (0.68,1.47) | 0.66 (0.48,0.91) | <0.001 | 1.00 (0.67,1.41) | 1.1–3.2 |
| Infection-related indices | ||||||||
| IL-6 (pg/ml) | 10 (4,26) | 9 (7,23) | 0.646 | 8 (6,13) | 12 (8,36) | 0.256 | 9 (6,21) | <7.0 |
| hs-CRP (mg/L) | 13.2 (5.0,40.0) | 59.0 (21,112) | <0.001 | 32.5 (11.2,79.1) | 94.3 (32.0,160.0) | <0.001 | 27.3 (5.0,74.3) | 0–3 |
| PCT (ng/ml) | 0.05 (0.03,0.10) | 0.06 (0.07,0.36) | 0.139 | 0.05 (0.05,0.09) | 0.15 (0.65,0.34) | 0.028 | 0.054 (0.050,0.143) | 0–0.052 |
Abbreviations: ALC, absolute lymphocyte count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; cTnI, cardiac troponin I; DBP, diastolic blood pressure; FBG, fasting blood glucose; GRAN %, Granulocyte percentage; Hemoglobin, Hb; HDL-c, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C reactive protein; IL-6, interleukin-6; LDL-c, low-density lipoprotein cholesterol; NT-proBNP, N-Terminal pro-brain natriuretic peptide; PCT, procalcitonin; SBP, systolic blood pressure; TC, total cholesterol; TG, triacylglycerol; UA, uric acid; WBC, white blood cell.
After further analysis, it was evident that patients who died in the ICU had higher SOFA, APACHE II, NRS score and mNUTRIC score (Table 2 ). The median mNUTRIC score in 211 ICU patients was 5 (4, 6). Of these, there were 129 low-risk patients (0–4) and 82 high-risk patients (5–9). The median time from admission to ICU transfer was one day for patients who survived and two days for patients who died. Moreover, patients who survived had fewer complications. During respiratory treatment, non-survivors more often adapted to mechanical ventilation and invasive mode than survivors. There was no difference in the proportion of ALB support between non-survivors and survivors. However, the ratio of parenteral nutrition (PN) therapy in non-survivors was greater than that in survivors (62.1% vs 10.3%, p < 0.001), and the time until onset of nutrition therapy was longer than that in survivors (Table 2).
Table 2.
Severity of illness scores and nutrition therapy of patients with COVID-19 in the ICU.
| Survivors, n = 116 | Non-survivors, n = 95 | All patients, n = 211 | p value | |
|---|---|---|---|---|
| SOFA score on day 1 | 4 (3,5) | 6 (4,8) | 5 (4,6) | <0.001 |
| APACHE Ⅱ score on day 1 | 15 (13,17) | 19 (16,21) | 16 (15,19) | <0.001 |
| NUTRIC score on day 1 | 3 (2,4) | 6 (4,7) | 4 (3,5) | <0.001 <0.001 |
| Low risk (0–4) | 108 (83.7%) | 21 (16.3%) | 129 (61.1%) | |
| High risk (5–9) | 8 (9.8%) | 74 (90.2%) | 82 (38.9%) | |
| NRS score on day 1 | 4 (3,6) | 5 (4,6) | 5 (3,6) | 0.01 |
| Time from hospital admission to ICU admission, days | 1 (0,2) | 2 (1,3) | 2 (1,3) | 0.009 |
| Complications, n | 1 (0,1) | 3 (1,3) | 2 (1,3) | <0.001 |
| Oxygen therapy on day 1 | <0.001 | |||
| Nasal cannula + mask | 50 (43.1%) | 10 (10.5%) | 60 (28.4%) | |
| NMV | 57 (49.1%) | 28 (29.5%) | 85 (40.3%) | |
| IMV | 9 (7.8%) | 57 (60%) | 66 (31.3%) | |
| Initial nutrition therapy mode | <0.001 | |||
| ONS | 9 (7.8%) | 4 (4.2%) | 13 (6.2%) | |
| EN | 34 (29.3%) | 12 (12.6%) | 46 (21.8%) | |
| PN | 12 (10.3%) | 59 (62.1%) | 71 (33.6%) | |
| Initial time of ICU nutrition support, days | 2 (1,3) | 1 (0,1) | 2 (1,3) | <0.001 <0.001 |
| ≤48 h | 95 (81.9%) | 36 (37.9%) | 131 (62.1%) | |
| >48 h | 21 (18.1%) | 59 (62.1%) | 80 (37.9%) | |
| Albumin support, % | 86 (74.1%) | 62 (65.3%) | 148 (70.1%) | 0.106 |
| Hospitalization time in ICU, days | 6 (4,8) | 13 (9,16) | 7 (5,12) | <0.001 |
Abbreviations: APACHE II: Acute Physiology and Chronic Health Evaluation II; EN: Enternal nutrition; IMV: Invasive mechanical ventilation; NMV: Noninvasive mechanical ventilation; NUTRIC score: Nutrition Risk in Critically ill score; ONS: Oral nutrition support; PN: Parenternal nutrition; SOFA: Sequential Organ Failure Assessment.
Binary logistic regression model was used to analyze the relationship between common nutrition metabolism indices and endpoint events, including ICU transfer and death. BMI, HDL-c, FBG, blood urea nitrogen, creatinine (Cr), uric acid, and plasma protein levels were significantly associated with the two endpoint events. Hemoglobin and total bilirubin levels were significantly related to ICU transfer, while total cholesterol and LDL-c levels were independently related to patient death (Table 3 ). However, among these indicators, only triacylglycerol level was not associated with death and ICU transfer (Table 3).
Table 3.
Bivariate logic regression of factors associated with end points.
| Death |
Transfer to ICU |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | |
| BMI | 0.827 | 0.768–0.891 | <0.001 | 0.791 | 0.742–0.844 | <0.001 |
| ≤20.5vs > 20.5 | 0.304 | 0.198–0.466 | <0.001 | 0.353 | 0.242–0.516 | <0.001 |
| Hb | 1.007 | 0.997–1.018 | 0.172 | 1.019 | 1.009–1.028 | <0.001 |
| TC | 0.708 | 0.527–0.951 | 0.022 | 0.817 | 0.647–1.030 | 0.087 |
| TG | 1.023 | 0.773–1.354 | 0.875 | 1.047 | 0.828–1.324 | 0.698 |
| LDL-c | 0.509 | 0.341–0.759 | 0.001 | 0.901 | 0.669–1.215 | 0.495 |
| HDL-c | 0.301 | 0.124–0.730 | 0.008 | 0.363 | 0.179–0.738 | 0.005 |
| FBG | 1.239 | 1.154–1.331 | <0.001 | 1.133 | 1.063–1.208 | <0.001 |
| BUN | 1.465 | 1.331–1.613 | <0.001 | 1.156 | 1.084–1.232 | <0.001 |
| Cr | 1.018 | 1.010–1.026 | <0.001 | 1.011 | 1.005–1.018 | <0.001 |
| UA | 0.997 | 0.994–0.999 | 0.012 | 0.995 | 0.993–0.998 | <0.001 |
| TB | 0.973 | 0.945–1.003 | 0.073 | 0.969 | 0.946–0.993 | 0.012 |
| Total protein | 0.805 | 0.770–0.841 | <0.001 | 0.890 | 0.865–0.915 | <0.001 |
| ≤35vs > 35 | 0.366 | 0.228–0.559 | <0.001 | 0.457 | 0.305–0.673 | <0.001 |
| Albumin, | 0.677 | 0.626–0.732 | <0.001 | 0.799 | 0.760–0.841 | <0.001 |
| Globulin | 0.670 | 0.616–0.729 | <0.001 | 0.820 | 0.776–0.867 | <0.001 |
| Prealbumin | 0.988 | 0.984–0.991 | <0.001 | 0.993 | 0.990–0.996 | <0.001 |
Multivariate Cox regression analysis showed that the mNUTRIC score was closely related to patient death and ICU stay time. In unadjusted variables, mNUTRIC scores were significantly correlated with hospital mortality and ICU stay time. After adjusting for age, sex, BMI, liver and kidney function index, inflammatory factors, therapies, and other variables, the mNUTRIC score was able to independently predict the risk of death in the hospital (OR = 1.197, 95%CI: 1.091–1.445, p = 0.006) and duration of the ICU stay (β = 0.566, 95%CI: 0.068–1.064, p = 0.026, Table 4 ). For each one-point increase in the mNUTRIC score, the risk of death in the ICU increased by nearly 20%. After adjusting for age and sex, for each standard deviation increase in BMI, the risk of in-hospital death was reduced by 13% (HR = 0.871, 95%CI:0.795–0.955, p = 0.003), while the risk of ICU transfer was reduced by 7% (HR = 0.932, 95%CI:0.885–0.981, p = 0.007, Table 4).
Table 4.
Cox regression of NUTRIC score and BMI associated with clinical adverse outcomes.
| mNUTRIC scores | Death |
Hospitalization days of ICU |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | β | 95%CI | p value | |
| Unadjusted | 1.343 | 1.170–1.541 | <0.001 | 1.534 | 1.143–1.926 | <0.001 |
| Model 1a | 1.219 | 1.040–1.429 | 0.014 | 1.090 | 0.592–1.587 | <0.001 |
| Model 2 | 1.348 | 1.137–1.597 | 0.001 | 1.029 | 0.530–1.528 | <0.001 |
| Model 3a |
1.197 |
1.091–1.445 |
0.006 |
0.566 |
0.068–1.064 |
0.026 |
| BMI |
Death |
Transfer to ICU |
||||
| HR per SD |
95%CI |
p value |
HR per SD |
95%CI |
p value |
|
| Unadjusted | 0.822 | 0.761–0.888 | <0.001 | 0.925 | 0.879–0.972 | 0.002 |
| Model 1 b | 0.871 | 0.795–0.955 | 0.003 | 0.932 | 0.885–0.981 | 0.007 |
| Model 2 | 0.936 | 0.868–1.009 | 0.083 | 0.923 | 0.876–0.973 | 0.003 |
| Model 3 b | 0.992 | 0.897–1.097 | 0.877 | 0.956 | 0.900–1.106 | 0.146 |
Model 1a: adjusted for age, gender and BMI.
Model 2: Model 1a + Hypertension, Diabetes mellitus and Coronary artery disease.
Model 3a: Model 2 + Initial nutrition therapy mode, Oxygen therapy mode, Duration of start nutrition therapy.
Model 1 b: adjusted for age, gender.
Model 2: Model 1 b + Hypertension, Diabetes mellitus and Coronary artery disease.
Model 3 b: Model 2 +Cr, PCT, ALC, cTnI, hs-CRP, LDL-c and FBG.
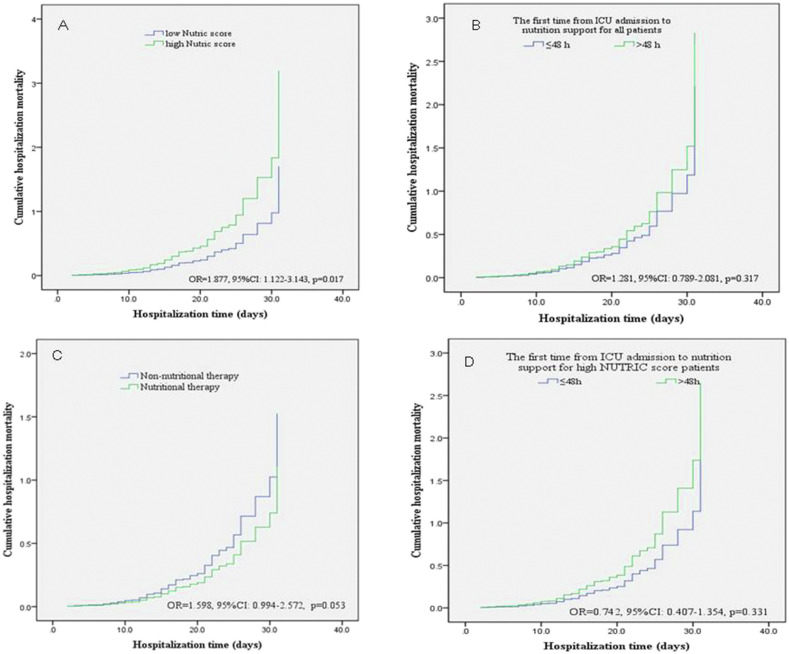
ICU patients were divided into low- and high-risk groups according to the mNUTRIC score. The Cox proportional risk model showed that after adjusting for age, sex, and other variables, patients with high nutritional risk (5–9 points) had a significantly increased risk of in-hospital death compared to patients with low nutritional risk (OR = 1.877, 95%CI: 1.122–3.143, p = 0.017, Fig. 1 a). Similarly, patients were divided into two groups according to the nutrition therapy start time. However, after adjusting for variables, there was no significant increase in the risk of in-hospital death in patients whose nutrition therapy started >48 h after ICU admission (OR = 1.281, 95%CI: 0.789–2.081, p = 0.317, Fig. 1b). Univariate logistic regression analysis results showed that the risk of death in ICU patients who did not receive nutritional treatment was significantly higher than that in patients who did (OR = 2.880, 95%CI: 1.878–4.414, p < 0.001). However, after adjusting for age, gender, BMI, and prior medical history, there was no significant difference in the risk of in-hospital death (OR = 1.598, 95%CI: 0.994–2.572, p = 0.053, Fig. 1c). In addition, there was no difference in the risk of in-hospital death between patients with high mNUTRIC scores who received nutritional support early and those who delayed (Fig. 1d).
Fig. 1.
Cumulative hospitalization mortality between the different groups of NUTRIC score and nutrition therapy timing.
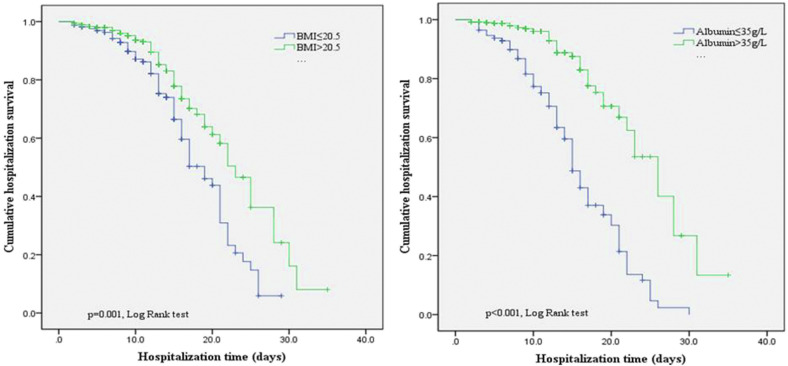
All patients were divided into two groups according to their BMI and ALB levels. The Kaplan–Meier curve analysis showed that the in-hospital survival time in patients with BMI ≤20.5 (18.1 d, 95% CI: 15.9–22.1, vs 22.5 d, 95% CI: 20.2–25.8, p = 0.001, Fig. 2 ) or ALB level ≤35 g/L (15.9 d, 95% CI: 13.7–16.3, vs 24.2 d, 95% CI: 22.3–29.7, p < 0.001, Fig. 2) was significantly lower.
Fig. 2.
Kaplan–Meier curves stratified according to BMI and albumin.
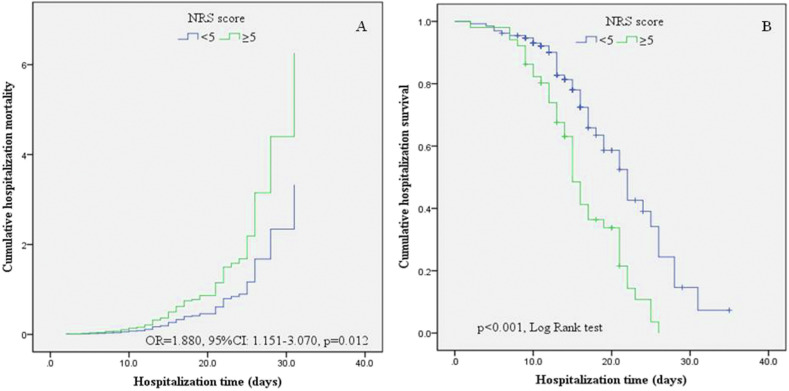
In addition, the NRS scores of the two grades also have significant differences in clinical outcomes. The Cox proportional risk model showed that after adjusting for age, sex, and other variables, patients with higher NRS score (≥5 points) had a significantly increased risk of in-hospital death compared to patients with low NRS score (OR = 1.880, 95%CI: 1.151–3.070, p = 0.012, Fig. 3 a).Those with higher NRS scores (≥5 points) had significant shorter in-hospital survival time (16.2 d, 95% CI: 14.6–17.8, vs 21.4 d, 95% CI: 19.4–23.5 d, p < 0.001, Fig. 3b). These results were confirmed by Kaplan–Meier survival curve estimates, which showed a higher likelihood for mortality with increasing NRS scores.
Fig. 3.
Kaplan–Meier analysis and Cox proportional risk model comparing elevated and low NRS scores.
4. Discussion
In this retrospective study of 523 severe and critical COVID-19 patients, those who were admitted to the ICU or died had a higher risk of malnutrition. Low BMI and protein levels were significantly associated with adverse events, such as death and ICU admission. The mNUTRIC score was able to independently predict the risk of death and duration of ICU stay in COVID-19 patients.
Nutritional support is an important part of the ARDS treatment [23]. In addition to affecting the lungs and inducing ARDS, coronavirus can also cause dysfunction in other organs, such as sepsis and myocardial damage [3,24]. The large amount of virus replication and amplification in vivo also leads to a dramatic increase in metabolism. Patients admitted to the ICU or those who died may have a higher nutritional risk and metabolic level due to a high viral load or extremely strong immune response in vivo. Some patients also have gastrointestinal symptoms [25], which further aggravate the nutritional risk. Nutritional support therapy can meet the patient needs for macronutrients, prevent the adverse effects of metabolism on diseases, reduce cell oxidative damage, and regulate the immune response [26]. The treatment of critical COVID-19 patients is a long process. Nutritional support is an important part of treatment and requires more attention.
The clinical characteristics of patients admitted to the ICU were further analyzed. Similar to other studies, the ICU patients had higher APACHE II and SOFA scores [6,21]. Most ICU patients received ALB support therapy. The European guidelines recommend that all hospitalized patients in the ICU consider medical nutrition treatment and that all patients who stay in the ICU for >48 h should receive early nutrition treatment [16]. Low-calorie nutrition (no more than 70% of energy consumption) should be used in the early stages of the acute disease [16]. If critical patients can eat normally, oral nutrition support (ONS) is better than enteral nutrition (EN) and PN. In the present study, only 6.2% of patients selected ONS as the initial nutrition support method. Most of them still preferred EN and PN. It is recommended that nutrition therapy be started within 48 h. However, many patients started nutrition therapy after >48 h. In the present study, although there was no difference in the risk of in-hospital death between patients who received early and delayed nutrition therapy, early nutrition therapy was still necessary.
BMI is widely used in cardiovascular, nutrition, and metabolic risk assessment [27,28]. The present study found that low BMI was significantly associated with an increased risk of death and ICU admission. The hospital survival time in patients with BMI ≤20.5 was significantly lower than that in patients with BMI >20.5. NRS recommends BMI of ≤20.5 to be an indicator for initial risk screening [15]. Obesity and morbid obesity are associated with lower mortality in patients with ARDS [29]. It is also considered important to maintain a certain weight during the COVID-19 outbreak.
It is necessary to use specific tools to screen for nutritional risk in ICU patients. The NUTRIC score was first proposed by Heylend et al., in 2011 and is also suitable for critically bedridden patients [18]. APACHE II and SOFA scores are the most widely used critical scales in the ICU. Based on the APACHE II and SOFA scores, the NUTRIC score added several simple indicators, such as age, number of complications, and time from hospital admission to ICU admission. Heyland et al. prospectively observed 597 patients in the ICU and found that the NUTRIC score was related to the mechanical ventilation time, 28-day mortality, and other prognostic indicators [18]. When the NUTRIC score was compared to traditional screening tools, a large variability was observed [16]. A limitation to this score is that no nutritional parameters are included. Furthermore, mortality is not the best outcome to assess the efficacy of a nutritional intervention considering the numerous factors influencing ICU mortality [16]. Viana et al. demonstrated that high NRS scores may identify patients at highest risk of poor outcome when exposed to underfeeding [17]. A single-center retrospective study in Wuhan found that COVID-19 patients with a high nutritional risk had a higher probability of death at ICU 28-day than those with a low nutritional risk. The mortality of ICU 28-day was significantly higher in the high mNUTRIC score group than in the low mNUTRIC score group [30]. Zhao et al. found that critically COVID-19 patients and those with higher NRS score had a higher risk of mortality and longer stay in hospital. In their logistic regression models, 1-unit increase in NRS score was associated with the risk of mortality increasing by 1.23 times [31]. In the present study, we found that higher NUTRIC and NRS scores were associated with poor outcomes in patients with COVID-19.Therefore, we thought that NUTRIC score and NRS score are convenient, provides objectivity, and might be the suitable nutritional risk assessment tool for COVID-19 patients.
ALB and PA levels are two classical laboratory indexes in traditional nutrition assessment [32,33]. They cannot be used as the evaluation indexes of nutritional status alone. However, they can directly reflect the approximate nutritional status or disease severity in clinical practice. The ALB level can also provide a sensitive reflection of the condition and prognosis of critical patients with acute lung injury [34]. Serum PA level is a useful tool in the assessment of malnutrition in hospitalized patients [21]. In the present study population, the average ALB and PA levels upon admission already indicated a state of mild malnutrition. Therefore, the timing of nutritional support for COVID-19 patients may need to be further advanced.
5. Limitations
This study had some limitations. First, only a retrospective nutritional risk assessment was conducted in the ICU patients. Other patients not admitted to the ICU did not undergo a nutritional risk assessment. Whether early nutritional risk screening and early intervention in all in-hospital COVID-19 patients could reduce mortality and proportion of conversion to critical illness is worth further investigation. Second, due to the nature of the study, we were unable to accurately assess the daily energy needs of the ICU patients, which may have affected the prognosis judgment.
6. Conclusions
Severe and critical COVID-19 patients have a high risk of malnutrition. Low BMI and protein levels are significantly associated with adverse events, such as death and ICU transfer. The NUTRIC score and NRS score can be used to assess the nutritional risk of COVID-19 patients in the ICU. Early nutritional risk screening and therapy are necessary for COVID-19 patients.
Contributors
Drs G. Li, C. Zhou, Y. Wang and B. Shong contributed equally and share first authorship. Drs X. Cheng, Q. Dong, and S. You collected the epidemiological and clinical data. G. Li, C. Zhou, and Y. Wang summarized all data. G. Li, and L. Wang drafted the manuscript. G. Li and Y. Ba revised the final manuscript.
Conflict of Interest
We declare no competing interests.
Funding
None.
Availability of data and materials
All data generated or analyzed in this study are included in this published article, and the datasets are available from the corresponding author within the limits imposed by ethical and legal dispositions.
Ethics approval and consent to participate
Exemption from the written informed consent was obtained. The study was approved by the ethics committee of Hubei Provincial Hospital of Traditional Chinese Medicine (HBZY2020-C14-01).
Consent for publication
Not applicable.
Acknowledgments
We thank all patients and their families involved in the study.
Abbreviations
- 2019-nCoV
2019 novel coronavirus
- ALB
albumin
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- ARDS
acute respiratory distress syndrome
- ASPEN
American Society for Parenteral and Enteral Nutrition
- BMI
body mass index
- COVID-19
Coronavirus Disease 2019
- EN
enteral nutrition
- FBG
fasting blood glucose
- GCS
Glasgow coma scale
- ICU
intensive care units
- IL-6
Interleukin-6
- LDL-c
low-density lipoprotein cholesterol
- NUTRIC
modified Nutrition Risk in the Critically ill
- NRS 2002
Nutritional Risk Screening 2002
- PA
prealbumin
- PN
parenteral nutrition
- SARS
severe acute respiratory syndrome
- SOFA
Sequential Organ Failure Assessment
References
- 1.World Health Organization Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation reports/
- 2.Zhang H., Josef M.P., Li Y., Zhong N.S., Arthur S.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. The Lancet Gastroenterology & Hepatology. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., Jennifer M.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Yang X.B., Yu Y., Xu J.Q., Shu H.Q., Xia J.A., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q.R., Yang K., Wang W.X., Jiang L.Y., Song J.X. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C.M., Chen X.Y., Cai Y.P., Xia J.A., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C.L., Wang Y.M., Li X.W., Ren L.L., Zhao J.P., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manjili R.H., Zarei M., Habibi M., Manjili M.H. COVID-19 as an acute inflammatory disease. J Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew C.C.H., Yandell R., Fraser R.J.L., Chua A.P., Chong M.F.F., Miller M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. J Parenter Enter Nutr. 2017;41(5):744–758. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 13.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Health Commission of the People’s Republic of China. Chinese management guideline for COVID-19 (version 7.0). Available at: http://www.chinadaily.com.cn/specials/diagnosisandtreatment-Africa.pdf. Accessed March 3, 2020.
- 15.Kondrup J., Rasmussen H., Hamberg O., Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 16.Singer P., Reintam-Blaser A., Berger M.M., Alhazzani W., Calder P.C., Casaer M., et al. ESPEN guidelines: nutrition in the ICU. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Viana M.V., Pantet O., Bagnoud G., Martinez A., Favre E., Charriere M., et al. Metabolic and nutritional characteristics of long-stay critically ill patients. J Clin Med. 2019;8:985. doi: 10.3390/jcm8070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyland D.K., Dhaliwal R., Jiang X., Day A.G. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.) J Parenter Enteral Nutr. 2016;40(2):159e211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 20.National Health Commission of the People's Republic of China home page. http://www.nhc.gov.cn [DOI] [PMC free article] [PubMed]
- 21.Wang D.W., Hu B., Hu C., Zhu F.F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman A., Hasan R.M., Agarwala R., Martin C., Day A.G., Heyland D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–162. doi: 10.1016/j.clnu.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Anna K., Melissa P., Lena M.N. Nutrition therapy for ALI and ARDS. Crit Care Clin. 2011;27:647–659. doi: 10.1016/j.ccc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R.H., Fan G.H., Liu Y., Liu Z.B., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClave S.A., Beth E.T., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) J Parenter Enter Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 27.Wilson W.F., D'Agostino R.B., Sullivan L. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 28.He Y., Jiang B., Wang J., Feng K., Chang Q., Zhu S.X., et al. BMI versus the metabolic syndrome in relation to cardiovascular risk in elderly Chinese individuals. Diabetes Care. 2007;30:2128–2134. doi: 10.2337/dc06-2402. [DOI] [PubMed] [Google Scholar]
- 29.Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P., He Z.G., Yu G., Peng D., Feng Y.K., Ling J.M., et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. 2021;40(2):534–541. doi: 10.1016/j.clnu.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID-19 patients. J Parenter Enter Nutr. 2020;0(0):1–11. doi: 10.1002/jpen.953. e-pub 01 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frederick B.K., Rosenthal T.C. Prealbumin: a marker for nutritional evaluation. Am Fam Physician. 2002;65:1575. [PubMed] [Google Scholar]
- 33.Don B.R., George K. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:6. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan G.J., Mumby S., Martin G.S., Bernard G.R., Gutteridge J.M.C., Evans T.W. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med. 2004;32:755–759. doi: 10.1097/01.ccm.0000114574.18641.5d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this published article, and the datasets are available from the corresponding author within the limits imposed by ethical and legal dispositions.