Abstract
Objectives
To determine the rate of complicated birth at term in women classified at low risk according to the National Institute for Health and Care Excellence guideline for intrapartum care (no pre-existing medical conditions, important obstetric history, or complications during pregnancy) and to assess if the risk classification can be improved by considering parity and the number of risk factors.
Design
Cohort study using linked electronic maternity records.
Participants
276 766 women with a singleton birth at term after a trial of labour in 87 NHS hospital trusts in England between April 2015 and March 2016.
Main outcome measure
A composite outcome of complicated birth, defined as a birth with use of an instrument, caesarean delivery, anal sphincter injury, postpartum haemorrhage, or Apgar score of 7 or less at five minutes.
Results
Multiparous women without a history of caesarean section had the lowest rates of complicated birth, varying from 8.8% (4879 of 55 426 women, 95% confidence interval 8.6% to 9.0%) in those without specific risk factors to 21.8% (613 of 2811 women, 20.2% to 23.4%) in those with three or more. The rate of complicated birth was higher in nulliparous women, with corresponding rates varying from 43.4% (25 805 of 59 413 women, 43.0% to 43.8%) to 64.3% (364 of 566 women, 60.3% to 68.3%); and highest in multiparous women with previous caesarean section, with corresponding rates varying from 42.9% (3426 of 7993 women, 41.8% to 44.0%) to 66.3% (554 of 836 women, 63.0% to 69.5%).
Conclusions
Nulliparous women without risk factors have substantially higher rates of complicated birth than multiparous women without a previous caesarean section even if the latter have multiple risk factors. Grouping women first according to parity and previous mode of birth, and then within these groups according to presence of specific risk factors would provide greater and more informed choice to women, better targeting of interventions, and fewer transfers during labour than according to the presence of risk factors alone.
Introduction
Risk assessment is an essential part of antenatal and intrapartum care. In middle and high income settings, women are typically considered to be at low risk of complications in pregnancy or at birth if they do not have specific conditions or comorbidities.1 2 3 4 5 6 7 8 9 10 11 12 In the United Kingdom, a national guideline for intrapartum care, developed by the National Institute for Health and Care Excellence, includes a set of risk factors that provide the basis of a risk classification system.4 These risk factors, including a woman’s age, body mass index (BMI), and the presence of specific clinical and obstetric conditions, are considered to identify women at increased risk of complications during labour and birth and to inform recommendations on place of birth.4
Women considered to be at low risk of complications are advised that a low risk setting such as their home or a midwife led unit is a suitable place for them to give birth,4 whereas women considered to be at increased risk are advised to give birth in an obstetric unit. The NICE guideline recommends that women with characteristics that indicate they are at a higher risk of complications, but who according to its risk classification do not fall into the increased risk group (referred to as the intermediate risk group in this paper) should have an individual discussion about place of birth with their obstetric and midwifery team. The risk classification according to the NICE guideline does not distinguish between women with a single risk factor and those with multiple risk factors. Importantly, the listed factors that identify women as being at increased risk do not include a reference to parity (except for women who have had four or more pregnancies), even though the rate of complications and interventions during labour are different between nulliparous and multiparous women.4
We evaluated the set of risk factors identified in the NICE guideline as a tool to identify women at increased risk of complications during labour and birth, using routinely collected maternity data. Firstly, we determined the numbers of women classified according to the NICE guideline as being at low, intermediate, and increased risk at the time of birth. Then we determined the rate of complications and interventions that are generally considered to require action by an obstetric or neonatal team, according to these risk groups. Finally, we assessed the extent to which the risk classification could be improved by considering the number of risk factors present in a woman and by distinguishing between nulliparous women and multiparous women with and without a previous caesarean section.
Methods
Data sources
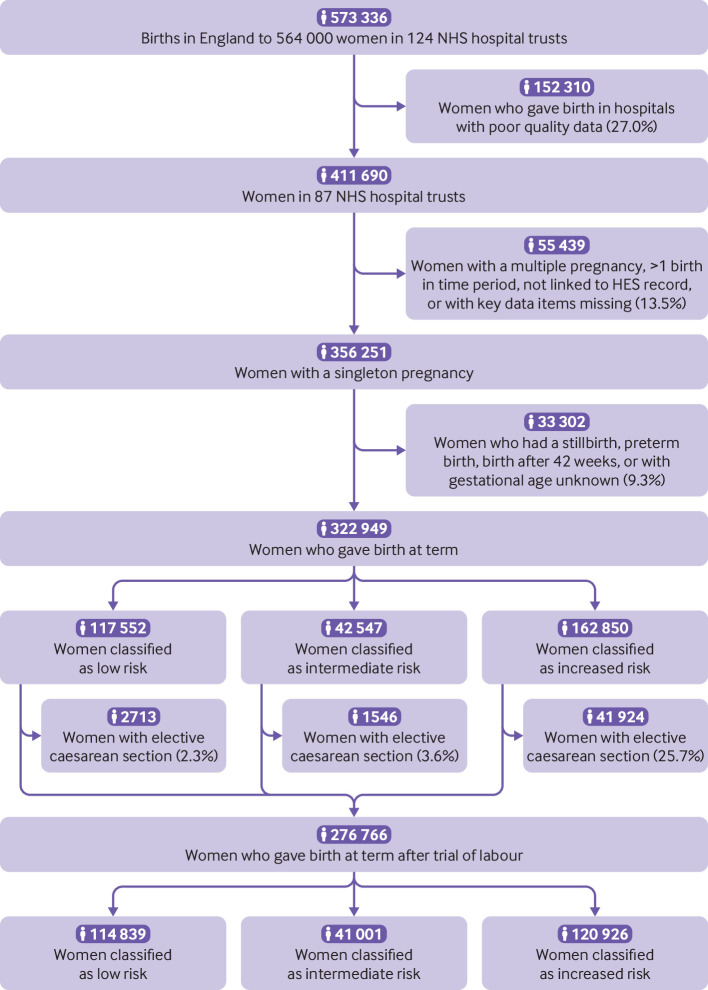
We used a national maternity dataset that was collated from extracts of the routinely collected electronic Maternity Information Systems (MIS) of English National Health Service hospitals by the National Maternity and Perinatal Audit.13 14 In England, more than 99% of births occur within the NHS.15 MIS extracts were provided by 124 of the 134 NHS hospital trusts that provide maternity services in England. After cleaning, the dataset contains information on mothers and babies for 573 336 babies born in England between 1 April 2015 and 31 March 2016 (85.9% of all babies born that year) to 564 000 women (fig 1).14
Fig 1.
Flowchart of cohort selection. NHS=national health service; HES=Hospital Episode Statistics
MIS data were linked at patient level to records from Hospital Episode Statistics (HES), an administrative database containing records of all admissions to English NHS hospitals. NHS Digital carried out the linkage using a deterministic algorithm based on the woman and baby’s NHS numbers, dates of birth, and postcode. Of the 573 336 MIS records, 536 924 (93.6%) could be linked. When information was available in both datasets, we used the MIS data. If a woman had given birth twice during the study period, we only considered the first birth.
Study population
We first restricted the study population to 411 690 women aged 15-45 who gave birth in the 87 hospital trusts with high levels of completeness of key data items (>70% of records complete on all of maternal BMI, maternal age, and gestational age) and within these hospitals to the 356 251 women with a record of a singleton pregnancy linked to HES with available data on these key data items (fig 1). We identified 322 949 women who gave birth at term (37+0 to 41+6 weeks gestation), and the 276 766 women who gave birth at term after a trial of labour (elective caesarean births excluded) were included in the analyses (fig 1). We compared the characteristics of included and non-included hospital trusts and the complete and incomplete records within those trusts.
Definition of risk group
From the NICE guideline, we derived a list of characteristics and diagnosis and procedure codes that can be used to identify women at an increased risk of birth complications.4 Diagnoses in HES records are coded using ICD-10 (international statistical classification of diseases and related health problems, 10th revision)16 and procedure codes using the operating procedure codes (OPCS) classification of interventions and procedures used by NHS hospitals in the UK (see supplementary table S1).17 When no precise match was possible for the condition defined, we included all diagnosis codes that could be used as a proxy for the condition. To assess concurrent validity, we also checked coded frequencies against known rates of particular conditions in the UK population (table 1, see full details in supplementary table S1).
Table 1.
Summary of risk classification derived from National Institute for Health and Care Excellence guideline CG90: intrapartum care for healthy women and babies4
| Risk factors | Risk group | ||
|---|---|---|---|
| Low | Intermediate | Increased | |
| Age (years) | <35 | ≥35 | – |
| Body mass index | <30 | 30-34.99 | ≥35 |
| Pre-existing medical conditions | Stable mild medical conditions (asthma, hypothyroidism) | Mild medical conditions | Comorbidity (eg, hypertension), previous uterine surgery (eg, myomectomy) |
| Important obstetric history | No important history | Parity of ≥4, previous events that are unlikely to recur (eg, stillbirth of known cause), previous mild complications not known to occur in this pregnancy (eg, mild pre-eclampsia) | Previous caesarean section, previous events that might recur (eg, severe pre-eclampsia or stillbirth of unknown cause) |
| Complications in current pregnancy | None | – | Conditions or suspected conditions in mother, such as pre-eclampsia, fetal complications such as anomaly, multiple pregnancy, or suspected macrosomia |
As it is not possible to define exactly when a diagnosis code was assigned in the HES records, we made assumptions about the time of onset of conditions. We assumed that illnesses of pregnancy such as gestational diabetes or pre-eclampsia occurred after booking but before birth.
For some types of medical conditions, most notably asthma, hypothyroidism, and cardiac disease, HES does not give enough information about severity. For these three conditions, the most common level of severity was assumed for all women. Therefore, we assumed women with asthma or hypothyroidism to be stable and at low risk and women with cardiac conditions to be at increased risk. We carried out a sensitivity analysis in which we classified women with asthma and hypothyroidism in the increased risk group.
We distinguished five types of risk factors: previous caesarean section, BMI of 35 or more, pre-existing medical conditions, important obstetric history, and complications in the current pregnancy (table 1).
Definition of outcomes
When possible we defined outcomes using previously published coding frameworks.18 19 A composite outcome of complicated birth was defined as a birth with any of the following events: use of an instrument (forceps or vacuum device), emergency caesarean delivery, obstetric anal sphincter injury, postpartum haemorrhage, or neonate born with an Apgar score of 7 or less at five minutes. We chose this composite outcome as it represents a birth that typically requires the attention of an obstetric or neonatal team. It also closely matches a definition of complicated birth used in a recent Danish study.20
As about 90% of stillbirths occur before the onset of labour and as information was not available on the timing of stillbirths in this dataset, we considered all stillbirths to be antepartum and therefore they were excluded.21 We carried out a sensitivity analysis in which we considered all stillbirths as intrapartum and included stillbirth as a component of the complicated birth outcome.
To assess the sensitivity of the results to the dominance of instrumental birth among nulliparous women, we conducted a sensitivity analysis with instrumental birth excluded from the outcome.
Statistical analysis
Firstly, we used proportions to describe the women’s characteristics. Then we compared frequencies and the 95% confidence intervals of complicated births in women who gave birth at term after a trial of labour in three groups: nulliparous women, multiparous women with previous vaginal deliveries only, and multiparous women with a previous caesarean section. We used Poisson regression with robust standard errors to estimate risk ratios comparing the likelihood of complicated birth compared with a reference group of multiparous women with no additional risk factors. Finally, for these three groups we compared frequencies of the individual components of the composite outcome between the risk groups separately.
Patient and public involvement
This study was motivated by discussions with the Women and Families Group of the National Maternity and Perinatal Audit, which represents women and their families accessing maternity care in the UK. This group helped to refine the research question.
Results
Risk classification groups at birth
Of the 322 949 women who gave birth at term, 117 552 (36.4%) were considered to be at low risk, 42 547 (13.2%) at intermediate risk, and 162 850 (50.4%) at increased risk at the time of birth according to the risk factors derived from the NICE guideline (table 2). Women in the low risk group were more often younger than 25 years (22.4%) and nulliparous (51.9%) than the women in the intermediate risk group (18.4% and 38.8%, respectively) and the increased risk group (16.3% and 31.8%, respectively). Women in the intermediate risk and increased risk groups were more often of black ethnicity and from more socioeconomically deprived backgrounds.
Table 2.
Characteristics of 322 949 women who gave birth at term, according to risk classification. Values are numbers (percentages) unless stated otherwise
| Characteristics | Risk group | ||
|---|---|---|---|
| Low (n=117 552) | Intermediate (n=42 547) | Increased (n=162 850) | |
| Age (years): | |||
| 15-24 | 26 284 (22.4) | 7836 (18.4) | 26 573 (16.3) |
| 25-34 | 91 268 (77.6) | 14 089 (33.1) | 105 838 (65.0) |
| 35-44 | – | 20 622 (48.5) | 30 439 (18.7) |
| Parity: | |||
| 0 (nulliparous) | 61 039 (51.9) | 16 491 (38.8) | 51 850 (31.8) |
| 1-3 | 56 513 (48.1) | 22 072 (51.9) | 103 523 (63.6) |
| ≥4 | – | 3984 (9.4) | 7477 (4.6) |
| IMD (national fifth)*: | |||
| 1st (least deprived) | 20 308 (18.5) | 7607 (19.0) | 25 181 (16.5) |
| 2nd | 16 497 (15.0) | 5821 (14.5) | 20 782 (13.7) |
| 3rd | 21 761 (19.8) | 7454 (18.6) | 28 476 (18.7) |
| 4th | 24 382 (22.2) | 8782 (21.9) | 33 909 (22.3) |
| 5th (most deprived) | 26 746 (24.5) | 10 409 (26.0) | 43 843 (28.8) |
| Missing | 7858 | 2474 | 10 659 |
| Ethnicity: | |||
| White | 85 553 (79.2) | 31 674 (80.0) | 116 010 (75.9) |
| Black | 5005 (4.6) | 2410 (6.1) | 11 006 (7.2) |
| Asian | 12 960 (12.0) | 3826 (9.7) | 19 857 (13.0) |
| Other | 4463 (4.1) | 1648 (4.2) | 5999 (3.9) |
| Unknown | 9571 | 2989 | 9978 |
| Body mass index: | |||
| <18 | – | – | 9361 (5.7) |
| 18-24.9 | 77 821 (66.2) | 15 039 (35.3) | 61 513 (37.8) |
| 25-29.9 | 39 731 (33.8) | 8385 (19.7) | 43 594 (26.8) |
| 30-34.9 | – | 19 123 (44.9) | 22 677 (13.9) |
| ≥35 | – | – | 25 705 (15.8) |
| Pre-existing medical conditions: | |||
| Cardiac | – | – | 2514 (1.5) |
| Endocrine/renal | – | – | 3245 (2.0) |
| Neurological | – | – | 9262 (5.7) |
| Psychiatric | – | – | 12 682 (7.8) |
| Haematological | – | – | 7473 (4.6) |
| Other | – | – | 65 583 (40.3) |
| None | 117 552 (100) | 42 547 (100) | 62 091 (38.1) |
| Important obstetric history: | |||
| Caesarean birth | – | – | 48 331 (29.7) |
| Uterine surgery | – | – | 6827 (4.2) |
| Previous tear | – | – | 5267 (3.2) |
| Other | – | – | 21 029 (12.9) |
| None | 117 552 (100) | 42 547 (100) | 91 018 (55.9) |
| Complications in current pregnancy: | |||
| Hypertension | – | – | 16 880 (10.4) |
| Fetal complication | – | – | 30 956 (19.0) |
| Diabetes | – | – | 15 690 (9.6) |
| Other | – | – | 45 471 (27.9) |
| None | 117 552 (100) | 42 547 (100) | 67 550 (41.5) |
IMD=index of multiple deprivation.
Comparisons between risk groups were made using two sided χ2 tests. All comparisons were statistically significant, at P<0.001.
Combines socioeconomic information about a postcode area.
Of the 162 850 women in the increased risk group, 25 705 (15.8%) had a BMI of 35 or higher, 100 759 (61.9%) had at least one pre-existing medical condition, and 95 300 (58.5%) had complications in the current pregnancy (table 2). In total, 64 410 women at increased risk (39.6%) had more than one of the five types of risk factors: previous caesarean section, BMI of ≥35, pre-existing medical conditions, important obstetric history, and complications in the current pregnancy.
Of the 322 949 women who gave birth at term, 46 183 (14.3%) had an elective caesarean section and 276 766 (86.7%) had a trial of labour (fig 1). The elective caesarean section rate was lowest in multiparous women in the low risk group (1.9%) and highest in multiparous women with a previous caesarean section (59.0%) (table 3). Induction rates increased according to risk group, both in nulliparous women (from 24.8% in the low risk group to 40.2% in the increased risk group) and in multiparous women without a previous caesarean section (from 18.3% in the low risk group to 37.8% in the increased risk group).
Table 3.
Mode of onset of labour according to risk group in 322 949 women who gave birth at term
| Onset of labour by parity | Risk group | ||
|---|---|---|---|
| Low (n=117 552) | Intermediate (n=42 547) | Increased (n=162 850) | |
| Nulliparous women | |||
| No in group: | n=61 039 | n=16 491 | n=51 850 |
| Spontaneous | 44 260 (72.5) | 10 372 (62.9) | 23 048 (44.5) |
| Induced | 15 153 (24.8) | 5 244 (31.8) | 20 854 (40.2) |
| Elective caesarean section | 1626 (2.7) | 875 (5.3) | 7948 (15.3) |
| Multiparous women | |||
| No previous caesarean section: | n=56 513 | n=26 056 | n=63 003 |
| Spontaneous | 45 075 (79.8) | 19 221 (73.8) | 33 506 (53.2) |
| Induced | 10 351 (18.3) | 6164 (23.7) | 23 824 (37.8) |
| Elective caesarean section | 1087 (1.9) | 671 (2.6) | 5673 (9.0) |
| Previous caesarean section: | 47 997 | ||
| Spontaneous | – | – | 13 979 (29.1) |
| Induced | – | – | 5715 (11.9) |
| Elective caesarean section | – | – | 28 303 (59.0) |
Outcomes in women who had a trial of labour, by risk group
Among the 276 766 women who gave birth at term after a trial of labour, multiparous women without a previous caesarean section had the lowest rates of complicated birth (table 4, fig 2), with rates varying from 8.8% (95% confidence interval 8.6% to 9.0%) in those at low risk to 21.8% (20.2% to 23.4%) in those at increased risk with three or more types of risk factor. The rate of complicated birth was higher in nulliparous women across all risk groups, with rates varying from 43.4% (43.0% to 43.8%) in those at low risk to 64.3% (60.3% to 68.3%) in those at increased risk with three or more types of risk factor. This is confirmed by risk ratios presented in supplementary table S3.
Table 4.
Rates of complicated birth in 276 766 women who gave birth at term after a trial of labour (elective caesarean births excluded*)
| Risk classification† | Nulliparous women | Multiparous women without a previous caesarean section | Multiparous women with a previous caesarean section | All women | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No in group | Complicated births; % (95% CI) | No in group | Complicated birth; % (95% CI) | No in group | Complicated births; % (95% CI) | No in group | Complicated births; % (95% CI) | ||||
| Low risk | 59 413 | 25 805; 43.4 (43.0 to 43.8) | 55 426 | 4879; 8.8 (8.6 to 9.0) | – | – | 114 839 | 30 684; 26.7 (26.5 to 27.0) | |||
| Intermediate risk | 15 616 | 7153; 45.8 (45.0 to 46.6) | 25 385 | 2712; 10.7 (10.3 to 11.1) | – | – | 41 001 | 9870; 24.1 (23.7 to 24.5) | |||
| BMI 30-34 alone | 6671 | 2933; 44.0 (42.9 to 45.2) | 8700 | 771; 8.9 (8.3 to 9.5) | – | – | – | – | |||
| Age ≥35 alone | 8134 | 3799; 46.7 (45.6 to 47.8) | 11 284 | 1449; 12.8 (12.2 to 13.5) | – | – | – | – | |||
| Parity ≥4 alone | 0 | 0 | 2022 | 100; 4.9 (4.0 to 5.9) | – | – | – | – | |||
| No of risk factor types: | |||||||||||
| 1 | 14 805 | 6732; 45.5 (44.7 to 46.3) | 22 006 | 2320; 10.5 (10.1 to 11.0) | – | – | – | – | |||
| 2 | 811 | 421; 51.9 (48.4 to 55.4) | 3032 | 364; 12.0 (10.9 to 13.2) | – | – | – | – | |||
| 3 | – | – | 347 | 28; 8.1 (5.4 to 11.5) | – | – | – | – | |||
| Increased risk | 43 902 | 25 230; 57.5 (57.0 to 57.9) | 57 330 | 9767; 17.0 (16.7 to 17.4) | 19 694 | 10 908; 55.4 (54.7 to 56.1) | 120 926 | 45 905; 38.0 (37.7 to 38.2) | |||
| Previous caesarean section alone | – | – | – | – | 7 993 | 3 426; 42.9 (41.8 to 44.0) | – | – | |||
| BMI ≥35 alone* | 7063 | 3260; 46.2 (45.0 to 47.3) | 8134 | 917; 11.3 (10.6 to 12.0) | 790 | 317; 40.1 (36.6 to 43.6) | – | – | |||
| Pre-existing medical conditions alone* | 9920 | 5098; 51.4 (50.4 to 52.4) | 11 446 | 1445; 12.6 (12.0 to 13.2) | 1217 | 571; 46.9 (44.1 to 49.8) | – | – | |||
| Important obstetric history alone* | – | – | 8415 | 1511; 18.0 (17.1 to 18.8) | 1110 | 505; 45.5 (42.5 to 48.5) | – | – | |||
| Complications in current pregnancy alone* | 19 464 | 12 456; 64.0 (63.3 to 64.7) | 13 230 | 2826; 21.4 (20.6 to 22.1) | 4601 | 3480; 75.6 (74.4 to 76.9) | – | – | |||
| No of risk factor types*: | |||||||||||
| 1 | 36 447 | 20 814; 57.1 (56.6 to 57.6) | 41 225 | 6709; 16.3 (15.9 to 16.6) | 7718 | 4873; 63.1 (62.0 to 64.2) | – | – | |||
| 2 | 6889 | 4052; 58.8 (57.6 to 60.0) | 13 294 | 2445; 18.4 (17.7 to 19.1) | 3147 | 2055; 65.3 (63.6 to 67.0) | – | – | |||
| ≥3 | 566 | 364; 64.3 (60.3 to 68.3) | 2811 | 613; 21.8 (20.2 to 23.4) | 836 | 554; 66.3 (63.0 to 69.5) | – | – | |||
| All | 118 931 | 58 188; 48.9 (48.6 to 49.2) | 138 141 | 17 358; 12.6 (12.4 to 12.7) | 19 694 | 10 908; 55.4 (54.7 to 56.1) | 276 766 | 86 459; 31.2 (31.1 to 31.4) | |||
In addition to previous caesarean section for multiparous women with previous caesarean section.
According to National Institute for Health and Care Excellence guidelines.
Fig 2.
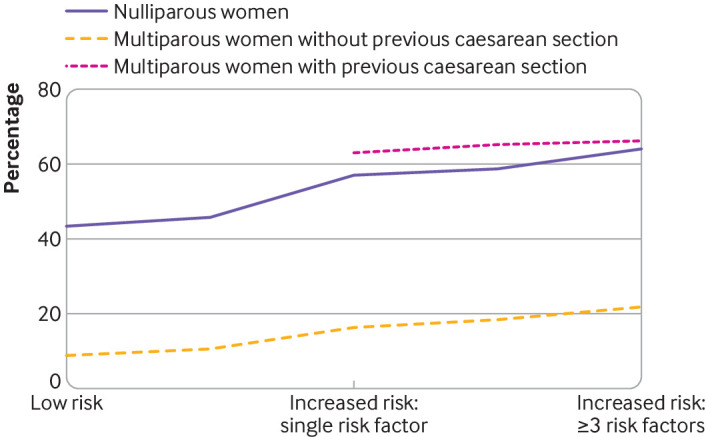
Association between number of types of risk factors and rates of complicated birth among women who gave birth at term after a trial of labour in England, 2015-16
Multiparous women with a previous caesarean section had the highest rates of complicated birth, with rates varying from 42.9% (41.8% to 44.0%) in those who had a previous caesarean section but no additional risk factors to 66.3% (63.0% to 69.5%) in those with three or more additional risk factors.
Figure 2 shows that parity and a previous caesarean section are the dominant risk factors for a complicated birth in women experiencing a trial of labour at term, with substantially higher rates of complicated birth in nulliparous women and multiparous women with a previous caesarean section compared with multiparous women without a previous caesarean section. In contrast, not taking into account parity and history of a caesarean section, women classified as low, intermediate, and increased risk using the risk factors identified in the NICE guideline had rates of complicated birth of 26.7% (26.5% to 27.0%), 24.1% (23.7% to 24.5%), and 38.0% (37.7% to 38.2%), respectively (table 4).
Components of the composite outcome
Table 5 presents the components of the composite outcome according to risk group. The most common components of the composite outcome were instrumental birth and emergency caesarean section, especially in nulliparous women and in multiparous women with a previous caesarean section. For example, the increase in the rate of complicated birth in nulliparous women from 43.4% in the low risk group to 57.5% in the increased risk group is mainly driven by emergency caesarean section. Also, more than 90% of the complicated births in multiparous women with a previous caesarean resulted from instrumental birth or emergency caesarean section (table 5). For multiparous women with no previous caesarean section, the risks were low for all components of the composite outcome.
Table 5.
Components of composite outcome by risk group, parity and obstetric history in 276 766 women who gave birth at term after a trial of labour (elective caesarean births excluded*)
| Components of composite outcome | Low risk | Intermediate risk | Increased risk | |||||
|---|---|---|---|---|---|---|---|---|
| No with outcome | % (95% CI) | No with outcome | % (95% CI) | No with outcome | % (95% CI) | |||
| Nulliparous with trial of labour | 59 413 | 15 616 | 43 902 | |||||
| Complicated birth: | 25 805 | 43.4 (43.0 to 43.8) | 7153 | 45.8 (45.0 to 46.6) | 25 230 | 57.5 (57.0 to 57.9) | ||
| Instrumental birth | 15 377 | 25.9 (25.5 to 26.3) | 3830 | 24.5 (23.8 to 25.2) | 11 935 | 27.2 (26.8 to 27.6) | ||
| Emergency caesarean birth | 7445 | 12.5 (12.2 to 12.9) | 2657 | 17.0 (16.4 to 17.6) | 11 274 | 25.7 (25.3 to 26.1) | ||
| Obstetric anal sphincter injury | 3565 | 6.0 (5.8 to 6.2) | 755 | 4.8 (4.5 to 5.2) | 2266 | 5.2 (5.0 to 5.4) | ||
| Postpartum haemorrhage | 1561 | 2.6 (2.5 to 2.8) | 468 | 3.0 (2.7 to 3.3) | 1903 | 4.3 (4.1 to 4.5) | ||
| Apgar score ≤7 at 5 minutes | 696 | 1.2 (1.0 to 1.3) | 194 | 1.2 (1.1 to 1.4) | 820 | 1.9 (1.7 to 2.0) | ||
| Multiple components (≥2) | 2839 | 4.8 (4.6 to 5.0) | 751 | 4.8 (4.5 to 5.2) | 2968 | 11.7 (11.4 to 12.2) | ||
| Multiparous no previous caesarean with trial of labour | 55 426 | 25 385 | 57 330 | |||||
| Complicated birth: | 4879 | 8.8 (8.6 to 9.0) | 2712 | 10.7 (10.3 to 11.1) | 9767 | 17.0 (16.9 to 17.2) | ||
| Instrumental birth | 2140 | 3.9 (3.7 to 4.0) | 1098 | 4.3 (4.1 to 4.6) | 3317 | 5.8 (5.6 to 6.0) | ||
| Emergency caesarean birth | 976 | 1.8 (1.6 to 1.9) | 749 | 3.0 (2.7 to 3.2) | 3589 | 6.3 (6.1 to 6.5) | ||
| Obstetric anal sphincter injury | 1125 | 2.0 (1.9 to 2.1) | 485 | 1.9 (1.7 to 2.1) | 1541 | 2.7 (2.6 to 2.8) | ||
| Postpartum haemorrhage | 689 | 1.2 (1.1 to 1.3) | 414 | 1.6 (1.5 to 1.7) | 1515 | 2.6 (2.5 to 2.8) | ||
| Apgar score ≤7 at 5 minutes | 318 | 0.6 (0.5 to 0.6) | 185 | 0.7 (0.6 to 0.8) | 645 | 1.1 (1.0 to 1.2) | ||
| Multiple components (≥2) | 369 | 0.7 (0.6 to 0.7) | 219 | 0.9 (0.8 to 1.0) | 840 | 1.5 (1.4 to 1.6) | ||
| Multiparous with previous caesarean with trial of labour | 19 694 | |||||||
| Complicated birth: | – | – | – | – | 10 908 | 55.4 (55.0 to 55.7) | ||
| Instrumental birth | – | – | – | – | 3416 | 17.3 (16.8 to 17.9) | ||
| Emergency caesarean birth | – | – | – | – | 6748 | 34.3 (33.6 to 34.9) | ||
| Obstetric anal sphincter injury | – | – | – | – | 747 | 3.8 (3.5 to 4.1) | ||
| Postpartum haemorrhage | – | – | – | – | 1322 | 6.7 (6.4 to 7.1) | ||
| Apgar score ≤7 at 5 minutes | – | – | – | – | 575 | 2.9 (2.7 to 3.2) | ||
| Multiple components (≥2) | – | – | – | – | 1900 | 9.6 (9.2 to 10.1) | ||
Sensitivity analyses
A sensitivity analysis was conducted to examine the impact of not having information about the severity of asthma and hypothyroidism. In this sensitivity analysis, 9672 women were classified as having unspecified asthma or hypothyroidism in the increased risk group rather than in the low risk group, as was done for the main analysis. This had little impact on the rate of a complicated birth according to the risk classification. The biggest changes were seen in nulliparous women with increased risk whose risk of a complicated birth decreased from 57.5% to 55.3% and in multiparous women at increased risk with no previous caesarean section whose risk decreased from 17.0% to 16.0%.
A further sensitivity analysis examined the impact of not having information about the timing of stillbirth in the dataset. In this sensitivity analysis, all 1271 stillbirths were considered to have occurred intrapartum rather than antepartum, and these stillbirths were therefore included in the analysis as a complicated birth. The rate of stillbirth was low among all women with a singleton pregnancy (0.36%). The stillbirth rate was highest in nulliparous women and thus the largest difference in results was seen in nulliparous women, with an associated increase in the rate of complicated birth from 43.4% to 43.6% in women at low risk, from 45.8% to 45.9% in those at intermediate risk, and from 57.5% to 57.7% in those at increased risk.
A final sensitivity analysis evaluated whether the dominance of instrumental delivery in the composite outcome influenced the pattern of associations for nulliparous women. If instrumental delivery was not included in the composite outcome, a similar pattern of results was, however, observed (supplementary table S4).
Discussion
In this study we found that parity and a history of caesarean section are considerably stronger determinants for risk of a complicated birth in women with a trial of labour at term than the other factors of the risk classification derived from the clinical guideline for intrapartum care developed by NICE in the UK. Low risk nulliparous women have substantially higher rates of complicated birth than increased risk multiparous women without a previous caesarean section even if the latter have multiple risk factors.
Methodological considerations
We used routinely collected data to determine the risk of complications during childbirth at the onset of term labour in the English NHS. About 60% of births in England could be included. Most exclusions were related to poor data completeness at hospital level rather than missing data at individual level. Comparison of included and excluded NHS hospital trusts showed similar maternal and hospital characteristics for number of deliveries each year and region. A comparison among women with a singleton term birth showed that women in the included hospital trusts were younger, more often of white ethnicity, and less often living in socioeconomically deprived areas (supplementary table S5). However, as these differences were small they will have had minimal impact on our findings.
We were not able to conduct any direct validation of the clinical information recorded in the MIS or HES data. It is possible that owing to under-recording of diagnostic and procedure data in routinely collected datasets, some women in the low risk group were misclassified. As a result, we might have overestimated the risk of a complicated birth in the low risk group, but it is unlikely that this will have had a major impact on our results given that the observed risk of complications in multiparous women classified as low risk is low (8.8%). Furthermore, the routinely collected maternity data included little information about severity of specific medical conditions. However, a sensitivity analysis assessing the impact of moving women with asthma and hypothyroidism from the low risk group to the increased risk group had little effect on our results.
A further limitation is that our data reflect real world practice and as a result the risk factors included in the NICE guideline will have influenced the women’s chosen place of birth. This will in turn have affected the rate of complicated birth that we report, given that the use of instrumental delivery or emergency caesarean section is higher in obstetric led care.13 This limitation cannot, however, explain the substantially higher rates of complicated birth we found in nulliparous women compared with multiparous women without a previous caesarean section. Nulliparous women were more often classified as low risk according to the risk factors derived from the NICE guideline (50.0% of those who had a trial of labour at term) than multiparous women without a previous caesarean section (40.1%).
Lastly, we did not have information about the timing of stillbirth and assumed for the main analyses that all stillbirths had occurred before the onset of labour. A sensitivity analysis that included stillbirths as if all had occurred after the onset of labour did not change our results appreciably. Importantly, the stillbirth rate varied little according to the risk classification and it was highest in nulliparous women. The results of this sensitivity analysis are in line with the main analysis, adding to the evidence that parity and a history of caesarean birth are stronger determinants of the rates of complicated birth than other risk factors identified in the NICE guideline.
Comparison with other studies
Other studies have classified women as low risk using a similar risk classification as recommended by NICE.1 2 3 5 6 7 8 9 10 11 12 22 A study of about 10 million births in the United States found that 38% of women were low risk according to the absence of risk factors at the time of birth and, similar to our results, 29% of these women had a complication requiring an obstetric or neonatal intervention.8 A Dutch study, using a similar risk classification, estimated the proportion of women at low risk of complications as 42.5%.10 This Dutch study did not, however, have access to information about BMI, which is a major reason why women in our study were classified in the intermediate risk or increased risk groups.
The Birthplace in England study, which included 45 000 births between April 2008 and April 2010, was designed to look at place of birth for women at low risk of complications, defined in a similar way as the risk classification recommended by NICE.23 The primary outcome in this study was a composite of perinatal mortality and adverse neonatal outcomes. The neonatal component of our outcome, a low Apgar score (≤7) at five minutes, was chosen as it represents a baby requiring additional involvement from the neonatal team. It is also a proxy for adverse neonatal outcomes. Evidence suggests that a low Apgar score at five minutes is associated with perinatal mortality,24 poorer cognitive development,25 26 and cerebral palsy.27
In line with our findings, the Birthplace in England study found that the chances of a baby having serious medical problems were higher for low risk nulliparous women (about 5 per 1000 births) than for low risk multiparous women (about 3 per 1000 births), irrespective of planned place of birth. For nulliparous women planning a home birth, this risk was even higher (about 10 per 1000 births). The study also found that about one in three nulliparous women who had planned to give birth in a midwife led unit were transferred before delivery to an obstetric unit compared with fewer than one in 10 multiparous women.23 These results support our findings that the risk of requiring the attention of an obstetric or neonatal team is much higher in nulliparous women than in multiparous women and highlight the importance of considering parity when planning place of birth.
Substantial literature evaluates the importance of previous mode of birth for multiparous women.28 29 30 In agreement with these studies, we found that the risk of a complicated birth for multiparous women with a previous caesarean section is similar to the risk observed in nulliparous women.29 The risk in women with a previous caesarean section who also had a vaginal delivery either before or after the caesarean section is, however, much lower, and offers opportunities for further refinement of a risk group based on parity and obstetric history.19
Implications
Our results indicate that it is appropriate to consider modifying the risk classification used to give advice to women who are planning where to give birth. For multiparous women without a previous caesarean section, the chance of a complicated birth is low, irrespective of whether or not they have additional risk factors, and planning to give birth in a midwife led setting is appropriate, even for women with additional risk factors. However, nulliparous women, including those without additional risk factors, have considerably higher risks of a complicated birth, and they could consider giving birth in a setting that enables rapid access to care by an obstetric or neonatal team, including midwife led units. These considerations are especially relevant for those women considering a home birth. There are no obvious reasons why these findings in the English NHS are not applicable to other countries with similar models of care.
Giving more weight to parity when assessing the risk in women giving birth at term might lead to substantial shifts in where women choose to give birth, which in turn could have substantial implications for workforce and capacity planning. For example, 45% of women who labour at term are multiparous without a history of caesarean section. In contrast, the risk classification system recommended by NICE identifies only 36% of women who labour at term as low risk and therefore as candidates for a low risk birth setting. These shifts would not only expand the proportion of women who are advised to consider giving birth in midwife led settings but would also have an impact on the proportion of women actually giving birth in these settings, given that the transfer rate during labour to obstetric led care is far lower for multiparous women than for nulliparous women.23
Conclusion
Parity and obstetric history are key determinants of the risk of a complicated birth in women who labour at term. Grouping women first according to parity and previous mode of birth, and then within these groups according to the presence of specific risk factors would allow better planning of place of birth and targeting of interventions, with greater and more informed choice for many women and fewer undesired transfers to obstetric led care after the onset of labour.
What is already known on this topic
In many countries, women at low risk of complications at birth are advised that it is safe to give birth at home or in a midwife led unit
A National Institute for Health and Care Excellence guideline on intrapartum care used a consensus approach to identify women with specific risk factors for whom care in an obstetric unit is expected to reduce risk to mother or baby
The NICE guideline does not distinguish between women in their first pregnancy and those who have previously given birth
What this study adds
Parity and history of a caesarean section are considerably stronger determinants of the risk of a complicated birth than other risk factors identified by NICE
Giving more weight to parity and obstetric history would provide greater choice for many women, expand the proportion of women advised to consider giving birth in a midwife led unit, and reduce transfers to obstetric led care after the onset of labour
Acknowledgments
This study was based on data collected by the National Maternity Perinatal Audit linked to Hospital Episode Statistics made available by NHS Digital (https://digital.nhs.uk/data-andinformation/data-tools-and-services/data-services/hospital-episode-statistics).
Web extra.
Extra material supplied by authors
Supplementary information: additional tables, S1 to S5
Contributors: JvdM, KW, and DP are joint senior authors and contributed equally to this study and manuscript. They supervised all phases of design and analysis and drafted and revised the paper. JJ conceived the idea, wrote the analysis plan, cleaned and analysed the data, drafted and revised the paper, and is guarantor. AB and HK reviewed the concept and analysis plan and revised the paper. IG-U reviewed the analysis plan and revised the paper. TH and JH reviewed the concept and revised the paper. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The National Maternity and Perinatal Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP; www.hqip.org.uk) as part of the National Clinical Audit and Patient Outcomes Programme and funded by NHS England and the Scottish and Welsh governments. Neither HQIP nor the funders had any involvement in designing the study; collecting, analysing, and interpreting the data; writing the report; or the decision to submit the article for publication. DP is also funded by Tommy’s Charity.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all individuals are or have been partially or wholly funded by the Healthcare Quality Improvement Partnership for their contribution to the submitted work. DP is also funded by Tommy’s Charity. All authors also declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. Dr Hawdon reports personal fees from expert medicolegal reporting, for defendant and claimant, on perinatal injury cases. The authors report no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study used data collected to evaluate service provision and performance and therefore was exempt from ethical review by the NHS Health Research Authority. The use of personal data without patients’ consent was approved by the NHS Health Research Authority (16/CAG/0058). This study has also been considered and approved by the London School of Hygiene and Tropical Medicine research ethics committee (reference 14544).
Data sharing: The data are available for further research and service evaluation after approval from the data controllers, which are the Healthcare Quality Improvement Partnership for the data derived from the maternity information systems and NHS Digital for Hospital Episode Statistics.
The lead author (JJ) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We disseminate results through patient organisations and representative groups of women giving birth in the UK.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Scarf VL, Rossiter C, Vedam S, et al. Maternal and perinatal outcomes by planned place of birth among women with low-risk pregnancies in high-income countries: A systematic review and meta-analysis. Midwifery 2018;62:240-55. 10.1016/j.midw.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 2. Halfdansdottir B, Hildingsson I, Smarason AK, Sveinsdottir H, Olafsdottir OA. Contraindications in planned home birth in Iceland: A retrospective cohort study. Sex Reprod Healthc 2018;15:10-7. 10.1016/j.srhc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO recommendations: intrapartum care for a positive childbirth experience. 2018. www.who.int/reproductivehealth/publications/intrapartum-care-guidelines/en/ [PubMed]
- 4.National Institute for Health and Care Excellence. Clinical Guideline 190: Intrapartum care for healthy women and babies 2014. nice.org.uk/guidance/cg190. [PubMed]
- 5. Hollowell J, Li Y, Bunch K, Brocklehurst P. A comparison of intrapartum interventions and adverse outcomes by parity in planned freestanding midwifery unit and alongside midwifery unit births: secondary analysis of ‘low risk’ births in the birthplace in England cohort. BMC Pregnancy Childbirth 2017;17:95. 10.1186/s12884-017-1271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Jonge A, Peters L, Geerts CC, et al. Mode of birth and medical interventions among women at low risk of complications: A cross-national comparison of birth settings in England and the Netherlands. PLoS One 2017;12:e0180846. 10.1371/journal.pone.0180846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavallaro FL, Cresswell JA, Ronsmans C. Obstetricians’ Opinions of the Optimal Caesarean Rate: A Global Survey. PLoS One 2016;11:e0152779. 10.1371/journal.pone.0152779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danilack VA, Nunes AP, Phipps MG. Unexpected complications of low-risk pregnancies in the United States. Am J Obstet Gynecol 2015;212:809. 10.1016/j.ajog.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lukasse M, Rowe R, Townend J, Knight M, Hollowell J. Immersion in water for pain relief and the risk of intrapartum transfer among low risk nulliparous women: secondary analysis of the Birthplace national prospective cohort study. BMC Pregnancy Childbirth 2014;14:60. 10.1186/1471-2393-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blix E, Huitfeldt AS, Øian P, Straume B, Kumle M. Outcomes of planned home births and planned hospital births in low-risk women in Norway between 1990 and 2007: a retrospective cohort study. Sex Reprod Healthc 2012;3:147-53. 10.1016/j.srhc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 11. de Jonge A, van der Goes BY, Ravelli AC, et al. Perinatal mortality and morbidity in a nationwide cohort of 529,688 low-risk planned home and hospital births. BJOG 2009;116:1177-84. 10.1111/j.1471-0528.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- 12. Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low‐risk pregnancy. Cochrane Datab Syst Rev, 2015, 10.1002/14651858.CD000934.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NMPA Project Team. National Maternity and Perinatal Audit: Organisational report 2017. Royal College of Obstetricians and Gynaecologists 2017. https://maternityaudit.org.uk/FilesUploaded/NMPA%20Organisational%20Report%202017.pdf
- 14.NMPA Project Team. National Maternity and Perinatal Audit Clinical report 2017: revised version. Royal College of Obstetricians and Gynaecologists 2018. https://maternityaudit.org.uk/FilesUploaded/NMPA%20Clinical%20Report%202018.pdf
- 15.Comptroller and Auditor General. Maternity services in England 2013. www.nao.org.uk/wp-content/uploads/2013/11/10259-001-Maternity-Services-Book-1.pdf
- 16.International Statistical Classification of Diseases and Related Health Problems 10th Revision. https://icd.who.int/browse10/2016/en
- 17. OPCS Classification of Interventions and Procedures (OPCS) www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp
- 18. Cromwell DA, Knight HE, Gurol-Urganci I. Parity derived for pregnant women using historical administrative hospital data: accuracy varied among patient groups. J Clin Epidemiol 2014;67:578-85. 10.1016/j.jclinepi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 19. Knight HE, Gurol-Urganci I, van der Meulen JH, et al. Vaginal birth after caesarean section: a cohort study investigating factors associated with its uptake and success. BJOG 2014;121:183-92. 10.1111/1471-0528.12508. [DOI] [PubMed] [Google Scholar]
- 20. Andersson CB, Flems C, Kesmodel US. The Danish National Quality Database for Births. Clin Epidemiol 2016;8:595-9. 10.2147/CLEP.S99492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draper E, Gallimore I, Smith L, et al. MBRRACE-UK Perinatal Mortality Surveillance Report for Births in 2017 - FINAL Revised.pdf. 2019.
- 22. Sandall J, Murrells T, Dodwell M, et al. The efficient use of the maternity workforce and the implications for safety and quality in maternity care: a population-based, cross-sectional study. Health Services and Delivery Research 2014;2:1-266. 10.3310/hsdr02380. [DOI] [PubMed] [Google Scholar]
- 23. Brocklehurst P, Hardy P, Hollowell J, et al. Birthplace in England Collaborative Group Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ 2011;343:d7400. 10.1136/bmj.d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iliodromiti S, Mackay DF, Smith GCS, Pell JP, Nelson SM. Apgar score and the risk of cause-specific infant mortality: a population-based cohort study. Lancet 2014;384:1749-55. 10.1016/S0140-6736(14)61135-1. [DOI] [PubMed] [Google Scholar]
- 25. Odd DE, Rasmussen F, Gunnell D, Lewis G, Whitelaw A. A cohort study of low Apgar scores and cognitive outcomes. Arch Dis Child Fetal Neonatal Ed 2008;93:F115-20. 10.1136/adc.2007.123745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stuart A, Otterblad Olausson P, Källen K. Apgar scores at 5 minutes after birth in relation to school performance at 16 years of age. Obstet Gynecol 2011;118:201-8. 10.1097/AOG.0b013e31822200eb. [DOI] [PubMed] [Google Scholar]
- 27. Persson M, Razaz N, Tedroff K, Joseph KS, Cnattingius S. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: population based cohort study in Sweden. BMJ 2018;360:k207. 10.1136/bmj.k207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA 2002;287:2684-90. 10.1001/jama.287.20.2684. [DOI] [PubMed] [Google Scholar]
- 29. Wu Y, Kataria Y, Wang Z, Ming WK, Ellervik C. Factors associated with successful vaginal birth after a cesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2019;19:360. 10.1186/s12884-019-2517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grobman WA, Lai Y, Landon MB, et al. National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol 2007;109:806-12. 10.1097/01.AOG.0000259312.36053.02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables, S1 to S5