Abstract
Background
Liver transplantation (LT) is a game changer in cirrhosis. Poor muscle mass defined as sarcopenia may potentially upset the LT scoreboard.
Aim
To assess the prevalence and impact of sarcopenia on the intraoperative and early postoperative outcomes in Indian patients undergoing LT.
Methods
Pre LT, single-slice routine computed tomography images at L3 vertebra of 115 LT recipients were analyzed, to obtain cross-sectional area of six skeletal muscles normalized for height in m2 – skeletal muscle index (SMI; cm2/m2). SMI< 52.4 in males and <38.5 in females was called sarcopenia. The intraoperative, postoperative outcome parameters and 90-day mortality were compared between sarcopenics and nonsarcopenics.
Results
Sarcopenia was found in 47.8% of patients [M (90.4%); age, 46.3 ± 10; BMI, 24.5 ± 4.3 kg/m2; child A:B:C = 1%:22%:77%; MELD, 20.6 ± 6.3; etiology alcohol: nonalchohol = 53%:47%; Charlson Comorbidity Index (CCI) > 3:≤3 = 56.5%:43.5%]. Sarcopenics vs. Nonsarcopenics; early postoperative complications: [sepsis, 49(89%) vs. 33(55%), P = 0.001; neurologic complications, 16(29.6%) vs. 5(8.8%), P = 0.040; Clavien-Dindo Classification ≥3–24 (43.6%):15 (25.4%),P = 0.041; ancillary parameters (days), duration of ventilation [median (range)] 1.5(1–3) vs. 1 (1–2), P = 0.021; intensive care unit (ICU) stay 12 (8–16) vs. 10 (8–12), P = 0.024; time to ambulation 9 (7–11) vs. 6 (5–7), P = 0.001; drain removal 18.7 ± 7.3 vs. 14.4 ± 6.2, P = 0.001; need for tracheostomy 5 (9%) vs. 0 (%), P = 0.017; preoperative prevalence of acute kidney injury, comorbidities and requirement for dialysis, intraoperative blood loss & inotropic support were significantly higher in sarcopenics. Ninety-day mortality was comparable between sarcopenics 5 (9.09%) and nonsarcopenics 4 (6.6%) P = 0.63. SMI (OR: 0.83; 95% CI: 0.71–0.97, P = 0.016; Acute on chronic liver failure (ACLF) presentation 12.5 (1.65–95.2), P = 0.015 and intraoperative blood loss 3.74 (0.96–14.6), P = 0.046 were predictors of 90-day mortality.
Conclusion
Almost 50% of LT recipients had sarcopenia, who had a higher incidence of postoperative sepsis, neurological complications, longer ICU stay and ventilatory support. Low SMI, ACLF presentation, and intraoperative blood loss were the independent predictors of early mortality.
Keywords: nutritional status, asian indian, chronic liver disease, skeletal muscle area, liver transplant outcome
Abbreviations: ACLF, Acute on chronic liver failure; AKI, Acute kidney injury; BCM, Body cell mass; BT, Blood transfusion; CCI, Charlson Comorbidity Index; CDC, Clavien-Dindo classification; CNS, Central nervous system; COPD, Chronic obstructive pulmonary disease; CT, Computed tomography; ECOG, Eastern Cooperative Oncology Group; EN, Enteral nutrition; ERAS, Enhanced recovery after surgery; ESLD, End-stage liver disease; EWGSOP, European Working Group on Sarcopenia in Older People; GRBWR, Graft recipient body weight ratio; HAT, Hepatic artery thrombosis; HE, Hepatic encephalopathy; HU, Hounsfield Unit; ICU, Intensive care unit; LDLT, Living donor liver transplant; LT, Liver transplantation; MELD, Model for end-stage liver disease; MHV, Middle hepatic vein; NIV, Noninvasive ventilation; ORS, Oral rehydration solution; PMI, Psoas muscle index; PVT, Portal vein thrombosis; SD, Standard deviation; SMA, Skeletal muscle area; SMI, Skeletal muscle index
Liver transplantation (LT) has become standard of care for patients with decompensated end-stage liver disease (ESLD). The success of LT depends on the profound impact of the primary pathology of chronic liver disease and the acute stress of this complex surgical procedure. Besides the hemodynamic and metabolic perturbances, severe loss of the muscle mass in a cirrhotic has been found to be one of the major factors associated with increased morbidity and mortality after LT.1 A progressive loss of muscle mass and function has been explicitly defined as “sarcopenia”. Studies have shown that patients with poor nutritional status as defined by a low body cell mass (BCM) have higher mortality rates after LT than those with a relatively preserved BCM.2 Sarcopenia even seems to capture the risk profile in patients with cirrhosis independent of Child-Pugh score and model for end-stage liver disease (MELD) score3 and thus may serve as an independent prognostic marker. Sarcopenia in patients with cirrhosis listed for LT has been assessed using various methods.4, 5, 6 Single-slice computed tomography (CT) image analysis has emerged as an imperative tool, to objectively assess muscle mass and aptly define sarcopenia in patients with ESLD. The aim of the present study was to assess the prevalence of sarcopenia in Indian patients undergoing LT and also to evaluate the impact of sarcopenia on the short-term post-LT outcome.
Patients and Methods
Prospective observational study including all adult patients who underwent living donor liver transplant (LDLT) between December 2013 and October 2015 at the Institute of Liver and Biliary Sciences (ILBSs) were enrolled. Suitability for LT was evaluated as per the standard protocol of the institute. Patients with acute liver failure, pediatric patients, and those without an abdominal CT scan were excluded from the study. Enrolled patients underwent assessment of sarcopenia before LT and were followed up to 90 days after LT. Before LT, all the patients were managed using standard protocols of the institute as described in the following sections. Approval for this study was taken from the Institutional Ethics Committee/Institutional Review Board (IEC/IRB No.32/7) of ILBS. Informed consent was taken from all the patients at the time of enrollment in the study.
Assessment of Sarcopenia
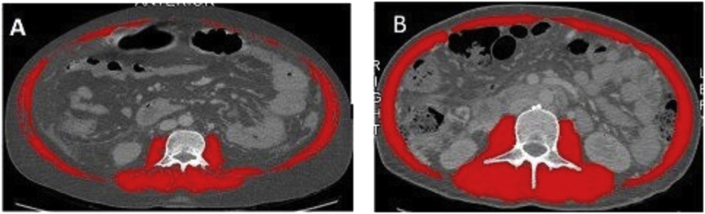
All abdominal CTs were performed on Discovery 750 HD-64 row spectral CT scanner (GE, USA). Only CT scans performed within a period of three months before LT were used. Single-slice CT images at third lumbar vertebra were analyzed using the image analysis software Slice-O-Matic, version 4.3 (Tomovision, Montreal, Canada). The muscles and fat were identified at a predefined CT density of −29 to +150 Hounsfield Unit (HU)7 and −190 to −30 HU8, respectively. The sum of the area of six abdominal muscles (erector spinae, quadrates lumborum, psoas, transversus abdominis, interior/exterior oblique, and rectus abdominis) was defined as skeletal muscle area (SMA, in cm2). SMA was normalized for height in m2 and expressed as skeletal muscle index (SMI) in cm2/m2. Sarcopenia was identified with a SMI <52.4 cm2/m2 for males and <38.5 cm2/m2 for females.9 (Figure 1).
Figure 1.
Muscle quantification by CT scan images at level of L3 vertebra. Muscle area at 3rd lumbar vertebra is highlighted in red. Patient with sarcopenia (A) (SMI 35 cm2/m2) and without sarcopenia (B) (SMI 53 cm2/m2), both patients had alcoholic liver disease, age 50 years, and BMI 26 kg/m2.
SMI, Skeletal Muscle Index; CT, Computed Tomography; BMI, Body Mass Index.
Post-LT Medical Management
Extubation
Conscious, oriented patients with stable hemodynamics (off inotropic support) and good respiratory effort requiring minimal pressure support were extubated. Postextubation intermittent noninvasive ventilation (NIV) was given to all the patients till maintenance of normal oxygen saturation and improvement in tachypnea and pleural effusion.
Ambulation
As per the clinical condition, early mobilization was performed, initially supine-to-sit then sit on chair with support, later patients were ambulated in the intensive care unit (ICU) itself, initially with support, and then independently.
Medication
All patients received immunosuppression as per the standard protocol (induction with steroids and addition of tacrolimus on postoperative day 1 or 2 based on clinical condition and laboratory parameters). Abdominal drain output replacement was carried out with intravenous albumin infusion in all patients. At a stable clinical condition i.e. without a need of intensive monitoring or organ support, patients were shifted out of ICU.
Medical Nutrition Therapy
Oral rehydration solution was given by an intragastric feeding tube to all the patients immediately after surgery and enteral nutrition (EN) was initiated early within 12 h after LT in all hemodynamically stable patients. Standard EN formulas were used to meet the requirements of 30–35 Kcal and 1.5–2.0 gm protein/Kg body weight/day,10 using continuous feed administration over 24 h. Multivitamins including the fat-soluble vitamins (A, D, E, and K) along with zinc and selenium were supplemented. Full fluid diets were introduced upon extubation, taken over by soft diet later within 24 h, however EN continued until oral intake was more than 60% of the requirement. Once feeding tube was removed, patients were given oral nutrition supplements along with a normal diet.
Discharge and Follow-up
Haemodynamically stable patients on immunosuppression were discharged from the hospital with initial follow-up on weekly basis in the first month and later on monthly follow-up till one year.
Definition of Outcome of LT
Preoperative Charlson Comorbidity Index
Modified Charlson Comorbidity Index (CCI) for patients undergoing LT was used to assess the medical comorbidity. A total of 9 comorbidities comprising the CCI were analyzed. The CCI was calculated by assigning a weight of 2 to diabetes, stroke, renal insufficiency, and malignancy, and a weight of 1 to the other comorbidities, as previously described.11
Intraoperative parameters
Duration of surgery, total blood loss, inotropic requirement at time of completion of surgery, and any aberration from usual surgical course were noted.
Postoperative parameters
Time to extubation, duration of NIV support, time of initiation of EN, time of initiation of mobilization, length of ICU stay, incidence of septic complications, length of hospital stay, morbidity, and 90-day mortality were assessed.
Assessment of postsurgical complications
Major surgical complications (morbidity) were defined as those corresponding to grade 3 (patient condition requiring surgical, endoscopic, or radiological intervention) and higher in accordance with the Clavien-Dindo classification (CDC).12
Statistical analysis
Data are presented as number (%), mean ± SD, or median (range) as appropriate. All the baseline, intraoperative, and postoperative parameters were compared between patients with and without sarcopenia. Student’s t test or Mann-Whitney test were used for comparing the continuous variables, and categorical variables were compared using chi square test and Fischer exact test. P value < 0.05 was considered statistically significant. Predictors of 90-day mortality were assessed using univariate and multivariate logistic regression analysis. Predictors of 28-day mortality were assessed using univariate and multivariate Cox regression analysis. All those parameters with P value of <0.2 in univariate analysis were considered for multivariate analysis. SPSS, version 20.0 (IBM, India) software was used for statistical analysis.
Results
From December 2013 to October 2015 living donor liver transplant (LDLT) was performed in 151 patients. Of these, pediatric transplants (n = 10), deceased donor LTs (n = 13), acute liver failure (n = 10), and patients without CT scans (n = 3) were excluded, and a total of 115 patients were enrolled in the study. The mean age of the patients was 45.75 ± 10.6 years, majority of them being males (90.4%), with Child status C (77%), having ethanol as the primary etiology (53%). Of 115 patients who had undergone LDLT, sarcopenia was seen in 55 (47.8%) of patients called sarcopenics and rest 60 (52.2%) were nonsarcopenics. The mean SMA and SMI of sarcopenics (122.5 ± 15.4 cm2; 43.9 ±4.2 cm2/m2) was significantly lower than that of nonsarcopenics (158.6 ± 26.2 cm2; 59.3 ± 8.5 cm2/m2), respectively.
Comparison of the Baseline Characteristics Between Sarcopenics and Nonsarcopenics
The baseline clinical characteristics compared between sarcopenics and nonsarcopenics are shown in Table 1. Age, gender, Child Pugh score, MELD score, and primary etiology, were comparable between patients with and without sarcopenia. There was no difference in the clinical presentation at the time of LT between both these groups. There was a trend toward higher prevalence of ascites among sarcopenics; however, both groups were comparable in terms of number of LVP sessions, history of spontaneous bacterial peritonitis, hepatic encephalopathy (HE), and hepatopulmonary syndrome. Nevertheless, the prevalence of acute kidney injury (AKI) and requirement for dialysis before LT was significantly higher in patients with sarcopenia as compared with those without sarcopenia. A higher percentage of patients had a CCI score >3 among sarcopenics as compared with nonsarcopenics.
Table 1.
Comparison of the Baseline Clinical Characteristics Between Patients With and Without Sarcopenia.
| Parameter | Overall (n = 115) | Sarcopenics (n = 55) | Nonsarcopenics (n = 60) | P value |
|---|---|---|---|---|
| Age (yrs) | 46.3 ± 10.2 | 45.2 ± 10.9 | 47.2 ± 9.3 | 0.51 |
| Gender (M:F) | 104 (90.4):11(9.6) | 51 (92.7): 4 (7.3) | 53(88.3): 7 (11.7) | 0.53 |
| BMI(Kg/m2) | 24.5 ± 4.3 | 23.1 ± 3.1 | 25.8 ± 4.9 | 0.14 |
| Disease severity scores | ||||
| Child-Pugh score | 10.6 ± 1.7 | 11.0 ± 1.6 | 10.2 ± 1.8 | 0.67 |
| Child A | 1 (0.87) | 0 (0.0) | 1(1.7) | 0.08 |
| Child B | 26 (22.6) | 8 (14.5) | 18(30.0) | |
| Child C | 88 (76.5) | 47 (85.5) | 41(68.3) | |
| MELD score | 20.6 ± 6.3 | 21.9 ± 7.0 | 19.5 ± 5.4 | 0.63 |
| MELD-Na score | 23.0 ± 6.6 | 24.8 ± 6.8 | 21.4 ± 5.9 | 0.39 |
| Primary Etiology | ||||
| Alcohol | 61 (53) | 26 (47.3) | 35 (58.4) | 0.66 |
| NASH | 34 (29.6) | 21 (38.2) | 13 (21.6) | |
| Viral | 12 (10.4) | 6 (11) | 6 (10) | |
| Others | 8(6.9) | 2 (3.5) | 6 (10) | |
| Clinical presentation | ||||
| Decompensated CLD | 97 (75.7) | 44 (80) | 53 (88.3) | 0.31 |
| ACLF | 18 (24.3) | 11 (20) | 7 (11.7) | |
| Presence of Ascites | 111 (96.5) | 55 (100) | 56 (93.3) | 0.051 |
| History of LVP | 57 (49.7) | 30 (54.5) | 27 (45) | 0.37 |
| Number of LVP sessions | ||||
| ≤2 sessions | – | 21 (38.1) | 25 (41.7) | 0.19 |
| ≥3 sessions | – | 9 (16.4) | 2 (3.3) | |
| History of complications | ||||
| SBP | 68 (59.1) | 26 (47.3) | 18 (30.0) | 0.12 |
| HE | 67 (58.2) | 35 (63.6) | 32 (53.3) | 0.26 |
| HPS | 45 (39.1) | 22 (40.0) | 23 (38.3) | 0.85 |
| AKI | 64 (55.7) | 37 (67.3) | 27 (45.0) | 0.016* |
| Requirement of dialysis | 9 (7.8) | 8 (14.5) | 1 (1.6) | 0.01* |
| Charlson Comorbidity Index (CCI) | ||||
| CCI score | 3.5 ± 1.4 | 3.8 ± 1.4 | 3.2 ± 1.3 | 0.026* |
| CCI score <3 | – | 26 (47.3) | 39 (65) | |
| CCI score ≥ 3 | – | 29 (52.7) | 21 (35) | 0.047* |
Data expressed as number (percentage) and mean ± SD.
*Significant at P < 0.05.
ACLF, Acute on chronic liver failure; MELD, Model for end-stage disease; NASH, Nonalcoholic steatohepatitis; HBV, Hepatitis B virus; HCV, Hepatitis C virus; CLD, Chronic liver disease; LVP, Large volume paracentesis; SBP, Spontaneous bacterial peritonitis; HE, Hepatic encephalopathy; HPS, Hepatopulmonary syndrome; AKI, Acute kidney injury; CCI, Charlson Comorbidity Index.
Comparison of Intraoperative Parameters Between Sarcopenics and Nonsarcopenics
Right lobe graft without the middle hepatic vein (MHV) was the most common liver graft type which was used in 91 patients. Left lobe graft was used in 21 patients, whereas right posterior sector was used in two and right lobe with partial MHV in one patient. Patients with and without sarcopenia were comparable in terms of total duration of surgery, graft recipient body weight ratio, cold ischemia time, and warm ischemia time. However, the intraoperative blood loss, requirement of blood transfusion (BT), and the requirement of inotropic support at shift out from operation theatre was significantly higher in sarcopenics compared with nonsarcopenics (Table 2).
Table 2.
Comparison of Intraoperative Parameters Between Sarcopenics and Nonsarcopenics.
| Parameter | Sarcopenia (n = 55) | No sarcopenia (n = 60) | P value |
|---|---|---|---|
| Operating time (min) | 935.6 ± 145.5 | 973.2 ± 178 | 0.38 |
| Blood loss (liter) | 3.1 (2–5.1) | 2.2 (1.4–4.4) | 0.036* |
| Blood transfused (units) | 6 (4–10) | 4 (2–10) | 0.044* |
| GRBWR | 1.0 ± 0.23 | 0.96 ± 0.21 | 0.24 |
| Cold ischemia time (min) | 93.0 ± 27.3 | 98.6 ± 44.2 | 0.73 |
| Warm ischemia time (min) | 38.6 ± 10.3 | 38.6 ± 8.4 | 0.77 |
| Inotropic support at shift out | |||
|
11.5 ± 4.9 | 8.6 ± 3.8 | 0.001* |
|
1.6 ± 0.84 | 1.0 ± 0.7 | <0.001* |
Data presented as mean ± standard deviation/median (IQR).
GRBWR, graft recipient body weight ratio.
*Significant at P < 0.05.
Comparison of Early Postoperative Complications Between Sarcopenics and Nonsarcopenics
Nine patients died in the early 90 days of post-transplant period, 5 of them were sarcopenics and 4 nonsarcopenics, the difference being statistically not significant (P = 0.63). Postoperative complications as per Clavien-Dindo classification with a score >3 was significantly higher among sarcopenics compared with nonsarcopenics. The requirement of BT, incidence of hepatic artery thrombosis, and portal vein thrombosis were comparable between the sarcopenics and the nonsarcopenics. However the incidence of post-LT sepsis and neurological complications were significantly higher in patients with sarcopenia (Table 3). Similarly, there was a trend toward significance for a higher requirement of exploratory laparotomy in the sarcopenia group.
Table 3.
Comparison of Early Postoperative Complications Between Sarcopenics and Nonsarcopenics.
| Parameter | Sarcopenics (n = 55) | Nonsarcopenics (n = 60) | P value |
|---|---|---|---|
| 90-day Mortality | 5 (9.09) | 4 (6.66) | 0.63 |
| Bleeding requiring BT | 2 (3.6) | 0 (0.0) | 0.22 |
| HAT | 2 (3.6) | 0 (0.0) | 0.14 |
| PVT | 1 (1.9) | 1 (1.8) | 1.0 |
| Exploratory laparotomy | 7 (12.7) | 2 (3.3) | 0.064 |
|
2 (28.6) | 1 (50.0) | |
|
1 (14.4) | 0 (0.0) | |
|
2 (28.6) | 0 (0.0) | |
|
2 (28.6) | 1(50.0) | |
| Sepsis | 49 (89) | 33 (55) | 0.001* |
|
9 (16.3) | 5 (8.3) | |
|
6 (10.9) | 4 (6.7) | |
|
22 (40) | 16 (26.7) | |
|
12 (21.8) | 8 (13.3) | |
| Neurologic complications | 16 (29.6) | 5 (8.8) | 0.040* |
| Clavien-Dindo score ≥3 | 24 (43.6) | 15 (25.4) | 0.041* |
Data presented as number (percentage).
*Significant at P < 0.05.
BT, Blood transfusion, HAT, Hepatic artery thrombosis; PVT, Portal vein thrombosis.
Comparison of Ancillary Outcome Parameters Between Sarcopenics and Nonsarcopenics
The overall duration of ventilator support, time to resumption of oral diet, time to ambulation with and without support, length of ICU stay, time of drain removal, and Eastern Cooperative Oncology Group performance status scores at discharge was significantly higher in patients with sarcopenia as compared with those without sarcopenia (Table 4). Similarly, the requirement for tracheostomy was also much higher in sarcopenics. In comparison with nonsarcopenics, patients with sarcopenia had a poorer performance status at discharge. A trend toward statistical significance was observed in the total duration of inotropic support in patients with sarcopenia. Nonetheless, the duration of NIV support, readmissions to the ICU, reintubation rates, incidence of rejection episode, total duration of hospital stay, and readmission within 90 days of LT were comparable between sarcopenics and nonsarcopenics.
Table 4.
Comparison of Ancillary Outcome Parameters Between Sarcopenics and Nonsarcopenics.
| Parameter | Sarcopenics (n = 55) | Nonsarcopenics (n = 60) | P value |
|---|---|---|---|
| Duration of inotropic support (hrs) | 19 (13.5–26.5) | 16(10–21) | 0.054 |
| Total ventilator support (days) | 1.5 (1–2.75) | 1 (1–2) | 0.021* |
| Duration of NIV (days) | 9.7 ± 4.9 | 8.1 ± 4.6 | 0.09 |
| Reintubation (n%) | 12 (22.6) | 7 (11.9) | 0.129 |
| Need for tracheostomy (n%) | 5 (9.1) | 0 (0.0) | 0.017* |
| Resumption of complete oral diet (days) | 9 (5–29) | 5 (4–6) | 0.013* |
| Ambulation with support (days) | 3 (3–5) | 3 (2–3) | 0.001* |
| Ambulation without support (days) | 9 (7–11) | 6(5–7) | 0.001* |
| ICU stay (days) | 12(8–16) | 10 (8–12) | 0.024* |
| Readmission to ICU (n%) | 8 (15.7) | 7 (12.1) | 0.58 |
| Drain removal (days) | 18.7 ± 7.3 | 14.4 ± 6.2 | 0.001* |
| Rejection episode (n%) | 9 (17.3) | 11 (19.6) | 0.09 |
| Hospital stay (days) | 23 (19–27) | 24 (20–31) | 0.099 |
| ECOG performance status on discharge | |||
|
7 (14.3) | 25 (44.6) | 0.001* |
|
20 (40.8) | 21 (37.5) | |
|
22 (44.9) | 10 (17.9) | |
| Readmission within 90 days of LT | 24 (43.6) | 20 (33.3) | 0.135 |
| Reason for readmission | |||
|
8 (33.3) | 11(55.0) | |
|
5 (20.8) | 2 (10.0) | |
|
2 (8.3) | 4 (20.0) | |
|
9 (37.5) | 3 (15.0) | |
Data presented as number (percentage), Mean ± SD; median (range).
*Significant at P < 0.05.
ECOG, Eastern Cooperative Oncology Group; LT, Liver transplantation; NIV, Noninvasive ventilation; ICU, Intensive care unit.
Note that sepsis and CDC >3 were the perfect predictors of mortality as all patients who died within 90 days of LT had sepsis and a CDC score >3, suggesting that development of complications and sepsis in the immediate postoperative period is associated with 100% mortality. However, on doing further analysis, baseline SMI, clinical presentation as acute on chronic liver failure (ACLF), and intraoperative blood loss emerged as predictors of 28 day (Table 6) as well as 90-day mortality in LT recipients. Each unit increase in SMI decreases mortality by 17%. Presentation as ACLF for LT increases the risk of mortality by 12.5 fold. Each unit increase in blood loss increases mortality risk by 3.74 fold (Table 5).
Table 6.
Perioperative Predictors of 28-day Mortality in Patients Undergoing LDLT Using Univariate and Multivariate Cox -regression Analysis.
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR(95% CI) | Pvalue | HR(95% CI) | Pvalue | |
| Pre-operative parameters | ||||
| Age | 1.02 (0.96–1.1) | 0.62 | ||
| BMI | 1.02 (0.87–1.2) | 0.84 | ||
| SMI | 0.82 (0.71–0.95) | 0.006 | 0.81 (0.68–0.96 | 0.017* |
| CTP | 1.24 (0.77–2.0) | 0.38 | ||
| MELD | 1.01 (0.91–1.13) | 0.81 | ||
| Etiology (alcohol versus others) | 0.53 (0.13–2.2) | 0.38 | ||
| Clinical presentation (ACLF vs. DLDa) | 3.3 (0.67–16.4) | 0.144 | 5.9 (0.9–39.5) | 0.06* |
| Intraoperative parameters | ||||
| Blood loss (lnb) | 3.3 (1.4–7.97) | 0.007 | 3.84 (1.43–10.4) | 0.008* |
| CIT | 1.0 (0.99–1.02) | 0.44 | ||
| WIT | 0.90 (0.81–1.0) | 0.073 | ||
| Inotropic support | 1.18 (1.03–1.36) | 0.019 | ||
| Postoperative parameters | ||||
| Neurological complication | 1.1 (0.12–9.5) | 0.96 | ||
| Re-exploration | 2.8 (0.31–25.1) | 0.36 | ||
| Total MV days | 1.02 (0.84–1.23) | 0.84 | ||
| Time to ambulate | 0.99 (0.81–1.2) | 0.93 | ||
Data presented as OR (95% CI).
BMI, Body mass index; SMI, Skeletal muscle index; CTP, Child Turcotte Pugh Score; MELD, Model of end-stage liver disease; ACLF, Acute on chronic liver disease; DLD, Decompensated liver disease; CIT, cold ischemia time; WIT, warm ischemia time; MV, Mechanical ventilation; OR, Odds ratio; CI, Confidence interval; LDLT, Living donor liver transplant.
* Significant at P < 0.05.
Reference category.
Log value.
Table 5.
Perioperative Predictors of 90-day Mortality in Patients Undergoing LDLT.
| Parameters | Univariate regression analysis | Multivariate regression analysis | ||
|---|---|---|---|---|
| OR(95% CI) | P value | OR(95% CI) | P value | |
| Preoperative parameters | ||||
| Age | 1.03 (0.96–1.1) | 0.42 | ||
| BMI | 1.03 (0.89–1.2) | 0.66 | ||
| SMI | 0.86 (0.77–0.97) | 0.012 | 0.83 (0.71–0.97) | 0.016* |
| CTP | 1.3 (0.91–2.2) | 0.26 | ||
| MELD | 1.02(0.91–1.1) | 0.77 | ||
| Etiology (alcohol versus others) | 1.5 (0.37–5.7) | 0.59 | ||
| Clinical presentation (ACLF vs. DLDa) | 5.3 (1.26–21.9) | 0.023 | 12.5 (1.65–95.2) | 0.015* |
| Intraoperative parameters | ||||
| Blood loss (lnb) | 2.75 (1.14–6.61) | 0.024 | 3.74 (0.96–14.6) | 0.046* |
| CIT | 1.0 (0.99–1.02) | 0.60 | ||
| WIT | 0.93 (0.84–1.03) | 0.15 | ||
| Inotropic support | 1.17 (1.01–1.36) | 0.033 | ||
| Postoperative parameters | ||||
| Neurological complication | 2.3 (0.39–13.4) | 0.36 | ||
| Re-exploration | 2.45 (0.25–25.6) | 0.44 | ||
| Total MV days | 1.0 (0.81–1.24) | 0.97 | ||
| Time to ambulate | 1.0 (0.86–1.2) | 0.99 | ||
Data presented as OR (95% CI).
BMI, Body mass index; SMI, Skeletal muscle index; CTP, Child Turcotte Pugh Score; MELD, Model of end-stage liver disease; ACLF, Acute on chronic liver disease; DLD, Decompensated liver disease; CIT, Cold ischemia time; WIT, Warm ischemia time; MV, Mechanical ventilation; OR, Odds ratio; CI, Confidence interval; LDLT, Living donor liver transplant.
* Significant at P < 0.05.
Reference category.
Log value.
Discussion
Notwithstanding the advancements made in the surgical and anesthesiological techniques, the outcome of LT remains questionable. Well the answer lies in the decades old clichéd problem of “malnutrition”, which as a more contemporary definition – “sarcopenia” has a sinister effect on the surgical outcome. As per the European Working Group on Sarcopenia in Older People, sarcopenia is characterized by a decrease in the muscle mass, muscle strength, and function.13 In the present study, we found that of 115 patients who underwent LT over the study period of two years, almost half (48%) were identified with sarcopenia. This figure although does not represent the true prevalence as the patients who did not undergo LT were not studied, yet it is quite alarming and needs to be given heed to, particularly in the LDLT setup which exerts major surgical risk to the donors apart from the obvious economic burden. Globally, the problem of sarcopenia ranges from 22% to 70% in patients awaiting LT.14,15 The choice of the method of assessment and the definitions used for defining sarcopenia can potentially influence these figures. Though most working groups recommend considering both muscle mass and muscle function for the diagnosis of sarcopenia, yet almost all studies in cirrhosis have used quantification of muscle mass alone as an indicator of sarcopenia which has been associated with poor prognosis.16,17,18 The method of assessment of muscle mass and the cutoffs used for the definition of sarcopenia can also influence the overall prevalence in any given population. A number of methods for the assessment of muscle mass have been described in the literature. Anthropometry, bioelectrical impedance analysis, and dual energy x-ray absorptiometry have been well established in this respect, but their measurements are influenced by the hydration status of the patients.19 Hence CT has emerged as the gold standard method20 for the objective assessment of the muscle mass in patients with cirrhosis. Muscle area determined from a single-slice abdominal CT scan is highly correlated with total-body skeletal muscle quantified by whole-body multislice analysis.21 Moreover CT scans are a routine part of the patient evaluation, hence it does not levy an extra financial burden on the patients.
In our study, disease severity, etiology, and clinical presentation at time of LT were comparable between sarcopenics and nonsarcopenics. However, the history of complications such as AKI, past requirement for dialysis, and comorbidities as assessed by the CCI were significantly higher in patients with sarcopenia as compared with those without sarcopenia. Besides the known factors such as poor nutritional intake, altered metabolism, hyperammonemia, inflammation, endotoxemia, and physical inactivity which orchestrate sarcopenia in cirrhosis, AKI alone impairs protein synthesis and promotes protein degradation.22 Dialysis also promotes protein degradation and reduces protein synthesis and such an effect even continues after 2 h of dialysis.23 Presence of comorbidities such as coronary disease, diabetes, chronic obstructive pulmonary disease, connective tissue disease, and renal insufficiency in the patients undergoing LT as apparent from a higher CCI score seen in patients with sarcopenia could be a plausible reason for a poor muscle mass other than the basic chronic liver disease.
On comparison of the intraoperative parameters, it was observed that sarcopenics had a significantly higher amount of blood loss, required higher units of BTs, and had greater requirements of vasopressors after LDLT procedure compared with nonsarcopenics. It is known that patients with LDLT are at a higher risk of developing septic shock due to massive blood losses, and use of immunosuppressants. Though the reason for a greater amount of blood loss seen among sarcopenics in the present study is not clear, however a large prospective study had a similar observation; but the reasons for the same remain largely unexplained.24
We observed a low 90-day mortality rate with almost 7.8% deaths at 3 months after LT. The difference in the mortality rates between sarcopenics and nonsarcopenics did not turn out to be significant. Nonetheless, all the patients who died had sepsis, hence sepsis was a perfect predictor of 90-day mortality in our study. Moreover, we also observed a higher incidence of postoperative sepsis among sarcopenics. Infections including sepsis are the most frequent cause of post-LT death, and sarcopenia has been identified as an independent predictor of postoperative sepsis.25 Factors such as intraoperative blood loss, requirement of BTs along with sarcopenia can potentially influence the LT outcomes, mainly development of sepsis and multiple organ failure.26,27.
We also observed that patients with sarcopenia developed more severe complications during the postoperative hospital stay as compared with nonsarcopenics. Similar observations have been made previously also, where sarcopenia as defined by a low psoas muscle index was found to be independently predictive of the postoperative complications.28 A low muscle mass could potentially influence the postoperative course through a reduced wound healing, reduced immune function due to reduced core body temperature, and also due to reduced levels of glutamine released from the muscle which indirectly retard the immune functions.26
The incidence of neurological complications during the early postoperative period was significantly higher in sarcopenics than nonsarcopenics. Central nervous system complications occur frequently after LT and their presence is associated with significantly increased morbidity and mortality. The incidence of these complications are though strongly associated with overt HE before LT. Complications such as ICU-acquired weakness is observed in almost 80% post-LT patients, wherein previous neurological complications and sarcopenia may be the predisposing factors.29 In general, protein calorie malnutrition and deficiency of vitamins and minerals have been implicated as one of the causes for the development of such neurological complications.30
In our observation, sarcopenics took longer time to ambulate both with and without support. Promotion of early ambulation and feeding are major components of the enhanced recovery after surgery (ERAS) guidelines31 in the gastrointestinal surgery and definitely LT is no exception. But sarcopenia in these transplanted patients seems to promote the frailty syndrome32 reflecting not only a poor muscle mass but also weak muscle function making the implementation of ERAS protocol difficult and thus affecting the LT outcome.33 Though a poor muscle mass does not always correlate with a poor muscle function but a poor muscle quality, i.e., infiltration of muscle with fat definitely does compromise the muscle quality which may prevent early ambulation in these patients. Among the ancillary outcome parameters, we found that patients with sarcopenia had longer period of total ICU stay, longer duration of mechanical ventilation, and also greater requirement of tracheostomy. Many studies have previously reported a prolonged hospital and ICU stay in post-LT patients with sarcopenia,34,35,36,37,38, suggesting that a poor muscle mass is associated with longer recovery period after LT, reflecting as a longer duration of total ICU stay, ventilation dependence, even requiring tracheostomy, as these patients require longer inpatient rehabilitation. A longer stay in the ICU and hospital also increases their susceptibility to nosocomial infections suggesting a decreased defiance of stress further affecting the functional reserve and LT outcomes in general.39 Both sepsis and CDC grade>3 were absolute predictors of 90-day mortality in our group of transplanted patients. Nevertheless, of the remaining parameters SMI, clinical presentation as ACLF, and blood loss during surgery have been found to be the independent factors associated with short-term mortality.
Sarcopenia has been associated with an increased risk of complications in patients with cirrhosis but it continues to cast a dark shadow on the life of a cirrhotic even after LT. Studies have shown that cirrhotics continue to lose muscle after LT,40,41 hence predisposing them to insulin resistance and metabolic syndrome particularly development of post-LT diabetes mellitus.42 Early EN therapy right after LT has been found effective in reducing the risk of sepsis in this group of patients.43 Though most of our patients were managed with an aggressive nutritional management post-LT yet the negative effects of a pre-LT poor nutritional status reflecting as frank sarcopenia cannot be handled with early post–LT-EN therapy. Sincere nutritional and physical activity interventions targeted for a pretransplant nutritional optimization is the need of the hour. We would not be wrong in stating that an early referral for LT would also be in favor of better outcome of LT as most of the cases in our study were patients with Child C cirrhosis, who are way beyond any kind of nutritional repair before surgery.
Thus, we conclude that Indian patients undergoing LT have a high prevalence of sarcopenia which negatively impacts the outcome of LT and the solution lies in early risk stratification and aggressive nutritional interventions.
Authors contributions
V. P. contributed to concept and design. V. K., V. P., K. G. S. B., P. K. S., J. B., and V. S. contributed to acquisition of data. G. K., V. K., V. S., and J. B. contributed to statistical analysis and interpretation. V. K., J. B., and V. S. contributed to literature search. V. K. and J. B. contributed to manuscript writing. J. B., V. P., K. G. S. B., and P. K. S. contributed in revising the article critically for important intellectual content. J. B. and V. P. contributed to the final approval of the version submitted.
Conflicts of interest
The authors have none to declare.
References
- 1.Meeks A.C., Madill J. Sarcopenia in liver transplantation: a review. Clin Nutr ESPEN. 2017 Dec;22:76–80. doi: 10.1016/j.clnesp.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Selberg O., Böttcher J., Tusch G., Pichlmayr R., Henkel E., Müller M.J. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997 Mar;25:652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 3.Montano-Loza A.J., Duarte-Rojo A., Meza-Junco J. Inclusion of sarcopenia within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015 Jul 16;6 doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montano-Loza A.J. Muscle wasting: a nutritional criterion to prioritize patients for liver transplantation. Curr Opin Clin Nutr Metab Care. 2014 May;17:219–225. doi: 10.1097/MCO.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 5.Peng S., Plank L.D., McCall J.L. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 6.Rubbieri G., Mossello E., Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:181–184. [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Sottier D., Petit J.M., Guiu S. Quantification of the visceral and subcutaneous fat by computed tomography: interobserver correlation of a single slice technique. Diagn Interv Imaging. 2013;94:879–884. doi: 10.1016/j.diii.2013.04.00. [DOI] [PubMed] [Google Scholar]
- 9.Prado C.M., Lieffers J.R., McCargar L.J. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 10.Plauth M., Cabré E., Riggio O. For DGEM (German society for nutritional medicine), ESPEN (European society for parenteral and enteral nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr. 2006;25:285-294. doi: 10.1016/j.clnu.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Volk M.L., Hernandez J.C., Lok A.S., Marrero J.A. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transplant. 2007 Nov;13:1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older People. European working group on sarcopenia in older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Vugt J.L., Levolger S., de Bruin R.W., van Rosmalen J., Metselaar H.J., IJzermans J.N. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016 Aug;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 15.Bhanji R.A., Takahashi N., Moynagh M.R. The evolution and impact of sarcopenia pre- and post-liver transplantation. Aliment Pharmacol Ther. 2019 Mar;49:807–813. doi: 10.1111/apt.15161. [DOI] [PubMed] [Google Scholar]
- 16.Montano-Loza A.J., Meza-Junco J., Prado C.M. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. doi: 10.1016/j.cgh.2011.08.028. 173. [DOI] [PubMed] [Google Scholar]
- 17.Englesbe M.J., Patel S.P., He K. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi Y., Kaido T., Okumura S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 19.Giusto M., Lattanzi B., Albanese C. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:328–334. doi: 10.1097/MEG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.J., Janssen I., Heymsfield S.B. Relation between whole body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80:1215–1221. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Mitch W.E. Mechanisms causing loss of muscle in acute uremia. Ren Fail. 1996 May;18:389–394. doi: 10.3109/08860229609052808. [DOI] [PubMed] [Google Scholar]
- 23.Ikizler T.A., Pupim L.B., Brouillette J.R. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002 Jan;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 24.Harimoto N., Yoshizumi T., Izumi T. Clinical outcomes of living liver transplantation according to the presence of sarcopenia as defined by skeletal muscle mass, hand grip, and gait speed. Transplant Proc. 2017 Nov;49:2144–2152. doi: 10.1016/j.transproceed.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Masuda T., Shirabe K., Ikegami T. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transplant. 2014 Apr;20:401–407. doi: 10.1002/lt.23811. Epub 2014 Jan 27. [DOI] [PubMed] [Google Scholar]
- 26.JB1 Cywinski, Alster J.M., Miller C., Vogt D.P., Parker B.M. Prediction of intraoperative transfusion requirements during orthotopic liver transplantation and the influence on postoperative patient survival. Anesth Analg. 2014 Feb;118:428–437. doi: 10.1213/ANE.0b013e3182a76f19. [DOI] [PubMed] [Google Scholar]
- 27.Mueller A., Platz K.P., Krause P. Perioperative factors influencing patient outcome after liver transplantation. Transpl Int. 2000;13(1):S158. doi: 10.1007/s001470050311. [DOI] [PubMed] [Google Scholar]
- 28.Izumi T., Watanabe J., Tohyama T., Takada Y. Impact of psoas muscle index on short-term outcome after living donor liver transplantation. Turk J Gastroenterol. 2016 Jul;27:382–388. doi: 10.5152/tjg.2016.16201. [DOI] [PubMed] [Google Scholar]
- 29.Weiss N., Thabut D. Neurological complications occurring after liver transplantation: role of risk factors, hepatic Encephalopathy, and acute (on chronic) brain injury. Liver Transplant. 2019 Mar;25:469–487. doi: 10.1002/lt.25420. [DOI] [PubMed] [Google Scholar]
- 30.Bemeur C. Neurological complications post-liver transplantation: impact of nutritional status. Metab Brain Dis. 2013 Jun;28:293–300. doi: 10.1007/s11011-012-9352-4. [DOI] [PubMed] [Google Scholar]
- 31.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery: a review. JAMA Surg. 2017 Mar 1;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 32.Jeon J.Y., Wang H.J., Ock S.Y. Newly developed sarcopenia as a prognostic factor for survival in patients who underwent liver transplantation. PLoS One. 2015 Nov 30;10 doi: 10.1371/journal.pone.0143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn J., Wagner D., Homfeld N., Müller H., Kniepeiss D., Schemmer P. Both sarcopenia and frailty determine suitability of patients for liver transplantation-A systematic review and meta-analysis of the literature. Clin Transplant. 2018 Apr;32 doi: 10.1111/ctr.13226. [DOI] [PubMed] [Google Scholar]
- 34.Kalafateli M., Mantzoukis K., Choi Yau Y. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-Stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. Epub 2016 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiMartini A., Cruz R.J., Jr., Dew M.A. Muscle mass predicts outcomes following liver transplantation. Liver Transplant. 2013;19:1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Englesbe M.J., Patel S.P., He K. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. Epub 2010 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamaguchi Y., Kaido T., Okumura S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 38.Masuda T., Shirabe K., Ikegami T. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transplant. 2014;20:401–407. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- 39.Montano-Loza A.J.1, Meza-Junco J., Baracos V.E., Prado C.M., Ma M., Meeberg G. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transplant. 2014 Jun;20:640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 40.Hussaini S.H., Oldroyd B., Stewart S.P. Effects of orthotopic liver transplantation on body composition. Liver. 1998;18:173–179. doi: 10.1111/j.1600-0676.1998.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 41.Plank L.D., Metzger D.J., McCall J.L. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001;234:245–255. doi: 10.1097/00000658-200108000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsien C., Garber A., Narayanan A. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda T., Shirabe K., Ikegami T. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transplant. 2014;20:401–407. doi: 10.1002/lt.23811. Epub 2014 Jan 27. [DOI] [PubMed] [Google Scholar]