Abstract
Introduction
We describe our technique of ex vivo organ perfusion and procurement in donation after deceased brain death (DBD) donors.
Material and methods
This technique comprises warm dissection of liver, kidneys, and heart, in hemodynamically stable DBD donors and perfusing them ex vivo. The cardiac and abdominal dissection can take place simultaneously. As a precaution, the iliac arteries and the abdominal aorta are dissected and kept ready for rapid cannulation and perfusion, should the donor become unstable at any stage.
The liver dissection is in principle similar to living donor hepatectomy, where portal dissection is combined with supra and infrahepatic caval dissection to completely mobilize liver to allow it to be removed and perfused ex vivo. The renal dissection is done after hepatic dissection is complete. The sequence of recovery of organ was modified where kidneys were procured first followed by hepatic and cardiac procurement simultaneously.
Results
Twelve multivisceral (liver and kidneys in all and heart in four) procurements have been performed. The average perfusion fluid volume for liver was 3.4 L. All recipients had uneventful postoperative course.
Conclusion
Our technique has not affected recipient outcomes and with benefits of less use of preservation solution, shortening bench surgery time, and decreasing the propensity of procurement injuries by avoiding cold-phase dissection.
Keywords: donation after brain death (DBD), liver, transplantation, living donor, organ perfusion
Abbreviations: CIT, cold ischemia time; DDLT, deceased donor liver transplantation; DBD, deceased brain dead; IVC, inferior vena cava; LDLT, living donor liver transplantation; UW, university of Wisconsin; WIT, warm ischemia time
In the early period of organ transplantation, the hilar dissection before perfusion (i.e the warm dissection technique) was the standard procedure for liver graft retrieval in deceased brain dead (DBD) donors.1,2 Thereafter, Neghim et al3 described the techniques of in situ flushing and sequential hilar dissection after perfusion (the cold dissection technique) for rapid procurement of all abdominal organs. At present, the cold dissection techniques are widely used for multiorgan procurement.4 With the evolution of living donor liver transplantation (LDLT), the surgical technique of donor hepatectomy has been well defined.5 Because of our vast experience in LDLT, we have applied the similar technique of living donor hepatectomy for warm dissection of organ procurement in selected DBD (donation after brain death) donors. In this study, we describe our technique of ex vivo perfusion after organ retrieval from DBD donors.
Materials and methods
From June 2010 to November 2018, 2008 cases of LDLT and 123 cases of deceased donor liver transplantation (DDLT) were performed at our institution. Of 123 DDLT, our technique of ex vivo organ perfusion was performed in 12 cases. The current technique has been used at our center since 2016 in all donors who met the criteria of this technique.
The selection criteria of DBD donor for ex vivo perfusion technique were as follows:
-
1.
Stable DBD donor (no or low levels of vasopressor support).
-
2.
Multiorgan retrieval: Liver, heart, and kidney (pancreas and small bowel are excluded).
The study has been approved by the institutional review board.
Surgical technique
Methods
This technique comprises warm dissection of liver, kidneys, and heart and perfusing them ex vivo. The longitudinal skin incision was made from xiphoid process to pubis and if needed a transverse cut at the level of umbilicus. The liver was inspected for its color, texture, edges, and wedge liver biopsy was performed selectively.
Step A
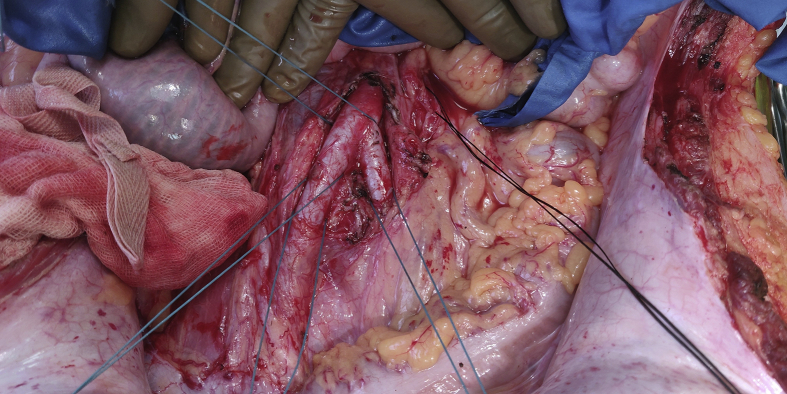
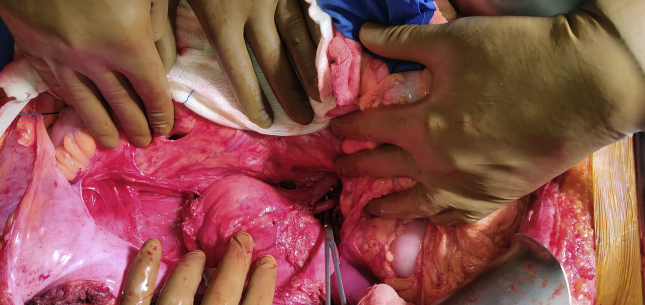
Dissection to Prepare for Emergency Perfusion (Figure 1)
Figure 1.
Dissection to prepare for emergency perfusion.
As a precaution, both right and left common iliac arteries, inferior mesenteric vein, and the supra celiac abdominal aorta are dissected and kept ready for rapid cannulation and perfusion, should the donor become unstable at any stage.
Cardiac dissection can take place simultaneously along with hepatic dissection, if heart procurement is also planned.
Step B
Hepatic Dissection
The liver dissection is in principle similar to living donor hepatectomy, where hepatic hilar dissection is combined with suprahepatic and infrahepatic caval dissection to completely mobilize liver to allow it to be removed and perfused ex vivo.
-
1.
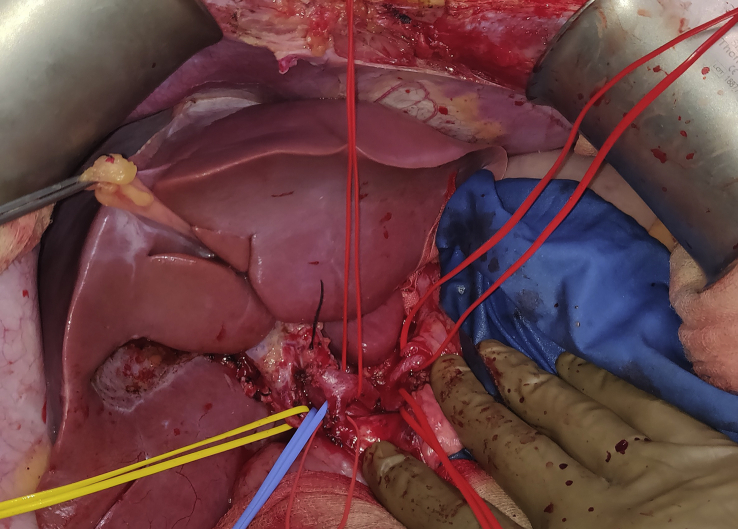
Hepatic hilar dissection – Cholecystectomy was performed and common bile duct was flushed with saline. The common hepatic artery was dissected down to the celiac axis preserving gastroduodenal artery, left gastric artery, and splenic artery. The main portal vein (MPV) was dissected till superior edge of pancreas and looped (Figure 2).
-
2.
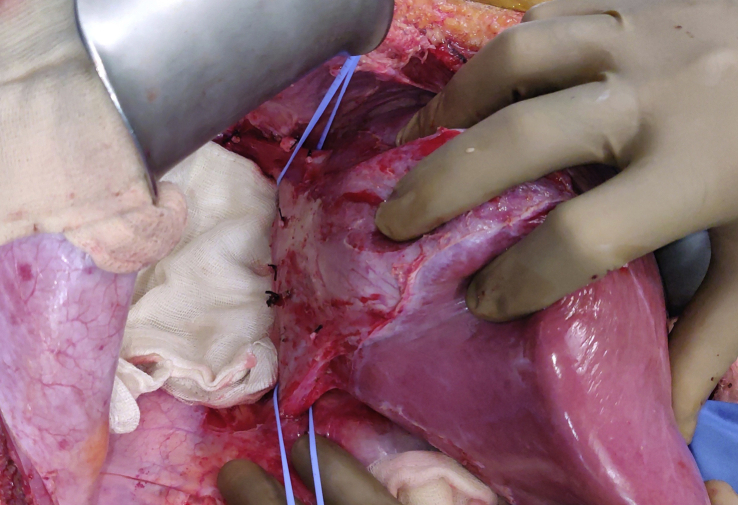
Hepatic mobilization – The liver was mobilized by dividing the falciform, left and right triangular ligaments.
-
3.
Infrahepatic IVC loop – Inferior vena cava (IVC) was dissected off the posterior abdominal wall, with looping of infrahepatic IVC superior to the renal veins.
-
4.
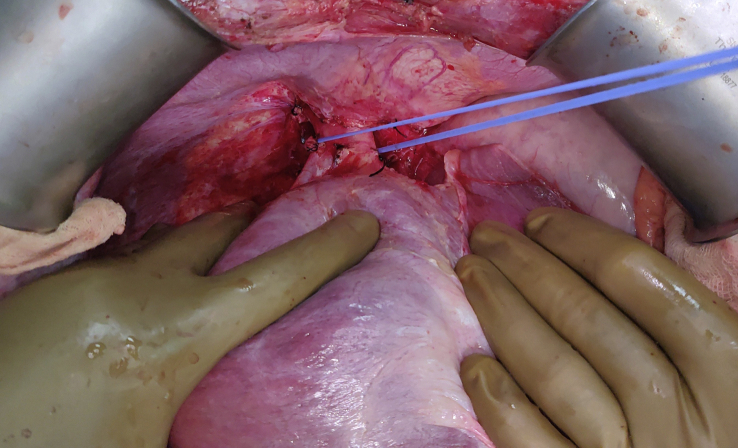
Suprahepatic IVC dissection – The suprahepatic IVC was dissected after ligating phrenic veins and opening the diaphragmatic hiatal opening and then it was looped (Figure 3, Figure 4).
Figure 2.
Hepatic hilar dissection.
Figure 3.
Suprahepatic inferior vena cava dissection.
Figure 4.
Complete hepatic mobilization.
Step C
Renal Dissection
The renal dissection was performed after hepatic dissection was complete and involved exposing both the kidneys along with their vessels and ureters (Figure 5).
Figure 5.
Renal dissection.
Step D
Organ Recovery
The donor was heparinized with 25000 units of heparin, and the sequence of organ recovery was modified. The kidneys were procured first, left followed by right, by ligating their respective arteries and veins in succession, followed by ureters. In case of short vascular stumps, their origin from aorta/opening into the IVC was sutured. They were perfused on bench similar to a living donor kidney transplant.
The hepatic and cardiac procurement then proceeded simultaneously, and after removal both organs were perfused on bench with respective fluids.
Hepatic Procurement
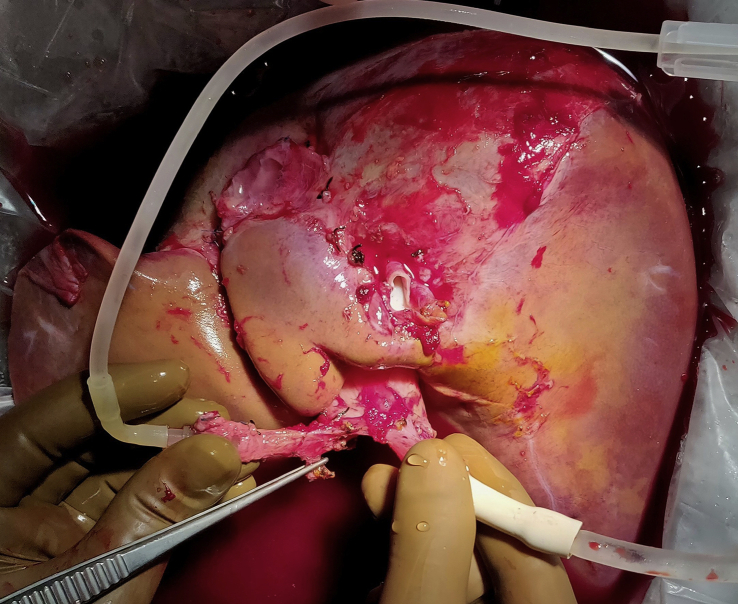
Hepatic hilar structures were divided by dividing the common bile duct, celiac trunk at its origin, and MPV above the pancreas. Subsequently, division of suprahepatic and infrahepatic IVC was carried out to remove the graft for perfusion (Figure 6).
Figure 6.
Liver perfusion on bench.
The procurement time after clamping of inflow artery and MPV was 2–3 min (warm ischemia time [WIT] in donor) in all cases. The liver graft was flushed with 3 ml/kg of graft weight (usually 3–4 L) of University of Wisconsin (UW) solution at 4 degree C through the portal vein and hepatic artery on the bench (Figure 3).
Statistical analysis
Patient baseline characteristics were expressed as mean standard deviation for continuous data and categorical variables were presented as number and percentage. All statistical analyses were performed using SPSS, version 22.0 for Windows statistical software.
Results
Twelve procurements (liver and kidney in all and heart in four DBD donor) have been performed using this technique. The donor characteristics are shown in Table 1. The mean age of DBD donor was 43.12 ± 11.25 years; 8 were males. The cause of brain death was head injury in ten donors and cerebrovascular accident in two. All the donors were in-house or our associate center within the city and hemodynamically stable with single vasopressor support. The mean time of retrieval of liver using standard technique among 111 donors was 47 ± 10 min, whereas it was 74 ± 12 min in 12 donors using ex vivo perfusion technique. The WIT during liver procurement was 2–3 min. The mean volume of UW solution used was 3.4 ± 0.42 L. There was no procurement related injuries to the graft. Similarly, there were no technique-related complications reported by cardiac and renal teams.
Table 1.
Donor Characteristics.
| Age (years, mean ± SD) | 43.12 ± 11.25 |
| Sex – male/female (n) | 8/4 |
| Cause of death(n) | |
| Brain trauma | 10 |
| Cerebrovascular accident | 2 |
| Blood test parameters (mean ± SD) | |
| Serum sodium (meq/L) | 140.83 ± 4.26 |
| AST (IU/L) | 43.33 ± 14.08 |
| ALT (IU/L) | 43.08 ± 9.27 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase.
The recipient characteristics are shown in Table 2. The mean age of recipients was 51.67 ± 6.35 years; all were male. The mean body mass index was 27.21 ± 3.21 kg/m2. Hepatitis C virus–related chronic liver disease was the most common etiology (n = 6). The mean Child-Turcotte-Pugh (CTP) and model for end-stage liver disease (MELD) scores of recipients were 9.23 ± 2.31 and 18.67 ± 3.49. The graft implantation was performed by standard piggy back technique with mean cold ischemia time (CIT) of 141 ± 32.25 min and WIT of 26.58 ± 3.08 min. The mean intraoperative blood loss was 975 + 160.26 ml. There were no vascular or biliary complications or cytomegalovirus (CMV) infection in the postoperative period. All the patients had good graft function. None of the patients developed graft dysfunction except one who had moderate acute cellular rejection on postoperative day 8 which responded to standard antirejection therapy. All the recipients were discharged in stable condition with median hospital stay of 12 days (range, 10–17 days).
Table 2.
Recipient Characteristics.
| Age (years, mean ± SD) | 51.67 ± 6.35 |
| Sex – male/female (n) | 12/0 |
| Etiology of liver disease(n) | |
| Hepatitis C virus | 6 |
| Hepatitis B virus | 2 |
| Alcoholic liver disease | 4 |
| CTP | 9.23 ± 2.31 |
| MELD | 18.67 ± 3.49 |
| Operation time (minutes, mean ± SD) | 392.08 ± 21.19 |
| Cold ischemia time (minutes, mean ± SD) | 141 ± 32.25 |
| Warm ischemia time ((minutes, mean ± SD) | 26.58 ± 3.08 |
| Blood loss (mL, mean ± SD) | 975 ± 160.26 |
Discussion
Living donor hepatectomy is the predominant form of liver transplantation at our center. Experience in right lobe LDLT encouraged us to attempt this ex vivo perfusion technique. The liver perfusion was performed in a controlled manner on bench. Our technique did not adversely affect the outcomes in recipients. There are several advantages of our technique. First, much less preservation solution is used, usually 3–4 L as compared with 10–12 L of preservation solution that is needed in the standard in situ perfusion technique. The average cost of a DDLT in our center is 38000 USD. Each liter of UW solution costs 350 USD. This means that approximately 2000 USD (more than 5% of the total cost of transplant) can be saved. The choice of perfusion fluid for our donors was UW solution. However, Histidine-Tryptophan-Ketoglutarate (HTK) or Institut Georges Lopez (IGL) or other solutions can be used as per the center's preferences in view of shorter CIT. This will alter the cost analysis. Second, it obviates the need of ice packing and cooling of the entire abdomen and chest.
Third, it reduces the incidence of procurement-related graft injuries during cold phase dissection. The teaching and training of young surgeon who wants to do LDLT is one of the another potential advantage of this technique. The dissection in warm phase, by virtue of blood flow maintained, not only allows a real life dissection which is good for teaching and training especially with LDLT dissection in mind but also reduces, and early identifies any vascular injury that might happen, when compared with dissection in cold phase on the bench. Graft injuries during organ retrieval are a known complication and can potentially influence morbidity and mortality in recipients. Several studies have reported retrieval associated injury to the donor liver grafts, both to the liver parenchyma and to hepatic arteries.6, 7, 8, 9, 10, 11 Nijkamp et al 8 reported that procurement injuries of donor livers were observed in approximately one-third of all cases without compromising overall graft survival. In our series, procurement-related graft injury was not seen.
The dissection of organ in ex vivo technique took about 45–90 min but overall time from donor incision to implantation was similar, as the in situ cannulation, perfusion, and cold dissection, as well as the bench surgery times were saved.
Pitfalls of the Technique
-
1
It should be performed only in hemodynamically stable donors, and it is not suitable if rapid retrieval is needed in case a donor is hemodynamically unstable.
-
2.
Although liver, kidney, and heart retrieval are possible, it cannot be used where pancreas and small bowel retrievals are also planned.
-
3.
The surgeon must have extensive experience in living donor hepatectomy to perform it smoothly and rapidly in DBD donors.
The liver and kidney transplant surgeons performing predominantly deceased donor transplantations might find adaptation to this technique difficult initially because (i) it is not possible to retrieve both kidneys en bloc, (ii) to retrieve kidneys with a patch of cava/aorta or retrieve liver with patches of the celiac/superior mesenteric artery without the inconvenience of having to place clamps/sutures on the donor side with their attendant risks of slippage/prolongation of retrieval time, (iii) although the kidney is the organ which can tolerate longer CIT, it is removed first to restore circulation before procuring the heart and liver, and (iv) the portal vein length procured is usually till the upper border of the pancreas which in our view is sufficient for the safe implantation in the recipients. A conventional cold-phase dissection until the retropancreatic or infrapancreatic superior mesenteric vein and the splenic vein junction if desired may be difficult.
The major limitation of the study is the use of this technique in small number of cases which is mainly because of scarcity of deceased donor in our part of world and we have started using this technique since 2016. However, the purpose of the study is to show the feasibility of this new ex vivo technique with acceptable post-transplant outcomes which has clearly been demonstrated in the study. Similarly, in the present study, the outcomes of patients with ex vivo technique have not been compared with those with classical technique of cold dissection performed at our center because of highly heterogeneous donor and recipient characteristics of the latter group. A further study from the centers with high volume of both LDLT and DDLT will be needed to validate this new technique.
In summary, we have described a novel technique of ex vivo perfusion for organ retrieval in DBD donors based on our experience in living donor hepatectomy, which can be used for liver, kidney, and heart retrieval in hemodynamically stable DBD donors. It has multiple benefits including less use of preservation solution, reducing the cost, shortening bench surgery time, and decreasing the propensity of procurement injuries by avoiding cold-phase dissection.
Author contributions
A.N.R., A.S.S., S.K.Y. designed the study and wrote the article. S.K.Y. collected the data and analyzed the data
Conflicts of interest
The authors have none to declare.
Funding
The authors received no financial support/grant for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.12.007.
Appendix A. Supplementary data
The following is/are the Supplementary data to this article:
References
- 1.Rosenthal J.T., Shaw B.W., Hardesty R.L., Griffith B.P., Starzl T.E., Hakala T.R. Principles of multiple organ procurement from cadaver donors. Ann Surg. 1983;198:617–621. doi: 10.1097/00000658-198311000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl T.E., Hakala T.R., Shaw B.W. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 3.Nghiem D.D. Rapid exenteration for multiorgan harvesting: a new technique for the unstable donor. Transplant Proc. 1996;28:256–257. [PubMed] [Google Scholar]
- 4.Abu-Elmagd K., Fung J., Bueno J. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232:680–687. doi: 10.1097/00000658-200011000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goja S., Yadav S.K., Saigal S., Soin A.S. Right lobe donor hepatectomy: is it safe? A retrospective study. Transpl Int. 2018;31:600–609. doi: 10.1111/tri.13092. [DOI] [PubMed] [Google Scholar]
- 6.Lerut J., Reding R., de Ville de Goyet J., Baranski A., Barker A., Otte J.B. Technical problems in shipped hepatic allografts: the UCL experience. Transpl Int. 1994;7:297–301. doi: 10.1007/BF00327160. [DOI] [PubMed] [Google Scholar]
- 7.Soliman T., Langer F., Puhalla H. Parenchymal liver injury in orthotopic liver transplantation. Transplantation. 2000;69:2079–2084. doi: 10.1097/00007890-200005270-00018. [DOI] [PubMed] [Google Scholar]
- 8.Nijkamp D.M., Slooff M.J.H., van der Hilst C.S. Surgical injuries of postmortem donor livers: incidence and impact on outcome after adult liver transplantation. Liver Transplant. 2006;12:1365–1370. doi: 10.1002/lt.20809. [DOI] [PubMed] [Google Scholar]
- 9.Ausania F., White S.A., Coates R., Hulme W., Manas D.M. Liver damage during organ donor procurement in donation after circulatory death compared with donation after brain death. Br J Surg. 2013;100:381–386. doi: 10.1002/bjs.9009. [DOI] [PubMed] [Google Scholar]
- 10.Jung S.W., Kim D.-S., Yu Y.D. Does procurement technique affect posttransplant graft function in deceased donor liver transplantation? Transplant Proc. 2013;45:2880–2885. doi: 10.1016/j.transproceed.2013.08.084. [DOI] [PubMed] [Google Scholar]
- 11.Jung D.-H., Hwang S., Ahn C.-S. Safety and usefulness of warm dissection technique during liver graft retrieval from deceased donors. Transplant Proc. 2015;47:576–579. doi: 10.1016/j.transproceed.2014.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.