Abstract
Citrullinaemia is a urea cycle defect that results from a deficiency of the enzyme arginosuccinate synthetase. Type 1 disease is diagnosed in childhood, whereas type 2 disease is adult onset. But, we report the outcome in a boy (13 years) with citrullinaemia type 2 who received a live donor liver transplant (LDLT) at our centre. One advantage of LDLT over deceased donor liver transplantation is the opportunity to schedule surgery, which beneficially affects neurological consequences. In conclusion, transplantation should be considered to be the definitive treatment for citrullinaemia type 2 at this stage, although some issues remain unresolved.
Keywords: urea cycle defect, living donor liver transplantation, citrullinaemia type II
Abbreviations: ASS, Arginosuccinate synthetase; CTLN2, Citrullinaemia type 2; FLR, Future liver remnant; LDLT, Living donor liver transplantation; LLV, Left lobe volume; MCT, Medium-chain triglycerides; RLV, Right lobe volume; TLV, Total liver volume; UCD, Urea cycle defects
Citrullinaemia is a urea cycle defect (UCD) that results from a deficiency of the enzyme arginosuccinate synthetase (ASS). Citrin deficiency (type 2 citrullinaemia) is a recessively inherited metabolic disorder with age-dependent clinical manifestations. It causes neonatal intrahepatic cholestasis (NICCD) and adult-onset type II citrullinaemia (CTLN2). Patients with NICCD present with intrahepatic cholestasis in the neonatal period and usually respond to the treatment with medium-chain triglyceride (MCT) supplement and lactose-restricted formula. In adulthood, CTLN2 develops in <10% of the patients showing hyperammonemic encephalopathy. Patients with CTLN2 required liver transplantation for the most promising prognosis; however, they were successfully treated with MCT supplement with a low-carbohydrate formula. Citrin deficiency is caused by mutations in SLC25A13 on chromosome 7q21.3. Citrin is an aspartate/glutamate transporter in mitochondria, a component of malate-aspartate nicotinamide adenine dinucleotide hydrogen shuttle, and is essential for the hepatic glycolysis.1
Case report
Our patient was a 13-year-old boy who came to us with growth retardation. His medical and childhood history was scant although both the patient and his father insist these were uneventful and that he had been an above average student in school. The patient presented to our hospital at the age of 12 years for growth retardation and fatiguability. He was described by his father that he was doing below average at school for the past 2 years. On evaluation, arterial ammonia (95 mmol/L) and lactate (2.5 mmol/L) levels were markedly elevated. However, other liver tests and imaging were not suggestive of liver cirrhosis and excluded the presence of any portosystemic shunt. Computerized tomography imaging revealed a replaced right hepatic artery from superior mesentric artery and hepatomegaly (Figure 1). Tests for hepatitis B, hepatitis C, HIV, Wilson disease, systemic autoimmune disease and syphilis were negative. In view of his significant behavioural change and elevated ammonia levels, he was screened for inborn errors of metabolism. Citrulline levels in the blood and urine were elevated fivefold. There was corresponding elevated ratios between plasma arginine and threonine and plasma serine. Liver biopsy showed diffuse bridging fibrosis, marked mixed steatosis, suggestive of metabolic aetiology. Genetic study revealed positive for a homozygous pathogenic variant, in exon 16 of the SLC25A13 gene which is associated with citrin deficiency. He was commenced on 1 g of l-arginine twice daily and with a low-carbohydrate formula. Orthotopic living donor liver transplantation (LDLT) from his father was performed in December 2018. The donor was tested for the same mutation and was found to be positive but heterozygous pathologic variant. USG abdomen showed no obvious abnormality. Intravenous contrast triphasic CT scan of the abdomen showed: Liver attenuation index: +7.4 TLV: 1252 cc, right lobe volume without MHV: 850 cc, left lobevolume with middle hepatic vein: 402 cc, expected graft to recipient weight ratio-1.37 and FLR -60%. MRCP showed normal type 1 intrahepatic biliary anatomy. Gall bladder was normal. Mean hepatic parenchymal fat fraction was −6.5%. Left lobe graft with middle hepatic vein was used. The graft weight was 454 gm and GRWR was 1.5, with cold ischaemia time of 105 min and warm ischaemia time of 45 min. The explanted liver was enlarged measuring 10 cm and weighing 856 kg. The gross cut sections appeared pale brown, and the liver emitted a peculiar sweetish odour (Figure 2). Histology showed Masson trichrome stain highlighting fibrosis around cirrhotic nodules and hepatocytes showing severe large and medium droplet steatosis (Figure 3) and patchy fine perisinusoidal fibrous bands appreciated (Figure 4). Immunosuppression consisted of prednisolone and tacrolimus. He has recovered well after LDLT and his arterial ammonia levels (20 umol/L) normalised less than 2 weeks after transplant. He is currently on tailing doses of tacrolimus. He continues to remain well more than 10 months after liver transplantation (LT) and has since returned to school. The donor was followed up too and has resumed his daily activities.
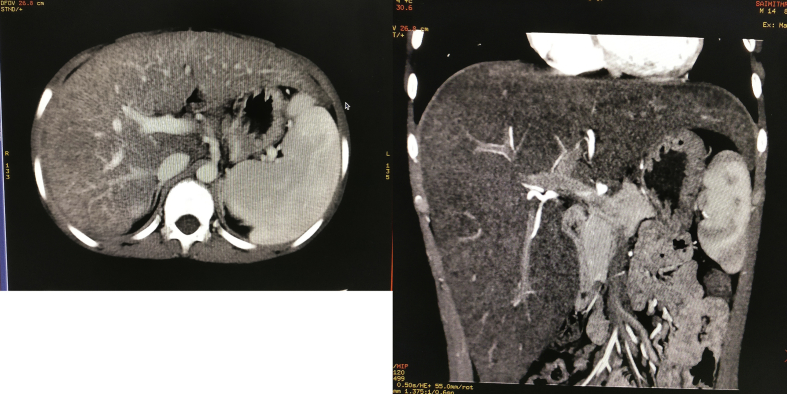
Figure 1.
CT imaging revealed a replaced right hepatic artery from SMA and hepatomegaly.
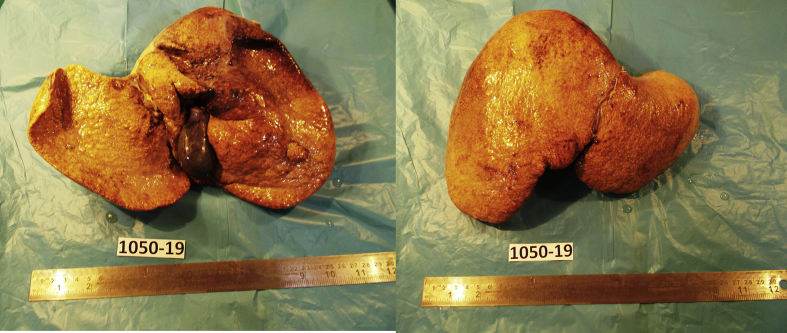
Figure 2.
Explanted liver from recepient.
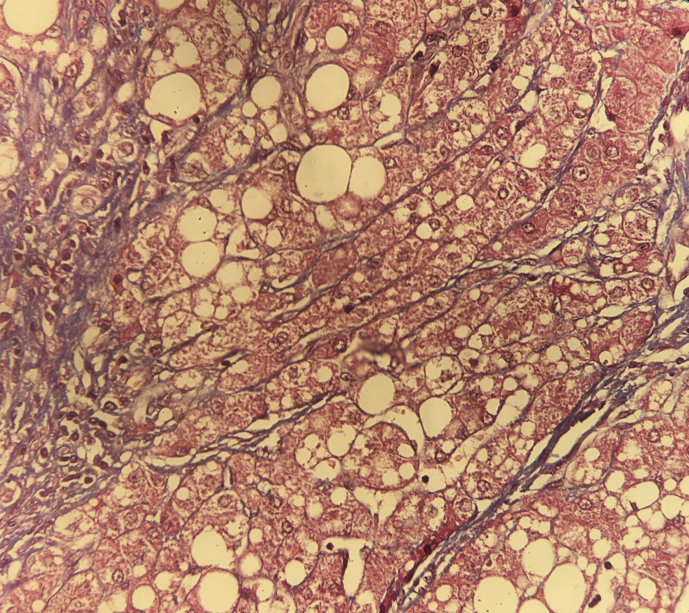
Figure 3.
Masson trichrome stain highlighting fibrosis around cirrhotic nodules and hepatocytes showing severe large and medium droplet steatosis.
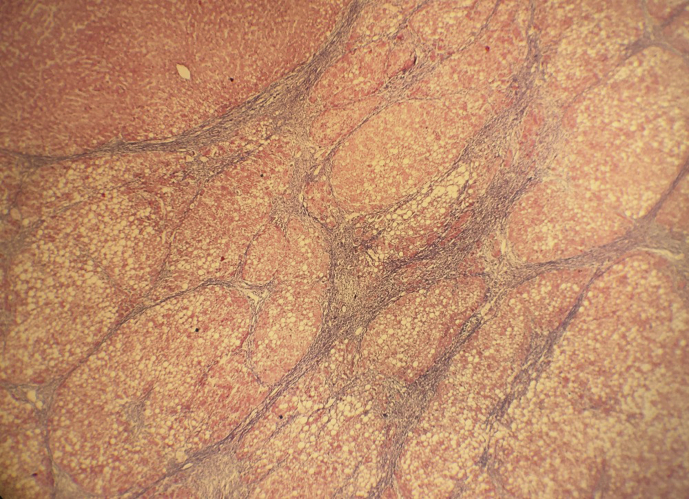
Figure 4.
Patchy fine perisinusoidal fibrous bands appreciated.
Discussion
Citrullinaemia is a UCD that results from a deficiency of the enzyme ASS. Nitrogen from enteral sources (dietary protein) and muscle is excreted from the body, as urea, via the urea cycle. ASS converts citrulline to arginosuccinic acid in the urea cycle, removing ammonia in the process. Deficiency of ASS results in citrullinaemia, an autosomal recessive disorder. The severity of disease and clinical presentation is proportionate to residual enzyme activity, dietary protein load and patient age.
Citrullinaemia type 2
Is adult onset and ASS deficiency limited to the liver 2? Patients with type 2 disease usually present between the ages of 20–50 years although patients younger or much older than this have also been reported. Type 2 disease is believed to be caused by mutations in the gene (SLC25A13) which encodes citrin, a mitochondrial aspartate glutamate carrier protein (AGC2) located on chromosome 7q21.3 Patients are described to be thin, with up to 40% having a body mass index under 17 and tend to have a predilection for protein-rich foods.4 Liver dysfunction is minimal or absent. Most patients present suddenly with hyperammonemia and associated neuropsychiatric symptoms such as altered conscious levels, irritability, seizures or coma. Cerebral oedema is the commonest mode of death and usually occurs several years after onset.
Management of the disease involves strategies to remove ammonia, maintenance of nitrogen excretion, reducing the frequency of intercurrent episodes and nutritional and fluid repletion. Although patients with UCDs are commonly treated with low-protein high-carbohydrate diets, this is harmful in patients with type 2 citrullinaemia. High carbohydrate intake increases NADH production, further disrupting the urea cycle and stimulating the citrate-malate shuttle, resulting in further hyperammonemia, hypertriglyceridaemia and fatty liver disease.5, 6, 7 The recommended management of CTLN2 is MCT supplement therapy with low-carbohydrate formula. 5,6 Sodium pyruvate administration with a low-carbohydrate diet was reported to decrease the frequency of hyperammonemic encephalopathy.8 However, this treatment did not prevent the relapse of encephalopathy nor improve the Fischer ratio and citrullinaemia.
Presently, only LT is curative of the disorder.9,10 With successful LT, hyperammonemic episodes cease, dietary restrictions are not required, and alternative pathway medication can be discontinued.11,12 Successful LT for type 2 disease has been reported with both deceased donor and living donor grafts. Auxiliary partial orthotopic LT has also been described for the treatment of UCDs. Such transplants using a left lobe graft have been described to provide sufficient enzyme supplementation and can correct citrullinaemia.13 However, this technique too has its complications, such as competition of blood inflow between the native liver remnant and the graft and higher morbidity rates compared with nonauxiliary transplanted recipients.14,15 Unlike in type 1 disease, the metabolic correction of type 2 disease with liver transplantation is complete, as the enzyme deficiency is limited to the liver. Optimum timing for LT is unknown although the aim is to correct the underlying metabolic error before irreversible brain damage occurs. Ikeda et al.16 reported that 1 of 7 citrullinaemia type 2 patients who underwent living-related liver transplantation in their series continued to be cognitively impaired despite transplantation, whereas the rest had recovered completely from their neuropsychiatric symptoms. This patient had CT and magnetic resonance imaging evidence of diffuse cortical atrophy of the brain and marked decreased radionuclide uptake on brain spectroscopy images.A review based on worldwide data of LT for urea cycle disorders suggested that neurological impairments were more likely to persist after LT in paediatric patients rather than adult patients and in patients receiving a deceased donor graft rather than a living donor graft.12
Ornithine transcarbamylase deficiency was subsequently diagnosed from the measurement of urea cycle enzymes and molecular analysis of donor liver tissue. Secondly, deceased donor grafts are scarce in many parts of the world, necessitating living donor liver transplantation instead. As living donor grafts are usually harvested from relatives, a thorough donor workup should be undertaken to exclude an ASS deficiency state in the donor because this is an inheritable disorder. Apart from measuring plasma citrulline and ammonia levels, some centres also biopsy donor liver tissue to measure urea cycle enzymatic activity. Hepatocyte transplants and gene transfers have been explored for the treatment of UCDs, including citrullinaemia. However, these are not without risks, and their current role remains in a research setting.
After the surgery, the patient remained well, and he is currently leading a normal life. Usually for citrullinaemia type2, diet plays the primary therapeutic role, while orthotopic liver transplantation is often considered as a last resort. Our case report and the recent literature data on the quality of life and prognosis of traditionally treated patients vs patients support LDLT as a primary intervention to prevent life-threatening acute episodes and chronic mental and growth impairment. Therefore, our present experience, together with the results obtained worldwide in patients with this type of metabolic disease, supports LDLT as soon as possible. The advantages for these children are an approximate 90% survival rate with normal mental, growth development and a better quality of life. So, LT should be considered to be the definitive treatment for UCDs, and thus more enterprising application of this procedure to patients with is acceptable. If deceased donor liver transplantation is unavailable, the selection of living donors must be initiated immediately.
Authors’ contribution
All authors contributed equally in writing the article.
Conflicts of interest
All authors have none to declare.
Acknowledgement
This case report did not receive any financial support. The research was performed at the Department of Surgical Gastroenterology and Liver transplantation, Global hospitals, Hyderabad.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.12.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1.
References
- 1.Hayasaka K, Numakura C .Adult-onset type II citrullinemia, Current insights and therapy [DOI] [PMC free article] [PubMed]
- 2.Häberle J., Pauli S., Linnebank M. Structure of the human argininosuccinate synthetase gene and an improved system for molecular diagnostics in patients with classical and mild citrullinemia’. Hum Genet. 2002;110:327–333. doi: 10.1007/s00439-002-0686-6. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K., Sinasac D.S., Iijima M. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999;22:159–163. doi: 10.1038/9667. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi K., Iijima M., Yasuda T., Ikeda S., Saheki T. Citrin deficiency. Journal of the Japan Pediatric Society. 2006;110:1057–1059. [Google Scholar]
- 5.Saheki T., Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD) J Hum Genet. 2002;47:333–341. doi: 10.1007/s100380200046. [DOI] [PubMed] [Google Scholar]
- 6.Imamura Y., Kobayashi K., Shibatou T. Effectiveness of carbohydrate-restricted diet and arginine granules therapy for adult-onset type II citrullinemia: a case report of siblings showing homozygous SLC25A13 mutation with and without the disease. Hepatol Res. 2003;26:68–72. doi: 10.1016/s1386-6346(02)00331-5. [DOI] [PubMed] [Google Scholar]
- 7.Saheki T., Iijima M., Meng X.L. Citrin/mitochondrial glycerol-3-phosphate dehydrogenase double knock-out mice recapitulate features of human citrin deficiency. J Biol Chem. 2007;282:25041–25052. doi: 10.1074/jbc.M702031200. [DOI] [PubMed] [Google Scholar]
- 8.Yazaki M., Fukushima K., Saheki T., Ikeda S. Program and Abstracts for the 3rd Asian Congress for Inherited Metabolic Diseases. The 55th Annual Meeting of the Japanese Society for Inherited Metabolic Diseases. Chiba; 2013. Therapeutic strategy for patients with adult onset type II citrullinemia (CTLN2) p. 101. [Google Scholar]
- 9.Burton B.K. Urea cycle disorders. Clin Liver Dis. 2000;4:815–830. doi: 10.1016/s1089-3261(05)70143-4. [DOI] [PubMed] [Google Scholar]
- 10.Whitington P.F., Alonso E.M., Boyle J.T. Liver transplantation for the treatment of urea cycle disorders. J Inherit Metab Dis. 1998;21(suppl 1):112–118. doi: 10.1023/a:1005317909946. [DOI] [PubMed] [Google Scholar]
- 11.Takenaka K., Yasuda I., Araki H. Type II citrullinemia in an elderly patient treated with living related partial liver transplantation. Intern Med. 2000;39:553–558. doi: 10.2169/internalmedicine.39.553. [DOI] [PubMed] [Google Scholar]
- 12.Morioka D., Kasahara M., Takada Y. Current role of liver transplantation for the treatment of urea cycle disorders: a review of the worldwide English literature and 13 cases at Kyoto University. Liver Transplant. 2005;11:1332–1342. doi: 10.1002/lt.20587. [DOI] [PubMed] [Google Scholar]
- 13.Yazaki M., Hashikura Y., Takei Y.I. Feasibility of auxiliary partial orthotopic liver transplantation from living donors for patients with adult-onset type II citrullinemia. Liver Transplant. 2004;10:550–554. doi: 10.1002/lt.20131. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara M., Takada Y., Egawa H. Auxiliary partial orthotopic living donor liver transplantation: Kyoto University experience. Am J Transplant. 2005;5:558–565. doi: 10.1111/j.1600-6143.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara M., Takada Y., Kozaki K. Functional portal flow competition after auxiliary partial orthotopic living donor liver transplantation in noncirrhotic metabolic liver disease. J Pediatr Surg. 2004;39:1138–1141. doi: 10.1016/j.jpedsurg.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S., Kawa S., Takei Y.I. Chronic pancreatitis associated with adult-onset type II citrullinemia: clinical and pathologic findings. Ann Intern Med. 2004;141:W109–W110. doi: 10.7326/0003-4819-141-7-200410050-00028-w1. [DOI] [PubMed] [Google Scholar]