Abstract
Mechanistic and functional studies by gene disruption or editing approaches often suffer from confounding effects like compensatory cellular adaptations generated by clonal selection. These issues become particularly relevant when studying factors directly involved in genetic or epigenetic maintenance. To provide a genetic tool for functional and mechanistic investigation of DNA-repair mediated active DNA demethylation, we generated experimental models in mice and murine embryonic stem cells (ESCs) based on a minigene of the thymine-DNA glycosylase (TDG). The loxP-flanked miniTdg is rapidly and reliably excised in mice and ESCs by tamoxifen-induced Cre activation, depleting TDG to undetectable levels within 24 hours. We describe the functionality of the engineered miniTdg in mouse and ESCs (TDGiKO ESCs) and validate the pluripotency and differentiation potential of TDGiKO ESCs as well as the phenotype of induced TDG depletion. The controlled and rapid depletion of TDG allows for a precise manipulation at any point in time of multistep experimental procedures as presented here for neuronal differentiation in vitro. Thus, we provide a tested and well-controlled genetic tool for the functional and mechanistic investigation of TDG in active DNA (de)methylation and/or DNA repair with minimal interference from adaptive effects and clonal selection.
Keywords: Embryonic Stem Cells, TDG, Active DNA Demethylation, Base Excision Repair, Neuronal Differentiation, Minigene, Cre/loxP, Tamoxifen
Introduction
To allow for the differentiation to a multitude of cell types during the development of multicellular organisms, stem cells need to respond to a variety of extrinsic and intrinsic developmental cues and integrate these into epigenetically stabilized gene expression patterns during lineage specification. The underlying mechanisms to ensure this necessary epigenetic plasticity are therefore active and highly dynamic in embryonic stem cells (ESC). One of those mechanisms includes the thymine DNA-glycosylase (TDG) and the downstream factors of DNA base excision repair (BER), which were shown to be involved in the dynamic, locus-specific regulation of DNA methylation. Following the activity of dioxygenases of the ten-eleven-translocation (TET) family that iteratively oxidize 5-methylcytosine (5-mC) to formyl- and carboxylcytosine (5-fC, 5-caC), TDG-induced BER provides a mechanism of active DNA demethylation and, thereby, impact gene regulation ( Cortázar et al., 2011; Cortellino et al., 2011; Schuermann et al., 2016; Weber et al., 2016). Studying the precise function of TDG (and BER) in epigenetic regulation during differentiation of stem cells, however, is challenging due to its multiple and interwoven interactions with other proteins like transcription factors and epigenetic modifiers ( Henry et al., 2016; Jacobs & Schär, 2012; Léger et al., 2014), the deregulation of which may cause a plethora of confounding effects. Also, genetic depletion of TDG itself is not compatible with embryonic development and alters the epigenetic state of cultured cells, causing a difficult-to-control drift of phenotype in long-term cultivated stem cell populations. To reduce and circumvent such confounders in functional studies of TDG (and BER) in murine ESCs, we established and validated a Tdg minigene, introduced it into mice and derived a series of different ESC variants. The minigene carries, among other features, a loxP-flanked coding region of Tdg, allowing for a controlled and fast, 4-hydroxytamoxifen (OHT)-inducible depletion of TDG in the background of a homozygous disruption of the endogenous Tdg. By eliminating phenotypic divergence between TDG proficient and deficient ESC, this versatile model facilitates precise functional and mechanistic investigations into TDG-mediated active DNA demethylation. This novel Tdg minigene introduced into mice as well as ESCs will facilitate future research in the field of active DNA demethylation in vivo and in vitro.
Methods
Ethics statement and Animal Work
All animal work was carried out in accordance with the Swiss Animal Welfare Act and the guidelines of the Swiss Federal Veterinary Office (SFVO) or with the UK Animals (Scientific Procedures) Act. Housing, breeding and experimentation of mice has been performed with the approval of the Cantonal Veterinary Office of Basel-Stadt (Licenses 10062-H, 1912) or was covered by a project license to Wolf Reik (80/1896) further regulated by the Babraham Institute Animal Welfare, Experimentation, and Ethics Committee. Mice were housed under specific pathogen free condition in individually ventilated cages under 12 h light/dark cycles at 22 ±2°C and 35-65% relative humidity. Animal and colony health was checked daily and quarterly, respectively. Sterilized diet (Kliba-Nafag Extrudate 3436) and water was provided ad libitum.
Tdg tm1Psch mice ( http://www.informatics.jax.org/allele/MGI:5487834) were generated from E14 ESC (RRID:CVCL_C320) and backcrossed for more than 10 generations with female C57BL/6JRj mice, obtained at 7-8 weeks of age from Janvier Labs. A Tdg expression cassette (pTCO2-mTDGi.0, Addgene Plasmid #149429) was introduced as single copy into C57BL/6JRj mice by pronuclear injection and crossed into Tdg tm1Psch mice. Genotyping was done by PCR with the HOT FIREPol (Solis BioDyne) on diluted crude DNA preparations (diluted by a factor of five with 10 mM Tris-HCl pH8) by boiling toe clips with 25 mM NaOH/0.2 mM EDTA for 1 h followed by neutralization with 40 mM Tris-HCl. Reactions were performed according to the manufacturer’s recommendation with 35 PCR cycles at 95°C for 30 s, 60°C for 30 s, and at 72°C for 60 s (for details about all used primers and annealing temperatures in PCR reactions, see Extended data Table S1) ( Schwarz, 2020).
Generation of ESCs
ESCs were derived from male blastocysts from timed matings of male miniTdg tg/tg// Tdg -/- mice with 2 super-ovulated female Tdg +/- mice and cultured on murine immortalized feeder cells in 2i medium with leukemia inhibitory factor LIF (Merck-Millipore) ( Ying et al., 2008).
To introduce the inducible Cre recombinase into the ROSA26 locus, 30 µg of the targeting vector (pROSA26-ERT2CreERT2, Addgene Plasmid #149436) was electroporated into 15×10 6 mESCs using a gene pulser Xcell (Bio-Rad) at 240 V and 475 μF. Transgenic cells were selected with 8 and 5 µg/ml blasticidin for a week each, before colonies were picked and then amplified without selection pressure. From blasticidin-resistant colonies, genomic DNA was extracted with the “QIamp DNA mini” kit (Qiagen) and screened for a targeted integration at the 3’ junction of the ROSA26 locus by PCR with the Phusion polymerase (NEB) (see Extended data, Table S1) ( Schwarz, 2020), applying a general three-step cycling protocol: initial denaturation 95°C for 30 s, 35 times 95°C for 10 s, annealing for 20 s, 72°C for 15–80 s. PCR reactions were analyzed by gel electrophoresis followed by image acquisition with a U:Genius 3 system (Version 3.0.12.0, Syngene).
To evoke a disruption of the Neo R reading frame by CRISPR/Cas9, two guide RNAs (5’-GCCGATCCCATATTGGCTGCAGG-3’, 5’-GAAGGCGATGCGCTGCGAATCGG-3’, IDT) were designed and transfected with TransIT-X2 (Mirus Bio) into the TDGiKO1 ESCs. RNP assembly was performed according to the manufacturers protocol (IDT). One day after transfection, single cells were sorted with a FACSaria IIIu (BD BioSciences) into 96-well plates coated with inactivated mouse fibroblasts, based on GFP expression from a co-transfected pEGFP-N1 plasmid (Clontech). Screening for the successful disruption of the Neo R gene was done by PCR as described above and assaying the loss of the neomycin resistance with the Cell Counting Kit-8, according to the manufacturer’s instructions (CCK-8, Dojindo).
Cell culture and differentiation
ESCs were cultured under a controlled atmosphere (37°C, 5% CO 2, 95% humidity) in serum-free 2i medium with 1000 U/ml LIF, without antibiotics unless indicated otherwise. The 4-OHT (Sigma-Aldrich H7904) was dissolved in DMSO (stock 10 mM) and administered at indicated concentrations for 2 h.
Neuronal differentiation was performed as described before ( Bibel et al., 2007; Cortázar et al., 2011; Steinacher et al., 2019). Differentiation towards cardiomyocytes was performed by culturing ESCs in classical ESC medium (ESM) with 15% FCS without LIF in non-adhesive petri dishes (Greiner) for at least 10–14 days.
All pictures were taken with a DM IL LED Fluo microscope and MC170 HD camera (Leica) at 100x magnification.
Molecular analysis of ROSA26 targeting and miniTdg excision
Probes for Southern blotting were generated by PCR amplification with Taq DNA Polymerase (Qiagen) from the ROSA26 targeting plasmid using the DIG DNA labeling mix (Roche). Southern blotting was done with 20 µg of genomic DNA, extracted by the “Genomic tip 100G” kit (Qiagen). Probes were hybridized according to the “DIG Application Manual for Filter Hybridization” by Roche. Signals were acquired by exposing the membrane to chemiluminescence detection films (Amersham/GE Healthcare) and digitalized by a CanoScan 8400F scanner with CanoScan Toolbox (Version 4.9.3).
Excision of the miniTdg gene by Cre was assessed by qPCR with the “Rotor-Gene SYBR Green PCR Kit” (Qiagen) on a Rotor Gene 3000 (Qiagen) system. After 95°C for 5 min, qPCR reactions followed a two-step cycling (40x) protocol: 95°C for 5 s, 60°C for 30 s, terminated by ramping from 60 to 95°C in 175 s to generate a melting curve. PCRs to exclude background recombination by Cre were done with NEB Phusion polymerase as described above.
Gene expression analyses
Total RNA was extracted with the RNAeasy Mini Kit (Qiagen), including on-column DNase digest, and reverse transcribed with RevertAid First Strand cDNA synthesis kit (ThermoFisher) using oligo-dT primers. qPCR was performed as described above with marker-specific primers (see Extended data, Table S1) ( Schwarz, 2020).
Protein levels were analyzed by western blotting of 50 µg of NP-40 (or SDS) whole-cell extracts, separated by SDS-PAGE, onto a nitrocellulose membrane (Amersham) and immunodetection with anti-mTDG antibody (rabbit polyclonal L58, Lab P.Schär, dilution 1:10’000, 1 h at 33°C) and anti-GAPDH antibody (Sigma-Aldrich Cat# G9545, RRID:AB_796208, dilution 1:20’000, 1 h at RT). Chemiluminescence signals were detected with a Fusion FX7 system (Software version 16.15.0.0, Vilber).
Detection of oxidized variants of 5-methylcytosine
DNA was extracted and purified with the Genomic tip 100G Kit (Qiagen). High-performance liquid chromatography–tandem mass spectrometry analysis was performed as described before ( Weber et al., 2016).
Statistical analysis
To test for statistical significance ( p ≤ 0.05), we performed two-tailed Student’s t-tests on the indicated number of replicates in Microsoft Excel (Version 16.0.4954.1000)
Results and discussion
Generation of a chimeric Tdg minigene and derivation of murine ESCs carrying a tamoxifen-inducible Cre recombinase
To facilitate genetic manipulation of the Tdg gene (~28 kb including the putative promoter), we first constructed a synthetic Tdg minigene ( miniTdg, ~11 kb) ( Figure 1A). The minigene consists of the endogenous Tdg promoter and terminator sequences flanking the Tdg coding sequence (CDS). It also includes the rabbit β-globin intron at the authentic position of the first intron of the Tdg transcript variant 2 (Genebank NM_172552.4). This intron allows for the expression of the two naturally occurring Tdg splice variants ( Um et al., 1998). In addition, the miniTdg contains two loxP sites in the same orientation for Cre-recombinase-mediated excision of the coding region. A plasmid carrying the miniTdg gene (pTCO2-mTDGi.0) was introduced into C57BL/6 mice by pronuclear injection and offspring carrying the minigene were crossed into heterozygous Tdg tm1Psch mutant mice ( Kunz et al., 2009). When breeding heterozygous mice for the miniTdg transgene and the Tdg KO allele, the genotypes of offspring followed the expected Mendelian pattern for two loci ( Figure 1B). The miniTdg allele segregated with a 3:1 ratio, consistent with a random integration into a single genomic locus. About 25% of born mice with the miniTdg gene were homozygous for the Tdg tm1Psch KO allele while no homozygous TDG KO mouse was obtained without the transgene. This shows that the miniTdg transgene is fully functional in complementing the developmental defects and lethality of homozygous Tdg tm1PschKO embryos ( Cortázar et al., 2011; Cortellino et al., 2011). MiniTdg complemented mice were healthy and fertile with normal lifespan.
Figure 1. miniTdg complementation of Tdg -/- mice and mESCs.
( A) Synthetic miniTdg gene on the plasmid integrated to a random genomic locus by pronuclear injection. miniTdg consisting of 6 kb endogenous Tdg promoter, the Tdg coding sequence (CDS) including a chimeric splice donor ( Tdg exon 1 of transcript variant 2 NM_172552.4, rabbit β-globin intron), to allow for alternative splicing, 3 kb of the 3’ untranslated region (3’ UTR) and terminator (term) of Tdg, and the bovine growth hormone terminator sequence (BGH!). loxP sites are indicated in red. ( B) Expected and obtained genotype distribution in offspring (n=116) from crosses of heterozygous mice. ( C) Top: Immunoblot for TDG of whole-cell SDS-extracts from derived ESCs with different genotype. Bottom: Loading control by Ponceau staining of the membrane. ( D) Accumulation of oxidized 5-mC derivates measured by HPLC-MS/MS in ESCs with the indicated genotype. Shown are means and standard deviation of two independent clones of each genotype. ( E) Scheme of the targeting construct pROSA26-ERT2CreERT2 for the ROSA26 locus with the tamoxifen-inducible Cre recombinase ( Matsuda & Cepko, 2007). ERT2-Cre-ERT2 expression is under the control of the synthetic cytomegalo-virus/chicken-actin/beta-globin promoter (CAG). Homology arms for ROSA26 targeting are indicated in light grey. A blasticidin resistance cassette (BlaR) for positive selection is under the control of the SV40 promoter and terminator. ( F) Predicted restriction pattern of ROSA26 WT and integration events (top) and fragments detected by Southern blotting (bottom). Left: The hybridization probe (P1) locating to the left ROSA26 homology arm detected a genomic fragment of 6.9 kb. The 4.4 kb fragment represents the wild-type ROSA26 allele. Right: Detection of possible off-target events using a second probe (P2) locating to the blasticidin selection marker within the targeting construct. See Underlying data for the raw data behind this figure ( Schwarz, 2020).
Three ESC populations were then derived from male blastocysts from crosses of miniTdg tg/tg// Tdg -/- with Tdg +/- mice ( Extended data, Table S2: Mm11, Mm01, Mm02) ( Schwarz, 2020). In these ESCs, TDG protein levels were similar to those in wild-type ESCs ( Figure 1C), indicating that the miniTdg gene is regulated as the endogenous Tdg. To assess functional integrity at the molecular level, we measured the accumulation of oxidized 5-methylcytosine (5-mC) derivates, 5-hydroxymethylcytosine (5-hmC) and 5-carboxylcytosine (5-caC), the substrate for excision by TDG ( Maiti & Drohat, 2011; Shen et al., 2013) ( Figure 1D). Mass spectrometry showed that TDG deficient ESCs accumulated 5-caC as expected, while ESCs expressing TDG solely from the miniTdg gene showed 5-caC levels similar to those in wild-type ESCs.
To allow for a controlled excision of the miniTdg coding region, we introduced a tamoxifen-inducible Cre recombinase expression cassette ( Figure 1E) into the non-essential ROSA26 locus ( Soriano, 1999). Screening 60 blasticidin-resistant colonies by PCR for targeted integration at 3’ ROSA26 locus yielded six clones (TDGiKO1-6). Southern blotting confirmed correct heterozygous integration of the complete ER T2-Cre-ER T2 cassette ( Matsuda & Cepko, 2007) for four of the six clones ( Figure 1F, left, asterisks). Potential additional off-target integrations were tested and excluded by hybridizing a second probe within the transgene ( Figure 1F, right). Original Southern blotting images are available as Underlying data ( Schwarz, 2020).
To ease further genetic manipulations, we eliminated the neomycin resistance gene, which was introduced at the endogenous Tdg locus to generate the original Tdg KO allele ( Kunz et al. 2009), by applying a CRISPR-guided Cas9 nuclease. Using two guide RNAs targeting the 5’ and 3’ end of the Neo R gene, we generated two ESC clones (TDGiKO1.1 and TDGiKO1.2) that bear a deletion of 776 and 767 bp, respectively, and are no longer resistant to neomycin ( Extended data, Figure S1A/B) ( Schwarz, 2020).
Taken together, we established a miniTdg gene that complements expression and function of the endogenous Tdg gene in vivo and in vitro. The functional integrity of the transgene is confirmed by rescuing phenotypes of Tdg KO defects at the systemic and cellular level, namely the rescue of embryonic lethality and suppression of 5-caC accumulation. The introduction of a Cre-recombinase to the ROSA26 locus, without off-targeted events, will allow conditional inactivation of the miniTdg in ESCs.
Induction of the Cre recombinase leads to fast and efficient depletion of TDG in ESCs
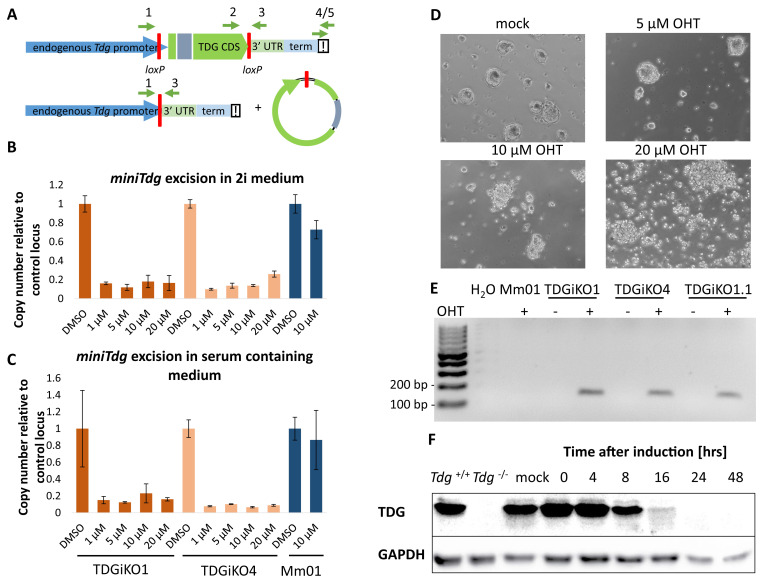
To make the inducible TDG depletion ( Figure 2A) applicable to differentiation experiments that often depend on complex and strict cell culture procedures, we aimed at establishing a short Cre-induction protocol minimizing any interference with standard differentiation conditions. Titrating 4-Hydroxytamoxifen (OHT) and measuring miniTdg excision by quantitative PCR, we identified OHT concentrations of 1-5 µM in serum-free 2i ( Figure 2B) and 5-10 µM OHT in serum-containing medium ( Figure 2C) to yield maximal excision efficiencies without impairing cell viability ( Figure 2D). While the miniTdg excision product is readily detectable by PCR in different TDGiKO ESCs after OHT treatment, no unintentional background activity of the Cre recombinase is detectable before OHT induction ( Figure 2E). Following the kinetics of TDG depletion by SDS-PAGE/immuno-detection, we found that TDG was reduced below detection limit (<2% of endogenous TDG) 24 h after Cre induction for 2 h ( Figure 2F and Extended data, Figure S1C) ( Schwarz, 2020).
Figure 2. Cre induction for two hours results in the depletion of TDG within 24 hours without affecting cell viability.
( A) Scheme of the miniTdg cassette before (top) and after (bottom) Cre-mediated recombination. Position of PCR primers are indicated with green arrows. ( B) Quantitation of miniTdg excision upon OHT administration in TDGiKO1, TDGiKO4 and parental ESCs without the Cre recombinase (Mm01). Genomic DNA of cells was extracted two days after OHT treatment with indicated concentrations for 2 hours. Copy number of the miniTdg is measured by qPCR using primers 2 and 3, normalized to the nearby terminator region (primers 4/5) and a control locus on chromosome two. Shown are means and standard error from technical quadruplicates per clone. ( C) Quantitation of miniTdg excision as in ( B), but Cre induction executed in ES medium containing 15% FCS. ( D) Phase-contrast images of TDGiKO1 ESCs in 2i medium, two days after OHT treatment for two hours at indicated concentrations. ( E) Detection of Cre recombination events (158 bp) by PCR using primers 1 and 3 ( A) before and after addition of OHT. ( F) Time-course assessment of TDG protein levels expressed from the miniTdg gene in 2i cultivated TDGiKO1 ES cells after Cre induction by 1 µM OHT for 2 h. See Underlying data for the raw data behind this figure ( Schwarz, 2020).
We also assessed the tissue specific induction of Cre-mediated miniTdg excision in vivo. We crossed miniTdg mice with mice expressing Cre-recombinase under the control of a FoxN1-promoter ( http://www.informatics.jax.org/allele/key/62500) which drives expression in thymic epithelial cells (TECs), but not in thymic cells (TCs). We observed that the mRNA and protein expressed from the miniTdg are reliably depleted in TECs expressing FoxN1-driven Cre , whereas TDG levels are much less affected in TCs ( Extended data, Figure S1D/E) ( Schwarz, 2020).
These data demonstrate the functionality and tight regulation of the inducible miniTdg KO model in murine ESCs as well as in mice. A short-time Cre-induction with 1–5 μM or 5–10 μM OHT (in 2i+LIF or 15% FCS-containing medium, respectively) is sufficient to mediate excision of the miniTdg, resulting in depletion of TDG below detection within 24 hours. This rapid depletion of TDG is presumably facilitated by the cell cycle-regulated proteasomal degradation of TDG via the ubiquitination at its PCNA-interacting peptide (PIP) motif ( Hardeland et al., 2007; Shibata et al., 2014).
Molecular and cellular phenotypes of TDG depletion in TDGiKO ESCs
To validate the molecular phenotype of TDG depletion in our newly derived TDGiKO ESC, we again assessed changes in levels of oxidized 5-mC derivates, 5-formylcytosine (5-fC) and 5-caC following Cre-mediated miniTdg deletion. One week after depletion of TDG, we measured a 4.4 and 4.2-fold accumulation of the TDG substrates 5-fC and 5-caC in TDGiKO ESCs, respectively, but no changes of 5-hydroxymethyl cytosine (5-hmC), which is not recognized by TDG ( Figure 3A). This accumulation reflects well the previously reported measurements in constitutive Tdg KO cells ( Shen et al., 2013; Steinacher et al., 2019).
Figure 3. TDGiKO ECSs express a functional TDG and are proficient for cell lineage commitment.
( A) Measurement of oxidized derivates of 5-mC in selected ESCs by HPLC-MS/MS, cultivated in 2i medium + LIF, one week after TDG depletion by 1 µM of OHT for 2 h. Shown are the means and standard deviation of 2-3 biological replica. ( B) Scheme of the neural differentiation protocol ( Bibel et al., 2007), adapted with a 16 h priming step in ESC medium for ESCs grown in 2i medium. Representative phase-contrast images of TDGiKO1 cells with (+OHT) and without (mock) TDG depletion at the differentiation stages indicated (arrows). ( C) Expression of pluripotency and germ layer marker genes were assessed by qRT-PCR in TDGiKO1 cells at the stages of differentiation as marked in ( B). Expression was normalized to the housekeeping genes Rps13 and Eef1a1. Shown are means and standard errors of 3 biological replicates. Asterisks indicate p-values of Student’s t-test: * p-value < 0.05, ***p-value < 0.001. See Underlying data for the raw data behind this figure ( Schwarz, 2020).
Finally, we re-tested the pluripotency and differentiation capacity of some of the engineered TDGiKO ESCs clones ( Extended data, Table S2) ( Schwarz, 2020). When subjecting TDGiKO1 ESCs to all-trans retinoic acid (RA) induced in vitro differentiation towards the neuronal lineage ( Bibel et al., 2007), we observed an upregulation of the neural marker Nestin ( Figure 3B, C) and, ultimately, the formation of neuron-like cells ( Figure 3B). As expected during embryoid body (EB) formation, LIF withdrawal resulted in an induction of lineage-specific genes of all three germ layers and the repression of the pluripotency marker gene Nanog. This indicates the ability of the TDGiKO ESCs to commit to cell types of all germ layers. In support of this, continued cultivation of TDGiKO1 and TDGiKO1.1 in ESM without LIF led to a spontaneous differentiation to cardiomyocytes (mesodermal cell type) forming beating organoids (see Extended data Videos 1 and 2) ( Schwarz, 2020). While the early initiation of lineage-specific genes seemed to be unaffected by TDG depletion prior to differentiation, the formation of neuron-like cells ( Figure 3B, bottom) was impaired. This deficiency to stably commit to and/or maintain the neuronal lineage was indicated by a low number of neuron-like cells formed and the loss of cells with Nestin expression following RA application. Upon loss of TDG, we furthermore observed a significant difference in the downregulation of the pluripotency marker Nanog as well as and dysregulation of the endodermal marker Gata6 ( Figure 3C).
Thus, when subjecting the TDGiKO ESCs to in vitro differentiation, we obtained data consistent with previous observations in constitutive Tdg KO ESCs ( Steinacher et al., 2019). This highlights the usability of the newly established ESCs in TDG and BER-related research, with the advantage of eliminating clonal divergences between TDG proficient and deficient cells. While here, we focused on the reproduction of known and published TDG KO phenotypes, the fast and reliable depletion of TDG in the TDGiKO ESC model now provides a tool for in-depth execution point analysis of TDG-BER mediated active DNA demethylation during cell differentiation or in any other context of interest. Furthermore, the loxP sites flanking the coding region of the miniTdg gene enable recombinase-mediated cassette exchange to introduce TDG separation of function variants devoid of catalytic function or harbour mutations that ablate posttranslational modifications ( Hardeland et al., 2000). Additionally, the availability of mice carrying the miniTdg, which can be combined with Cre-driver mice of interest by crossing, allows the translation of in vitro observations into in vivo settings. All in all, we provide a versatile, well-controlled and validated toolbox ( Extended data, Table S2) ( Schwarz, 2020) to facilitate functional and mechanistic research on TDG-mediated active DNA demethylation and BER in cell culture and animal models.
Data availability
Underlying data
Zenodo: Inducible TDG knockout models to study epigenetic regulation. http://doi.org/10.5281/zenodo.3979375 ( Schwarz, 2020).
This project contains the following underlying data:
Raw_Data_Figure1.zip. Database excerpt for genotyping of mouse crossings, original pictures of Southern and western blots and MS-quantification of oxidized mC derivates (also for figure 3).
Raw_Data_Figure2.zip. qPCR data of miniTDG excision, original pictures of OHT-treated ESCs and western blots.
Raw_Data_Figure3.zip. Original pictures of differentiating cells and qPCR data of pluripotency and germ line markers.
Raw_Data_Extended_Figure.zip. Sequencing data for Neo R excision, absorption values of G418-treated ESCs, qPCR data for miniTDG excision and original pictures of western blots.
Extended data
Zenodo: Inducible TDG knockout models to study epigenetic regulation. http://doi.org/10.5281/zenodo.3979375 ( Schwarz, 2020).
This project contains the following extended data:
Extended Figure and Tables.pdf. Supplementary information as referenced in the text.
Extended_Video_1_TDGiKO1.mp4. Video of “beating bodies” from an ESC clone.
Extended_Video_2_TDGiKO1.2.mp4. Video of “beating bodies” from a second ESC clone.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgments
We want to express our thanks to the laboratory of Wolf Reik and the transgenics facility at the Babraham Institute (Cambridge, UK) for performing the pronuclear injection. We also thank the animal facility at the DBM Mattenstrasse and the transgenic core facility of the University of Basel for their help in breeding the mouse strains and establishing ESCs. Additional thanks goes to Carlos Mayer from George Holländer’s Lab (DBM) for the sorting of the thymic cells.
Funding Statement
This work was supported by the Swiss National Science Foundation, grant 156467.
[version 1; peer review: 3 approved]
References
- Bibel M, Richter J, Lacroix E, et al. : Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2(5):1034–1043. 10.1038/nprot.2007.147 [DOI] [PubMed] [Google Scholar]
- Cortázar D, Kunz C, Selfridge J, et al. : Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature. 2011;470(7334):419–423. 10.1038/nature09672 [DOI] [PubMed] [Google Scholar]
- Cortellino S, Xu J, Sannai M, et al. : Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146(1):67–79. 10.1016/j.cell.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland U, Bentele M, Jiricny J, et al. : Separating substrate recognition from base hydrolysis in human thymine DNA glycosylase by mutational analysis. J Biol Chem. 2000;275(43):33449–33456. 10.1074/jbc.M005095200 [DOI] [PubMed] [Google Scholar]
- Hardeland U, Kunz C, Focke F, et al. : Cell cycle regulation as a mechanism for functional separation of the apparently redundant uracil DNA glycosylases TDG and UNG2. Nucleic Acids Res. 2007;35(11):3859–3867. 10.1093/nar/gkm337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RA, Mancuso P, Kuo YM, et al. : Interaction with the DNA Repair Protein Thymine DNA Glycosylase Regulates Histone Acetylation by p300. Biochemistry. 2016;55(49):6766–6775. 10.1021/acs.biochem.6b00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AL, Schär P: DNA glycosylases: In DNA repair and beyond. Chromosoma. 2012;121(1):1–20. 10.1007/s00412-011-0347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz C, Focke F, Saito Y, et al. : Base excision by thymine DNA glycosylase mediates DNA-directed cytotoxicity of 5-fluorouracil. PLoS Biol. 2009;7(4):e91. 10.1371/journal.pbio.1000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger H, Smet-Nocca C, Attmane-Elakeb A, et al. : A TDG/CBP/RARα ternary complex mediates the retinoic acid-dependent expression of DNA methylation-sensitive genes. Genom Proteom Bioinf. 2014;12(1):8–18. 10.1016/j.gpb.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti A, Drohat AC: Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem. 2011;286(41):35334–35338. 10.1074/jbc.C111.284620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL: Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A. 2007;104(3):1027–1032. 10.1073/pnas.0610155104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann D, Weber AR, Schär P: Active DNA demethylation by DNA repair: Facts and uncertainties. DNA Repair (Amst). 2016;44:92–102. 10.1016/j.dnarep.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Schwarz SD: Inducible TDG knockout models to study epigenetic regulation [Data set].F1000 Research. Zenodo.2020. 10.5281/zenodo.3979375 [DOI] [PMC free article] [PubMed]
- Shen L, Wu H, Diep D, et al. : Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153(3):692–706. 10.1016/j.cell.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E, Dar A, Dutta A: CRL4Cdt2 E3 ubiquitin ligase and Proliferating Cell Nuclear Antigen (PCNA) cooperate to degrade thymine DNA glycosylase in S phase. J Biol Chem. 2014;289(33):23056–23064. 10.1074/jbc.M114.574210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Steinacher R, Barekati Z, Botev P, et al. : SUMOylation coordinates BERosome assembly in active DNA demethylation during cell differentiation. EMBO J. 2019;38(1):e99242. 10.15252/embj.201899242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um S, Harbers M, Benecke A, et al. : Retinoic Acid Receptors Interact Physically and Functionally with the T:G Mismatch-specific Thymine-DNA Glycosylase. J Biol Chem. 1998;273(33):20728–36. 10.1074/jbc.273.33.20728 [DOI] [PubMed] [Google Scholar]
- Weber AR, Krawczyk C, Robertson AB, et al. : Biochemical reconstitution of TET1-TDG-BER-dependent active DNA demethylation reveals a highly coordinated mechanism. Nat Commun. 2016;7:10806. 10.1038/ncomms10806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q, Wray J, Nichols J, et al. : The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–524. 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]