Abstract
Increased access to and improved sensitivities of methods for diagnosing Mycobacterium tuberculosis infection and detecting rifampicin and isoniazid resistance are needed. Herein, the performance of the new cobas MTB assay for use on cobas 6800/8800 Systems (Roche) was assessed and compared with two other commercial assays: RealTime MTB (Abbott), and Xpert MTB/RIF (Cepheid). Molecular PCR-based assays were conducted on sputum specimens from individuals with presumptive and confirmed tuberculosis (n = 294) from two clinical facilities in South Africa between December 2016 and October 2017. Liquid mycobacterial culture was the reference. Test sensitivities were 94.7% (95% CI, 88%–98%), 92.6% (95% CI, 85%–97%), and 91.6% (95% CI, 84%–96%) for cobas MTB, RealTime MTB, and Xpert MTB/RIF assays, respectively. cobas MTB sensitivity was unaffected by HIV coinfection (95.7%; 95% CI, 88%–99%; n = 176) and sediment testing (94.7%; 95% CI, 88%–98%). Sensitivities were 81.8% (95% CI, 60%–95%), 72.7% (95% CI, 50%–89%), and 72.7% (95% CI, 50%–89%) among smear-negative, culture-positive individuals (n = 221) for cobas MTB, RealTime MTB, and Xpert MTB/RIF assays, respectively. cobas MTB specificity was 95.7% (95% CI, 89%–99%) and 99% (95% CI, 94%–100%) among HIV coinfected and uninfected individuals, respectively. The cobas 6800/8800 system is already implemented in South Africa for high-throughput HIV viral load testing, making it suitable for integrated HIV/tuberculosis diagnostics.
The estimated incidence of tuberculosis (TB) in South Africa in 2017 was 567/100,000, and 60% of TB cases were co-infected with HIV.1 In 2011, the Xpert MTB/RIF (Cepheid, Sunnyvale, CA) test was implemented2 as the initial screening test for pulmonary TB disease in adults,3 followed later by its use on specimens from children4 and extrapulmonary specimens.5 In 2017, the Xpert was replaced with the Xpert MTB/RIF Ultra (Cepheid). The average annual rate of detection of Mycobacterium tuberculosis (MTB) infection reported nationally using the Xpert laboratory test was 10%, which increased to 12% once the more sensitive Ultra6 was implemented. Before molecular testing, the estimated detection rates were 4.6% in 2012 to 2014 and 3.4% in 2001 to 2002.7 The Xpert and Ultra identify approximately 5% of TB-positive cases with resistance to rifampicin (RIF). Although non–molecular-based methods are available for TB diagnosis, approximately 2.3 million Ultra tests were performed in 2018 in South Africa.
Molecular tests are also widely utilized as part of the national HIV program for diagnosing HIV infection (5.1 million HIV viral load tests and 0.6 million early infant PCR HIV diagnostic tests were performed in 2018). This volume of testing requires centralized laboratory systems with automated workflow, capable of high throughput in addition to good logistics support. Systems currently in use for HIV viral load testing are the cobas 6800/8800 (up to 1000 specimens/8-hour capacity; Roche Molecular Diagnostics, Pleasanton, CA),8 the COBAS AmpliPrep/COBAS TaqMan (45 results reported/day; Roche Molecular Diagnostics),9 and m2000rt/m2000sp (93 tests/8 hours; Abbott Molecular, Des Plaines, IL).9 For TB diagnostics, the Xpert XVI (64 tests/8 hours; Cepheid) and the Xpert Infinity-80 (320 tests/8 hours; Cepheid) systems are used.10 In response to the need for HIV and TB integration of care11 and to address gaps in access to pathology and laboratory health services in low-resource settings,12 manufacturing companies are increasing the number and types of diagnostic tests available on molecular diagnostic platforms.9,10,13,14 Following this approach, the cobas MTB assay was developed for integration on to the fully automated, high-throughput cobas 6800/8800 system, which was initially only used for HIV viral load monitoring. The cobas MTB assay is an automated RT-PCR–based test used for the detection of M. tuberculosis complex (MTBC) DNA in sputum specimens. Simultaneous testing for RIF and isoniazid (INH) resistance as well as testing for nontuberculosis infections can be conducted using the cobas MTB-RIF/INH test and the cobas MAI assays, respectively. These tests are all currently CE IVD only.8,15,16 The characteristics of the cobas MTB, cobas MTB-RIF/INH, and other commercially available comparator assays [RealTime MTB assay (Abbott, Des Plaines, IL) and Xpert MTB/RIF and Xpert MTB/RIF Ultra (both from Cepheid)] are outlined in Table 1.
Table 1.
Characteristics of Four Molecular Assays Designed for the Screening of Mycobacterium tuberculosis Disease, Including the Detection of RIF and INH Susceptibility
| Assay name | cobas MTB and cobas MTB-RIF/INH | Abbott RealTime (MTB, RIF/INH)17, 18, 19 | Xpert MTB/RIF20 | Xpert MTB/RIF Ultra21 |
|---|---|---|---|---|
| Regulatory status | CE-IVD | CE-IVD | CE-IVD | |
| Platform | cobas 6800/8800 | m2000 system | Xpert Infinity-48s/80 | |
| Target in MTBC | MTBC: 16S rRNA and 5 esx RIF-INH: rpoB, katG, and inhA |
MTBC: IS6110 and pab gene RIF-INH: rpoB, katG, and inhA |
rpoB with 5 probes | IS6110, IS1081, and rpoB with 4 probes |
| LOD | MTBC: 7.6–8.8 CFU/mL | MTBC: 2.5–35 CFU/mL RIF-INH: 60 CFU/mL |
50–165 CFU/mL | 5–25 CFU/mL |
| Specimen type and volume | Sputum: 0.4–1.2 mL Sediment: 0.2–0.6 mL |
Sputum or sediment: 0.5 mL | Sputum: 1 mL Sediment: 0.5 mL |
|
| Specimen processing and time | 1. Specimen + MIS (1:2) (1 minute/specimen) 2. Vortex (20–30 seconds) 3. Inactivation (1 hour) 4. Sonication (1 minute/specimen) 5. Centrifugation (1 minute at 3000 rcf) 4. Test (∼2 hours 20 minutes) |
1. Specimen + IR (1:3) (1 minute/specimen) 2. Vortex (20–30 seconds) 3. Inactivation (1 hour) 4. Test (∼3 hours for DNA extraction and 2 hours 30 minutes for amplification and detection) |
1. Specimen + SR (1:2) (1 minute/specimen) 2. Vortex (10 seconds) 3. Inactivation (15 minutes) 4. Test (1 hour 48 minutes) |
1. Specimen + SR (1:2) (2 minutes/specimen) 2. Vortex (10 seconds) 3. Inactivation (15 minutes) 4. Test (65 minutes negative results, 77 minutes positive results) |
| Extraction method | Chemical and mechanical (sonication) combined with magnetic glass particle technology for DNA capture | Chemical (guanidium thiocyanate) and magnetic microparticle technology for DNA capture | Closed cartridge, intact bacteria retained on a filter membrane, and DNA extracted with mechanical ultrasonication | |
| Amplification and detection | PCR volume = 25 μL Qualitative real-time PCR Fluorescent-labeled target-specific probes |
PCR volume = 50 μL Fluorescence-based real-time PCR Fluorescent-labeled target-specific probes |
PCR volume = 25 μL Heminested real-time PCR Molecular beacons |
PCR volume = 50 μL Semiquantitative, nested real-time PCR; SMB |
| Testing time | 8 hours/45 specimens (MTB detection and resistance profiling)∗ | 8 hours/93 specimens (MTB detection) + 3 hours/24 specimens (resistance detection) | ∼2 hours/specimen | ∼1–1.5 hours/specimen |
| Reported result | 1. MTBC: MTB positive/negative 2. RIF-INH: invalid/RIF positive/RIF negative/INH positive/INH negative |
1. MTBC: MTB positive/negative 2. RIF-INH: RIF R-; INH R-/RIF R det; INH high R/RIF R det; INH low R/RIF R det; INH low R/RIF R det; INH R/RIF R det; INH R/RIF Indet; INH R-/RIF R-; INH Indet/RIF R det; INH Indet/RIF Indet; INH Indet/Below LOD |
1. MTBC: MTB not detected/MTB detected (very low/low/medium/high) 2. RIF: RIF resistance (not detected/detected/indeterminate) 3. Error 4. Invalid 5. No result |
1. MTBC: MTB not detected/MTB detected (very low/low/medium/high/trace) 2. RIF: RIF resistance (not detected/detected/indeterminate) 3. Error 4. Invalid 5. No result |
CFU, colony-forming unit; Indet, indeterminate; INH, isoniazid; IR, inactivation reagent; LOD, limit of detection; MIS, mycobacterial inactivation solution; MTB, M. tuberculosis; MTBC, MTB complex; rcf, relative centrifugal force; R, resistant; R-, resistance not detected; R det, resistance detected; RIF, rifampicin; SMB, sticky molecular beacon; SR, sample reagent.
Throughput based on current study conditions, which can increase with scaled front-end sonication to system capacity (cobas 6800 system has capacity for 384 results/8 hours and cobas 8800 system has capacity for 960 results/8 hours).
The Xpert MTB/RIF assay was endorsed by the World Health Organization (WHO) in 201022 and can be applied in both decentralized and centralized testing settings.23 The system has a closed cartridge approach, in direct contrast to the cobas MTB and RealTime MTB, which are multi–plate-based platforms. The Xpert MTB/RIF, however, is comparable on throughput when used in the Xpert Infinity-48s/80 system, because of the modular approach of the Xpert system. The Ultra cartridge, on the other hand, requires a software change (version 4.7b) for testing on an existing Xpert system, but all other processes remain unchanged. Determining RIF resistance and whether high-dose INH is required or exclusion of it17 is important as part of a multi–drug-resistant treatment regimen. This depends on detecting the presence of the rpoB, katG, and inhA genes. The Xpert and Ultra assays are limited in this regard because they are only able to detect RIF resistance genes. This, however, has been addressed by Roche and Abbott with the cobas MTB and RealTime MTB reflex tests and the cobas MTB-RIF/INH and RealTime MTB RIF/INH tests, respectively, which are able to detect both RIF and INH resistance. The RealTime MTB and RealTime MTB RIF/INH tests require two separate testing areas (extraction on the m2000sp and amplification on the m2000rt platforms), whereas the cobas MTB requires additional front-end equipment for sonication and centrifugation. All three high-throughput systems require operation by a technician, and all systems are capable of interfacing with a laboratory information system.
In this study, the cobas MTB and cobas RIF/INH assays were evaluated for use on the cobas 6800/8800 system high-throughput multi-analyte platform in comparison with the standard of care (SOC) Cepheid Xpert MTB/RIF assay available at the time of study in South Africa, as well as the commercially available Abbott RealTime MTB for the detection of MTB from sputum and sediment specimens in a high-burden HIV patient population accessing TB care.
Materials and Methods
Participant Recruitment and Study Procedures
Participants were enrolled at two clinical facilities in South Africa from December 2016 to October 2017: in Soweto at Chris Hani Baragwanath Hospital in Gauteng Province and at Tshepong Hospital in the Matlosana Municipality of the North West Province. Eligible participants were aged ≥18 years and were symptomatic for TB. Symptoms included weight loss, fever, cough for >2 weeks, and night sweats. The participants would additionally be willing and able to have an HIV test and to return for a second visit. Participants who had received any TB treatment in the 6 months before their second study visit were not eligible for the study. Some participants already diagnosed with pulmonary TB by public sector testing and referred to the research sites were also tested (before the initiation of TB treatment). All individuals provided written informed consent. Participants were required to provide two raw sputum specimens at two scheduled clinical visits, a minimum of 24 hours apart. Participants who did not provide all four specimens were excluded from this analysis, as were those who started TB treatment after the first sampling. SOC testing was prioritized, and all participants with a positive Xpert MTB/RIF or a mycobacteria growth indicator tube (MGIT) positive test were recontacted and started on TB treatment as per the South African TB management guidelines (National Tuberculosis Management Guidelines, Department of Health, Republic of South Africa, http://www.tbonline.info/media/uploads/documents/ntcp_adult_tb-guidelines-27.5.2014.pdf, last accessed May 4, 2020). Results from the cobas MTB and Abbott RealTime MTB were not used for clinical decision-making.
Laboratory Procedures
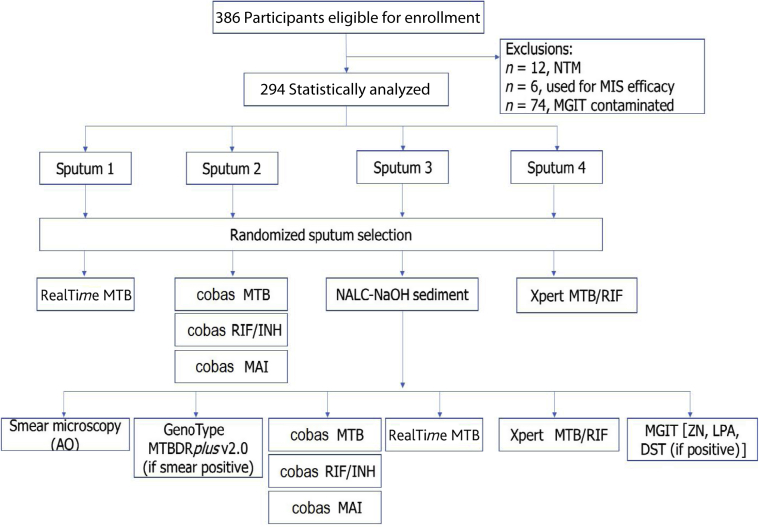
All specimens were transported to the clinical testing laboratory in Braamfontein (Johannesburg), which is accredited by the Division of AIDS and South African National Accreditation Service, for SOC and research testing. Patient results were routinely returned to the clinical testing sites for patient management. The four raw sputum specimens from each participant were randomly selected for TB testing using the Xpert MTB/RIF (n = 1), the cobas MTB (and corresponding cobas RIF/INH) (n = 1), or the RealTime MTB (n = 1) or were processed with N-acetyl-l-cysteine sodium hydroxide to prepare a sediment (n = 1) and smear microscopy, as outlined in Figure 1.
Figure 1.
Description of the study and laboratory testing. Analysis of the cobas MAI is not reported. AO, auramine O; DST, drug susceptibility test; INH, isoniazid; LPA, line probe assay; MAI, Mycobacterium avium and Mycobacterium intracellulare; MGIT, mycobacteria growth indicator tube; MIS, microbial inactivation solution; MTB, Mycobacterium tuberculosis; MTBDR, Mycobacterium tuberculosis drug resistant; NALC-NaOH, N-acetyl-l-cysteine sodium hydroxide; NTM, nontubercular mycobacteria; RIF, rifampicin; ZN, Ziehl-Neelsen staining.
Sediments were tested for acid-fast bacillus (AFB) by smear microscopy (auramine O fluorescence staining) and graded according to international guidelines (https://medicalguidelines.msf.org/viewport/TUB/latest/appendix-2-sputum-smear-process-20323719.html, last accessed May 4, 2020). Results were reported as negative (0) for no AFB seen per one length, scanty for 1 to 19 AFBs per one length, + for 20 to 199 AFBs per one length, ++ for 5 to 50 AFBs per one field, and +++ for >50 AFBs per field. MGIT liquid culture, MGIT drug susceptibility testing (DST), and the GenoType MTBDRplus version 2.0 line probe assay (Hain Lifescience GmbH, Nehren, Germany) were performed as part of SOC. These tests were performed as per the manufacturer's instructions and as previously described.10 Briefly, for liquid culture, 500 μL of the concentrated pellet was added to prepared MGIT (Becton Dickinson & Co, Franklin Lakes, NJ) and loaded onto the Bactec MGIT 960 mycobacterial detection system (Becton Dickinson & Co). The tubes were incubated until either a positive result was noted or 42 days had passed without a positive result (MGIT culture negative). Positive results or contamination was confirmed using blood agar and Ziehl-Neelsen staining. MGIT DST [Bactec MGIT 960 SIRE (streptomycin, isoniazid, rifampin, and ethambutol) and pyrazinamide; Becton Dickinson & Co] was performed on positive cultures. Species identification and genotypic first-line DST was performed using the GenoType MTBDRplus version 2 assay (Hain LifeScience GmbH) on smear-positive sputum specimens. The presence/absence of MTBC and resistance/susceptibility to RIF and INH were visually reported. The cobas MTB, RealTime MTB, and Xpert MTB/RIF assays were performed on sputum and processed sediment.
The cobas microbial inactivation solution (MIS; Roche Molecular Diagnostics) is a component of the cobas MTB and the cobas MTB-RIF/INH assays, used before testing on the cobas 6800/8800 system, to render any MTBC inactive and ensure specimens are safe for testing outside a biosafety level 3 laboratory environment. An in-house verification inactivation study was performed, to confirm the performance of the MIS as follows: three laboratory-derived isolates from MGIT tubes, confirmed to be positive for MTBC using the line probe assay, were selected and testing was conducted using a neat (undiluted), 1:10, and 1:100 aliquots of each culture. In addition, a positive control (without the addition of the inactivation solution) was processed for each dilution. Culture aliquots were inactivated according to manufacturer's instructions, where inactivation solution was added to each aliquot in a 1:4 (culture/inactivation solution) ratio. The MGIT-positive controls showed growth in 3 to 5 days, thereby indicating that a heavy initial inoculum was used (approximately 105 to 106 colony-forming units/mL). Once the MIS was proved effective using culture isolates, research specimens from six eligible participants in this study were set aside for inclusion in a small study to confirm that the Roche MIS reagent is able to inactivate MTB in sputum specimens.
Statistical Analysis
Data analysis included calculation of: sensitivity, specificity, reproducibility, positive percentage agreement, negative percentage agreement, and overall percentage agreement; the calculated two-sided 95% CIs for positive percentage agreement, negative percentage agreement, and overall percentage agreement for each test were applied, and Standards for Reporting of Diagnostic Accuracy Studies guidelines were followed (Equator Network, https://www.equator-network.org/reporting-guidelines/stard, last accessed May 7, 2020). Performance indicators were tabulated and represented in radar plots for the full data set as well as subanalyses stratified by HIV and smear status. Any significant differences in participants recruited from the two clinical sites was determined using a t-test and χ2 statistical test for continuous numbers or proportions, respectively. The Levene test was applied to determine the equality of variances for the different characteristics across the sites. The CT PCR quantitative variable from the cobas MTB assay was also recorded and graphically represented in a scatterplot alongside smear grade (−, scanty, +, ++, and +++) and the time (days) to positivity (TTP) of an MGIT culture. The scatterplot included trend analysis parameters (r, linear equation, and R2) of the CT values sorted by increasing TTP.
To compare the performance of the cobas MTB, RT-MTB, and Xpert MTB/RIF, only specimens that generated valid results across all assays (molecular, smear, and culture) were included in the statistical method comparison analysis. MGIT cultures that were positive for contaminating organisms or did not have an MTBC-positive culture result were excluded from the statistical analyses to measure assay performance. In addition, some distinguishing qualitative features of the Xpert Infinity-48s/80, the cobas 6800/8800, and the m2000sp/m2000rt molecular systems were recognized by study personnel during the testing period. These features are categorized into four sections (specimen processing, maintenance requirements, result interpretation, and overall ease of use) and are presented in Table 2. Some features would contribute to increased hands-on time, potential for contamination, reliance on the manufacturer for troubleshooting, and differences in interpretation of results.
Table 2.
Comparison of the Different Molecular Instruments and Assay Utility
| Features | GeneXpert Infinity-48s/80 | m2000sp/m2000rt | cobas 6800/8800 | |
|---|---|---|---|---|
| Processing | Access | Random access | No random access | No random access |
| Express lane available | ||||
| Reagents | External storage | Reagent reconstitution required and wasted if not a full run | Ready-to-use reagents (no thawing, mixing, or pouring) | |
| Inactivation | Inactivation with a 5–15 minutes incubation step | Preparation of the inactivation reagent is required. Specimens should be incubated with the inactivation reagent for ≥1 hour. | Specimens should be incubated with the inactivation reagent for ≥1 hour | |
| Additional front-end processing | Cartridge barcodes scanned individually | Manual air bubble check and removal before testing | Sonication step integrated into pre-analytic workflow would benefit from full automation. | |
| Manual PCR plate sealing | PCR plate sealed and automated pipette tightness check by instrument | |||
| Sample preservation | Cartridges can only be loaded once. Specimen is lost if not processed. Any remaining reagent-treated specimen can be kept up to 4 hours at 2°C to 8°C. | Residual DNA can be reflexed to resistance assay and stored | Residual DNA extracted material unavailable and discarded. Smaller input of patient specimen may enable more runs per available specimen. | |
| Maintenance required | Frequency | Daily, weekly, monthly | Daily, weekly, monthly | Weekly (minimal) |
| Result interpretation | Flags for problematic specimens | Problematic specimens have defined flags (eg, MTB invalid/no result; SPC: fail/no result; probe check: pass/fail) on result report | Problematic specimens have defined flags on result report (eg, target CN is less than minimum) | Problematic specimens have flags on result report, but with less definition than comparators (ie, result invalid) |
| Graph settings | Graph settings and overview for assay targets (melting temperature, CT) are accessible | Individual result analysis accessible (graph settings, target baseline, control baseline, threshold, cycle number, maximum ratio) | Graph settings and overview for assay targets not available (only CT provided) | |
| Result interpretation | Result interpretation is simple: | Result interpretation is detailed, with the MTB +/−, the mutation and interpretation column reported: eg, | Result interpretation is complex: | |
| MTB detected, RIF resistance detected | MTB_POS, rpoB wt; katG wt; inhA wt, RIF R-, INH R-, no details of mutations incurring resistance provided | MTB +/−/invalid | ||
| MTB detected, RIF resistance not detected | MTB-RIF/INH: INH (target 1) +/−/invalid, RIF targets 2, 3, 4: +/−/invalid | |||
| MTB detected, RIF resistance indeterminate | ||||
| MTB not detected | No details of mutations incurring resistance | |||
| Invalid, error, no result | ||||
| Functionality | USB/CD options | USB function available | No USB function, only CD-ROM accepted | No USB function |
| Troubleshooting | Troubleshooting requires manufacturer input | Operator manual contains detailed troubleshooting steps | Troubleshooting requires manufacturer input | |
| Storage | External storage is required | Storage cabinet on instrument | On-board reagent storage for life of the reagents. No requirement to unload each day. | |
CD, compact disc; CN, cycle number; INH, isoniazid; inhA wt, enoyl acyl carrier protein reductase InhA (isoniazid)A wild type; INH R-, isoniazid resistance not detected; katG wt, catalase-peroxidase wild type; MTB, Mycobacterium tuberculosis; MTB_POS, Mycobacterium tuberculosis positive; RIF, rifampicin; RIF R-, rifampicin resistance not detected; rpoB wt, RNA Polymerase Beta Subunit wild type; SPC, internal control; USB, Universal Serial Bus.
Study Ethics Approval
Ethics approval for the study was obtained from the Human Research Ethics Committee at the University of Witwatersrand, Johannesburg, South Africa (REF number M1511110).
Results
Cohort Characteristics
Participant characteristics for those included in the statistical analysis (n = 294) are detailed in Table 3. The mean participant age was 41 years (interquartile range, 18–73 years); 160 (54%) were male and 176 (60%) were HIV positive. Participant enrollment was balanced between the two study sites; however, there was a higher proportion of HIV-positive patients in Soweto (64% HIV+) versus Matlosana (58%). The proportion on antiretroviral therapy was notably lower in Soweto (22%) versus Matlosana (70%). More than 96% of participants reported more than one symptom of TB infection, and 71% reported a loss of weight within the ≥7 days before their first clinic visit. Approximately 3% of individuals reported having a previous TB infection. Table 4 further describes the biological classification of the specimens received. On the basis of the Xpert MTB/RIF test results, the overall TB positivity was 31%, with 6.5% resistant to RIF.
Table 3.
Study Participant Characteristics and Distribution of Specimens Collected from Each Clinical Site
| Characteristic | Participants (n = 294) | Matlosana (n = 193) | Soweto (n = 101) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (range), years | 41 (18–73) | 42 (18–73) | 39 (19–68) | 0.048 |
| Male sex, n (%) | 160 (54.5) | 104 (53.8) | 58 (55.4) | 0.799 |
| HIV-related information | ||||
| HIV negative, n (%) | 118 (40.1) | 82 (42.5) | 36 (35.6) | 0.254 |
| HIV positive, n (%) | 176 (59.9) | 111 (58) | 65 (64.3) | 0.254 |
| CD4 cell count, mean (range), cells/μL | 293 (4–1307) | 319 (4–1307) | 252 (9–833) | 0.097 |
| Receiving ARV therapy, n/total (%) | 92/175 (52.6) | 78/111 (70.2) | 14/64 (21.9) | 0.0001 |
| TB history | ||||
| Previously diagnosed with TB, n (%) | 10 (3) | 4 (1) | 6 (2) | <0.0001 |
| Clinical signs and symptoms at presentation (n = 294) | ||||
|---|---|---|---|---|
| Reported weight loss, n (%) | 210 (71) | |||
| Reported fever, n (%) | 104 (35) | |||
| Reported cough >2 weeks, n (%) | 151 (51) | |||
| Reported night sweats, n (%) | 286 (97) | |||
ARV, antiretroviral; TB, tuberculosis.
Table 4.
Characterization of Specimens by Laboratory Methods
| Bacteriologic classification | |
| Smear and culture negative | 199 (68) |
| Smear negative and culture positive | 22 (7) |
| Smear and culture positive | 73 (25) |
| Scanty | 11 (15) |
| + | 14 (19) |
| ++ | 15 (21) |
| +++ | 33 (45) |
| Molecular classification of TB by Xpert MTB/RIF (SOC, raw sputum) | |
| Xpert MTB/RIF MTB positive | 92 (31) |
| Xpert MTB/RIF RIF resistance | 6/92 (6.5) |
Data are given as number (percentage).
+, 1 to 9 acid-fast bacilli per 100 fields; ++, 1 to 9 acid-fast bacilli per 10 fields; +++, 1 to 9 acid-fast bacilli per 1 field; MTB, Mycobacterium tuberculosis; RIF, rifampicin; SOC, standard of care; TB, tuberculosis.
Diagnostic Performance of the cobas MTB Assay Compared with SOC
Before testing on the cobas 6800, the ability of the cobas MIS to inactivate MGIT inoculum was shown by 17 of 18 MIS-treated cultures, demonstrating no growth of MTBC after the 42-day incubation period. One culture was visually contaminated with mold growth.
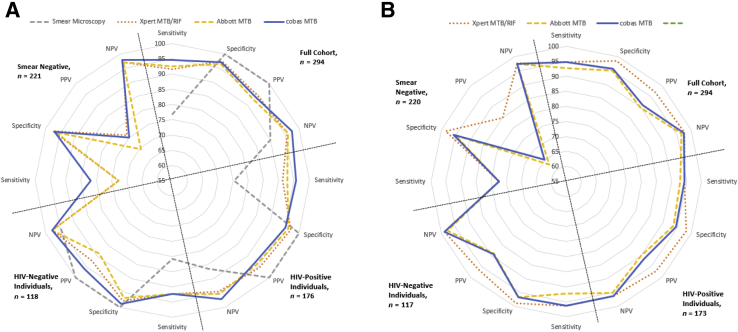
The performance of the cobas MTB, Xpert MTB/RIF, and RealTime MTB assays on their respective systems for both sputum and decontaminated sediment specimens is compared with the reference method of liquid culture and, additionally, stratified by smear status, as outlined in Table 5 and Table 6 as well as in Figure 2 for 294 specimen results. Among the cohort (n = 294), the cobas MTB test on sputum samples (Table 5) generated the highest sensitivity of 94.7%, followed by 92.6% for RealTime MTB and 91.6% for Xpert MTB/RIF, with overlapping CIs in all cases. The specificity, however, remained similar at approximately 97% for all the molecular tests. Overall, the negative predictive value for cobas MTB (97.5%) was slightly higher than both Xpert MTB/RIF (96%) and RealTime MTB (96.5%). Xpert MTB/RIF (94.6%), on the other hand, had the highest PPV. Similarly among HIV co-infected individuals, the cobas MTB assays showed improved sensitivity of 95.7%. The negative predictive value for cobas MTB (97.1%) also showed improvement over Xpert MTB/RIF (94.5%) and RealTime MTB (95.4%). The cobas MTB, however, had a lower specificity and PPV than the other assays analyzed. The PPV among HIV-negative individuals for cobas MTB was increased (96.0%) in comparison to the other assays.
Table 5.
The Performance (Including 95% CIs) of Smear Microcopy and Molecular TB Assays Compared with the Reference Method (Liquid Culture), for Sputum
| Variable | Smear microscopy |
Xpert MTB/RIF |
RealTime MTB |
cobas MTB |
|---|---|---|---|---|
| Sputum | ||||
| All samples (n = 294) | ||||
| Sensitivity, % | 76.8 (67.1–84.9) | 91.6 (84.1–96.3) | 92.6 (85.4–97.0) | 94.7 (88.1–98.3) |
| Specificity, % | 100 (98.2–100) | 97.5 (94.1–99.2) | 96.5 (92.9–98.6) | 97.0 (93.6–98.9) |
| PPV, % | 100 (95.1–100) | 94.6 (87.8–98.2) | 92.6 (85.4–97.0) | 93.8 (86.9–97.7) |
| NPV, % | 90.0 (85.3–93.7) | 96.0 (92.3–98.3) | 96.5 (92.9–98.6) | 97.5 (94.2–99.2) |
| Specimens from HIV-positive individuals (n = 176) | ||||
| Sensitivity, % | 75.4 (63.5–84.9) | 91.3 (82.0–96.7) | 92.8 (83.9–97.6) | 95.7 (87.8–99.1) |
| Specificity, % | 100 (96.6–100) | 97.2 (92.0–99.4) | 96.3 (90.7–99.0) | 95.3 (89.4–98.5) |
| PPV, % | 100 (93.0–100) | 95.5 (87.3–99.1) | 94.1 (85.6–98.4) | 93.0 (84.3–97.7) |
| NPV, % | 86.3 (79.0–91.8) | 94.5 (88.5–98.0) | 95.4 (89.5–98.5) | 97.1 (91.9–99.4) |
| Specimens from HIV-negative individuals (n = 118) | ||||
| Sensitivity, % | 80.8 (60.6–93.4) | 92.3 (74.9–99.1) | 92.3 (74.9–99.1) | 92.3 (74.9–99.1) |
| Specificity, % | 100 (96.1–100) | 97.8 (92.4–99.7) | 96.7 (90.8–99.3) | 98.9 (94.1–100) |
| PPV, % | 100 (83.9–100) | 92.3 (74.9–99.1) | 88.9 (70.8–97.6) | 96.0 (79.6–99.9) |
| NPV, % | 94.8 (88.4–98.3) | 97.8 (92.4–99.7) | 97.8 (92.3–99.7) | 97.8 (92.4–99.7) |
| Smear microscopy–negative specimens (n = 221) | ||||
| Sensitivity, % | NA | 72.7 (49.8–89.3) | 72.7 (49.8–89.3) | 81.8 (59.7–94.8) |
| Specificity, % | 97.5 (94.2–99.2) | 96.5 (92.9–98.6) | 97.0 (93.6–98.9) | |
| PPV, % | 76.2 (52.8–91.8) | 69.6 (47.1–86.8) | 75.0 (53.3–90.2) | |
| NPV, % | 97.0 (93.6–98.9) | 97.0 (93.5–98.9) | 98.0 (94.9–99.4) | |
MTB, Mycobacterium tuberculosis; NA, not applicable; NPV, negative predictive value; PPV, positive predictive value; RIF, rifampicin; TB, tuberculosis.
Table 6.
The Performance (Including 95% CIs) of Smear Microcopy and Molecular TB Assays Compared with the Reference Method (Liquid Culture), for Decontaminated (Sediment) Specimens
| Variable | Xpert MTB/RIF |
RealTime MTB |
cobas MTB |
|---|---|---|---|
| Sediment | |||
| All samples (n = 294) | |||
| Sensitivity, % | 94.7 (88.1–98.3) | 92.6 (85.4–97.0) | 94.7 (88.1–98.3) |
| Specificity, % | 98.5 (95.6–99.7) | 95.0 (91.0–97.6) | 95.5 (91.6–97.9) |
| PPV, % | 96.8 (90.9–99.3) | 89.8 (82.0–95.0) | 90.9 (83.4–95.8) |
| NPV, % | 97.5 (94.3–99.2) | 96.4 (92.8–98.6) | 97.4 (94.1–99.2) |
| Specimens from HIV-positive individuals (n = 176) | |||
| Sensitivity, % | 94.2 (85.8–98.4) | 92.8 (83.9–97.6) | 94.2 (85.8–98.4) |
| Specificity, % | 98.1 (93.4–99.8) | 93.5 (87.0–97.3) | 94.4 (88.2–97.9) |
| PPV, % | 97.1 (89.6–99.6) | 90.1 (80.7–95.9) | 91.5 (82.5–96.8) |
| NPV, % | 96.3 (90.9–99.0) | 95.2 (89.2–98.4) | 96.2 (90.5–99.0) |
| Specimens from HIV-negative individuals (n = 118) | |||
| Sensitivity, % | 96.2 (80.4–99.9) | 92.3 (74.9–99.1) | 96.2 (80.4–98.9) |
| Specificity, % | 98.9 (94.0–100) | 96.7 (90.8–99.3) | 96.7 (90.8–99.3) |
| PPV, % | 96.2 (80.4–99.9) | 88.9 (70.8–97.6) | 89.3 (71.8–97.7) |
| NPV, % | 98.9 (94.0–100) | 97.8 (92.3–99.7) | 98.9 (94.0–100) |
| Smear microscopy–negative specimens (n = 221) | |||
| Sensitivity, % | 77.3 (54.6–92.2) | 77.3 (54.6–92.2) | 77.3 (54.6–92.2) |
| Specificity, % | 98.5 (95.6–99.7) | 95.0 (91.0–97.6) | 95.5 (91.6–97.9) |
| PPV, % | 85.0 (62.1–96.8) | 63.0 (42.4–80.6) | 65.4 (44.3–82.8) |
| NPV, % | 97.5 (94.3–99.2) | 97.4 (94.1–99.2) | 97.4 (94.1–99.2) |
MTB, Mycobacterium tuberculosis; NPV, negative predictive value; PPV, positive predictive value; RIF, rifampicin; TB, tuberculosis.
Figure 2.
Radar plots illustrating the overall performance indicators of standard of care and molecular assays compared with liquid culture as the reference for sputum specimens (A) and decontaminated specimens (sediment; B). MTB, Mycobacterium tuberculosis; NPV, negative predictive value; PPV, positive predictive value; RIF, rifampicin.
To investigate the reduced specificity of these molecular assays compared with liquid culture, those patients identified with MTBC using molecular assays are further characterized in Table 7. Twelve patients (Table 7) were identified as having MTB DNA in their sputum, but no growth by MGIT. These results therefore contributed to a reduced specificity in the molecular assays compared with the liquid culture as a reference. Four patients (Patients 1, 2, 3, and 5) were identified by two or more assays (Xpert, RealTime, and cobas), and three of these were co-infected with HIV and therefore more likely to be a true positive. Four patients only identified by either cobas or RealTime MTB were HIV co-infected and reported on antiretroviral therapy, and more likely to be true positives. Only one patient (Patient 9) identified by the RealTime MTB assay only reported a single symptom and was not recorded as a new patient, and therefore more likely to be a false positive, and detecting residual MTB DNA. The remaining five patients all reported three or more symptoms, and two were co-infected with HIV, therefore also more likely to be true positives; however, initiation on treatment and post-study follow-up was not investigated.
Table 7.
List of Patients in Whom MTB Was Identified by at Least One Molecular Assay and MGIT Culture Showed No Growth
| Patient | Xpert MTB/RIF (specificity, 97.5%), CT value | RealTime MTB (specificity, 96.5%), CT value | cobas MTB (specificity, 97%), CT value | Clinical information |
|---|---|---|---|---|
| 1 | 32.3 | 29.9 | 35.3 | NP, NS, fever, WL, cough, HIV infected, not on ARVs, CD4 = 106 cells/μL |
| 2 | 20.4 | 26.1 | NS, WL, cough | |
| 3 | 29.4 | 35.1 | NP, NS, fever, WL, cough, HIV infected, not on ARVs, CD4 = 48 cells/μL | |
| 4 | 25.0 | NS, fever, cough | ||
| 5 | 29.5 | 36.0 | 30.1 | WL, cough, HIV infected, on ARVs, CD4 = 43 cells/μL |
| 6 | 39.8 | WL, cough, HIV infected, on ARVs, CD4 = 13 cells/μL | ||
| 7 | 39.4 | NS, WL, cough | ||
| 8 | 39.5 | NS, fever, WL, cough, HIV infected, on ARVs, CD4 = 265 cells/μL | ||
| 9 | 39.9 | Cough | ||
| 10 | 28.1 | NS, fever, cough | ||
| 11 | 33.1 | NS, cough, HIV infected, on ARVs | ||
| 12 | 19.4 | WL, cough, HIV infected, on ARVs, CD4 = 745 cells/μL |
ARV, antiretroviral; MGIT, mycobacteria growth indicator tube; MTB, Mycobacterium tuberculosis; NP, new patient; NS, night sweats; WL, weight loss.
Irrespective of HIV status, the performance of all three molecular assays decreased in specimens from patients with a smear-negative result (n = 221). The cobas MTB assay had a sensitivity of 81.8% (95% CI, 59.7%–94.8%), 9% higher than either the Xpert MTB/RIF or the RealTime MTB assays (both 72.7%; 95% CI, 49.8%–89.3%) in smear-negative cases. For processed sediments, although similar sensitivities were seen for all specimen types tested on both the cobas MTB and the Xpert MTB/RIF, the specificities, PPV, and NPP were higher with the Xpert MTB/RIF assay. No significant differences were, however, observed in the performance of the three assays. The radar plots in Figure 2 illustrate the distinction between the more sensitive molecular assays compared with smear microscopy. The plots further give a visual representation of how the different molecular assays compare with each other.
The percentage agreement of the cobas MTB with the Xpert MTB/RIF and RealTime MTB was similar in both sputum and sediment specimens, as outlined in Table 8. Compared with the Xpert MTB/RIF, the cobas MTB had a positive percentage agreement and a negative percentage agreement of 95.7% and 96.0% in sputum specimens and 98.9% and 96.5% in sediment specimens, respectively. Compared with the RealTime MTB test, the cobas MTB test had a positive percentage agreement and a negative percentage agreement of 92.6% and 96.0% in raw sputum samples and 90.8% and 94.9% in sediment samples, respectively.
Table 8.
Percentage Agreements of the cobas MTB Results with the Xpert MTB/RIF and RealTime MTB Tests
| Comparator test | Specimen type | Agreement | Result, % (n/total) | 95% CI, % |
|
|---|---|---|---|---|---|
| Clopper-Pearson | Score | ||||
| Xpert MTB/RIF | Sputum | PPA | 95.7 (88/92) | 89.2–98.8 | 89.3–98.3 |
| NPA | 96.0 (194/202) | 92.3–98.3 | 92.4–98.0 | ||
| OPA | 95.9 (282/294) | 93.0–97.9 | 93.0–97.6 | ||
| Sediment | PPA | 98.9 (92/93) | 94.2–100.0 | 94.2–99.8 | |
| NPA | 96.5 (194/201) | 93.0–98.6 | 93.0–98.3 | ||
| OPA | 97.3 (286/294) | 94.7–98.8 | 94.7–98.6 | ||
| RealTime MTB | Sputum | PPA | 92.6 (88/95) | 85.4–97.0 | 85.6–96.4 |
| NPA | 96.0 (191/199) | 92.2–98.2 | 92.3–97.9 | ||
| OPA | 94.9 (279/294) | 91.7–97.1 | 91.8–96.9 | ||
| Sediment | PPA | 90.8 (89/98) | 83.3–95.7 | 83.5–95.1 | |
| NPA | 94.9 (186/196) | 90.8–97.5 | 90.9–97.2 | ||
| OPA | 93.5 (275/294) | 90.1–96.1 | 90.1–95.8 | ||
MTB, Mycobacterium tuberculosis; NPA, negative percentage agreement; OPA, overall percentage agreement; PPA, positive percentage agreement; RIF, rifampicin.
In addition to evaluating assay performance and determining agreement among the different molecular platforms, an observation was made on the number of results reported using molecular testing, where the initial liquid culture reported contamination. A total of 23 liquid cultures required redecontamination. Of these specimens, four raw sputum and five sediment specimens tested positive for MTB by multiple molecular methods, whereas repeated culture demonstrated MTB in only three specimens. This further demonstrates the advantage of direct molecular testing for early and rapid diagnosis of TB over liquid culture.
Trend Analysis of the cobas MTB Assay CT
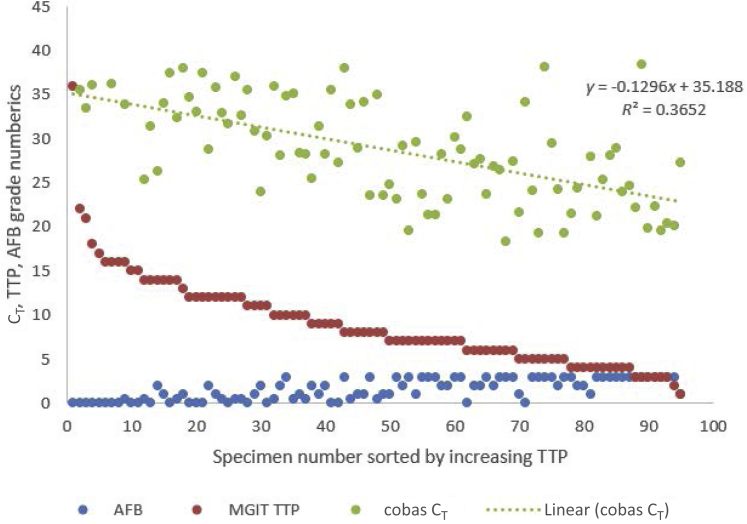
Figure 3 graphically represents the CT values, MGIT TTP, and smear grade from sputum specimens that flagged positive (n = 95) for liquid culture up to a period of 42 days. A significant trend (r = 0.6213; P < 0.0001) is evident between decreasing MGIT TTP and reductions in the CT, indicating an overall increase in bacterial load in participant's sputum.
Figure 3.
Scatterplot illustrating acid-fast bacillus (AFB) smear status (−, scanty, +, ++, and +++), mycobacteria growth indicator tube (MGIT) time (days) to positivity (TTP), and molecular CT on sputum specimens processed by cobas MTB. The equation of the line and R2 are represented on the plot. Specimen results are sorted by decreasing TTP.
Evaluation of the cobas MTB-RIF/INH
Although the RIF and INH drug resistance in this cohort was low (6/92; 6.5%), the cobas MTB-RIF/INH detected six of six RIF-resistant specimens, yielding a 100% agreement with Xpert MTB/RIF and MGIT DST. The INH performance was similar on RealTime MTB RIF/INH (three of five) and cobas MTB-RIF/INH (four of five). The specificity of RIF resistance on sputum was 100% (95% CI, 95.4%–100%; 79/79), and specificity of INH resistance on sputum was 97.5% (95% CI, 91.3%–99.7%; 78/80).
Discussion
To achieve the 2030 targets set in the Sustainable Development Goals and the WHO's End TB Strategy, the rate of reduction in TB incidence must accelerate.24 Gaps in service delivery must be addressed across the entire health care value chain, of which diagnostics play a big role.25 The use of molecular technologies is contributing toward the 1.9% annual decline in TB incidence24 by providing earlier diagnosis of TB disease. This is evident from the recent meta-analysis,26 showing the plausible reduction in 6-month all-cause mortality estimate at 12%, associated with the use of Xpert MTB/RIF as the initial diagnostic test for pulmonary TB. However, the Xpert technology is still not widely implemented; in spite of Xpert's increased sensitivity compared with smear microscopy, many countries still rely on the use of smear microscopy as their initial diagnostic tool.27 South Africa has a ratio of numbers of testing smear microscopy: Xpert cartridge use of 1:6, which is significantly lower than most countries.28 This is attributable to its successful molecular testing implementation package (GeneXpert Implementation in South Africa Public Sector, http://www.stoptb.org/wg/gli/assets/html/day%203/Stevens%20-%20South%20Africa.pdf, last accessed May 7, 2020), including a program of clinical and laboratory training, a quality assessment program,29 and strong engagement with key opinion leaders and funders. Although areas for improvement in TB diagnostics remain, including true point-of-care testing to identify active TB disease,30 the testing volumes required by the South African program demand high-throughput testing systems.
The Global TB Report in 2017 highlighted the expanding diagnostic development pipeline of molecular diagnostic assays (Global Tuberculosis Report World Health Organization, https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf, last accessed May 7, 2020). There are currently four molecular assays for TB detection on the market that have not yet been submitted to the WHO for evaluation and a further seven molecular assays in development. Multiple molecular technologies are, however, currently under WHO evaluation, and these include the cobas MTB and cobas MTB-RIF/INH assays as well as the RealTime MTB. This expanding pipeline along with other nonmolecular, culture-based microscopy and radiology innovations may help to increase access to testing and identifying infections earlier, ensure quality testing for difficult-to-diagnose groups (such as HIV co-infected individuals), expand the spectrum of drug-susceptibility testing, and reduce costs.24 In South Africa, 60% of TB cases have HIV co-infection1 and thus TB and HIV clinical and laboratory services cannot operate in isolation.
Previous studies reported the overall sensitivity of the Xpert MTB/RIF and Ultra as 85% (95% CI, 82%–88%) and 88% (95% CI, 85%–91%), with specificities of 98% (95% CI, 97%–98%) and 96% (95% CI, 94%–97%), respectively.31 The RealTime MTB has previously been reported with overall sensitivities of 83% (95% CI, 67%–93%) and specificities of 93% (95% CI, 86%–97%).10,32 The sensitivity of the Xpert MTB/RIF Ultra for RIF resistance detection is reported at 95% (95% CI, 90%–98%), with a specificity of 98% (95% CI, 97%–99%),6 and the performance of the Abbott RIF/INH reflex test concurred with SOC testing using phenotypic DST.33, 34, 35, 36 The current study introduces the cobas 6800/8800 system as an integrated HIV/TB diagnostic system that is an alternative highly automated and centralized option37 to the Xpert technology and in contrast to a point-of-care approach.38 The cobas MTB assay was shown to have comparable performance to the Xpert MTB/RIF and RealTime MTB, on both raw sputum and sediment specimens, with an overall higher sensitivity (94.7%) in detecting MTB DNA. The sensitivity was unaffected (95.7%) in patients co-infected with HIV and, although the cobas MTB yielded a lower sensitivity (82%) among smear-negative, culture-positive specimens, overall it generated 9% greater sensitivity than Xpert MTB/RIF (73%) and RealTime MTB (73%) assays. The specificity of the cobas MTB was comparable to existing molecular tests and ranged from 95% to 98% among HIV co-infected and uninfected individuals, respectively. The cobas MTB-RIF/INH assay also showed comparable performance to the Xpert MTB/RIF and RealTime MTB assay in identifying RIF- and RIF/INH-resistant MTB directly from sputum; however, the study was limited by few specimens with resistant MTB.
Specimen processing was shown to be safe, making the cobas platform not reliant on a biosafety level 3 laboratory environment for direct processing of raw sputum. The trend of increasing cobas MTB CT with increasing TTP of liquid culture is similarly reflected by other molecular assays,10 and a positive correlation between mycobacterial burden in patient sputum and CT values may have an off-label value as a marker of TB treatment response.39 However, although CT values are beneficial in their ability to provide a comparison of numbers over time, the inability of molecular assays to differentiate between viable and nonviable bacilli is a limiting factor in their use for treatment monitoring of patients.
The operation of the cobas 6800 system in this study was simple to perform; however, the front-end specimen processing relied on a sonication instrument with a low throughput, making this step in the workflow a bottleneck for operators. The manufacturer is addressing this aspect in the workflow. The throughput for this platform was not rigorously tested in this study, but the cobas 6800 system is designed to report 384 test results/8-hour period, whereas the cobas 8800 system can report 960 results/8 hours. An advantage of the cobas 6800/8800 system is the ability to perform several tests simultaneously, such as MTB, hepatitis B virus, hepatitis C virus, and HIV viral load. The combination of cobas MTB, cobas MTB-RIF/INH, and cobas MAI adds a further dimension to important patient management decisions, which for MAI is often challenging,40 especially in settings with high burden of disease, such as HIV,41 and wide geographic variation in species.42
Investment in research and development of new molecular TB diagnostics for both point-of-care and highly centralized testing systems continues to be a global focus,43 and more guidance is now available from WHO for studies to generate high-quality evidence44 through improved evaluation study design and key issues beyond accuracy of new TB diagnostics. The choice and implementation model for placement of new systems is complex and must consider factors, including sensitivity, throughput, ease of use, cost, time to reportable result, connectivity to a laboratory information system, the landscape of current testing, and clinical diagnostic algorithms. In South Africa, for example, the initial test for diagnosing pulmonary TB changed in 2017 to the Ultra test, with a reduced limit of detection to 15.6 colony-forming units/mL,23 and 17% higher sensitivity among smear-negative, culture-positive TB and 12% higher sensitivity in HIV co-infected individuals than the Xpert MTB/RIF assay.6 The cobas MTB assay therefore remains to be further evaluated against the Xpert Ultra test in this setting; however, current guidance for evaluation studies is to continue to include smear microscopy and Xpert MTB/RIF as comparators to allow broader comparability and generalizability of results.45 Collectively, this evaluation demonstrates that advances in MTB diagnostics continue to provide new strategies for combating TB disease.
Acknowledgments
We thank the patients who were included in the study; Trish Kahamba (medical scientist) and the Clinical Laboratory Service TB group, including Dr. Pedro da Silva, for pathology testing and oversight; and Elements Communications Ltd (Westerham, UK), funded by Roche Molecular Solutions (Pleasanton, CA), for editing support.
Footnotes
Supported by Roche Molecular Solutions (Pleasanton, CA); the South African Medical Research Council [with funds received from the South African National Department of Health (W.S., L.S., and G.E.); the UK Medical Research Council, with funds received from the UK Government’s Newton Fund under the UK/South Africa Newton Fund grant 015NEWTON TB (W.S., L.S., and G.E.)]; and the Bill & Melinda Gates Foundation grant OPP1171455 (W.S., L.S., and A.D.). Patient care was provided by the Gauteng and North West Department of Health.
Disclosures: L.S. reports having a patent issued (USP 8709712) that relates to quality control material and program for molecular TB diagnostics quality management. Roche Molecular Solutions provided funding but did not have any input in the study design or presentation of results contained in the article.
Current address of E.M., Quest Diagnostics, San Juan Capistrano, CA.
References
- 1.World Health Organization . 2018. Global Tuberculosis Report 2018. Geneva, Switzerland; 2018. [Google Scholar]
- 2.Page-Shipp L., Stevens W., Clark D., Scott L., Olsen F., Kisbey-Green H., Mametja D., Churchyard G. Successes, challenges and lessons from a novel deployment of Xpert((R)) MTB/RIF at a major South African public event. Int J Tuberc Lung Dis. 2014;18:438–440. doi: 10.5588/ijtld.13.0638. [short communication] [DOI] [PubMed] [Google Scholar]
- 3.Scott L.E., McCarthy K., Gous N., Nduna M., Van Rie A., Sanne I., Venter W.F., Duse A., Stevens W. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med. 2011;8:e1001061. doi: 10.1371/journal.pmed.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gous N., Scott L.E., Khan S., Reubenson G., Coovadia A., Stevens W. Diagnosing childhood pulmonary tuberculosis using a single sputum specimen on Xpert MTB/RIF at point of care. S Afr Med J. 2015;105:1044–1048. doi: 10.7196/SAMJ.2015.v105i12.8585. [DOI] [PubMed] [Google Scholar]
- 5.Scott L.E., Beylis N., Nicol M., Nkuna G., Molapo S., Berrie L., Duse A., Stevens W.S. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. J Clin Microbiol. 2014;52:1818–1823. doi: 10.1128/JCM.03553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorman S.E., Schumacher S.G., Alland D., Nabeta P., Armstrong D.T., King B., Hall S.L., Chakravorty S., Cirillo D.M., Tukvadze N., Bablishvili N., Stevens W., Scott L., Rodrigues C., Kazi M.I., Joloba M., Nakiyingi L., Nicol M.P., Ghebrekristos Y., Anyango I., Murithi W., Dietze R., Lyrio Peres R., Skrahina A., Auchynka V., Chopra K.K., Hanif M., Liu X., Yuan X., Boehme C.C., Ellner J.J., Denkinger C.M., study team. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail N.A., Mvusi L., Nanoo A., Dreyer A., Omar S.V., Babatunde S., Molebatsi T., van der Walt M., Adelekan A., Deyde V., Ihekweazu C., Madhi S.A. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18:779–787. doi: 10.1016/S1473-3099(18)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesmann F., Ehret R., Naeth G., Daumer M., Fuhrmann J., Kaiser R., Noah C., Obermeier M., Schalasta G., Tiemann C., Wolf E., Knechten H., Braun P. Multicenter evaluation of two next-generation HIV-1 quantitation assays, Aptima Quant Dx and Cobas 6800, in comparison to the RealTime HIV-1 reference assay. J Clin Microbiol. 2018;56:e00292-18. doi: 10.1128/JCM.00292-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott L.E., Noble L.D., Moloi J., Erasmus L., Venter W.D., Stevens W. Evaluation of the Abbott m2000 RealTime human immunodeficiency virus type 1 (HIV-1) assay for HIV load monitoring in South Africa compared to the Roche Cobas AmpliPrep-Cobas Amplicor, Roche Cobas AmpliPrep-Cobas TaqMan HIV-1, and BioMerieux NucliSENS EasyQ HIV-1 assays. J Clin Microbiol. 2009;47:2209–2217. doi: 10.1128/JCM.01761-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott L., David A., Noble L., Nduna M., Da Silva P., Black A., Venter F., Stevens W. Performance of the Abbott RealTime MTB and MTB RIF/INH assays in a setting of high tuberculosis and HIV coinfection in South Africa. J Clin Microbiol. 2017;55:2491–2501. doi: 10.1128/JCM.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odjidja E.N., Gatasi G., Duric P. Delivery of integrated infectious disease control services under the new antenatal care guidelines: a service availability and readiness assessment of health facilities in Tanzania. BMC Health Serv Res. 2019;19:153. doi: 10.1186/s12913-019-3990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson M.L., Fleming K.A., Kuti M.A., Looi L.M., Lago N., Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet. 2018;391:1927–1938. doi: 10.1016/S0140-6736(18)30458-6. [DOI] [PubMed] [Google Scholar]
- 13.Ndlovu Z., Fajardo E., Mbofana E., Maparo T., Garone D., Metcalf C., Bygrave H., Kao K., Zinyowera S. Multidisease testing for HIV and TB using the GeneXpert platform: a feasibility study in rural Zimbabwe. PLoS One. 2018;13:e0193577. doi: 10.1371/journal.pone.0193577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gous N., Scott L., Berrie L., Stevens W. Options to expand HIV viral load testing in South Africa: evaluation of the GeneXpert(R) HIV-1 viral load assay. PLoS One. 2016;11:e0168244. doi: 10.1371/journal.pone.0168244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan N.K., Carrington D., Pope C.F. Verification of the Roche cobas((R)) 6800 PCR 200 microl and 500 microl protocols for the quantification of HIV-1 RNA, HBV DNA and HCV RNA and evaluation with COBAS((R)) Ampliprep/COBAS((R)) TaqMan((R)) assays. J Med Microbiol. 2018;67:1711–1717. doi: 10.1099/jmm.0.000838. [DOI] [PubMed] [Google Scholar]
- 16.Wirden M., Larrouy L., Mahjoub N., Todesco E., Damond F., Delagreverie H., Akhavan S., Charpentier C., Chaix M.L., Descamps D., Calvez V., Marcelin A.G. Multicenter comparison of the new Cobas 6800 system with Cobas Ampliprep/Cobas TaqMan and Abbott RealTime for the quantification of HIV, HBV and HCV viral load. J Clin Virol. 2017;96:49–53. doi: 10.1016/j.jcv.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Tang N., Frank A., Pahalawatta V., Lampinen J., Coblenz-Korte A., Dunn C., Li C., Cloherty G., Abravaya K., Leckie G. Analytical and clinical performance of Abbott RealTime MTB, an assay for detection of Mycobacterium tuberculosis in pulmonary specimens. Tuberculosis (Edinb) 2015;95:613–619. doi: 10.1016/j.tube.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Vinuesa V., Navarro D., Poujois S., Zaragoza S., Borras R. Performance characteristics of the new Abbott Real Time MTB assay for detection of Mycobacterium tuberculosis complex in respiratory specimens. Diagn Microbiol Infect Dis. 2016;84:212–214. doi: 10.1016/j.diagmicrobio.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen J.H., She K.K., Kwong T.C., Wong O.Y., Siu G.K., Leung C.C., Chang K.C., Tam C.M., Ho P.L., Cheng V.C., Yuen K.Y., Yam W.C. Performance of the new automated Abbott RealTime MTB assay for rapid detection of Mycobacterium tuberculosis complex in respiratory specimens. Eur J Clin Microbiol Infect Dis. 2015;34:1827–1832. doi: 10.1007/s10096-015-2419-5. [DOI] [PubMed] [Google Scholar]
- 20.Blakemore R., Story E., Helb D., Kop J., Banada P., Owens M.R., Chakravorty S., Jones M., Alland D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravorty S., Simmons A.M., Rowneki M., Parmar H., Cao Y., Ryan J., Banada P.P., Deshpande S., Shenai S., Gall A., Glass J., Krieswirth B., Schumacher S.G., Nabeta P., Tukvadze N., Rodrigues C., Skrahina A., Tagliani E., Cirillo D.M., Davidow A., Denkinger C.M., Persing D., Kwiatkowski R., Jones M., Alland D. The New Xpert MTB/RIF ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio. 2017;8:1–12. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . 2011. Tuberculosis Diagnostics Xpert MTB/RIF Test WHO Endorsement and Recommendations.https://apps.who.int/iris/bitstream/handle/10665/44593/9789241501569_eng.pdf?sequence=1 Geneva, Switzerland: World Health Organization. Available at. [Google Scholar]
- 23.Hanrahan C.F., Clouse K., Bassett J., Mutunga L., Selibas K., Stevens W., Scott L., Sanne I., Van Rie A. The patient impact of point-of-care vs. laboratory placement of Xpert((R)) MTB/RIF. Int J Tuberc Lung Dis. 2015;19:811–816. doi: 10.5588/ijtld.15.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floyd K., Glaziou P., Zumla A., Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the end TB era. Lancet Respir Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 25.MacLean E., Huddart S., Pai M. Molecular diagnosis of tuberculosis: we need solutions that span the healthcare value chain. Expert Rev Mol Diagn. 2017;17:5–7. doi: 10.1080/14737159.2017.1265889. [DOI] [PubMed] [Google Scholar]
- 26.Di Tanna G.L., Khaki A.R., Theron G., McCarthy K., Cox H., Mupfumi L., Trajman A., Zijenah L.S., Mason P., Bandason T., Durovni B., Bara W., Hoelscher M., Clowes P., Mangu C., Chanda D., Pym A., Mwaba P., Cobelens F., Nicol M.P., Dheda K., Churchyard G., Fielding K., Metcalfe J.Z. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Health. 2019;7:e191–e199. doi: 10.1016/S2214-109X(18)30458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai M., Furin J. Tuberculosis innovations mean little if they cannot save lives. Elife. 2017;6:e25956. doi: 10.7554/eLife.25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Z.Z., Pai M., Van Gemert W., Sahu S., Ghiasi M., Creswell J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur Respir J. 2015;45:549–554. doi: 10.1183/09031936.00147714. [DOI] [PubMed] [Google Scholar]
- 29.Scott L.E., Gous N., Cunningham B.E., Kana B.D., Perovic O., Erasmus L., Coetzee G.J., Koornhof H., Stevens W. Dried culture spots for Xpert MTB/RIF external quality assessment: results of a phase 1 pilot study in South Africa. J Clin Microbiol. 2011;49:4356–4360. doi: 10.1128/JCM.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNerney R., Daley P. Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nat Rev Microbiol. 2011;9:204–213. doi: 10.1038/nrmicro2521. [DOI] [PubMed] [Google Scholar]
- 31.Horne D.J., Kohli M., Zifodya J.S., Schiller I., Dendukuri N., Tollefson D., Schumacher S.G., Ochodo E.A., Pai M., Steingart K.R. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;6:CD009593. doi: 10.1002/14651858.CD009593.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berhanu R.H., David A., da Silva P., Shearer K., Sanne I., Stevens W., Scott L. Performance of Xpert MTB/RIF, Xpert ultra, and Abbott RealTime MTB for diagnosis of pulmonary tuberculosis in a high-HIV-burden setting. J Clin Microbiol. 2018;56:e00560-18. doi: 10.1128/JCM.00560-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann-Thiel S., Molodtsov N., Duffner C., Kadyrov A., Kalmambetova G., Kabirov O., Rajabov A., Parpieva N., Sayfutdinov Z., Vogel M., Vogel H., Antonenka U., Hoffmann H. Capacity of Abbott RealTime MTB RIF/INH to detect rifampicin- and isoniazid-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23:458–464. doi: 10.5588/ijtld.18.0615. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann-Thiel S., Molodtsov N., Antonenka U., Hoffmann H. Evaluation of the Abbott RealTime MTB and RealTime MTB INH/RIF assays for direct detection of Mycobacterium tuberculosis complex and resistance markers in respiratory and extrapulmonary specimens. J Clin Microbiol. 2016;54:3022–3027. doi: 10.1128/JCM.01144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostera J., Leckie G., Abravaya K., Wang H. Performance of the Abbott RealTime MTB RIF/INH resistance assay when used to test Mycobacterium tuberculosis specimens from Bangladesh. Infect Drug Resist. 2018;11:695–699. doi: 10.2147/IDR.S158953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostera J., Leckie G., Tang N., Lampinen J., Szostak M., Abravaya K., Wang H. Analytical and clinical performance characteristics of the Abbott RealTime MTB RIF/INH Resistance, an assay for the detection of rifampicin and isoniazid resistant Mycobacterium tuberculosis in pulmonary specimens. Tuberculosis (Edinb) 2016;101:137–143. doi: 10.1016/j.tube.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Laczin J.A. The shift to a centralized lab approach. Appl Clin Trials. 2013;22 [Google Scholar]
- 38.Drain P.K., Hyle E.P., Noubary F., Freedberg K.A., Wilson D., Bishai W.R., Rodriguez W., Bassett I. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange B., Khan P., Kalmambetova G., Al-Darraji H.A., Alland D., Antonenka U., Brown T., Balcells M.E., Blakemore R., Denkinger C.M., Dheda K., Hoffmann H., Kadyrov A., Lemaitre N., Miller M.B., Nikolayevskyy V., Ntinginya E.N., Ozkutuk N., Palacios J.J., Popowitch E.B., Porcel J.M., Teo J., Theron G., Kranzer K. Diagnostic accuracy of the Xpert((R)) MTB/RIF cycle threshold level to predict smear positivity: a meta-analysis. Int J Tuberc Lung Dis. 2017;21:493–502. doi: 10.5588/ijtld.16.0702. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin S.L., Larsen S.E., Ordway D., Cassell G., Coler R.N. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl Trop Dis. 2019;13:e0007083. doi: 10.1371/journal.pntd.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suwanagool S., Kolladarungkri T., Leelarasamee A., Chuenarom V., Jearanaisilavong J., Chaiprasert A. Prolonged fever due to Mycobacterium avium complex (MAC) disease in advanced HIV infection: a public health concern. J Med Assoc Thai. 1998;81:893–905. [PubMed] [Google Scholar]
- 42.Sookan L., Coovadia Y.M. A laboratory-based study to identify and speciate non-tuberculous mycobacteria isolated from specimens submitted to a central tuberculosis laboratory from throughout KwaZulu-Natal Province, South Africa. South Afr Med J. 2014;104:766–768. doi: 10.7196/samj.8017. [DOI] [PubMed] [Google Scholar]
- 43.Marais B.J., Raviglione M.C., Donald P.R., Harries A.D., Kritski A.L., Graham S.M., El-Sadr W.M., Harrington M., Churchyard G., Mwaba P., Sanne I., Kaufmann S.H., Whitty C.J., Atun R., Zumla A. Scale-up of services and research priorities for diagnosis, management, and control of tuberculosis: a call to action. Lancet. 2010;375:2179–2191. doi: 10.1016/S0140-6736(10)60554-5. [DOI] [PubMed] [Google Scholar]
- 44.Denkinger C.M., Schumacher S.G., Gilpin C., Korobitsyn A., Wells W.A., Pai M., Leeflang M., Steingart K.R., Bulterys M., Schunemann H., Glaziou P., Weyer K. Guidance for the evaluation of tuberculosis diagnostics that meet the World Health Organization (WHO) target product profiles: an introduction to WHO process and study design principles. J Infect Dis. 2019;220:S91–S98. doi: 10.1093/infdis/jiz097. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher S.G., Wells W.A., Nicol M.P., Steingart K.R., Theron G., Dorman S.E., Pai M., Churchyard G., Scott L., Stevens W., Nabeta P., Alland D., Weyer K., Denkinger C.M., Gilpin C. Guidance for studies evaluating the accuracy of sputum-based tests to diagnose tuberculosis. J Infect Dis. 2019;220:S99–S107. doi: 10.1093/infdis/jiz258. [DOI] [PMC free article] [PubMed] [Google Scholar]