Key Points
Question
What features prognosticate vascular risk and response to low-dose rivaroxaban and aspirin treatment among patients with symptomatic lower extremity peripheral artery disease?
Findings
In this secondary analysis of a randomized clinical trial, the risk of major vascular events was greater than 10% over 30 months for patients with previous peripheral revascularization, previous amputation, or Fontaine III or IV symptoms and patients with comorbidities of kidney dysfunction, heart failure, diabetes, or polyvascular disease. These patients at high risk had an estimated 4.2% absolute risk reduction for major vascular events when treated with combination rivaroxaban and aspirin vs aspirin alone.
Meaning
Per this analysis, patients with high-risk lower extremity peripheral artery disease limb presentations or comorbidities, who are not at high bleeding risk, should be considered for treatment with combination rivaroxaban and aspirin.
This secondary analysis of a randomized clinical trial assesses the clinical features of symptomatic lower extremity peripheral artery disease that may prognosticate major vascular events and whether affected patients may benefit from low-dose rivaroxaban and aspirin.
Abstract
Importance
Patients with symptomatic lower extremity peripheral artery disease (LE-PAD) experience an increased risk of major vascular events. There is limited information on what clinical features of symptomatic LE-PAD prognosticate major vascular events and whether patients at high risk have a greater absolute benefit from low-dose rivaroxaban and aspirin.
Objective
To quantify the risk of major vascular events and investigate the response to treatment with low-dose rivaroxaban and aspirin among patients with symptomatic LE-PAD based on clinical presentation and comorbidities.
Design, Setting, and Participants
This is a subanalysis of a previously reported subgroup of patients with symptomatic LE-PAD who were enrolled in a large, double-blind, placebo-controlled randomized clinical trial (Cardiovascular Outcomes for People Using Anticoagulation Strategies [COMPASS]) in 602 centers in 33 countries from March 2013 to January 2020. Data analysis was completed from May 2016 to June 2020.
Interventions
A combination of low-dose rivaroxaban and aspirin compared with aspirin alone.
Main Outcomes and Measures
Thirty-month incidence risk of myocardial infarction, stroke and cardiovascular death (MACE), major adverse limb events (MALE) including major vascular amputation, and bleeding.
Results
The COMPASS trial enrolled 4129 patients with symptomatic LE-PAD (mean [SD] age, 66.8 [8.8] years; 2932 men [71.0%]). The 30-month Kaplan-Meier incidence risk of MACE or MALE, including major amputation, was 22.6% in those with prior amputation (this outcome was observed in 54 patients), 17.6% (n = 15) in those with Fontaine III or IV symptoms, and 11.8% (n = 142) in those with previous peripheral artery revascularization, classifying these features as high-risk limb presentations. The 30-month incidence risk of MACE or MALE, including major amputation, was 14.1% (n = 118) in those with kidney dysfunction, 13.5% (n = 67) in those with heart failure, 13.4% (n = 199) in those with diabetes, and 12.8% (n = 222) in those with polyvascular disease, classifying these features as high-risk comorbidities. Among patients with either high-risk limb presentations or high-risk comorbidities, treatment with rivaroxaban and aspirin compared with aspirin alone was associated with an estimated 4.2% (95% CI, 1.9%-6.2%) absolute risk reduction for MACE or MALE, including major amputation, at 30 months. Although the estimated absolute risk increase of major bleeding was higher with rivaroxaban and aspirin in combination than aspirin alone (2.0% [95% CI, 0.5%-3.9%]) for patients with either high-risk limb presentation or high-risk comorbidity, the estimated absolute risk increase of fatal or critical organ bleeding was low in this high-risk group (0.4% [95% CI, 0.2%-1.8%]), such that the net clinical benefit was estimated to be 3.2% (95% CI, 0.6%-5.3%).
Conclusions and Relevance
Patients with LE-PAD with high-risk limb presentations or high-risk comorbidities had a high incidence of major vascular events. For these patients, treatment with rivaroxaban and aspirin in combination compared with aspirin alone led to a large absolute reduction in vascular risk.
Introduction
Patients with peripheral artery disease (PAD) have widespread atherosclerosis and experience a high risk of major adverse cardiac events (MACE) as well as major adverse limb events (MALE).1 The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) double-blind randomized clinical trial, which included 27 395 patients with coronary artery disease (CAD) and/or PAD, demonstrated that low-dose rivaroxaban combined with aspirin decreased the risk of MACE by 24% and total mortality by 18% compared with aspirin alone.2 Furthermore, a recent analysis of the COMPASS trial demonstrated that patients with high-risk comorbidities, including polyvascular disease, heart failure, kidney insufficiency, and diabetes, derived a greater absolute risk reduction in MACE and MALE when treated with rivaroxaban and aspirin together compared with aspirin alone, than did patients at lower risk who did not have these comorbidities.3
Among the 7470 patients with chronic, stable PAD enrolled in COMPASS, a 28% reduction in MACE and a 46% reduction in MALE were observed with the combination of rivaroxaban and aspirin compared with aspirin alone.4 More than half of the patients in COMPASS who had PAD (n = 4129) had symptomatic lower extremity PAD (LE-PAD), as opposed to those with asymptomatic PAD (n = 1422) or carotid artery disease (n = 1919). Patients with symptomatic LE-PAD experience a particularly high risk of cardiovascular events5 and are typically undertreated with medical therapies.6,7 In this investigation, among patients with symptomatic LE-PAD, we sought to identify the patients at highest risk by limb presentations and comorbidities and examine the efficacy and safety of the combination of rivaroxaban and aspirin compared with aspirin alone across these risk groups.
Methods
Trial Design and Population
COMPASS was a multicenter, double-blind, placebo-controlled randomized clinical trial that enrolled 27 395 patients with CAD and/or PAD, comparing the combination of (1) 2.5 mg of rivaroxaban twice daily plus 100 mg of aspirin once per day, (2) 5 mg of rivaroxaban twice daily, and (3) 100 mg of aspirin once per day, for the prevention of MACE, defined as cardiovascular death, myocardial infarction, or stroke. The details of the inclusion criteria, exclusion criteria, adjudication process, and definitions of outcomes have been previously published.2,4,8,9 The protocol for the parent study was approved by health authorities and institutional review boards in all participating countries, and written informed consent was obtained from all participants. The trial was stopped early for efficacy following a recommendation by the independent data and safety monitoring board.2 This is a subanalysis of the previously reported PAD subgroup of patients (n = 7470) from the COMPASS trial, focusing on patients with symptomatic LE-PAD (n = 4129). The entire cohort of patients with symptomatic LE-PAD are described for event rates, and for treatment outcomes only, data comparing those who received the combination of rivaroxaban and aspirin with those who received aspirin alone are shown.
Participants with symptomatic LE-PAD included those with a history of aortofemoral bypass surgery, limb bypass surgery, or percutaneous transluminal angioplasty revascularization of the iliac or infrainguinal arteries; limb or foot amputation for arterial vascular disease; or intermittent claudication with an ankle brachial index of less than 0.90 or a peripheral artery stenosis (≥50%) documented by angiography or duplex ultrasonography.4 Fontaine classification was used to assess symptoms of limb ischemia at baseline. High-risk limb presentations of PAD were defined as an incidence risk of MACE or MALE, including major amputation, greater than 10% over 30 months. High-risk comorbidities (ie, polyvascular disease [vascular disease in 2 or more vascular beds]; history of diabetes; history of heart failure; and kidney insufficiency, defined as an estimated glomerular filtration rate <60 mL/min) were based on a published risk stratification analysis of the entire COMPASS cohort.3
Outcomes
We examined the estimates of the 30-month Kaplan-Meier incidence risk of MACE and the composite of limb outcomes of severe limb ischemia leading to an intervention, also known as MALE, including major vascular amputation.4 We also report the composite outcome of myocardial infarction, ischemic stroke, cardiovascular death, acute limb ischemia, or major amputation. For safety, we report the 30-month incidence risk of major bleeding, defined using the modified International Society of Thrombosis and Hemostasis definition, and severe bleeding, defined as fatal or critical organ bleeding.2 We also calculated the prespecified net clinical benefit outcome for patients with PAD, which includes the composite of MACE or MALE, including major amputation, and fatal or critical organ bleeding.4
Statistical Analysis
All efficacy analyses were done according to an intention-to-treat principle, and all clinical events that occurred between randomization and the end of observation date were included in the analysis. Annual event rates were estimated as the number of first events per 100 person-years of outcome-specific follow-up (ie, time from randomization until either the first occurrence of the outcome or the last follow-up with no outcome), and the 30-month Kaplan-Meier incidence risk was reported for all main outcomes. Traditional stratified Cox proportional hazards models were used to estimate traditional hazard ratios (HRs) and 95% CIs. Shrinkage estimates of the treatment effect, along with 95% credible intervals (CrIs), for the symptomatic LE-PAD population were obtained via bayesian hierarchical modeling analysis, considering the estimates from the PAD subgroups that made up the overall COMPASS population (ie, symptomatic LE-PAD, asymptomatic LE-PAD, carotid arterial disease, and patients with CAD and no PAD). The shrinkage HR and CrI estimates were reported and used to estimate the absolute risk reduction and corresponding 95% CIs in the symptomatic PAD-LE risk groups over 30 months.10,11,12 The shrinkage analysis was performed using the “bayesmeta” package in R version 3.5.1 (R Foundation for Statistical Computing), and all other analyses were conducted using SAS version 9.4 (SAS Institute).
Results
We enrolled 4129 patients with symptomatic LE-PAD in the COMPASS trial (mean [SD] age, 66.8 [8.8] years; 2932 men [71.0%]) (Table 1). Most patients (71.8%; n = 2966) had a history of intermittent claudication, 41.9% (n = 1729) had previous peripheral artery revascularization surgery, and 7.7% (n = 316) had a previous limb or foot amputation. Of patients with limb symptoms, most endorsed Fontaine stage IIa symptoms (42.1%; n = 1740) or stage IIb symptoms (26.8%; n = 1107), whereas the severe symptoms of rest pain and ischemic ulcers were reported by 5.0% (205 participants; 164 with Fontaine III and 41 with Fontaine IV symptoms). Most patients with symptomatic LE-PAD had at least 1 high-risk comorbidity (84.3%; n = 3481); specifically, 56.6% (n = 2335) had polyvascular disease, 47.0% (n = 1940) had diabetes, 16.6% (n = 686) had a history of heart failure, and 26.9% (n = 1112) had kidney insufficiency (Table 1).
Table 1. Baseline Characteristics of Patients With Symptomatic Lower Extremity Peripheral Artery Disease (LE-PAD) Within the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) Trial.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Overall | Rivaroxaban, 2.5 mg, twice a day, plus aspirin, 100 mg, once a day | Rivaroxaban, 5 mg, twice a day | Aspirin, 100 mg, once a day | |
| Randomized, No. | 4129 | 1409 | 1361 | 1359 |
| Age, mean (SD), y | 66.8 (8.8) | 67.1 (8.7) | 66.5 (8.8) | 66.7 (8.8) |
| Male | 2932 (71.0) | 995 (70.6) | 977 (71.8) | 960 (70.6) |
| Currently smoking | 1318 (31.9) | 421 (29.9) | 455 (33.4) | 442 (32.5) |
| History | ||||
| Diabetes | 1940 (47.0) | 664 (47.1) | 632 (46.4) | 644 (47.4) |
| Heart failure | 686 (16.6) | 232 (16.5) | 228 (16.8) | 226 (16.6) |
| Kidney insufficiency at baselinea | 1112 (26.9) | 383 (27.2) | 358 (26.3) | 371 (27.3) |
| High-risk comorbidity | 3481 (84.3) | 1205 (85.5) | 1133 (83.2) | 1143 (84.1) |
| ≥2 Vascular beds affected | 2335 (56.6) | 810 (57.5) | 764 (56.1) | 761 (56.0) |
| Coronary artery disease | 2212 (53.6) | 773 (54.9) | 714 (52.5) | 725 (53.4) |
| Cerebrovascular disease | 214 (5.2) | 69 (0.9) | 80 (5.9) | 65 (4.7) |
| Ankle-Brachial Index score, mean (SD) | 0.94 (0.2) | 0.95 (0.2) | 0.94 (0.2) | 0.94 (0.21) |
| Type of LE-PAD for inclusion | ||||
| Peripheral artery bypass surgery | 694 (16.8) | 229 (16.3) | 248 (18.2) | 217 (16.0) |
| Peripheral percutaneous angioplasty | 1211 (29.3) | 396 (28.1) | 401 (29.5) | 414 (30.5) |
| Previous peripheral artery revascularization or surgery | 1729 (41.9) | 576 (40.9) | 590 (43.4) | 563 (41.4) |
| Limb or foot amputation | 316 (7.7) | 111 (7.9) | 102 (7.5) | 103 (7.6) |
| Intermittent claudication | 2966 (71.8) | 1009 (71.6) | 981 (72.1) | 976 (71.8) |
| Fontaine classification | ||||
| IIa | 1740 (42.1) | 596 (42.3) | 565 (41.5) | 579 (42.6) |
| IIb | 1107 (26.8) | 389 (27.6) | 354 (26.0) | 364 (26.8) |
| III | 164 (4.0) | 47 (3.3) | 73 (5.4) | 44 (3.2) |
| IV | 41 (1.0) | 16 (1.1) | 13 (1.0) | 12 (0.9) |
| Use of angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker | 2780 (67.3) | 928 (65.9) | 932 (68.5) | 920 (67.7) |
| Use of lipid-lowering agent | 3248 (78.7) | 1124 (79.8) | 1073 (78.8) | 1051 (77.3) |
Defined as an estimated glomerular filtration rate less than 60 mL/min.
Incidence Risk in High-risk Groups
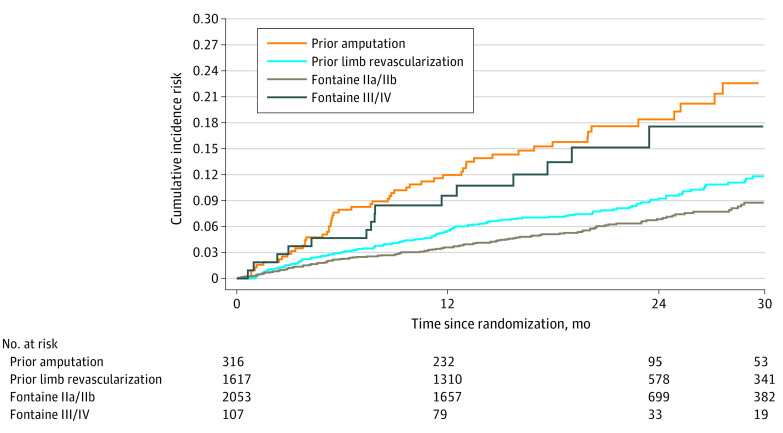
The risk of ischemic outcomes varied between subtypes of symptomatic LE-PAD based on severity of PAD (Table 2). Among all patients (N = 4129) with symptomatic LE-PAD, the 30-month Kaplan-Meier incidence risk of MACE or MALE, including major amputation, during follow-up was highest in patients with prior amputation (22.6%; this outcome was observed in 54 patients), followed by patients with Fontaine III or IV symptoms (17.6%; 15 patients) and patients with previous lower limb revascularization (11.8%; 142 patients). Patients with milder symptoms (Fontaine IIb or IIa and no history of revascularization) had a lower incidence of ischemic vascular outcomes, with an incidence risk of MACE or MALE, including major amputation, over 30 months of 8.8% (128 patients) (Figure 1). Thus, patients with previous amputation, Fontaine III or IV status, or previous lower limb revascularization were classified as having high-risk limb presentation of PAD, with incidence risks of MACE or MALE, including major amputation, greater than 10% over 30 months.
Table 2. Incidence Risk of Ischemic Outcomes and Severe Bleeding at 30 Months, Stratified by High-risk Limb Presentation and Presence of High-risk Comorbidity Among All Patients With Symptomatic Lower Extremity Peripheral Artery Disease (N = 4129).
| Outcome | Patients, No. | MACE | MALE, No. (%) | MACE or MALE, including major amputation, No. (%) | Bleeding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, No. (%) | Kaplan-Meier incidence risk at 30 mo, % | Patients, No. (%) | Kaplan-Meier incidence risk at 30 mo, % | Patients, No. (%) | Kaplan-Meier incidence risk at 30 mo, % | Fatal or critical organ, No. (%) | Major per modified ISTH, No. (%) | ||||
| Patients, No. (%) | Kaplan-Meier incidence risk at 30 mo, % | Patients, No. (%) | Kaplan-Meier incidence risk at 30 mo, % | ||||||||
| Any high-risk limb presentation | 2040 | 137 (6.7) | 9.2 | 81 (4.0) | 5.1 | 211 (10.3) | 13.7 | 17 (0.8) | 1.0 | 67 (3.3) | 4.7 |
| Amputation | 316 | 32 (10.1) | 13.8 | 23 (7.3) | 9.8 | 54 (17.1) | 22.6 | 2 (0.6) | 0.6 | 3 (0.9) | 1.1 |
| Previous revascularizationa | 1617 | 98 (6.1) | 8.4 | 50 (3.1) | 3.8 | 142 (8.8) | 11.8 | 15 (0.9) | 1.1 | 64 (4.0) | 5.6 |
| Fontaine classificationb | |||||||||||
| III or IV | 107 | 7 (6.5) | 7.1 | 8 (7.5) | 10.7 | 15 (14.0) | 17.6 | 0 | 0.0 | 0 | 0.0 |
| IIa or IIb | 2053 | 109 (5.3) | 7.3 | 22 (1.1) | 1.7 | 128 (6.2) | 8.8 | 15 (0.7) | 1.1 | 40 (1.9) | 3.0 |
| Any high-risk comorbidity | 3481 | 241 (6.9) | 9.5 | 86 (2.5) | 3.4 | 317 (9.1) | 12.4 | 30 (0.9) | 1.2 | 92 (2.6) | 4.0 |
| Polyvascular disease | 2335 | 175 (7.5) | 10.2 | 53 (2.3) | 2.9 | 222 (9.5) | 12.8 | 22 (0.9) | 1.4 | 73 (3.1) | 4.6 |
| History | |||||||||||
| Diabetes | 1940 | 146 (7.5) | 9.8 | 58 (3.0) | 4.1 | 199 (10.3) | 13.4 | 16 (0.8) | 1.0 | 41 (2.1) | 3.0 |
| Heart failure | 686 | 55 (8.0) | 11.1 | 13 (1.9) | 2.8 | 67 (9.8) | 13.5 | 4 (0.6) | 0.9 | 12 (1.7) | 4.0 |
| Kidney insufficiency | 1112 | 93 (8.4) | 11.2 | 26 (2.3) | 3.1 | 118 (10.6) | 14.1 | 10 (0.9) | 1.3 | 35 (3.1) | 5.0 |
| Both high-risk limb presentation and high-risk comorbidity | 1665 | 130 (7.8) | 10.4 | 66 (4.0) | 4.9 | 189 (11.4) | 14.7 | 15 (0.9) | 1.1 | 55 (3.3) | 4.9 |
| Neither high-risk limb presentation nor high-risk comorbidity | 273 | 5 (1.8) | 2.0 | 3 (1.1) | 1.1 | 8 (2.9) | 3.1 | 1 (0.4) | 0.4 | 4 (1.5) | 2.0 |
Abbreviations: ISTH, International Society of Thrombosis and Hemostasis; MACE, major adverse cardiac event; MALE, major adverse limb event.
Without prior amputation.
Without prior amputation or prior limb revascularization.
Figure 1. Major Adverse Cardiovascular Event or Major Adverse Limb Event, Including Major Amputation, Stratified by Subtype of Peripheral Artery Disease.
As expected, the incidence risk for ischemic events was also higher among patients with high-risk comorbidities.3 The 30-month incidence risk of MACE or MALE, including major amputation, was 12.8% among patients with polyvascular disease (this outcome was observed in 222 patients), 13.4% (199 patients) among those with diabetes, 13.5% (67 patients) among those with heart failure, and 14.1% (118 patients) among those with kidney insufficiency (Table 2).
Considering high-risk status overall, among the 4129 patients with LE-PAD, approximately half (49.4%; n = 2040) had a high-risk limb presentation of PAD, 84.3% (n = 3481) had high-risk comorbidities, and 40.3% (n = 1665) had both a high-risk limb presentation and a high-risk comorbidity. Importantly, only 6.6% (n = 273) with LE-PAD had neither a high-risk limb presentation nor a high-risk comorbidity. Among all patients with symptomatic LE-PAD, the 30-month incidence risk of MACE or MALE, including major amputation, was 13.7% among those with high-risk limb presentation (this outcome was observed in 211 patients), 12.4% (317 patients) among those with high-risk comorbidities, and 14.7% (189 patients) among those with both high-risk limb presentation and high-risk comorbidities, with a lower incidence risk of 3.1% (8 patients) among those with neither high-risk limb presentation or high-risk comorbidities (Table 2; eFigure 1 in the Supplement). A similar pattern of risk was observed for patients treated only with aspirin (eFigure 1 in the Supplement). The 30-month incidence risks of major bleeding and its subset, fatal or critical organ bleeding, were similar among patients with high-risk limb presentation (4.7%; 67 patients), high-risk comorbidities (4.0%; 92 patients), or both (4.9%; 55 patients), and lowest among those patients with neither high-risk limb presentation nor high-risk comorbidities (2.0%; 4 patients) (Table 2).
Treatment Outcome
Considering the shrinkage estimates, among the patients with symptomatic LE-PAD in COMPASS, those randomized to the combination of low-dose rivaroxaban and aspirin had a 26% lower risk of developing MACE (HR, 0.74 [95% CrI, 0.58-0.92]) and a 45% decreased risk of developing MALE, including major amputation (HR, 0.55 [95% CrI, 0.35-0.85]), compared with patients receiving aspirin alone. Furthermore, the composite of MACE or MALE, including major amputation, was reduced by 29% (HR, 0.71 [95% CrI, 0.57-0.87]; eFigure 2 in the Supplement) and the composite of cardiovascular death, myocardial infarction, ischemic stroke, acute limb ischemia, or major amputation by 28% (HR, 0.72 [95% CrI, 0.58-0.89]) in favor of the combination of rivaroxaban and aspirin compared with aspirin alone. Major bleeding was increased with the combination of rivaroxaban and aspirin compared with aspirin alone (HR, 1.69 [95% CrI, 1.18-2.40]). Fatal or critical organ bleeding was numerically increased with combination therapy, but this was not statistically significant (HR, 1.56 [95% CrI, 0.78-3.39]; Table 3).
Table 3. Efficacy and Safety of Rivaroxaban and Aspirin in Combination vs Aspirin Alone Among Patients With Symptomatic Lower Extremity Peripheral Artery Diseasea.
| Outcome | Rivaroxaban plus aspirin (n = 1409) | Aspirin alone (n = 1359) | Rivaroxaban plus aspirin vs aspirin alone | |||||
|---|---|---|---|---|---|---|---|---|
| First events, No. (%) | Annual rate, % per y | 30-mo Kaplan-Meier incidence risk, % | First events, No. (%) | Annual rate, % per y | 30 mo Kaplan-Meier incidence risk, % | Hazard ratio (95% CI)b | Shrinkage estimates hazard ratio (95% credible interval)c | |
| MACEd | 73 (5.2) | 2.9 | 6.9 | 98 (7.2) | 4.1 | 10.8 | 0.71 (0.53-0.97) | 0.74 (0.58-0.92) |
| Cardiovascular death, myocardial infarction, ischemic stroke, acute limb ischemia, or major amputation | 88 (6.2) | 3.6 | 8.1 | 120 (8.8) | 5.1 | 13.0 | 0.70 (0.53-0.93) | 0.72 (0.58-0.89) |
| MALE, including major amputation | 26 (1.8) | 1.0 | 2.5 | 46 (3.4) | 1.9 | 4.7 | 0.55 (0.34-0.88) | 0.55 (0.35-0.85) |
| MACE or MALE, including major amputation | 98 (7.0) | 4.0 | 9.2 | 136 (10.0) | 5.8 | 14.6 | 0.69 (0.53-0.89) | 0.71 (0.57-0.87) |
| Major bleeding | 46 (3.3) | 1.9 | 4.5 | 26 (1.9) | 1.1 | 2.8 | 1.71 (1.06-2.77) | 1.69 (1.18-2.40) |
| Fatal or critical organ bleeding | 15 (1.1) | 0.6 | 1.2 | 7 (0.5) | 0.3 | 0.8 | 2.06 (0.84-5.05) | 1.56 (0.78-3.39) |
| Net clinical benefite | 107 (7.6) | 4.4 | 9.6 | 137 (10.1) | 6.0 | 14.4 | 0.75 (0.58-0.96) | 0.78 (0.63-0.95) |
Abbreviations: MACE, major adverse cardiac events; MALE, major adverse limb event.
Percentage is the proportion of patients with an outcome. Percentage per year is the rate per 100 patient-years of follow-up.
Hazard ratios (95% CI) are from the stratified Cox proportional hazards models.
Shrinkage estimates were obtained via bayesian hierarchical modeling.
MACE was defined as a myocardial infarction, stroke, or cardiovascular death.
Net clinical benefit was defined as MACE, MALE (including major amputation), or fatal or critical organ bleeding.
At 30 months, the estimated absolute risk reduction with low-dose rivaroxaban and aspirin, compared with aspirin alone, was 2.7% (95% CI, 0.83%-4.4%) for MACE, 2.1% (95% CI, 0.68%-3.0%) for MALE, including major amputation, and 4.0% (95% CI, 1.8%-6.0%) for MACE or MALE, including major amputation, corresponding to 27, 21, and 40 events prevented per 1000 patients treated, respectively.
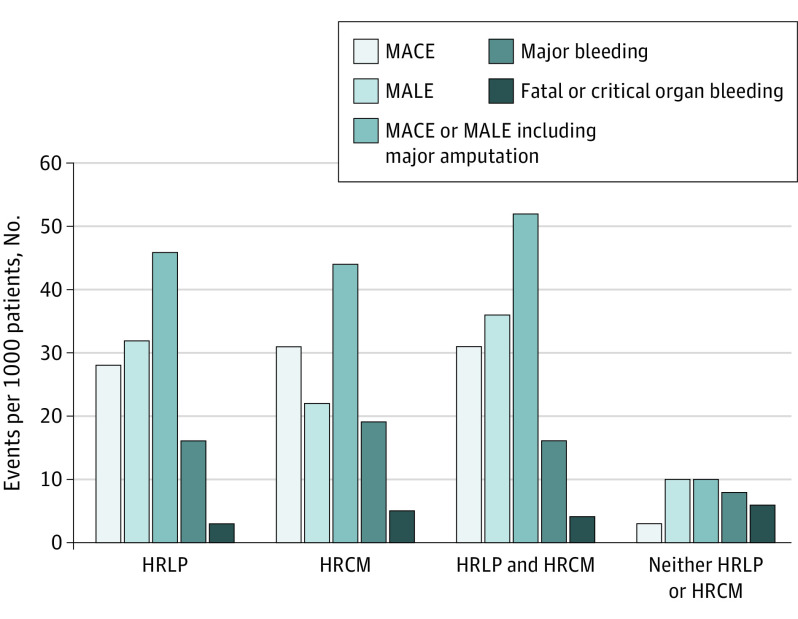
The magnitude of treatment effect with rivaroxaban and aspirin was similar for those at highest risk. Patients with either high-risk limb presentation or high-risk comorbidities demonstrated a similarly large estimated absolute risk reduction in the composite of MACE or MALE, including major amputation (4.2% [95% CI, 1.9%-6.2%]), when treated with rivaroxaban and aspirin as opposed to aspirin alone over a 30-month period; however, the estimated absolute risk reduction for MALE, including major amputation, was more pronounced in patients with high-risk limb presentation than for those with high-risk comorbidity over 30 months (3.2% [95% CI, 1.0%-4.7%] vs 2.2% [95% CI, 0.7%-3.2%]), while the 30-month estimated absolute risk reduction for MACE was slightly more pronounced in patients with high-risk comorbidities compared with those with high-risk limb presentation (3.1% [95% CI, 0.9%-4.9%] vs 2.8% [95% CI, 0.8%-4.4%]) (Figure 2). In contrast, patients without high-risk limb presentation or high-risk comorbidities demonstrated an estimated 0.3% (95% CI, 0.1%-0.5%) absolute risk reduction in MACE, an estimated 1.0% (95% CI, 0.3%-1.5%) absolute risk reduction in MALE, including major amputation, and an estimated 1.0% (95% CI, 0.4%-1.4%) in MACE or MALE, including major amputation, at 30 months.
Figure 2. Estimated Number of Ischemic Events Prevented and Bleeding Events Caused per 1000 Patients When Treated With Rivaroxaban and Aspirin in Combination, Compared With Aspirin Alone Over 30 Months.
HRCM indicates high-risk comorbidity; HRLP, high-risk limb presentation; MACE, major adverse cardiovascular event; MALE, major adverse limb event.
The 30-month estimated absolute risk increase of major bleeding is shown by high-risk limb presentation or high-risk comorbidity in Figure 2. The estimated risk increase in major bleeding was numerically higher in patients with high-risk limb presentation (1.6% [95% CI, 0.4%-3.3%]), a high-risk comorbidity (1.9% [95% CI, 0.5%-3.9%]), both a high-risk limb presentation and comorbidity (1.6% [95% CI, 0.4%-3.3%]), or either a high-risk limb presentation or comorbidity (2.0% [95% CI, 0.5%-3.9%]), compared with neither (0.8% [95% CI, 0.2%-1.5%]). The estimated absolute risk increase in fatal or critical organ bleeding was low among compared risk groups, being 0.3% (95% CI, 0.1%-1.4%) among patients with a high-risk limb presentation, 0.5% (95% CI, 0.2%-1.9%) among patients with high-risk comorbidities, 0.4% (95% CI, 0.2%-1.6%) among patients with both high-risk limb presentations and comorbidities, 0.4% (95% CI, 0.2%-1.8%) among those with either a high-risk limb presentation or comorbidity, and 0.6% (95% CI, 0.2%-2.6%) among patients with neither.
Overall, the net clinical benefit, which combined MACE or MALE, including major amputation or fatal or critical organ bleeding, remained in favor of rivaroxaban and aspirin compared with aspirin alone (HR, 0.78 [95% CrI, 0.63-0.95]) (eFigure 2 in the Supplement), equivalent to an estimated 31 events prevented per 1000 patients treated over 30 months. The magnitude of net clinical benefit was similar in patients with high-risk limb presentation (estimated absolute risk reduction, 3.5% [95% CI, 0.7%-5.9%]), high-risk comorbidity (estimated absolute risk reduction, 3.3% [95% CI, 0.7%-5.6%]), and either a high-risk limb presentation or comorbidity (estimated absolute risk reduction, 3.2% [95% CI, 0.6%-5.3%]), and less pronounced in patients with neither (estimated absolute risk reduction, 0.7% [95% CI, 0.2%-1.2%]).
Discussion
Among patients with symptomatic LE-PAD, we show that the 30-month incidence risk of MACE or MALE, including major amputation, is highest in patients with prior amputation, previous revascularization surgery, or severe symptoms of PAD, and these patients have a substantial absolute risk reduction when treated with rivaroxaban and aspirin compared with aspirin alone. In addition, we verify that patients with LE-PAD with at least 1 high-risk comorbidity, including polyvascular disease, diabetes, heart failure, or kidney insufficiency, have a similarly high incidence risk and substantial absolute risk reduction when treated with rivaroxaban and aspirin. Accordingly, the net clinical benefit in those with either high-risk limb presentation or high-risk comorbidities strongly favors combination rivaroxaban and aspirin over aspirin use alone.
Patients with LE-PAD are at greater risk for ischemic events and cardiovascular death compared with their counterparts with isolated CAD.13 Yet LE-PAD encompasses a wide spectrum of disease, with clinical manifestations ranging from asymptomatic atherosclerosis to limb ischemia requiring amputation.14 In the current investigation, we show that the highest risk subtypes of patients with symptomatic LE-PAD are those with previous amputation, Fontaine stage III or IV status, and previous revascularization, with all 3 groups having an incidence risk of MACE or MALE, including major amputation, greater than 10% over 30 months. To our knowledge, this is the first report from a large cohort of patients that characterizes rates of cardiac events and limb events according to clinical presentation of symptomatic LE-PAD. While it is not unexpected that those previously experiencing severe PAD symptoms or severe ischemia requiring surgical intervention had the highest risk of subsequent vascular ischemia, the magnitude of risk is quite high, with a 30-month incidence risk of 13.7% for MACE or MALE, including major amputation. Furthermore, these more severe forms of PAD are at particularly high risk for MALE. While the treatment benefit of the combination rivaroxaban and aspirin is apparent in the overall symptomatic LE-PAD cohort, the estimated absolute risk reduction at 30 months is greatest for those at the highest baseline risk, as shown in Figure 2.
Clinicians managing patients with LE-PAD desire guidance when making decisions regarding antithrombotic therapy.15 Among patients with LE-PAD, both PAD severity and comorbidities are important in this consideration. We demonstrate that half of the patients with symptomatic LE-PAD have the high-risk limb presentation of Fontaine III or IV symptoms, previous amputation or previous lower limb revascularization, while 4 of 5 patients have the high-risk comorbidities of polyvascular disease, diabetes, heart failure, or kidney insufficiency. Importantly, only 6.6% of patients had neither high-risk limb presentation or high-risk comorbidity, and these individuals expectedly had a lower incidence risk of MACE or MALE, including major amputation (eFigure 1 in the Supplement; Table 2).
Among patients with symptomatic LE-PAD, consistent with the overall COMPASS trial results, treatment with combination rivaroxaban and aspirin reduced the incidence of MACE or MALE, including major amputation, among patients with high-risk limb presentation or high-risk comorbidity, with approximately 42 events prevented per 1000 patients treated over 30 months. Interestingly, patients with PAD with a high-risk limb presentation demonstrated a greater reduction in MALE, while patients with high-risk comorbidity demonstrated a greater reduction in MACE. Furthermore, while ischemic risks varied among differing manifestations of symptomatic LE-PAD, the estimated absolute increase in fatal or critical organ bleeding was similarly low among patients with high-risk limb presentation, high-risk comorbidity, or neither, acknowledging the limitations of comparisons between small subgroups. Therefore, the net clinical benefit for patients with high-risk limb presentation or high-risk comorbidity receiving combination rivaroxaban and aspirin compared with aspirin alone prevented nearly 32 events per 1000 patients treated over 30 months. This large and clinically important risk reduction was similar to the benefit of warfarin for stroke prophylaxis in high-risk atrial fibrillation.16
Lower extremity PAD is a common manifestation of atherosclerosis, with more than 200 million people affected worldwide.17 Surgery is required in a minority of patients with symptomatic LE-PAD, with the hallmark of therapy being efficacious medical treatment.18,19 Now that 2.5 mg of rivaroxaban twice a day in combination with aspirin has received endorsement by multiple international agencies granting medication approval, uptake of this combination is increasing and being incorporated into contemporary international guidance statements.18,20,21 However, clinicians’ perceptions of the applicability of COMPASS results to patients with PAD remains a barrier to evidence-based therapy.22 Our analysis argues for strong consideration of combination rivaroxaban and aspirin for patients with symptomatic LE-PAD with high-risk limb presentation and/or comorbidities, to prevent vascular events, as long as this is not limited by undue bleeding risk. Patients with high-risk limb presentations in particular may also require dedicated strategies for limb preservation, given their higher risk of MALE.
The recent VOYAGER PAD trial23 (NCT02504216) also evaluated the efficacy of combination rivaroxaban and aspirin compared with aspirin alone, with or without short-term clopidogrel, shortly after infrainguinal revascularization in patients with symptomatic LE-PAD. The combination of rivaroxaban and aspirin yielded a significant 15% reduction in the composite outcome of myocardial infarction, ischemic stroke, or cardiovascular death, acute limb ischemia, or major vascular amputation. While there was not a statistically significant increase in major bleeding per Thrombolysis in Myocardial Infarction classifications, there was significant increase in major bleeding per International Society of Thrombosis and Hemostasis classifications. As with COMPASS, however, there was no excess in severe bleeding, including fatal or intracranial hemorrhage. Thus, VOYAGER PAD confirms our observation of the efficacy of combination rivaroxaban and aspirin compared with aspirin alone in patients with symptomatic LE-PAD.
Strengths of our investigation include large numbers of patients with well-characterized data, receiving contemporary medical therapy in the setting of a randomized clinical trial with adjudicated events. In addition, we used conservative shrinkage estimates of treatment effect, which are more robust than traditional subgroup analysis treatment estimates.10,11,12
Limitations
A potential limitation of our investigation is that the high degree of risk factor control within the COMPASS trial may underestimate the ischemic risk reduction that could be achieved with combination rivaroxaban and aspirin in clinical practice. Patients in COMPASS were relatively well treated, in that approximately 80% of patients were receiving statin therapy and 70% were receiving renin angiotensin aldosterone–system inhibitors. This is substantially higher secondary prevention therapy than that observed in routine clinical practice, where approximately 30% of patients are prescribed vascular protective agents.13 Suboptimal risk factor control is associated with a higher incidence risk of ischemic events and a greater absolute benefit of combination rivaroxaban and aspirin.24 This is analogous to the overall COMPASS trial, in which the ischemic risk was significantly lower than the risk observed within the general population (tabulated within the Reduction of Atherosclerosis for Continued Health [REACH] registry).25 The length of follow-up of patients in COMPASS may also underestimate the longer-term absolute risk reduction conferred by rivaroxaban and aspirin in combination, since the net clinical benefit has been shown to increase overtime.26 Even so, we demonstrate that in patients with symptomatic LE-PAD with nearly optimized medical therapy, the risks of MACE and MALE remain high and the use of combination rivaroxaban and aspirin yields large absolute risk reductions in ischemic events.
Conclusions
In patients with symptomatic LE-PAD, rates of ischemic events are particularly high for patients with high-risk limb presentations of PAD, including Fontaine III or IV symptoms, previous revascularization, or previous amputation, as well as for patients with high-risk comorbidities, such as polyvascular disease, diabetes, heart failure, and kidney insufficiency. For these patients, treatment with combination rivaroxaban and aspirin leads to a large and clinically important decrease in ischemic vascular events.
eFigure 1. Incidence Risk of MACE or MALE Including Major Amputation Among All Patients With Symptomatic Lower Extremity PAD
eFigure 2. Incidence Risk of MACE or MALE including Major Amputation (A) as Well as Net Clinical Benefit (B) Among Patients Randomized to Aspirin and Rivaroxaban in Combination or Aspirin Alone
References
- 1.Hess CN, Norgren L, Ansel GM, et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) initiative. Circulation. 2017;135(25):2534-2555. doi: 10.1161/CIRCULATIONAHA.117.024469 [DOI] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 3.Anand SS, Eikelboom JW, Dyal L, et al. ; COMPASS Trial Investigators . Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol. 2019;73(25):3271-3280. doi: 10.1016/j.jacc.2019.02.079 [DOI] [PubMed] [Google Scholar]
- 4.Anand SS, Bosch J, Eikelboom JW, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219-229. doi: 10.1016/S0140-6736(17)32409-1 [DOI] [PubMed] [Google Scholar]
- 5.Jones WS, Baumgartner I, Hiatt WR, et al. ; International Steering Committee and Investigators of the EUCLID Trial . Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135(3):241-250. doi: 10.1161/CIRCULATIONAHA.116.025880 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong EJ, Chen DC, Westin GG, et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3(2):e000697. doi: 10.1161/JAHA.113.000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaca MP, Gutierrez JA, Creager MA, et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis-thrombolysis in myocardial infarction 50 (TRA2°P-TIMI 50). Circulation. 2016;133(10):997-1005. doi: 10.1161/CIRCULATIONAHA.115.019355 [DOI] [PubMed] [Google Scholar]
- 8.Bosch J, Eikelboom JW, Connolly SJ, et al. Rationale, design and baseline characteristics of participants in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial. Can J Cardiol. 2017;33(8):1027-1035. doi: 10.1016/j.cjca.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Eikelboom JW, Bosch J, et al. ; COMPASS investigators . Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):205-218. doi: 10.1016/S0140-6736(17)32458-3 [DOI] [PubMed] [Google Scholar]
- 10.Jones HE, Ohlssen DI, Neuenschwander B, Racine A, Branson M. Bayesian models for subgroup analysis in clinical trials. Clin Trials. 2011;8(2):129-143. doi: 10.1177/1740774510396933 [DOI] [PubMed] [Google Scholar]
- 11.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Vol 13 John Wiley & Sons; 2004. [Google Scholar]
- 12.Pennello G. Bayesian hierarchical models for subgroup analysis in clinical studies: Biopharmaceutical Applied Statistics Symposium (BASS) program. Published 2013. Accessed August 20, 2020.http://www.bassconference.org/tutorials/BASS%202013%20Pennello.pdf
- 13.Welten GM, Schouten O, Hoeks SE, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51(16):1588-1596. doi: 10.1016/j.jacc.2007.11.077 [DOI] [PubMed] [Google Scholar]
- 14.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509-1526. doi: 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 15.Jones WS, Patel MR. Antithrombotic therapy in peripheral artery disease: generating and translating evidence into practice. J Am Coll Cardiol. 2018;71(3):352-362. doi: 10.1016/j.jacc.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 16.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297-305. doi: 10.7326/0003-4819-151-5-200909010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329-1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V, Ricco JB, Bartelink MEL, et al. ; ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by the European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC), and the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763-816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 19.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):e71-e126. doi: 10.1016/j.jacc.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 20.Conte MS, Bradbury AW, Kolh P, et al. ; GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS) . Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58(1S):S1-S109, 109.e33. doi: 10.1016/j.ejvs.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 22.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82-93. doi: 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- 23.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994-2004. doi: 10.1056/NEJMoa2000052 [DOI] [PubMed] [Google Scholar]
- 24.Vanassche T, Verhamme P, Anand SS, et al. Risk factors and clinical outcomes in chronic coronary and peripheral artery disease: an analysis of the randomized, double-blind COMPASS trial. Eur J Prev Cardiol. 2020;27(3):296-307. doi: 10.1177/2047487319882154 [DOI] [PubMed] [Google Scholar]
- 25.Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39(9):750-757a. doi: 10.1093/eurheartj/ehx658 [DOI] [PubMed] [Google Scholar]
- 26.Steffel J, Eikelboom JW, Anand SS, Shestakovska O, Yusuf S, Fox KAA. The COMPASS trial: net clinical benefit of low-dose rivaroxaban plus aspirin as compared with aspirin in patients with chronic vascular disease. Circulation. 2020;142(1):40-48. doi: 10.1161/CIRCULATIONAHA.120.046048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Incidence Risk of MACE or MALE Including Major Amputation Among All Patients With Symptomatic Lower Extremity PAD
eFigure 2. Incidence Risk of MACE or MALE including Major Amputation (A) as Well as Net Clinical Benefit (B) Among Patients Randomized to Aspirin and Rivaroxaban in Combination or Aspirin Alone