Key Points
Question
Can tofacitinib, an oral Janus kinase inhibitor, be used as a safe and effective treatment for nail involvement and palmoplantar pustulosis in synovitis, acne, pustulosis, hyperostosis, and osteomyelitis (SAPHO) syndrome?
Findings
In this case series study, tofacitinib treatment was associated with significant remission of nail lesions and palmoplantar pustulosis accompanied by an improvement in the quality of life in patients with SAPHO syndrome.
Meaning
Use of tofacitinib for SAPHO-induced nail involvement may offer a novel treatment method for patients with this syndrome.
Abstract
Importance
Nail involvement is common in synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome, which has a strong association with quality of life in patients with SAPHO. Tofacitinib is an oral Janus kinase inhibitor that has been previously shown to be effective for nail psoriasis.
Objective
To assess the efficacy and safety of tofacitinib for the treatment of nail involvement in SAPHO syndrome.
Interventions
Participants received tofacitinib, 5 mg, twice daily, for 12 weeks.
Design, Setting, and Participants
This open-label, single-arm, prospective pilot study included 13 patients with SAPHO syndrome accompanied by nail lesions and active palmoplantar pustulosis who were recruited from Peking Union Medical College Hospital from September 2019 to December 2019. Follow-up was completed in March 2020. Analysis began March 2020.
Main Outcomes and Measures
The primary end point was the percentage of the change from baseline in Nail Psoriasis Severity Index scores at week 12. Secondary end points included the percentage of the change from baseline in Palmoplantar Psoriasis Area and Severity Index scores, change from baseline in Visual Analogue Scale scores in global osteoarticular pain, Dermatology Life Quality Index scores, and inflammatory markers. Adverse events were recorded throughout the study.
Results
Thirteen female Asian patients (means [SD] age, 39.7 [12.3] years) were included, all of whom completed the study. At week 12, significant improvements were observed in Nail Psoriasis Severity Index scores (median, −67% [interquartile range (IQR), −56% to −77%]; P < .001) and Palmoplantar Psoriasis Area and Severity Index scores (median, −71% [IQR, −58% to −78%]; P < .001). Significant improvement was also noted in Dermatology Life Quality Index scores (median, −12 [IQR, −8.5 to −15]; P < .001) at week 12. A significant decrease in Visual Analogue Scale scores in global osteoarticular pain was observed at week 8 (median, −4 [IQR, 0 to −5]; P = .02) but was not significant at week 12. Inflammatory marker levels were decreased, as indicated by erythrocyte sedimentation rate (median, −8 mm/h [IQR, −4 mm/h to −11 mm/h]; P < .001) and high-sensitivity C-reactive protein levels (median, −1.6 [IQR, −0.3 to −4.1]; P = .01). No severe adverse events were observed.
Conclusions and Relevance
In this pilot study, tofacitinib yielded significant remission of nail lesions and palmoplantar psoriasis accompanied by an improvement in quality of life in patients with SAPHO syndrome. Additional follow-up studies to evaluate the long-term efficacy and safety of tofacitinib for nail involvement in SAPHO syndrome are warranted.
Trial Registration
Chinese Clinical Trial Registry number: ChiCTR1900025941
This case series study examines the use of tofacitinib for treating nail involvement in Chinese women with synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome.
Introduction
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is an inflammatory disease characterized by sterile osteitis and cutaneous manifestations. Palmoplantar pustulosis (PPP) is reported to be the most common manifestation,1,2,3,4 affecting 50% to 90% of the patients who develop skin lesions. Nail involvement, which often occurs following PPP, is a common manifestation in SAPHO syndrome5 and has a significant association with quality of life.6
Tofacitinib is an oral pan Janus-kinase (JAK) inhibitor, with preferential inhibition of JAK1 and JAK3. We previously reported the case of a 44-year-old woman with SAPHO syndrome who was successfully treated with tofacitinib.7 To our knowledge, the efficacy and safety of tofacitinib in patients with SAPHO syndrome remains to be confirmed.
Methods
This was a single-center, open-label, single-arm, 12-week prospective pilot study approved by the ethics committee of Peking Union Medical College Hospital and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Patients from a dynamic cohort of individuals with SAPHO syndrome2,8 were screened from September 2019 to December 2019 to determine inclusion in the study. Individuals aged 18 to 70 years who had received a diagnosis of SAPHO syndrome9 that was accompanied by nail lesions (defined as baseline overall Nail Psoriasis Severity Index [NAPSI] score of >14) and active palmoplantar psoriasis (PPP; defined as a baseline PPP Area and Severity Index [PPPASI] score of ≥8 with at least 10% of the total surface of palms and soles affected) were eligible. After a washout period of 3 months, participants received tofacitinib, 5 mg, twice daily, for 12 weeks, and were permitted to take nonsteroidal anti-inflammatory drugs (NSAIDs) at fixed doses during the study. Clinical responses to treatment and safety were evaluated at baseline and weeks 2, 4, 8, and 12.
The primary efficacy end point was the percentage of change from baseline in NAPSI scores10 at week 12. Secondary efficacy end points included the percentage of change from baseline in PPPASI scores; the proportion of patients achieving NAPSI50 (50% decrease of NAPSI scores) and NAPSI75 (75% decrease of NAPSI scores) and PPPASI50 (50% decrease of PPPASI scores) and PPPASI75 (75% decrease of PPPASI scores); the change from baseline in Visual Analogue Scale (VAS) scores in global osteoarticular pain; the change from baseline in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores; Dermatology Life Quality Index (DLQI) scores; and inflammatory markers, including erythrocyte sedimentation rate and high-sensitivity C-reactive protein levels. Safety and tolerability assessments comprised laboratory analyses and clinical manifestations.
Because of the small sample size, continuous variables were compared by a nonparametric test (Friedman test). For normally distributed data, analysis of covariance was performed in parallel. Bonferroni correction was applied to all multiple comparisons. A P value of <.05 was considered statistically significant. Statistical analyses were performed using SPSS, version 20.0 (IBM).
Results
Among 158 patients who were screened, 17 patients who met the inclusion criteria were invited to participate in the study. For financial reasons, 4 of the eligible patients declined to participate. A final total of 13 patients were included. The demographic characteristics and clinical features of these 13 patients are presented in the Table. During the washout period and throughout the study, 3 patients (23%) continued to receive a fixed dose of NSAIDs. Apart from NSAIDs, no patient took any other medications or received topical treatments. All enrolled patients completed the 12-week follow-up.
Table. Baseline Patient Characteristics.
| Patient No./sex/age, y | Disease duration, mo | Manifestations | Affected nails | NAPSI | PPPASI | Previous treatments | |
|---|---|---|---|---|---|---|---|
| Osteoarticular | Dermatological | ||||||
| 1/F/30s | 27 | Anterior chest wall, sacroiliac joint, cervical vertebra | PPP on the palms and soles | Fingernails and toenails | 30 | 13.5 | TwHF, NSAIDs |
| 2/F/30s | 3 | Anterior chest wall, sacroiliac joint, lumbar vertebra, shoulder joint | PPP on the palms and soles, PV | Fingernails and toenails | 41 | 19.2 | NSAIDs, systemic corticosteroids |
| 3/F/40s | 17 | Anterior chest wall, ankle joint | PPP on the palms and soles | Fingernails and toenails | 48 | 12.8 | NSAIDs, adalimumab |
| 4/F/50s | 12 | Anterior chest wall, thoracic vertebra, shoulder joint, knee joint | PPP on the palms and soles | Fingernails and toenails | 40 | 8.1 | TwHF, MTX, NSAIDS, systemic corticosteroids |
| 5/F/30s | 24 | Anterior chest wall, knee joint, ankle joint | PPP on the palms and soles | Fingernails and toenails | 51 | 15.1 | MTX, IEF, TwHF, NSAIDs |
| 6/F/50s | 16 | Anterior chest wall, sacroiliac joint | PPP on the palms and soles, PV | Fingernails and toenails | 141 | 52.2 | Etanercept, NSAIDs |
| 7/F/50s | 12 | Anterior chest wall | PPP on the palms and soles, PV | Fingernails and toenails | 90 | 24.6 | MTX |
| 8/F/20s | 60 | Anterior chest wall, hip joint, metacarpophalangeal joints | PPP on the palms and soles, PV | Fingernails and toenails | 86 | 10.8 | Cyclosporin |
| 9/F/30s | 180 | Anterior chest wall, shoulder joint | PPP on the palms | Fingernails | 63 | 16.8 | MTX, SASP, THD, TwHF, NSAIDs, systemic corticosteroids |
| 10/F/20s | 6 | Anterior chest wall, sacroiliac joint | PPP on the palms and soles, PV | Fingernails and toenails | 53 | 26.8 | BPs, SASP, NSAIDs |
| 11/F/50s | 10 | Anterior chest wall, shoulder joint, knee joint | PPP on the palms and soles, PV | Fingernails and toenails | 34 | 9.2 | Systemic corticosteroids |
| 12/F/40s | 4 | Anterior chest wall, lumbar vertebra | PPP on the palms and soles | Fingernails and toenails | 37 | 15.8 | None |
| 13/F/10s | 1 | Anterior chest wall, cervical vertebra | PPP on the palms | Fingernails | 33 | 9.6 | None |
Abbreviations: BPs, bisphosphonates; IEF, leflunomide; MTX, methotrexate; NAPSI, nail psoriasis severity index; NSAIDs, nonsteroidal anti-inflammatory drugs; PPP, palmoplantar psoriasis; PPPASI, palmoplantar psoriasis area and severity index; PV, psoriasis vulgaris; SASP, salazosulfapyridine; THD, thalidomide; TwHF, tripterygium wilfordii Hook F.
At week 12, significant improvements were observed in NAPSI (median, −67% [interquartile range (IQR), −56% to −77%]; P < .001, Figure 1A) and PPPASI scores (median, −71% [IQR, −58% to −78%]; P < .001, Figure 1B). All patients achieved NAPSI50 and 3 (23%) achieved NAPSI75. Eleven (85%) and 4 patients (31%) achieved PPPASI50 and PPPASI75, respectively. Significant improvements with tofacitinib were also observed in DLQI scores (median, −12 [IQR, −8.5 to −15]; P < .001, Figure 1C) at week 12. Significant improvement in VAS in global osteoarticular pain was observed at week 8 (median, −4 [IQR, 0 to −5]; P = .02, Figure 1D) but was not significant at week 12. No statistically significant difference in BASDAI scores between baseline and week 12 were observed (eFigure 1 in the Supplement). Levels of inflammatory markers were decreased as indicated by ESR (median, −8 mm/h [IQR, −4 mm/h to −11 mm/h]; P < .001, Figure 1E) and levels of high-sensitivity C-reactive protein (median, −1.6 [IQR, −0.3 to −4.1]; P = .01; Figure 1F). The results of analysis of covariance are presented in eFigure 2 in the Supplement. Typical photographs of nail lesions during the treatment are shown in Figure 2, and photographs of the nail and skin lesions of all patients before and after tofacitinib treatment are presented in eFigures 3 through 15 in the Supplement.
Figure 1. Clinical Response of Nail Involvement, Palmoplantar Pustulosis (PPP), Osteoarticular Manifestations, and Inflammatory Parameters in Patients With Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis Syndrome Treated With Tofacitinib.
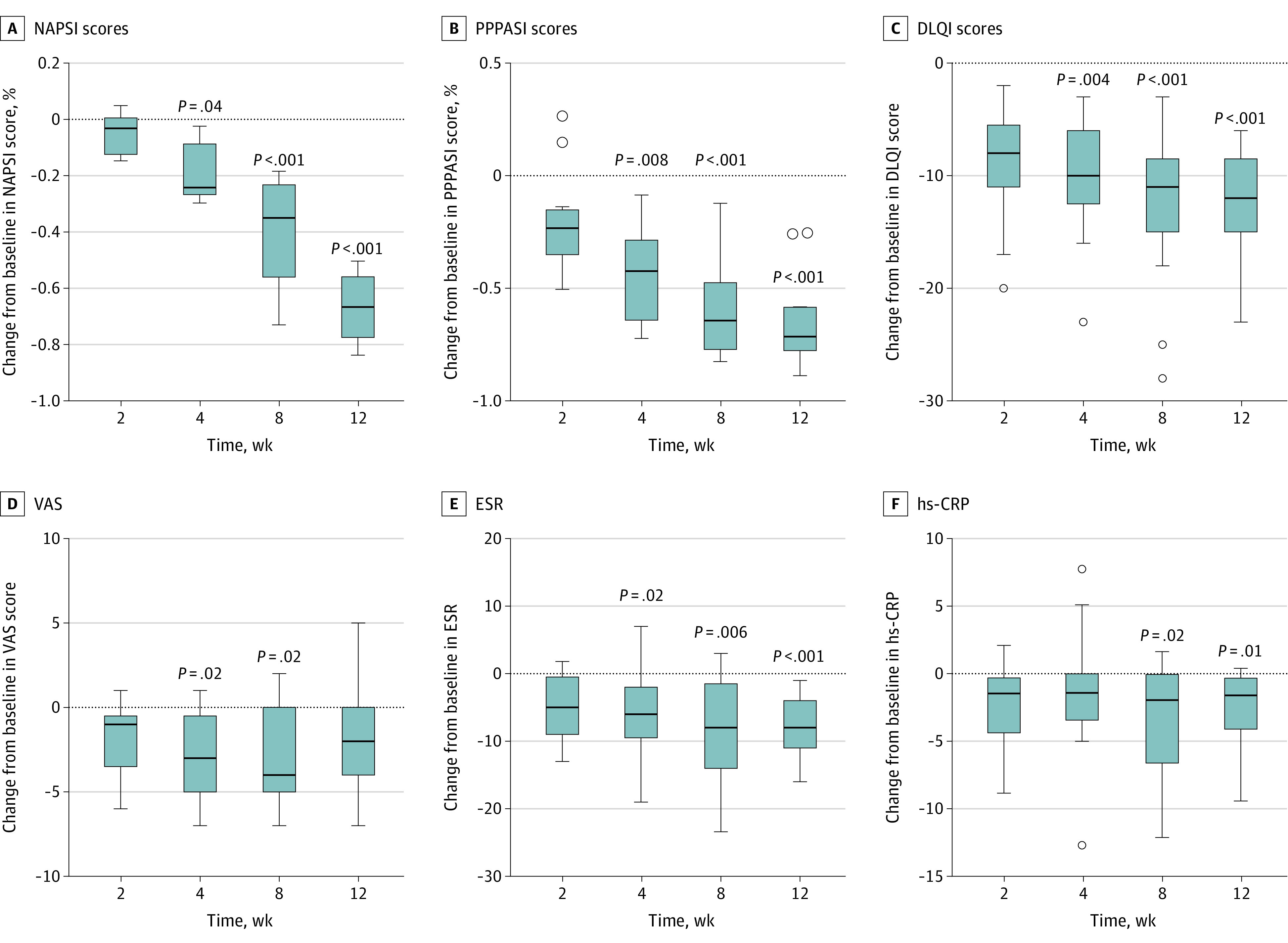
Box plots illustrate the change from baseline in Nail Psoriasis Severity Index (NAPSI) scores (A), PPP Area and Severity Index (PPPASI) scores (B), Dermatology Life Quality Index (DLQI) scores (C), Visual Analogue Scale (VAS) scores in global osteoarticular pain (D), erythrocyte sedimentation rate (ESR) (E), and high-sensitivity C-reactive protein (hs-CRP) levels (F) during the 12-week treatment. Boxes include the median and interquartile range. Whiskers are within the 1.5 interquartile range, and circles are outliers.
Figure 2. Changes in Nail Lesions During Tofacitinib Treatment in Patient 8 With Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis Syndrome.
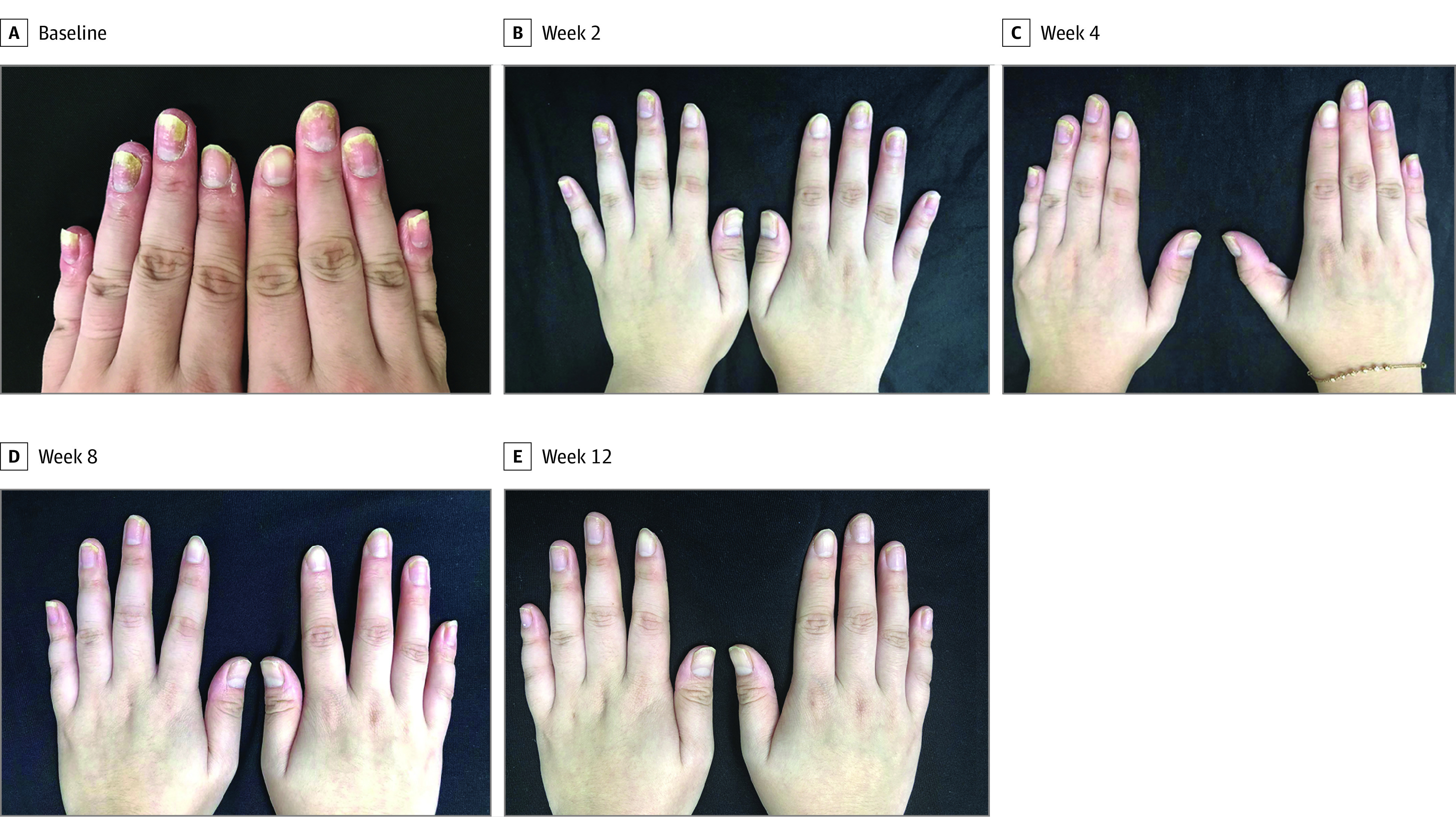
A, Baseline, and weeks 2 (B), 4 (C), 8 (D), and 12 (E).
Overall, tofacitinib therapy was well tolerated by the study participants. One patient (7.7%) reported acute tonsillitis during the 12-week treatment period. The remaining patients had no clinical adverse events. Abnormal serum laboratory test results included elevated low-density lipoprotein cholesterol levels (2 of 13 [15.4%]), elevated high-density lipoprotein cholesterol levels (1 of 13 [7.7%]), slightly elevated (<1.13 mg/dL; to convert to micromoles per liter, multiply by 88.4) serum creatinine levels (1 of 13 [7.7%]), slightly elevated (<50 U/L; to convert to microkatals per liter, multiply by 0.0167) aspartate aminotransferase levels (1 of 13 [7.7%]), and decreased neutrophil counts (0.5-1.5 × 109/L; 1 of 13 [7.7%]).
Discussion
In this study, we observed rapid and significant improvements in nail lesions and PPP of study participants as early as 4 weeks after treatment initiation compared with baseline. Moreover, we detected marked improvements in the quality of life of patients, as evidenced by improvements in DLQI scores from baseline.
The improvements in tofacitinib-induced nail lesions seen in this study appear to be greater for SAPHO syndrome than those reported in an analysis of pooled data from 2 phase 3 trials investigating the use of tofacitinib for nail psoriasis.11 In this study, we recorded a 67% reduction in NAPSI scores and 23% NAPSI75 at week 12, while 16.5% and 16.6% were attained at week 16 in the previous study, respectively. However, 10.3% of the patients in the previous study achieved a NAPSI score of 100 at week 16, while none achieved complete remission in our study. A possible explanation for this disparity may be differences in the respective study participants. Patients in our study had more severe nail lesions at baseline than those in previous studies (mean NAPSI score of 54.9 vs 27.1), which means a larger margin for improvement but may also mean greater difficulties in reaching complete remission.
In the few reported cases in which PPP was treated with tofacitinib, tofacitinib has been found to alleviate PPP induced by tumor necrosis factor–α inhibitors in patients with rheumatoid arthritis12 and Crohn disease.13 A patient with psoriatic arthritis showed resolution of refractory PPP within 2 weeks of tofacitinib initiation.14 However, Shibata et al15 reported a case in which tofacitinib induced PPP-like lesions in a patient with juvenile idiopathic arthritis that were alleviated after tofacitinib discontinuation. The potential risk of paradoxical exacerbation of PPP induced by tofacitinib should be closely monitored.
Elevated levels of low-density lipoprotein cholesterol in the serum were observed in 2 patients (15.4%). Longer-term monitoring is needed to determine the risk of cardiac events in patients with SAPHO receiving tofacitinib. Close monitoring of infection and thrombotic risk is also recommended during tofacitinib treatment.
Limitations
There are several limitations to this study. First, this was a single-center, single-arm, open-label pilot study. A randomized placebo-controlled trial with a larger sample size is needed to confirm the results. Second, all the participants included in this study were female, which may partially reflect the fact that patients with SAPHO with active nail lesions and PPP are predominantly women. Third, the follow-up duration was relatively short.
Conclusions
In this pilot study, tofacitinib yielded remission of nail lesions and PPP accompanied by an improvement in inflammatory markers and quality of life in patients with SAPHO syndrome. Further studies are needed to confirm the results.
eFigure 1. Change from Baseline in BASDAI in Patients with SAPHO Syndrome Treated with Tofacitinib.
eFigure 2. Clinical Response of Nail Involvement, Palmoplantar Pustulosis, Osteoarticular Manifestations, and Inflammatory Parameters in Patients with SAPHO Syndrome Treated with Tofacitinib.
eFigure 3. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 1.
eFigure 4. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 2.
eFigure 5. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 3.
eFigure 6. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 4.
eFigure 7. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 5.
eFigure 8. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 6.
eFigure 9. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 7.
eFigure 10. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 8.
eFigure 11. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 9.
eFigure 12. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 10.
eFigure 13. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 11.
eFigure 14. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 12.
eFigure 15. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 13.
References
- 1.Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254-265. doi: 10.1016/j.semarthrit.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Li C, Zuo Y, Wu N, et al. Synovitis, acne, pustulosis, hyperostosis and osteitis syndrome: a single centre study of a cohort of 164 patients. Rheumatology (Oxford). 2016;55(6):1023-1030. doi: 10.1093/rheumatology/kew015 [DOI] [PubMed] [Google Scholar]
- 3.Aljuhani F, Tournadre A, Tatar Z, et al. The SAPHO syndrome: a single-center study of 41 adult patients. J Rheumatol. 2015;42(2):329-334. doi: 10.3899/jrheum.140342 [DOI] [PubMed] [Google Scholar]
- 4.Okuno H, Watanuki M, Kuwahara Y, et al. Clinical features and radiological findings of 67 patients with SAPHO syndrome. Mod Rheumatol. 2018;28(4):703-708. doi: 10.1080/14397595.2017.1372874 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Li C, Wu N, et al. Demographic, clinical, and scintigraphic comparison of patients affected by palmoplantar pustulosis and severe acne: a retrospective study. Clin Rheumatol. 2020;39(6):1989-1996. doi: 10.1007/s10067-019-04904-8 [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Li C, Cao Y, Xiang Y, Li Y, Zhang W. Quality of life in patients with SAPHO syndrome: a single-center survey of 588 patients. Accessed April 28, 2020. https://acrabstracts.org/abstract/quality-of-life-in-patients-with-sapho-syndrome-a-single-center-survey-of-588-patients/
- 7.Yang Q, Zhao Y, Li C, Luo Y, Hao W, Zhang W. Case report: successful treatment of refractory SAPHO syndrome with the JAK inhibitor tofacitinib. Medicine (Baltimore). 2018;97(25):e11149. doi: 10.1097/MD.0000000000011149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Li C, Xu W, et al. Spinal and sacroiliac involvement in SAPHO syndrome: a single center study of a cohort of 354 patients. Semin Arthritis Rheum. 2019;48(6):990-996. doi: 10.1016/j.semarthrit.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Hayem G. Le syndrome SAPHO. Rev Prat. 2004;54(15):1635-1636. [PubMed] [Google Scholar]
- 10.Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206-212. doi: 10.1067/S0190-9622(03)00910-1 [DOI] [PubMed] [Google Scholar]
- 11.Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: Two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79-87.e1. doi: 10.1016/j.jaad.2017.01.053 [DOI] [PubMed] [Google Scholar]
- 12.Koga T, Sato T, Umeda M, et al. Successful treatment of palmoplantar pustulosis with rheumatoid arthritis, with tofacitinib: impact of this JAK inhibitor on T-cell differentiation. Clin Immunol. 2016;173:147-148. doi: 10.1016/j.clim.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 13.Haynes D, Topham C, Hagstrom E, Greiling T. Tofacitinib for the treatment of recalcitrant palmoplantar pustulosis: A case report. Australas J Dermatol. 2020;61(1):e108-e110. doi: 10.1111/ajd.13117 [DOI] [PubMed] [Google Scholar]
- 14.Wang YA, Rosenbach M. Successful treatment of refractory tumor necrosis factor inhibitor-induced palmoplantar pustulosis with tofacitinib: report of case. JAAD Case Rep. 2020;6(2):115-118. doi: 10.1016/j.jdcr.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata T, Muto J, Hirano Y, et al. Palmoplantar pustulosis-like eruption following tofacitinib therapy for juvenile idiopathic arthritis. JAAD Case Rep. 2019;5(6):518-521. doi: 10.1016/j.jdcr.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Change from Baseline in BASDAI in Patients with SAPHO Syndrome Treated with Tofacitinib.
eFigure 2. Clinical Response of Nail Involvement, Palmoplantar Pustulosis, Osteoarticular Manifestations, and Inflammatory Parameters in Patients with SAPHO Syndrome Treated with Tofacitinib.
eFigure 3. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 1.
eFigure 4. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 2.
eFigure 5. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 3.
eFigure 6. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 4.
eFigure 7. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 5.
eFigure 8. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 6.
eFigure 9. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 7.
eFigure 10. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 8.
eFigure 11. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 9.
eFigure 12. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 10.
eFigure 13. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 11.
eFigure 14. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 12.
eFigure 15. Nail and Skin Lesions in Patients with SAPHO Syndrome Before (Left Column) and After (Right Column) Tofacitinib Treatment: Case 13.


