Abstract
Angiopoietin-like protein 3 (ANGPTL3) is an important inhibitor of lipoprotein lipase and endothelial lipase that plays critical roles in lipoprotein metabolism. It specifically expresses in the liver and undergoes proprotein convertase-mediated cleavage during secretion, which generates an N-terminal coiled-coil domain and C-terminal fibrinogen-like domain that has been considered as the activation step for its function. Previous studies have reported that the polypeptide GalNAc-transferase GALNT2 mediates the O-glycosylation of the ANGPTL3 near the cleavage site, which inhibits the proprotein convertase (PC)-mediated cleavage in vitro and in cultured cells. However, loss-of-function mutation for GALNT2 has no effect on ANGPTL3 cleavage in human. Thus whether GALNT2 regulates the cleavage of ANGPTL3 in vivo is unclear. In present study, we systematically characterized the cleavage of Angptl3 in cultured cells and in vivo of mice. We found that endogenous Angptl3 is cleaved in primary hepatocytes and in vivo of mice, and this cleavage can be blocked by Galnt2 overexpression or PC inhibition. Moreover, suppressing galnt2 expression increases the cleavage of Angptl3 in mice dramatically. Thus, our results support the conclusion that Galnt2 is a key endogenous regulator for Angptl3 cleavage both in vitro and in vivo.
Subject terms: Cardiovascular diseases, Lipoproteins, Glycobiology
Introduction
Protein glycosylation is one of the most important posttranslational modification (PTM) that dramatically increases the diversity of protein functions. More than 50% of the proteome are glycosylated in a number of different ways1. N-linked glycosylation of asparagine (Asn) and O-linked glycosylation of serine (Ser) and threonine (Thr) amino acids are the most abundant forms of protein glycosylation2,3. O-glycosylation may also occur on the tyrosine (Tyr) and hydroxylysine (Hyl) residues4–6. In humans, there are several different types of O-glycosylation including O-GlcNAc, which is mainly found on cytosolic protein7, and O-GalNAc (mucin-type), O-mannose, O-xylose, O-fucose, O-Glucose, O-galactose, which are mainly found on proteins passing through the secretory pathway5,8–12.
In human, all types of protein glycosylation are initiated by one or two enzymes except the GalNAc type O-glycosylation. Human expresses up to 20 isoforms of UDP-GalNAc: polypeptide N-acetylgalactosaminyl transferases (GALNTs), which catalyze the initiation step where GalNAc is attached to Ser and Thr residues in proteins. Those GALNTs have different tissue distributions and substrate specificities, with some of them may be partially overlapped13. The large amount of GALNTs mediates the site-specific protein GalNAc O-glycosylation that can be differentially regulated and play different roles in cellular functions. Among different functions, the site-specific protein GalNAc O-glycosylation emerges as an important regulating step for proprotein processing by the proprotein convertase (PC) families13.
The mammalian PC is a family of serine proteinases that play central roles in the processing of various protein precursors ranging from hormones and growth factors to bacterial toxins and viral glycoproteins. The PC families includes seven basic amino acid-specific convertases known as PC1/PC3, PC2, furin, PC4, PACE4, PC5/PC6, PC7 and two enzymes processing at nonbasic residues SKI-1/S1P and NARC-1/PCSK914–18. The multi-basic recognition sites for basic amino acid-specific convertases exhibit the general motif (K/R)-(X)n-(K/R)↓, where X is any amino acid except Cys and n = 0, 2, 4 or 619. The enzyme S1P recognizes the motif (R/K)-X-(L, I, V)-Z↓, where Z is any amino acid except Pro, Cys, Glu and Val. PCSK9 processes its own prosegment at the motif VFAQ↓SIP, with Val being the most critical residue. This prosegment cleavage is critical for PCSK9 secretion and until now no other substrate has been found for PCSK919,20.
GALNT2 encodes the GalNAc-transferase isoform 2. SNPs in the GALNT2 locus are strongly associated with triglyceride (TAG) and HDL cholesterol (HDL-C) levels in multi-cohorts of genome wide association studies (GWAS)21–24. However, the specific mechanisms of how GLANT2 regulates blood lipid levels are not fully understood. Glycoproteomics studies identified dozes of substrates for GALNT2 and many of them play critical roles in lipoprotein metabolism, such as ANGPTL3, APOC-III, PLTP, APOE and LIPC et al.25,26. These proteins either positively or negatively regulate blood lipid levels and the impact of O-glycosylation for their activities remains largely unknown.
Angiopoietin-like protein 3 (ANGPTL3) is one of the best characterized substrates for GALNT2. It specifically expresses in the liver and secrets into circulation that regulate lipid metabolisms mainly through inhibiting lipoprotein lipase (LPL) and endothelial lipase (EL)27–29. Loss-of-function mutations in ANGPTL3 are strongly associated with panhypolipidemia and protect against coronary artery disease in humans30,31. ANGPTL3 contains a signal peptide, an extended N-terminal coiled-coil domain (CCD) and a C-terminal fibrinogen-like domain (FLD). During secretion, ANGPTL3 is cleaved at the proprotein convertase processing site (RAPR224↓TT) that localizes between the CCD and FLD domains28,32. This cleavage is primarily mediated by furin and PACE433–35. The N-terminal domain of ANGPTL3 contains the conserved LPL binding motif and was shown to be more effective in increasing circulating triglyceride (TAG) level when overexpressed in mice28. This PC mediated cleavage has been thought as the activation step for ANGPTL3. However, recent study showed that the N-terminal domain or full length ANGPTL3 have similar ability in inhibiting LPL activity with or without its cofactor ANGTPL8 in vitro36.
Previous studies showed that ANGPTL3 was glycosylated at Thr225 or Thr226 by GALNT2, which blocks the PC cleavage of ANGPTL3 in vitro and in cultured cells26,34. However, most of these studies were performed in vitro or using reporter system by overexpressing in cultured cells26,34,37. In contrast, loss-of-function mutation in GALNT2 has no impact on ANGPTL3 cleavage in human38. Thus whether GALNT2 regulates the cleavage of endogenous ANGPTL3, especially in vivo, remains unclear.
In present study, we systematically characterized the cleavage of Angptl3 and their dependence on GALNT2 and PC both in vitro of cultured cells and in vivo of mice. We first confirmed that the cleavage of Angptl3 is regulated by GALNT2 and PC in cultured cells. Then we checked the cleavage of endogenous Angptl3 in vivo of mice and also in mouse primary hepatocytes. We found that Galnt2 overexpressing or PC inhibition dramatically inhibits Angptl3 cleavage, whereas suppressing galnt2 expression dramatically promotes its cleavage in vivo. Thus, our results support the conclusion that Galnt2 is a key endogenous regulator of Angptl3 cleavage both in vitro and in vivo. To our knowledge, this is the first report to show that Galnt2 regulates Angptl3 cleavage in vivo.
Results
ANGPTL3 undergoes cleavage in vitro and in vivo
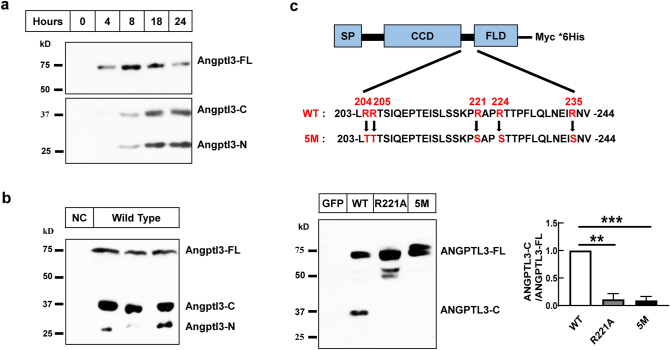
ANGPTL3 is specifically expressed in the liver. We first checked the cleavage of Angptl3 in mice and also in primary hepatocytes. We found that the endogenous Angptl3 is efficiently cleaved in both mouse primary hepatocytes and in vivo of mice (Fig. 1a,b). Previous reports have shown that ANGPTL3 is cleaved at the proprotein convertase processing site (RAPR224↓TT)28. We then tested whether mutation of this site can block the cleavage of ANGPTL3. As shown in Fig. 1c, the wild-type human ANGPTL3 (WT) is efficiently cleaved when overexpressed in Hepa1-6 cells. However, mutant the Arginine at 221 position alone (R221A) or mutant all five Arginine at 204, 205, 221, 224, 235 positions (5 M) completely blocks the cleavage of ANGPTL3 (Fig. 1c), which is consistent with previous reports28,33. These results confirm that both endogenous and exogenous ANGPTL3 undergoes cleavage at the proprotein convertase processing site.
Figure 1.
Angptl3 is cleaved in cultured cells and in vivo of mice. (a) Angptl3 protein levels in medium of mouse primary hepatocytes. Mouse primary hepatocytes were isolated as indicated in the “Methods”. Mediums were collected at indicated time after plating and were subjected to western blot analysis with a polyclonal antibody that recognize both N terminal and C terminal domains of Angptl3. (b) Angptl3 protein levels in serum of mice. Serum was collected at refeeding and subjected to western blot analysis using the same antibody as in A (Male, 8 weeks). NC was serum of angptl3 knocking down mice and was used as negative control. (c) ANGPTL3 protein levels in medium of cultured cells. Hepa1-6 cells were transfected with plasmids expressing the wild-type (WT) or cleavage defective human ANGPTL3 (R221A, or 5M: R204T, R205T, R221S, R224S, R235S) tagged with Myc/His at the C terminal. 48 h after transfection, medium was collected and subjected to western blot analysis with an anti-His antibody. Quantification of ANGPTL3-C versus ANGPTL3-FL immunoblots was shown at bottom right from three independent experiments. SP: signal peptide; CCD: Coiled-coil domain; FLD: Fibrinogen-like domain. ANGPTL3-FL, ANGPTL3-C, ANGPTL3-N stand for full length, C terminal and N terminal domains of ANGPTL3 respectively. Experiments were repeated for at least three times with similar results. **P < 0.01, ***P < 0.001.
ANGPTL3 cleavage is regulated by Galnt2 and proprotein convertases in cultured hepatocytes
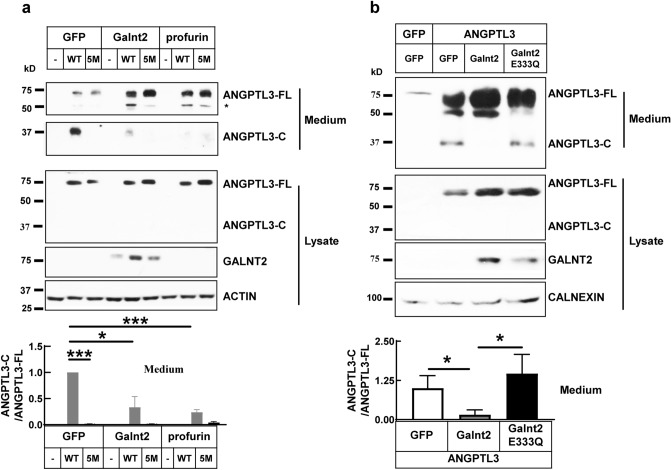
ANGPTL3 contains the proprotein convertase consensus site 221RAPR224 and is cleaved after R224 primarily by furin and PACE4. Profurin is the prosegment of human furin and is a strong inhibitor for proprotein convertases. We found that overexpressing profurin strongly decreases the cleaved forms of ANGPTL3 in the medium of mouse hepatocytes Hepla1-6 (Fig. 2a), which is consistent with previous reports34,35. Moreover, the 5 M mutation of ANGPTL3, which cannot be cleavage by PC family, is not responsive to profurin overexpression (Fig. 2a, Supplementary Fig. S1). ANGPTL3 is glycosylated at Thr225 or Thr226 by GALNT2 in vitro and the glycosylation inhibits its cleavage34. GALNT2 deficiency increases the cleavage of ANGPTL3 in HepG2 cells26. We found that overexpressing wild type Galnt2 significantly decreases the cleavage of ANGPTL3 in the medium (Fig. 2a,b). However, overexpressing the enzymatic dead mutation of Galnt2 (E333Q) has no effect (Fig. 2b), which suggests that Galnt2 suppresses ANGPTL3 cleavage through glycosylation modification25. In contrast to the medium, we could not see detectable cleaved forms of ANGPTL3 in cell lysate, which suggests that the decreased cleavage in medium is not due to secretion defect.
Figure 2.
Galnt2 or profurin overexpressing suppresses the cleavage of ANGPTL3 in cultured cells. (a) ANGPTL3 protein levels in medium and cell lysate of Hepa1-6 cells that transfected with indicated plasmids. GFP was used as negative control; WT: wild type ANGPTL3; 5M: cleavage defective ANGPTL3 (R204T, R205T, R221S, R224S, R235S). Quantification of medium ANGPTL3-C versus ANGPTL3-FL immunoblots was shown at the bottom from three independent experiments. *Indicates the pre-mature form of ANGPTL3 in cell lysate. (b) Enzymatic activity of Galnt2 is required for suppressing ANGPTL3 cleavage. Hepa1-6 cells were transfected with ANGPTL3-Myc/His together with wild-type or enzyme dead mutant Galnt2 (E333Q). Medium was collected and subjected to western blot analysis with anti-His antibody. Quantification of ANGPTL3-C versus ANGPTL3-FL immunoblots was shown at the bottom from three independent experiments. ANGPTL3-FL, ANGPTL3-C and ANGPTL3-N stand for full length, C-terminal and N-terminal domains of ANGPTL3 respectively. Experiments were repeated for at least three times with similar results. *P < 0.05, ***P < 0.001.
Angptl3 cleavage is regulated by the activity of Galnt2 and PC family in vivo of mice
Humans with homozygous loss-of-function mutations in GALNT2 have decreased glycosylation level at ANGPTL3 (Thr226), which suggests that GALNT2 deficiency may lead to increased cleavage of ANGPTL3 in vivo25. However, the heterozygous loss-of-function mutation of D314A, which disrupts the enzymatic activity of GALNT2, has no effect on the levels of either full length or N-terminal fragment of ANGPTL3 in human38. This leads us to question whether GALNT2 regulates ANGPTL3 cleavage in vivo.
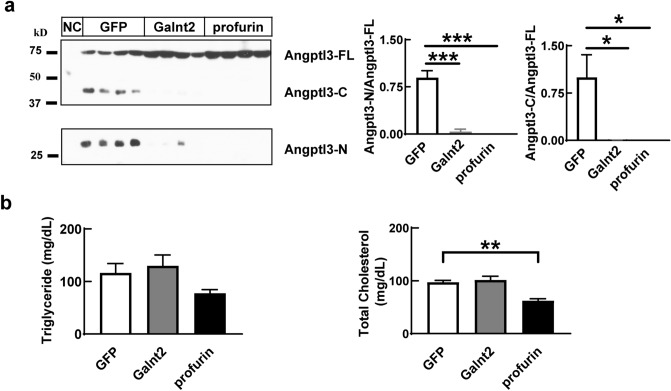
Consistent with results in cultured cells, overexpressing galnt2 or profurin in mouse liver dramatically decrease the cleavage of Angptl3 in serum (Fig. 3a, Supplementary Fig. S2a), which suggests that both Galnt2 and PC family regulate the cleavage of Angptl3 in vivo. We also checked the Angptl3 protein levels in liver lysate. Consistent with cultured cells, we can only detected full length Angptl3, which suggests that the decreased cleaved forms in the serum are not due to secretion defect (Supplementary Fig. S2b). Mutations of Galnt2 are strongly associated with blood cholesterol levels in human. However, we did not find significant changes for blood TAG level and total cholesterol level in Galnt2 overexpressing mice (Fig. 3b). In contrast, profurin overexpressing significantly decreases the total cholesterol level in mice (Fig. 3b).
Figure 3.
Galnt2 and PC families regulate the cleavage of Angptl3 in vivo. (a) Angptl3 protein levels in serum of mice overexpressing Galnt2 or profurin. Adeno-associated virus expressing Galnt2 or profurin were infused into mice through tail vein. 14 days later, serum was collected at refeeding and was subjected to analysis as indicated in “Methods” (N = 5/group, Male, 8 weeks). NC was serum collected from Angptl3 knocking down mice and was used as negative control. Quantifications of the ANGPTL3-C versus ANGPTL3-FL or ANGPTL3-N versus ANGPTL3-FL immunoblots were shown at the right. (b) Serum levels of triglyceride and total cholesterol in mice used in A. Experiments were repeated for at least three times with similar results. *P < 0.05, **P < 0.01, ***P < 0.001.
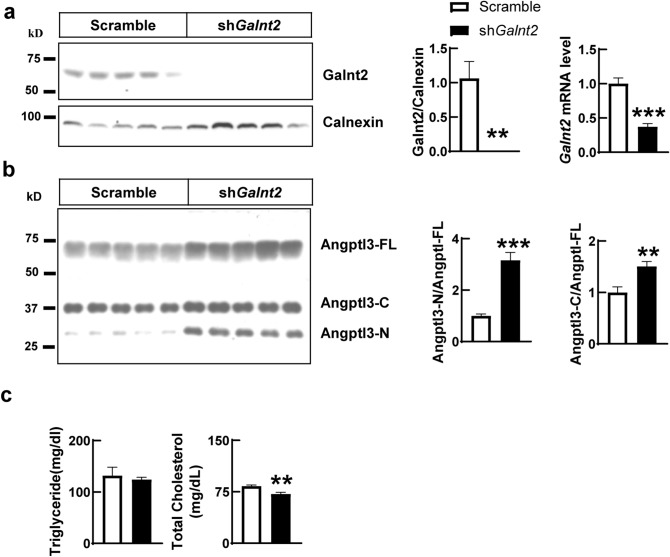
Previous report has shown that liver specific galnt2 knockout mice have normal TAG level but with decreased HDL-C level25. To further test whether Galnt2 does regulates blood lipid levels, we knocked down galnt2 in livers of mice through adeno-associated virus (AAVs) mediated shRNA delivery. As shown in Fig. 4, suppressing galnt2 expression dramatically increases the cleavage of Angptl3 in serum but not in liver lysate, which suggest that Galnt2 is not only sufficient, but also necessary in regulating Angptl3 cleavage in vivo. Moreover, knocking down galnt2 in mice does decrease the total cholesterol and HDL-C levels but has no effect on blood TAG level (Fig. 4, Supplementary Fig. S3), which are consistent with previous reports25.
Figure 4.
Suppressing galnt2 expression increases the cleavage of Angptl3 in vivo. (a) Hepatic levels of galnt2 protein and mRNA in galnt2 knocking down or control mice. Wildtype mice were infused with adeno-associated virus that expressing scramble or shRNA against galnt2. 14 days later, livers were collected at refeeding and were subjected to analysis as indicated in “Methods” (N = 5, Male, 8 weeks). Quantification of galnt2 immunoblot was shown at the middle. (b) Serum levels of Angptl3 in mice used in A. Quantifications of the ANGPTL3-C versus ANGPTL3-FL or ANGPTL3-N versus ANGPTL3-FL immunoblots were shown at the right. (c) Serum levels of triglyceride and total cholesterol in mice used in A. Experiments were repeated for at least three times with similar results. **P < 0.01, ***P < 0.001.
Discussion
Cleavage of ANGPTL3 has long been thought as the activation step for its inhibitory effect on LPL. Previous studies showed that GALNT2 catalyzes the mucin-type (GalNAc-type) O-glycosylation on ANGPTL3, which inhibits the PC-mediated cleavage of ANGPTL3 in vitro and in cultured cells26,34. However, loss-of-function mutations of GALNT2 in human have no effect on ANGPTL3 cleavage38. In present study, we systematically characterized the functions of Galnt2 and PC family on the cleavage of ANGPTL3. Our results clear demonstrate that both Galnt2 and PC family play critical roles in ANGPTL3 cleavage in both cultured cells and in vivo of mice. The enzymatic activity of Galnt2 is required for suppressing ANGPTL3 cleavage, which suggests that this process requires the glycosylation of ANGPTL3.
Proprotein convertase-mediated cleavage of target proteins ranges from hormones and growth factors to bacterial toxins and viral glycoproteins. Site-specific O-glycosylation is emerging as a novel mechanism that regulates the action of PC-mediated processing of proteins26,34,39. GALNT2 has been shown catalyzing the mucin-type O-glycosylation on ANGPTL3, APOC-III, PLTP, APOE and LIPC et al.25,26,34,38. This mucin-type O-glycosylation of ANGPTL3 is on Thr225 or Thr226, which is close to the PC cleavage site and blocks its cleavage. Our current results, together with previous reports firmly demonstrate that this regulation is sufficient and necessary for ANGPTL3 cleavage both in cultured cell and in vivo of mice.
SNPs in GALNT2 locus are strongly associated with triglyceride and HDL-C levels in different cohorts of GWAS21–24. People with Loss-of-function mutations in GALNT2 have decreased levels of TAG, HDL-C and to a less extent for LDL-C25,38. Genetic disruption of Galnt2 also leads to decreased level of HDL-C, but with variable changes for TAG, in rodents and nonhuman primates25. However, the specific mechanisms of how GLANT2 regulates blood lipid levels are not fully understood. Mechanistic studies suggest that the substrates of GALNT2, including PLTP, APOC-III and ANGPTL3, all contribute to the changes of HDL-C or TAG, but may have species difference25,38. Moreover, glycoproteomics studies identified dozens of extra substrates for GALNT2 and many of the target proteins are well known regulators for lipoprotein metabolism, including APOE, APOA1 and LIPC et al.25,26. These proteins either positively or negatively regulate blood lipid levels and the functional consequence of O-glycosylation modification on those proteins remains largely unknown. Our current study, together with previous reports, clearly demonstrates that GALNT2 regulates the cleavage of ANGPTL3 in vivo. However, whether this regulation mediates the functions of ANGPTL3, especially on blood lipids regulation, remains to be investigated.
Angptl3 was first discovered as an important player in lipid metabolism through mouse genetics. The mouse strain KK/San has extremely low level of blood TAG and genetic mapping found that the KK/San mice harbor Angptl3 mutation that produces no detectable protein27. Later on, a number of human genetic studies found that mutations in ANGPTL3 are strongly associated with dyslipidemia22,40–46. People with loss-of-function mutations in ANGPTL3 have significantly lower plasma levels of TAG, HDL-C, LDL-C and lower risk for coronary artery disease31,44,47. People with complete ANGPTL3 deficiency have extremely low plasma levels of TAG, LDL-C and HDL-C, without other notable abnormity30, which makes it an attractive target for lipid lowering drugs47,48.
ANGPTL3 modulates TAG metabolism mainly through inhibiting LPL42. LPL is the master intravascular TAG lipase that sitting on the endothelial surface and mediates the clearance of TAG from circulation. ANGPTL3 contains a conserved LPL binding motif at the N terminal and cleavage of ANGPTL3 was long thought as the activation step for LPL inhibition28. However, we found that there is no change in blood TAG level in mice, no matter increasing or decreasing the cleavage of Angptl3 by changing the level of Galnt2 or PC activity (Figs. 3, 4). Interestingly, the liver specific galnt2 knockout mice also have normal TAG level25. GALNT2 has dozens of substrates, including ANGPTL3, APOC-III, PLTP, APOE, APOA1 and LIPC25,26,34,38. All these proteins play important roles in regulating TAG metabolism. It could be that GALNT2 modifies multiple substrates at the same time and counterbalance each other in regulating blood TAG level. However, recent study also suggests that N-terminal domain of ANGPTL3 and cleavage defective ANGPTL3 have similar ability in LPL inhibition with or without its cofactor ANGTPL8 in vitro36.
ANGPTL3 modulates HDL-C and LDL-C metabolism mainly through inhibiting EL42,49–51. Galnt2 knocking down upregulates Angptl3 cleavage dramatically in mice, together with decreased levels of total cholesterol and HDL-C (Fig. 4, Supplementary Fig. S3), which are consistent with previous report25. However, whether the decreased cholesterol is caused by increased Angptl3 cleavage remains to be determined. Many of the GALNT2 substrates are well-known regulators of lipoprotein metabolism. New animal models will be needed to dissect the physiological functions for Galnt2 mediated O-glycosylation on each substrate. Our finding that Galnt2 and PC do regulate Angptl3 cleavage in vivo is the key step for further mechanistic studies.
Methods
Materials
Cell culture medium was obtained from life technologies (Staley Rd Island, USA), and FBS was obtained from Pan seratech (Aidenbach, Bavaria, Germany). EDTA-free protein inhibitor cocktail was purchased from Bimake (Houston, USA), Polyethylenimine (PEI) were obtained from Polysciences (Warrington, PA).
The following primary antibodies were used: Anti-ANGPTL3 antibody (AF136, R&D Systems, Minneapolis, USA), Anti-His antibody (Catalog: M20008F, Abmart, Berkeley Heights, US), Anti-Beta actin antibody (Catalog: 20536-1-AP, Proteintech, Chicago, IL), Anti-Calnexin antibody (Catalog: 10427-2-AP, Proteintech, Chicago, IL). The following secondary antibodies were used: HRP conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Jackson ImmunoResearch), bovine anti-goat IgG (Proteintech, Chicago, IL).
Mice
All mice are C57BL/6J background. The animal protocols were approved by the Institutional Animal Care and Use Committee of Wuhan University. The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations. Mice were housed in the specific pathogen-free animal facility with controlled environment (12-h light/12-h dark daily cycle, 23 ± 1 °C, 60–70% humidity) at Wuhan University. Mice were fed with standard chow diet unless otherwise indicated. In some experiments, mice were synchronized with 3 days training for food intake by daytime feeding (8:00 a.m. to 8:00 p.m.) and night time fasting (8:00 p.m. to 8:00 a.m.). Mice were sacrificed on day4 at 6 h after refeeding and samples were frozen at − 80 °C or subjected to analysis immediately.
Cell culture
Hepa1-6 cells were purchased from China Center for Type Culture Collection (CCTCC) and were cultured in high glucose DMEM medium containing 10% FBS and 100 units/ml penicillin G/streptomycin. Cells were infected with lentivirus for transgenes expression. Two days later, cells were changed with serum-free medium. On day 3, medium and cell pellet were collected and subjected to immunoblot or RT-PCR analysis.
Mouse primary hepatocytes were isolated as described previously52 and cultured in low-glucose DMEM medium containing 5% FBS and 100 units/ml penicillin G/streptomycin. Three hours after plating, cells were changed with serum-free medium and samples were harvested at indicated time points.
Plasmids and virus production
Human ANGPTL3 tagged with MYC/His at the C terminal was cloned into pShuttle vectors. The R221A mutant and 5 M mutant (R204T, R205T, R221S, R224S, R235S) were made with QuickChange site-directed mutagenesis according to the manufacturer’s instructions. Mouse galnt2 tagged with Flag at the C terminal was cloned into pShuttle vectors. The enzymatic-dead mutation of galnt2 (E333Q) was made with QuickChange site-directed mutagenesis. Profurin was cloned into pShuttle vectors. The presence of the desired mutation and the integrity of each construct were verified by DNA sequencing. shRNA sequence against galnt2 is: 5′-CGGATGGAGTTGTTGGAATTT-3′. Lenti-virus and adeno-associated virus were produced exactly the same as described before53.
Immunoblotting
Liver or cell pellet were homogenized in lysis buffer (1% v/v TritonX-100, 50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 5 mM EDTA plus protease inhibitor). Protein concentrations in the supernatants were measured using BCA methods. Equal amounts of protein were subjected to SDS-PAGE and immunoblot analysis as previously described54. Mouse serum was first diluted for tenfold with sterile saline and then added with sample buffer and heated at 95 °C for 5 min. Equal volumes of samples were subjected to SDS-PAGE analysis.
Real-time PCR
The real-time PCR were performed as described before55. Briefly speaking, the total RNA from mouse liver or cells was isolated by homogenization in Trizol (Sigma, Catlog T9424). cDNA was made from 2 μg of RNA using TaqMan (thermo scientific) with random hexamer primers. Oligonucleotides specific for each transcript were used to amplify by PCR in 2 × SYBR Master Mix (Yeasen, China) according to the manufacturer’s instructions. The mRNAs levels were normalized to the levels of cyclophilin or 36B4. The following primers were used in this study: Profurin: forward: 5′-AGGGCCAGAAGGTCTTCACC-3′; reverse: 5′-TTCGTCACTCCTCGATGCCAG-3′; Galnt2: forward, 5′-CGCCCTCTGCCTCCCTCTTTC-3′; reverse, 5′-TGATTGCTGCTTGCCCACTTGTTC-3′; 36b4: forward, 5′-CACTGGTCTAGGACCCGAGAAG-3′; reverse, 5′-GGTGCCTCTGGAGATTTTCG-3′; cyclophilin: forward, 5′-TGGAGAGCACCAAGACAGACA-3′; reverse, 5′-TGCCGGAGTCGACAATGAT-3’.
Blood chemistry
Plasma or serum was collected from the supernatant after centrifugation (4000 rpm X 10 min). In some experiments, plasma lipoproteins were size fractionated by Fast Protein Liquid Chromatography (FPLC) using a Superose 6 column (GE Healthcare). Levels of TAG and cholesterol were measured with commercial kits (Kehua Bio-engineering, Shanghai, China).
Statistical analysis
Data were analyzed using GraphPad Prism 6 and are presented as the mean ± SEM. Statistical analysis was performed using a 2-tailed Student’s t test. A P value less than 0.05 was considered significant.
Supplementary information
Acknowledgements
The authors would like to thank all members of Dr. Wang’s laboratory and the core facility at the College of Life Sciences, Wuhan University. This work was supported by grants from the National Natural Science Foundation of China (31771304, 91754101), the National Key Research and Development Program of China (2016YFA0500100, 2018YFA0800700) and the 111 Project (B16036).
Author contributions
Y.W. conceived the project. Y.W. and X.D.L. wrote the paper. X.D.L., Y.L.Z. and M.Z.Z. conducted the experiments.
Data availability
The datasets generated during the present study are available from the corresponding authors upon request. Most of the reagents used during these studies are commercially available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73388-3.
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochem. Biophys. Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 3.Stanley P. Golgi glycosylation. Cold Spring Harbor Perspect. Biol. 2011;3:a005199. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halim A, et al. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 2011;108:11848–11853. doi: 10.1073/pnas.1102664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiro RG. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J. Biol. Chem. 1969;244:602–612. [PubMed] [Google Scholar]
- 6.Steentoft C, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 7.Holt GD, Haltiwanger RS, Torres CR, Hart GW. Erythrocytes contain cytoplasmic glycoproteins. O-linked GlcNAc on Band 4.1. J. Biol. Chem. 1987;262:14847–14850. [PubMed] [Google Scholar]
- 8.Hanisch FG. O-glycosylation of the mucin type. Biol. Chem. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 9.Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (dystroglycan) purified from sheep brain. J. Biol. Chem. 1998;273:23698–23703. doi: 10.1074/jbc.273.37.23698. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, et al. Identification of a disaccharide (Xyl-Glc) and a trisaccharide (Xyl2-Glc) O-glycosidically linked to a serine residue in the first epidermal growth factor-like domain of human factors VII and IX and protein Z and bovine protein Z. J. Biol. Chem. 1989;264:20320–20325. [PubMed] [Google Scholar]
- 11.Harris RJ, Spellman MW. O-linked fucose and other post-translational modifications unique to EGF modules. Glycobiology. 1993;3:219–224. doi: 10.1093/glycob/3.3.219. [DOI] [PubMed] [Google Scholar]
- 12.Bjoern S, et al. Human plasma and recombinant factor VII. Characterization of O-glycosylations at serine residues 52 and 60 and effects of site-directed mutagenesis of serine 52 to alanine. J. Biol. Chem. 1991;266:11051–11057. [PubMed] [Google Scholar]
- 13.Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing—deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochem. Biophys. Acta. 1820;2079–2094:2012. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Seidah NG, Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 16.Seidah NG, et al. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc. Natl. Acad. Sci. USA. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai J, et al. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol. Cell. 1998;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- 18.Seidah NG, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 20.Benjannet S, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004;279:48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 21.Kathiresan S, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willer CJ, et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu DJ, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017;49:1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khetarpal SA, et al. Loss of function of GALNT2 lowers high-density lipoproteins in humans, nonhuman primates, and rodents. Cell Metab. 2016;24:234–245. doi: 10.1016/j.cmet.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schjoldager KT, et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. Proc. Natl. Acad. Sci. USA. 2012;109:9893–9898. doi: 10.1073/pnas.1203563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koishi R, et al. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 28.Ono M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003;278:41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl. Acad. Sci. USA. 2015;112:11630–11635. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musunuru K, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stitziel NO, et al. ANGPTL3 deficiency and protection against coronary artery disease. J. Am. Coll. Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 2013;110:16109–16114. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essalmani R, et al. Furin is the primary in vivo convertase of angiopoietin-like 3 and endothelial lipase in hepatocytes. J. Biol. Chem. 2013;288:26410–26418. doi: 10.1074/jbc.M113.501304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schjoldager KT, et al. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J. Biol. Chem. 2010;285:36293–36303. doi: 10.1074/jbc.M110.156950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin W, et al. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 2007;6:129–136. doi: 10.1016/j.cmet.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi X, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol. Metab. 2017;6:1137–1149. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song L, Bachert C, Schjoldager KT, Clausen H, Linstedt AD. Development of isoform-specific sensors of polypeptide GalNAc-transferase activity. J. Biol. Chem. 2014;289:30556–30566. doi: 10.1074/jbc.M114.599563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holleboom AG, et al. Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man. Cell Metab. 2011;14:811–818. doi: 10.1016/j.cmet.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 40.Romeo S, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robciuc MR, et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2013;33:1706–1713. doi: 10.1161/ATVBAHA.113.301397. [DOI] [PubMed] [Google Scholar]
- 42.Dijk W, Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 2016;27:249–256. doi: 10.1097/MOL.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 43.Minicocci I, et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 2012;97:E1266–1275. doi: 10.1210/jc.2012-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pisciotta L, et al. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ. Cardiovasc. Genet. 2012;5:42–50. doi: 10.1161/CIRCGENETICS.111.960674. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Campos JM, et al. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin. Chim. Acta Int. J. Clin. Chem. 2012;413:552–555. doi: 10.1016/j.cca.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Noto D, et al. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 2012;32:805–809. doi: 10.1161/ATVBAHA.111.238766. [DOI] [PubMed] [Google Scholar]
- 47.Dewey FE, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham MJ, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 49.Ando Y, et al. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipids Res. 2003;44:1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, et al. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J. Lipids Res. 2015;56:1296–1307. doi: 10.1194/jlr.M054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam RC, et al. Angiopoietin-like protein 3 (ANGPTL3) governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipids Res. 2020 doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones C, et al. Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia. J. Clin. Investig. 2007;117:165–174. doi: 10.1172/JCI29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han FF, Liu X, Chen CF, Liu YN, Du MK, Zhou Y, Liu Y, Song BL, He HH, Wang Y. Hypercholesterolemia risk-associated GPR146 is an orphan G-protein coupled receptor that regulates blood cholesterol levels in humans and mice. Cell Res. 2020;30(4):363–365. doi: 10.1038/s41422-020-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu T, Guan Y, Xu J, Wang Y. APP, APLP2 and LRP1 interact with PCSK9 but are not required for PCSK9-mediated degradation of the LDLR in vivo. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:883–889. doi: 10.1016/j.bbalip.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quagliarini F, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the present study are available from the corresponding authors upon request. Most of the reagents used during these studies are commercially available.