Abstract
Background
Ghŗ̥ta mūrcchana is a process of pre-treatment recommended in Ayurveda to purify ghee before it can be used for siddha ghŗ̥ta which is claimed to improve the properties of the ghee in general and that of the prepared siddha ghŗ̥ta.
Objective
This work is aimed at studying the physiochemical properties of ghee and mūrcchita ghŗ̥ta in order to understand the impact of ghŗ̥ta mūrcchana process.
Materials and methods
Ghee and mūrcchita ghŗ̥ta were prepared from the milk of local Pahadi, Jersey and Holstein cows. The samples were characterized by FTIR spectroscopy, differential scanning calorimetry and free fatty acid measurements.
Results
Among the samples studied, the Holstein cow ghee was found to contain the least amount of free acid (1.34%) whereas ghŗ̥ta mūrcchana process led to further decrease in the free acid content polymorphism was observed in the samples as evidenced by multiple melting points. In most cases, mūrcchita ghŗ̥ta was found to contain less solid fat than the corresponding ghee implying that the high melting compound was converted to low melting one during the process.
Conclusion
The observed lowering of free fatty acid and solid fat contents in the ghee samples may provide a possible validation to the performance enhancement of the ghŗ̥ta mūrcchana process.
Keywords: Ayurveda, Ghee, Mūrcchana, Mūrcchita ghŗ̥ta, Polymorphism
1. Introduction
Ghee is a specialized form of clarified butter indigenous to South Asia, usually prepared from milk of cows or buffalos [1]. Several commercial products (such as clarified butter, butter-oil and other indigenous milk products) commonly used in different regions are closely related to ghee. However, as demonstrated by Sawaya et al. [2] and Baker et al. [3], they must be understood as distinct products. Ghee is mainly used as food additive for pleasant smell and taste. It can substitute oil for frying, especially that involves high temperature as it has higher smoke point than the traditional oil. Nevertheless, due to higher cost of ghee than oil, its use in cooking is limited only to high economic status population [4]. On the other hand, consumption of saturated-fat-rich food is usually associated with chronic heart disease and insulin resistance in certain population, although the impact of trace components on these effects are yet to be ascertained [5,6]. It could be of interest to develop ghee to decrease its cost and make it available to more important population.
Ayurveda, the traditional medicinal practice in South Asia, considers ghee as a superior food. Ghee (alone or with other herbs), preferably obtained from cow milk, has been recommended in Ayurveda as medicine to improve memory, digestion and intellectual performance [7,8]. Moreover, ghee is used in combination with different kinds of herbs for formulating wide variety of drugs while maintaining its inherent qualities [9]. Ghee is a component of two common Aāyurvedika mixtures: pañcagavya which is a blend of five cow products namely milk, yoghurt, urine, dung and ghee; and pañcāmta which comprises milk, yoghurt, honey, sugar and ghee. Old ghee, 100 years or older, is believed to have enhanced remedial properties and can be applied topically to cure the ailments of skin, ear, head and reproductive organs [10]. Ghee based formulations have also been found effective as antibiotic to prevent surgical site infection [11].
Traditionally, ghee is obtained from milk using extensive procedure including fermentation of boiled milk (with cream included) with curd containing Lactobacillus followed by churning of the yoghurt hence obtained. The butter is then separated from buttermilk and simmered to obtain ghee. We have to notice that there are also other methods used to prepare ghee which may have different compositions than the traditional one [12,13]. Moreover, the origin of milk (goat, cow and buffalo [[14], [15], [16]]), the choice of the processing technique, and the storage method, leads to different fatty acids distribution and as a consequence to different final product in term of taste. For instance, β-carotene present in cow’s ghee gives it characteristic yellow tinge, which is lacking in ghee from buffalo milk. It has been observed that, not only among cattle, quality of ghee also differs among the breeds of cows. Sindhi cow ghee was found to be more stable than that from Jersey cow although the latter was found to be superior while in fresh state [17]. It is of interest to know that Sindhi cow ghee have more unsaturated fatty acid than Thari cow ghee [18]. Due to differences in methods used in above works, Sindhi and Thari cow ghee, however, could not be precisely compared.
Our interest in the present work lies in the process of sneha kalpanā, which is a drug formulation procedure, by boiling herbs with ghee, oil, animal fat or bone marrow as substrate [19], ghee being the most preferred choice [20]. Siddha ghŗ̥ta so prepared is used in a wide variety of conditions [21]. However, before its use, Ayurveda demands the ghee be purified. Ghr̥ta mūrcchana is the process of purification of ghee by treatment with certain herbs to obtain mūrcchita ghŗ̥ta, the ghee suitable to be used for preparing drug formulations [9]. Ghr̥ta mūrcchana is believed to improve the extraction power and shelf-life of ghee as well as to improve the efficacy of medicinal formulations and to remove any unpleasant odour [9]. Although clinical impacts of ghŗ̥ta mūrcchana have been studied [22,23], only limited attempts have been made to determine the changes that occur during the mūrcchana process. Further, no attempts have been made to understand the dynamics of this process and to establish structure–property relationship.
This study aims at understanding the physiochemical properties of the ghees and mūrcchita ghŗ̥ta in order to provide reliable evidence for or against the claimed effects [9] of ghŗ̥ta mūrcchana process.
2. Methodology
Three different cow breeds viz. Holstein, Jersey and Local Pahadi reared on same farm was selected for milk collection. The collected milk was boiled and allowed to cool to room temperature. 100 mg of thermophilic culture (Chr Hansen STI-13 containing Streptococcus thermophilus) was added and incubated at 30 °C for 12 h to form yoghurt. Commercial culture was chosen to ensure reproducibility. Lactobaciullus is generally found to be used in such experiment.
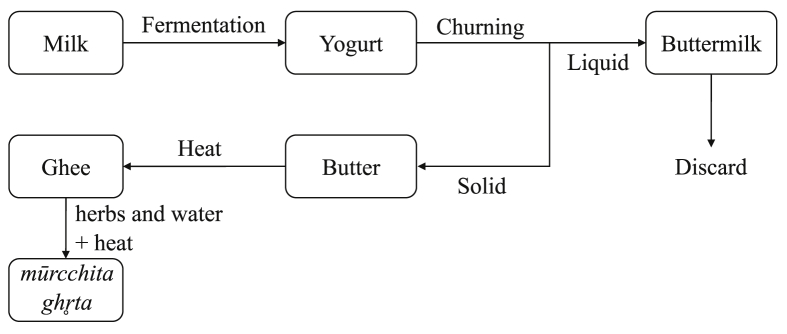
S. thermophilus was preferred over Lactobacillus in the present case as the former was more widely used culture in local market and hence easily available. Diluted yoghurt was then churned at 1400 rpm and butter separated manually. The butter was simmered at 115 °C until the boiling stopped and residue turned brown. The mixture was filtered and the filtrate obtained was the required ghee sample. The sample preparation overview is shown in Fig. 1. Commercial product from Kathmandu, the Sita Ram’s Pure Ghee Batch No 3/2017, was used as reference.
Fig. 1.
Schematic diagram showing the different stages for preparation of ghee and mūrcchita ghr̥ta samples.
Mūrcchita ghŗ̥ta was prepared from the ghee sample by boiling ghee with herbs in the proportion mentioned in Table 1 using standard method described in bhaiṣajya kalpanā vijñāna [9]. Briefly, Ghee was heated with constant stirring until the typical boiling sound stopped. Curcuma longa suspension was added and the ghee was boiled until its volume was reduced to one-fourth. Same process was repeated with Emblica officinalis juice. Remaining herbs and water were then added. The heat was cut off when water vapour disappeared and herbs could be rolled into wick. The mixture was filtered to obtain mūrcchita ghŗ̥ta as filtrate. Hence, four mūrcchita ghŗ̥ta samples were obtained from four ghee samples, which are indexed in Table 2.
Table 1.
Ingredients used in the present work for mūrcchita ghr̥ta preparation and their concentration.
| S.N. | Nepali Name | English/Botanical Name | Parts by weight |
|---|---|---|---|
| 1 | ghyu | Ghee | 100.00 |
| 2 | pānī | Water | 400.00 |
| 3 | amalā | Emblica officinalis | 6.25 |
| 4 | haledo | Curcuma longa | 6.25 |
| 5 | nāgaramothe | Cyprus rotandus | 6.25 |
| 6 | harro | Terminalia chebula | 6.25 |
| 7 | barro | Terminalia bellirica | 6.25 |
| 8 | kāgatī | Citrus limon | 6.25 |
Table 2.
Designation of the investigated samples with their specimen codes, relevant descriptions and free fatty acid (FFA) contents.
| Code | Description | FFA (% of oleic acid) | Yield (% from milk) | Yield (% from ghee) |
|---|---|---|---|---|
| CG | Commercial ghee | 2.49 | – | – |
| LG | Ghee from Local cow milk | 1.45 | 2.86 | – |
| JG | Ghee from Jersey cow milk | 1.43 | 2.68 | – |
| HG | Ghee from Holstein cow milk | 1.34 | 2.19 | – |
| CM (from CG) | mūrcchita ghr̥ta from CG | 1.09 | – | 59.4 |
| LM (from LG) | mūrcchita ghr̥ta from LG | 0.80 | 1.81 | 63.2 |
| JM (from JG) | mūrcchita ghr̥ta from JG | 1.61 | 1.48 | 55.2 |
| HM (from HG) | mūrcchita ghr̥ta from HG | 0.87 | 0.87 | 39.7 |
The samples were characterized by free fatty acid (FFA) content analysis, Fourier Transform infrared (FTIR) spectroscopy and differential scanning calorimetry (DSC).
FFA was determined by IUPAC standard methods for the analysis of oils, fats and derivatives with some modification [24]. Briefly, 0.5 g of sample was dissolved in 50 mL of 1:1 (v/v) mixture of ethanol and diethyl ether. The solution was titrated against 0.01 N NaOH solution with 1% phenolphthalein as indicator to determine the equivalent free fatty acid value as mass percentage equivalent of oleic acid.
FTIR spectra of solid samples were obtained from 400 cm−1 to 4000 cm−1 with 2 cm−1 resolution. IRTracer-100 from Shimadzu was used to obtain the FTIR spectra using pellets of sample with KBr. Mettler Toledo DSC12E was used to obtain the calorimetric data. The specimen was heated on hermetically sealed aluminium crucible with air as reference. DSC heating curve was obtained from with a heating rate of 5 °C min−1. The sample was previously heated to 80 °C, held for 15 min, cooled and held at 10 °C to remove thermal memory of solid. Heating, cooling and holding time was same in each step. Solid fat content (SFC), the mass percentage of fat present as solid, was determined by partial integration of DSC curve [25,26].
3. Result and discussion
The yield of each product during ghee preparation with respect to boiled milk is shown in Table 2. Ghee from Local cow milk (LG) was obtained with the yield of 2.86%, which is the highest yield obtained among the test samples. All the breeds of cow gave the yield of ghee between 2% and 3% with Holstein cow having the least yield and Jersey cow the intermediate yield.
All the samples showed decrease in mass after ghŗ̥ta mūrcchana despite addition of herb components during the ghee processing. The yield of mūrcchita ghŗ̥ta also followed the same trend as that obtained for the ghee of corresponding cow with Local cow (LM) giving the highest yield of mūrcchita ghŗ̥ta (1.81%). Local cow’s ghee (LG) also gave the highest yield with respect to mass of ghee (63.2%) while Holstein cow’s ghee gave the least yield (39.7%). The samples can easily be grouped in two categories with the raw ghee with high yield and mūrcchita ghŗ̥ta with low yield. Lower yield of mūrcchita ghŗ̥ta than that of corresponding ghee indicates some sort of decomposition process occurring during mūrcchana process. However, no new functional groups are obtained as demonstrated by the lack of new peak in the FTIR spectrum. Hence, it can be assumed that ghŗ̥ta mūrcchana process results in changes hat the decomposition products are lost through evaporation and/or decant as solid residue. The photographs of the prepared samples are in Supplementary Information.
FFA values, which represent the fraction of unesterified fatty acids, are displayed in Table 2. Holstein cow ghee (HG) was found be least acidic with 1.35% oleic acid equivalent while market ghee (CG) was found to contain highest free acid (2.49%). Decrease in FFA value was seen in all samples after mūrcchana except on the ghee of Jersey cow. After mūrcchana the mūrcchita ghŗ̥ta of Local cow ghee (LM) had the lowest free fatty acid. Free fatty acid in fat promotes microbial growth and decreases the shelf life of fat.
The lowering of fat content might be the reason why mūrcchana process is claimed to increase the shelf-life of the fat. Thus, it justifies the recommendation of the mūrcchana process for increasing of shelf-life of the ghee in Ayurveda [14]. Although higher liquid fraction in mūrcchita ghŗ̥ta can make mūrcchita ghŗ̥ta more susceptible to contamination than the corresponding ghee, this is not a big issue as the liquid phase is non-aqueous where microbial growth is highly inhibited [27,28].
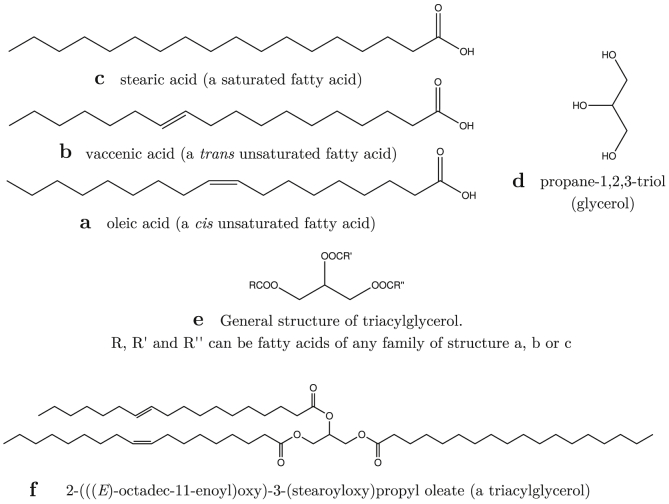
FTIR spectra of the investigated samples are shown on Fig. 2. As the sample is rich in fat, we can expect the peaks from C–H and C–C bonds in the carbon skeleton. These include peaks at 723 cm−1, 1169 cm−1, 1238 cm−1, 1377 cm−1, 1463 cm−1, 2852 cm−1 and 2922 cm−1 for C–H vibrations of –CH2- and -CH3 and groups. The peaks at 968 cm−1, 1417 cm−1, 1651 cm−1 and 3006 cm−1 indicates the sample also consists of (Fig. 3) unsaturated fatty acids such as ones depicted in Fig. 3b and c [29]. Indeed, among many types of fats, triacylglycerols are the most common ones, which are esters of glycerol with three different fatty acids. The nature of fatty acid chains of triacylglycerols determine the physical and chemical properties of the fats [30].
Fig. 2.
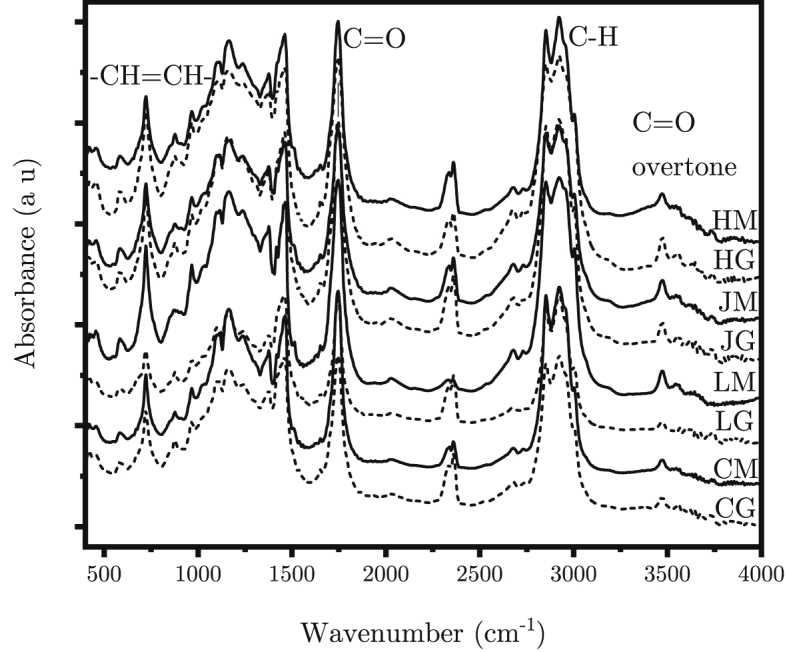
FTIR spectra of ghee (solid) and mūrcchita ghr̥ta (dotted) from milk of Holstein (HG/HM), Jersey (JG/JM) and Local (LG/LM) cow and commercial ghee (CG/CM).
Fig. 3.
Schematic structures of some important chemical compounds associated with ghee.
The C O stretching of ester at 1745 cm−1 and its overtone at 3468 cm−1 confirms the presence of triacylglycerols [31]. The C–O stretching peak at 1238 cm−1 further supports presence of ester group. Presence of shoulder around 1703 cm−1 for the samples indicates the presence of free acids [32]. Absence of broad peak in the range of 3200 cm−1 to 3700 cm−1 shows that the sample is moisture free. The peaks centred at 2337 cm−1 are due to atmospheric CO2 [33,34]. Thus, no peak appears or disappears after ghŗ̥ta mūrcchana, which indicates that mūrcchana does not change the functional groups.
DSC curves are shown on Fig. 4. The signals obtained show a series of endothermic and exothermic events. This is a complex signal already obtained on such complicated substances [25]. It is possible to distinguish two melting temperature domains with a crystallization session in between. Both melting domains are in fact a succession of multiple endothermic peaks. This is because many different polymorphic crystalline forms of fat are reached during sample preparation. The DSC curve resembles the curve obtained by Lopez et al. [35] which also has two melting domains but does not have a crystallization session.
Fig. 4.
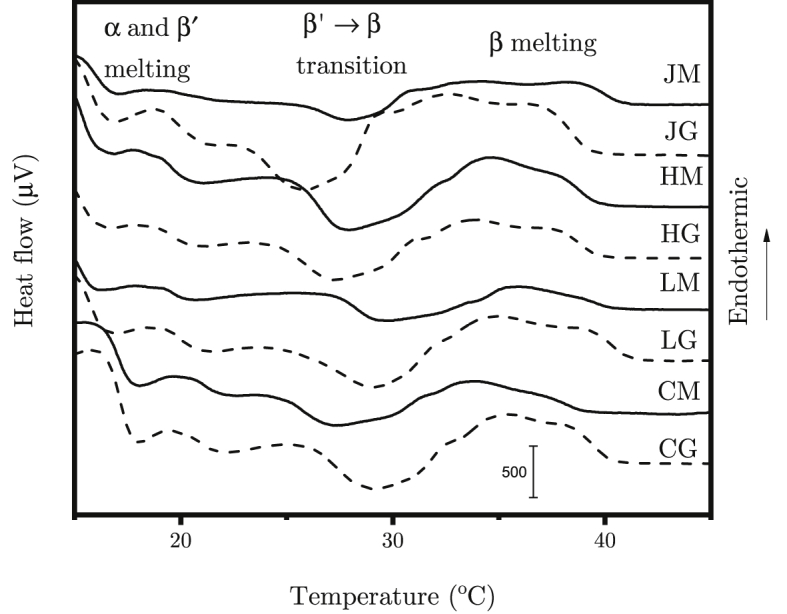
DSC curve of ghee (solid) and mūrcchita ghr̥ta (dotted) from milk of Holstein (HG/HM), Jersey (JG/JM) and Local (LG/LM) cow and commercial ghee (CG/CM).
In fact, three crystal structures called: α, β and β′ can be achieved from triacylglycerols [36]. The α-form has hexagonal cell for which the adjacent carbon chains can oscillate between parallel and perpendicular arrangements. It’s melting temperature is expected to appear from 17 °C to 22 °C and transition to β′ from 0 °C to 22 °C. The β′ crystal has orthorhombic cell with each carbon chain is perpendicular to the adjacent one. It’s melting range is 20 °C–27 °C and β′ to β transition occurs between 5 °C and 27 °C. Finally, β form is triclinic cell with all carbon chain parallel. It melts between 29 °C and 34 °C. α is the least stable while the stability of β and β′ depends on the type of triacylglycerol. Usually for milk fats β′ is more stable than β. When heating, the crystals can melt directly to liquid or transition from α to β′ to β in that order [37,38]. Thus, the DSC curve obtained show the melting of α and β′ between 15 °C and 25 °C, a transformation of β′ to β around 30 °C and β melting above 35 °C. Finally, we observe that the same event occurs for all the samples in the same temperature domains, only the magnitude of each event seems different.
The SFC procedure performed on the DSC data (Fig. 4) gives the curve presented in Fig. 5. It can be immediately concluded that the mūrcchana process decreases the SFC at all the temperatures of all ghee except Jersey cow ghee. As low SFC improves the extraction efficiency of the ghee and promotes emulsification, mūrcchita ghŗ̥ta can improve the medicine’s efficacy. At body temperature (37 °C), Jersey cow ghee has been found to contain only 10.6% solid fat which is the least among the ghee samples making it easiest to digest [39]. However, among the mūrcchita ghŗ̥ta samples, mūrcchita ghŗ̥ta from commercial ghee had the least solid fat at body temperature (3.7%). The β recrystallization from β′ can be clearly seen as a maximum in SFC curve (Fig. 5).
Fig. 5.
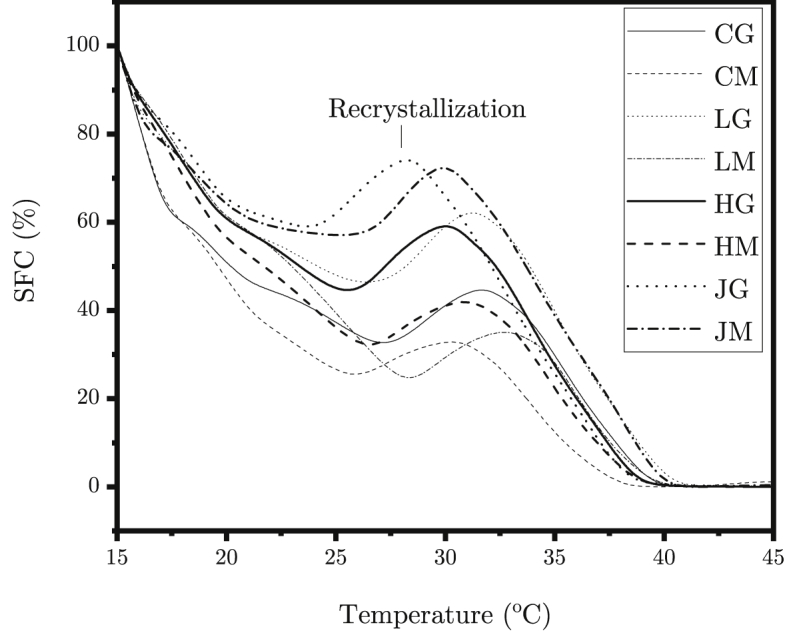
SFC curve of ghee (solid) and mūrcchita ghr̥ta (dotted) from milk of Holstein (HG/HM), Jersey (JG/JM) and Local (LG/LM) cow and commercial ghee (CG/CM).
4. Conclusion
Ghee and mūrcchita ghŗ̥ta samples prepared showed general similarity among each other with significant specific differences. The laboratory prepared samples contained less free acids than the commercial samples and the amount of free acids was found to decrease after ghŗ̥ta mūrcchana process. As mūrcchita ghŗ̥ta contains less free fatty acid and solid fat than the corresponding ghee, the traditional through the mūrcchana process can be justified. However, further extensive work with large sample size, full fatty acid profile and the effect of bacterial strain in fat composition is needed to further understand the dynamics of ghŗ̥ta mūrcchana.
Sources of funding
None.
Conflicts of Interest
None.
Acknowledgements
We would like to acknowledge Nepal Academy of Science and Technology (NAST), Khumaltar, Nepal for FTIR spectroscopy of the samples. We gratefully thank Mettler-Toledo International Inc., Switzerland for donating DSC instrument to Nepal Polymer Institute (NPI). We gratefully acknowledge the Mettler-Toledo GmbH, Greifensee, Switzerland for the donation of the DSC12E.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2020.06.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Oxford University Press ghee [Internet]. 2017 [cited 2017 Dec 2] https://en.oxforddictionaries.com/definition/ghee Available from:
- 2.Sawaya W.N., Khan P., Al-Shalhat A.F. Physical and chemical characteristics of ghee and butter from goat’s and sheep’s milk. Food Chem. 1984;14:227–232. [Google Scholar]
- 3.Baker G.W., Taubes S. Fluorescence of milk and butter in ultraviolet light. Analyst. 1932;57:375–376. [Google Scholar]
- 4.Dutta S. Sardar Patel University; 2011. Modernization of traditional dairy products sector techno economic assessment and strategy formulation for up gradation of Chhana manufacturing.http://hdl.handle.net/10603/75511 [Internet] [cited 2017 Dec 2]. Available from: [Google Scholar]
- 5.Singh R.B., Niaz M.A., Ghosh S., Beegom R., Rastogi V., Sharma J.P., et al. Association of trans fatty acids (vegetable ghee) and clarified butter (Indian ghee) intake with higher risk of coronary artery disease in rural and urban populations with low fat consumption. Int J Cardiol. 1996;56:289–298. doi: 10.1016/0167-5273(96)02760-x. [DOI] [PubMed] [Google Scholar]
- 6.Lovejoy J.C., Smith S.R., Champagne C.M., Most M.M., Lefevre M., DeLany J.P., et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care. 2002;25:1283–1288. doi: 10.2337/diacare.25.8.1283. [DOI] [PubMed] [Google Scholar]
- 7.Pandeya G.S., editor. Gadanigraha of sri vaidya sodhala, snehadhikaara, verse 26. Chowkhamba Sanskrit Series Office; Varanasi: 1969. p. 744. [Google Scholar]
- 8.Vatsya, editor. Kasyapa samhita, kalpa sthana; lashunakalpa, verse 81-90. Chowkhamba Sanskrit Series Office; Varanasi: 1953. p. 179. [Google Scholar]
- 9.Kaushik G.S., editor. Tejkumar Press; Lucknow: 1957. pp. 51–53. (Bhaisajyaratnavali of govinda dasji, atha jworadhkara, verse 258-272). [Google Scholar]
- 10.Vaidya L., editor. Motilal Banarasidas Publishers Private Limited; Varanasi: 1963. p. 36. (Ashtangahridayam of vagbhata, sootra sthana; dravadravya vidnyaniya). [Chapter 5], Verse 37-40. [Google Scholar]
- 11.Yadav S., Jain S., Chaudhary J., Bansal R., Sharma M. The role of Ayurveda management in preventing surgical site infections instead of surgical antibiotic prophylaxis. J Ayurveda Integr Med. 2017;8:263–265. doi: 10.1016/j.jaim.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguli N.C., Jain M.K. Ghee: its chemistry, processing and technology. J Dairy Sci. 1973;56:19–25. [Google Scholar]
- 13.Joshi K.S. Docosahexaenoic acid content is significantly higher in ghrita prepared by traditional Ayurvedic method. J Ayurveda Integr Med. 2014;5:85–88. doi: 10.4103/0975-9476.131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achaya K.T., Ghee In. Bangalore Press; Bangalore: 1948. The chemistry & manufacture of Indian dairy products; pp. 95–105.http://krishikosh.egranth.ac.in/bitstream/1/2025683/1/BPT19544.pdf [Internet][cited 2017 Dec 2] Available from: [Google Scholar]
- 15.Kumar M., Sharma V.I., Lal D., Kumar A., Seth R. A comparison of the physico-chemical properties of low-cholesterol ghee with standard ghee from cow and buffalo creams. Int J Dairy Technol. 2010;63:252–255. [Google Scholar]
- 16.Bindal M.P., Wadhwa B.K. Compositional differences between goat milk fat and that of cows and buffaloes. Small Rumin Res. 1993;12:79–88. [Google Scholar]
- 17.Kothavalla Z.R., Doctor N.S. Studies in the quality of the Indian and the Western table butter. Indian J Vet Sci Anim Husb. 1939;9:151–164. http://digital.nls.uk/75246200 [Internet] Available from: [Google Scholar]
- 18.Talpur F.N., Bhanger M.I., Khuhawar M.Y. Comparison of fatty acids and cholesterol content in the milk of Pakistani cow breeds. J Food Compos Anal. 2006;19:698–703. [Google Scholar]
- 19.Vidhyanath R., Sahasrayoga Nishteswar K., Varanasi Ghrita Prakarana. Chowkhamba Sanskrit Series Office; 2008. [Google Scholar]
- 20.Charaka Samhita, Sootra Sthana; Chapter 13, verse 12-13. Varanasi: Bhargav Pustakalaya; 157 p.
- 21.Bhishagratna K.L., editor. Sushruta samhita, chikitsha sthana. 1911. p. 349. [Chapter 9], Verse 9. Calcutta. [Google Scholar]
- 22.Choudhary N., Narode S. vol. 3. 2015. pp. 37–40.http://www.ijapr.in/index.php/ijapr/article/view/144 (Ghrita murchana with respect to comparative physico-chemical analysis of plain ghrita and murchita ghrita. Int J ayurveda pharma res [internet]). Available from: [Google Scholar]
- 23.Shrestha S., Bedarkar P., Chaudhari S., Galib R., Patgiri B.J., et al. Facets of sneha murchana sanskara - a review. J Ayurveda Holist Med. 2017;5(1):52–59. http://jahm.in/index.php/JAHM/article/view/584 [Internet]. [cited 2017 Dec 2]. Available from: [Google Scholar]
- 24.IUPAC Standard methods for the analysis of oils, fats and derivatives. Pure Appl Chem. 1979;51:2503–2525. [Google Scholar]
- 25.Vuillequez A., Koza L., Youssef B., Bridier M., Saiter J.M. Thermal and structural behavior of palm oil. Influence of cooling rate on fat crystallization. Macromol Symp. 2010;290:137–145. [Google Scholar]
- 26.Menard K.F., Sichina W.J. PerkinElmer Instruments; 2000. Prediction of solid fat index (SFI) values of food fats using DSC [internet]https://www.perkinelmer.com.cn/Content/applicationnotes/app_thermalsfivaluesfoodfats.pdf Available from: [Google Scholar]
- 27.Smart R., Spooner D.F. Microbiological spoilage in pharmaceuticals and cosmetics. J Soc Cosmet Chem. 1972;23:721–737. [Google Scholar]
- 28.Mossel D.A.A., Ingeam M. The physiology of the microbial spoilage of foods. J Appl Bacteriol. 1955;18:232–268. [Google Scholar]
- 29.Olsen E.F., Rukke E.-O., Egelandsdal B., Isaksson T. Determination of omega-6 and omega-3 fatty acids in pork adipose tissue with nondestructive Raman and Fourier Transform infrared spectroscopy. Appl Spectrosc. 2008;62:968–974. doi: 10.1366/000370208785793371. [DOI] [PubMed] [Google Scholar]
- 30.Nelson D.L., Cox M.M. W. H. Freeman and Company; New York: 2013. Lehninger principles of biochemistry. [Google Scholar]
- 31.Minato A., Yano J., Ueno S., Smith K., Sato K. FT–IR study on microscopic structures and conformations of POP–PPO and POP–OPO molecular compounds. Chem Phys Lipids. 1997;88:63–71. [Google Scholar]
- 32.Guillén M.D., Cabo N. Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agric. 1997;75:1–11. [Google Scholar]
- 33.Banwell C.N., McCash E.M. Tata McGraw-Hill; New Delhi: 1995. Fundamentals of molecular spectroscopy. [Google Scholar]
- 34.Silverstein R.M., Webster F.X., Kiemle D.J., Bryce D.L. John wiley & sons; Hoboke: 2014. Spectrometric identification of organic compounds. [Google Scholar]
- 35.Lopez C., Ollivon M. Triglycerides obtained by dry fractionation of milk fat 2. Thermal properties and polymorphic evolutions on heating. Chem Phys Lipids. 2009;159:1–12. doi: 10.1016/j.chemphyslip.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Timms R.E. Phase behaviour of fats and their mixtures. Prog Lipid Res. 1984;23:1–38. doi: 10.1016/0163-7827(84)90004-3. [DOI] [PubMed] [Google Scholar]
- 37.ten Grotenhuis E., van Aken G.A., van Malssen K.F., Schenk H. Polymorphism of milk fat studied by differential scanning calorimetry and real-time X-ray powder diffraction. J Am Oil Chem Soc. 1999;76:1031–1039. [Google Scholar]
- 38.Lawler P.J., Dimick P.S. In: Food lipids: chemistry, nutrition, and biotechnology. 3rd ed. Akoh C.C., Min D.B., editors. CRC Press; 2008. Crystallization and polymorphism of fats; pp. 245–266. [Google Scholar]
- 39.Carey M.C., Small D.M., Bliss C.M. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.