Abstract
BACKGROUND:
The orexin (hypocretin) system is important for reward-driven motivation but has not been implicated in the expression of a multiphenotype addicted state.
METHODS:
Rats were assessed for economic demand for cocaine before and after 14 days of short access, long access, or intermittent access (IntA) to cocaine. Rats were also assessed for a number of other DSM-5–relevant addiction criteria following differential access conditions. Orexin system function was assessed by quantification of numbers and activity of orexin cells, pharmacological blockade of the orexin-1 receptor, and subregion-specific knockdown of orexin cell populations.
RESULTS:
IntA produced a cluster of addiction-like behaviors that closely recapitulate key diagnostic criteria for addiction to a greater extent than long access or short access. IntA was accompanied by an increase in number and activity of orexin-expressing neurons within the lateral hypothalamic subregion. This increase in orexin cell number and activity persisted during protracted withdrawal from cocaine for at least 150 days and was accompanied by enhanced incubation of craving in the same rats. Selective knockdown of lateral hypothalamic orexin neurons reduced motivation for cocaine, and orexin-1 receptor signaling played a larger role in drug seeking after IntA.
CONCLUSIONS:
We provide the first evidence that lateral hypothalamic orexin system function extends beyond general reward seeking to play a critical role in expression of a multiphenotype addiction-like state. Thus, the orexin system is a potential novel target for pharmacotherapies designed to treat cocaine addiction. In addition, these data point to the IntA model as a preferred approach to modeling addiction-like behavior in rats.
Keywords: Behavioral economics, Cocaine, Hypothalamus, Intermittent access, Long access, Plasticity
The hypothalamic orexin (hypocretin) system is a promising target for pharmacotherapies to treat addiction (1–7). Orexin peptides are produced in neurons in caudal hypothalamus, including dorsomedial hypothalamus (DMH), perifornical hypothalamus (PF), and lateral hypothalamus (LH) proper (8,9); evidence indicates that LH orexin cells in particular underlie drug-seeking behaviors. LH orexin neurons are preferentially activated by cocaine-associated or morphine-associated stimuli, and stimulation of LH orexin neurons drives drug seeking (4,10–12). In contrast, DMH/PF orexin neurons are activated by stress, waking, or general arousal (13–15).
Pharmacological studies support a role for the orexin system in drug seeking, as compounds that block signaling at the orexin-1 receptor (OxR1) reduce seeking of multiple drugs of abuse (1). Moreover, orexin peptides regulate cocaine-induced plasticity at midbrain dopamine synapses (16), and alcohol or cocaine can change orexin peptide or receptor messenger RNA levels, respectively (17,18). Importantly, however, these behavioral and plasticity studies have almost exclusively utilized limited access models of drug self-administration that do not recapitulate pathological drug seeking and/or taking in human addicts. Furthermore, these studies typically focus on single addiction endophenotypes, such as sensitization or reinstatement, which do not manifest in isolation in addicts (19). Thus, a role for orexin in a clinically relevant, multifaceted addicted state characterized by disordered and pathological drug seeking has not been reported.
The transition from casual drug use to an addicted state in humans is often attributed to a gradual escalation of drug intake (tolerance, or shift in hedonic set-point) with repeated use (20). Thus, to promote an addiction-like phenotype, laboratory animals are often given unrestricted, continuous access to cocaine in extended sessions (≥6 hours), i.e., long access (LgA) (21). Recent evidence, however, indicates that the pattern of drug use, rather than the amount of drug intake, might be more important for addiction in humans (22). Experienced cocaine users report an intermittent pattern of intake, where even within a single binge, cocaine uses are separated by significant periods of time, resulting in a spiking pattern of cocaine levels in the brain (22,23). This spiking pattern can be mimicked in rats by giving intermittent access (IntA) to cocaine in daily sessions; Zimmer et al. (24) first reported that this pattern of intake was associated with enhanced drug motivation, and later studies by the same group reported that IntA is associated with enhanced dopamine release and uptake in nucleus accumbens (25,26). Recently it was reported that IntA is associated with the emergence of several addiction-relevant endophenotypes, including escalation of cocaine intake, higher break points on a progressive ratio test, increased economic demand for cocaine, and higher levels of compulsive drug seeking (25,27–29). It is currently unclear, however, how the IntA model compares with the more commonly used LgA model in terms of promoting these and other DSM-5–relevant behaviors in the same animal.
In this study, we used a behavioral economics task to measure changes in cocaine demand following IntA versus LgA or short access (ShA). We also used the behavioral economics task to track changes in free cocaine consumption, an index of hedonic set-point in animals (30). Several DSM-5– relevant addiction endophenotypes were also assessed, including escalation of intake, compulsive (punished) responding for cocaine, relapse, incubation of craving, and emergence of negative emotional states following abstinence. We hypothesized that IntA would promote a cluster of addiction-relevant endophenotypes to a greater extent than LgA. We also hypothesized that the well-documented shift in hedonic set-point following LgA (21) does not necessarily confer greater motivation for cocaine. Moreover, we predicted that the IntA-induced addicted-like state would be accompanied by persistent plasticity of orexin neurons, particularly those in the LH subpopulation, and that the addiction phenotype could be reduced by selective knockdown of these cells or pharmacological blockade of OxR1 signaling.
METHODS AND MATERIALS
All experiments were conducted in accordance with procedures established by the Institutional Animal Care and Use Committee of Rutgers University and the Guide for the Care and Use of Laboratory Animals (National Research Council).
IntA, LgA, and ShA Paradigms
In a single IntA session, cocaine was available in 12 epochs of 5 minutes each that were separated by 25-minute periods during which the levers were retracted. During the epochs of cocaine availability, responding was reinforced on a fixed ratio (FR1) schedule, whereby each response resulted in a 1-second infusion of drug (0.055 mg), which was paired with simultaneous (1 second) light and tone cues. As per previous reports (24), no time-out periods were imposed during the 5-minute epochs of cocaine availability apart from when the pump was active. To signal the return of cocaine availability following each 25-minute period of cocaine nonavailability, the light and tone cues were presented for 5 seconds and animals were given a single priming infusion of cocaine (1 second, 0.055 mg intravenous injection) before the levers were reinserted into the operant chamber. IntA continued for 14 days. Rats in the LgA group were given continuous access to cocaine (0.2 mg/infusion) on an FR1 schedule during daily 6-hour sessions for 14 days. ShA consisted of continuous access to cocaine (0.2 mg/infusion) on an FR1 schedule during 1-hour daily sessions for 14 days. In both the ShA and LgA paradigms, cocaine infusions (3.6 seconds) were paired with simultaneous light and tone cues, followed by a 20-second time-out period signaled by extinguishing the houselight. All animals were trained 5 to 6 days/week.
Behavioral Economics Procedure
Rats were trained on a within-session behavioral economics threshold procedure (31) previously described by our laboratory (30,32,33). In 110-minute sessions, rats received access to decreasing doses of cocaine in successive 10-minute intervals on a quarter logarithmic scale, which was achieved by decreasing the duration of the pump infusion. For the duration of each infusion, the houselight was turned off and light and tone cues were presented. During this time, presses on the active lever were recorded but did not elicit a second infusion, and a new infusion could be initiated as soon as the previous infusion was completed. By fitting an exponential demand equation to the data (34), we determined demand elasticity (α), an inverse measure of motivation, the maximum effort expended to defend preferred brain level of cocaine (calculated Pmax), and consumption at null cost (Q0) values (details are in Supplemental Methods).
Experimental Overview
Following self-administration training, rats were run on the behavioral economics threshold procedure daily for a minimum of 6 days and until the last 3 sessions produced stable α and Q0 values (<25% variability); these values were used as baseline values. Rats were then trained on IntA, LgA, or ShA and then immediately retested on the threshold procedure. Tests with the OxR1 antagonist SB-334867 (SB) began once animals achieved stability, and there was a minimum of three sessions between tests (0, 10, 30 mg/kg, intraperitoneal injection). Rats were then tested for compulsive (punished) responding (Supplemental Methods). In a subset of animals that maintained catheter patency, values from a threshold behavioral economics test 50 to 55 days following the final IntA, LgA, or ShA session (day 50) were obtained following tests of compulsive responding; these animals were then tested for demand following vehicle or SB30 treatment. For all other rats not tested at day 50, animals underwent extinction training and were tested for cued and/or primed reinstatement under SB conditions (0, 10, 30 mg/kg). A subgroup of rats then underwent 4 weeks of homecage abstinence, at which time they were tested for anxiety-like and depression-like behavior. Following 3 months of abstinence, incubation of craving was assessed under cued reinstatement conditions. Another subgroup of rats was tested for locomotor activity following SB (0, 10, 30 mg/kg). For Fos and orexin cell count studies, separate groups of rats were returned to the self-administration room (but remained in their homecages) either 1 day or 150 days following IntA or ShA training and perfused 90 minutes later. Tissue from these animals was also used to quantify the number of neurons expressing melanin-concentrating hormone, a neuropeptide expressed by nonorexin cells in the same hypothalamic regions. For orexin morpholino antisense (OX-AS) studies, a separate group of rats was tested for baseline cocaine demand and then again each day following OX-AS infusions into either DMH/PF or LH (Supplemental Methods); demand values analyzed are the values collected 6 days after OX-AS infusion, as this is the time of peak orexin knockdown (10,35). Details of animals, drug preparations, surgical procedures, extinction and reinstatement testing, mood assays, histology, imaging and cell counts, locomotor testing, and statistical analyses are included in Supplemental Methods.
RESULTS
IntA to Cocaine Promotes a Multiphenotype Addicted State
Escalation of first-hour drug consumption was observed in IntA and LgA animals, but not ShA animals (Figure 1A). Overall escalation of first-hour drug intake was similar between IntA and LgA animals (Figure 1B and Supplemental Figure S1A, B). IntA was associated with an increase in nonrewarded responding across the training period that was not observed following LgA or ShA (Supplemental Figure S1D, E). This stronger drug-seeking profile in IntA animals was observed despite much higher total cocaine consumption in LgA animals (Supplemental Figure S1F).
Figure 1.
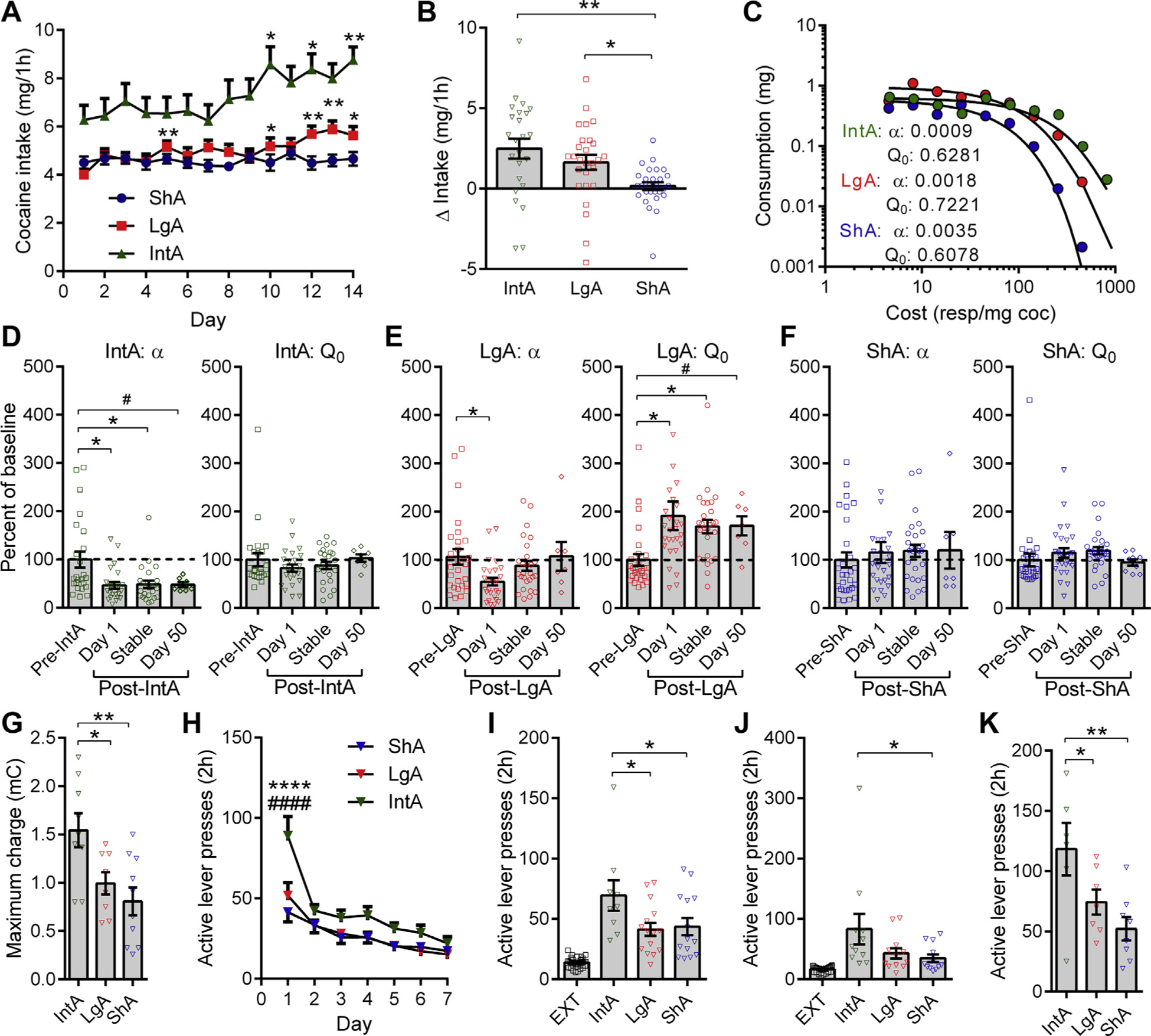
Intermittent access (IntA) produces a multiendophenotype addiction-like state. (A) IntA animals escalated their intake of cocaine across training (F13,335 = 6.788, p < .0001). Long access (LgA) animals also exhibited first-hour escalation of intake (F13,377 = 2.922, p = .0115). There was no change in cocaine intake in short access (ShA) rats (F13,391 = 0.7945, p = .5567). Asterisks denote significant difference relative to day 1, Holm-Sidak multiple comparisons. IntA, n = 24; LgA, n = 27; ShA, n = 28. (B) Delta intake (day 14 minus day 1) was similar between IntA and LgA rats and was significantly greater than ShA rats in both cases (F2,76 = 6.805, p = .0019; IntA vs. ShA: t76 = 3.617, p = .0016; LgA vs. ShA: t76 = 2.361, p = .0411; Holm-Sidak post hoc tests). IntA, n = 24; LgA, n = 27; ShA, n = 28. (C) Representative individual demand curves for treatment groups. (D) IntA was associated with decreased demand elasticity (α) values (increased motivation: F2,71 = 6.275, p = .0176) at day 1 (t23 = 2.629, p = .0298; Holm-Sidak post hoc test) and approximately day 7 (stable: t23 = 2.445, p = .0298; Holm-Sidak post hoc test). A separate analysis on data collected from animals tested at day 50 after IntA revealed that α values remained significantly lower at this time point (Wilcoxon test = −24, p = .0469). No changes were observed in consumption at null cost (Q0) at any time point. Pre-IntA, day 1, stable, n = 24; day 50, n = 7. #p < .05 vs. pre-IntA values. (E) LgA only transiently decreased α (F2,80 = 4.320, p = .0422) at day 1 (t26 = 2.434, p = .0438; Holm-Sidak post hoc test) despite the hedonic set-point for cocaine (Q0) being increased for up to 50 days (t6 = 2.448, p = .0499; separate paired t test). Day 1, stable, n = 27; day 50, n = 7. #p < .05 vs. pre-LgA values. (F) ShA had no effect on α or Q0 values at any of the time points measured. Day 1, stable, n = 28; day 50, n = 7. (G) IntA rats accepted a higher maximum electrical charge to defend their preferred brain cocaine levels (F2,24 = 6.717, p = .0048; IntA vs. LgA: t24 = 2.520, p = .0188; IntA vs. ShA: t24 = 3.563, p = .0032; Holm-Sidak post hoc comparisons). IntA, n = 9; LgA, n = 8; ShA, n = 10. (H) Active lever responding on the first day of extinction training was highest in IntA rats (F12,462 = 2.643, p = .0020; p < .0001 compared with both LgA and ShA rats, Holm-Sidak post hoc comparisons). IntA, n = 26; LgA, n = 23; ShA, n = 20. ****p < .0001 vs. ShA; ####p < .0001 vs. LgA. (I) Cued reinstatement following extinction training was significantly higher in IntA animals (F2,35 = 3.270, p = .0499) compared with LgA (t35 = 2.398, p = .0434) and ShA (t35 = 2.178, p = 0.0434; Holm-Sidak post hoc tests) groups. IntA, n = 9; LgA, n = 15; ShA, n = 14. (J) Cocaine-primed reinstatement following extinction training was highest in IntA animals (nonparametric Kruskal-Wallis test = 6.578, p = .0373; IntA vs. ShA, Dunn’s rank 10.84, p = .0224, post hoc test). IntA, n = 9; LgA, n = 15; ShA, n = 14. (K) Cued reinstatement following 12 weeks of home cage abstinence was highest in IntA rats (F2,18 = 5.703, p = .0121; IntA vs. LgA, t18 = 2.168, p = .0438; IntA vs. ShA, t18 = 3.357, p = .0070, Holm-Sidak post hoc tests). IntA, n = 6; LgA, n = 7; ShA, n = 8. Error bars are SEM. *p < .05; **p < .01. coc, cocaine; EXT, pooled extinction values across all groups; mC, millicoulombs; resp, response.
To assess motivation for drug, we tested animals on a behavioral economics task that measures consumption of cocaine across increasing prices (27,30,33). Baseline α (an inverse index of motivation) and Q0 (cocaine consumption at null cost) values were similar across groups before ShA, LgA, or IntA (Supplemental Figure S1G, H). IntA was associated with a robust decrease in α (increased motivation) (Figure 1C, D) and increase in Pmax values (maximum effort [price paid] to defend preferred brain cocaine concentrations) (Supplemental Figure S2A). Separate analyses of animals tested at day 50 after IntA indicated that these changes were persistent (Figure 1D and Supplemental Figure S2A). We observed no change in Q0 (free cocaine consumption) at any time point measured in IntA animals (Figure 1D). LgA was associated with only a transient decrease in α (Figure 1E) and a trend toward increased Pmax (Supplemental Figure S2B) that dissipated within 1 week. In contrast, the increase in Q0 values persisted at 50 days in LgA rats (Figure 1E), reflecting an enduring increase in preferred brain cocaine concentrations (tolerance). No changes in α, Pmax, or Q0 were observed in ShA animals (Figure 1F and Supplemental Figure S2C).
In a test of punished responding for drug, where cocaine infusions were paired with footshocks of increasing amplitude, IntA rats accepted a higher maximum electrical charge to defend their preferred brain levels of cocaine compared with LgA or ShA subjects (Figure 1G), revealing the development of a strong compulsive drug-seeking phenotype in IntA animals. IntA rats exhibited higher levels of drug seeking on the first day of extinction training (Figure 1H) and on tests of cued (Figure 1I) or cocaine-primed (Figure 1J) reinstatement of extinguished seeking. All groups showed robust incubation of craving as assessed by a second cued-reinstatement test following 3 months of abstinence, but responding in this test was highest in IntA animals (Figure 1K). In all reinstatement tests, responding on the inactive lever did not differ significantly between groups (all p > .05).
Clinical reports indicate that protracted withdrawal from drug is associated with negative emotional states that drive relapse of drug taking through negative reinforcement processes (36). To test for these affective changes, after IntA, LgA, or ShA treatments, animals remained in their homecage for 4 weeks of abstinence and then were tested with a forced swim test, saccharin preference test, or open field test. Compared with ShA or LgA subjects, IntA animals exhibited heightened depressive-like behaviors on the forced swim test and saccharin preference test and enhanced anxiety-like behavior on the open field test (Figure 2). Lower saccharin preference scores were associated with higher cued cocaine seeking following abstinence in IntA animals (R2 = −2.914, p = .0299), but not ShA or LgA rats.
Figure 2.
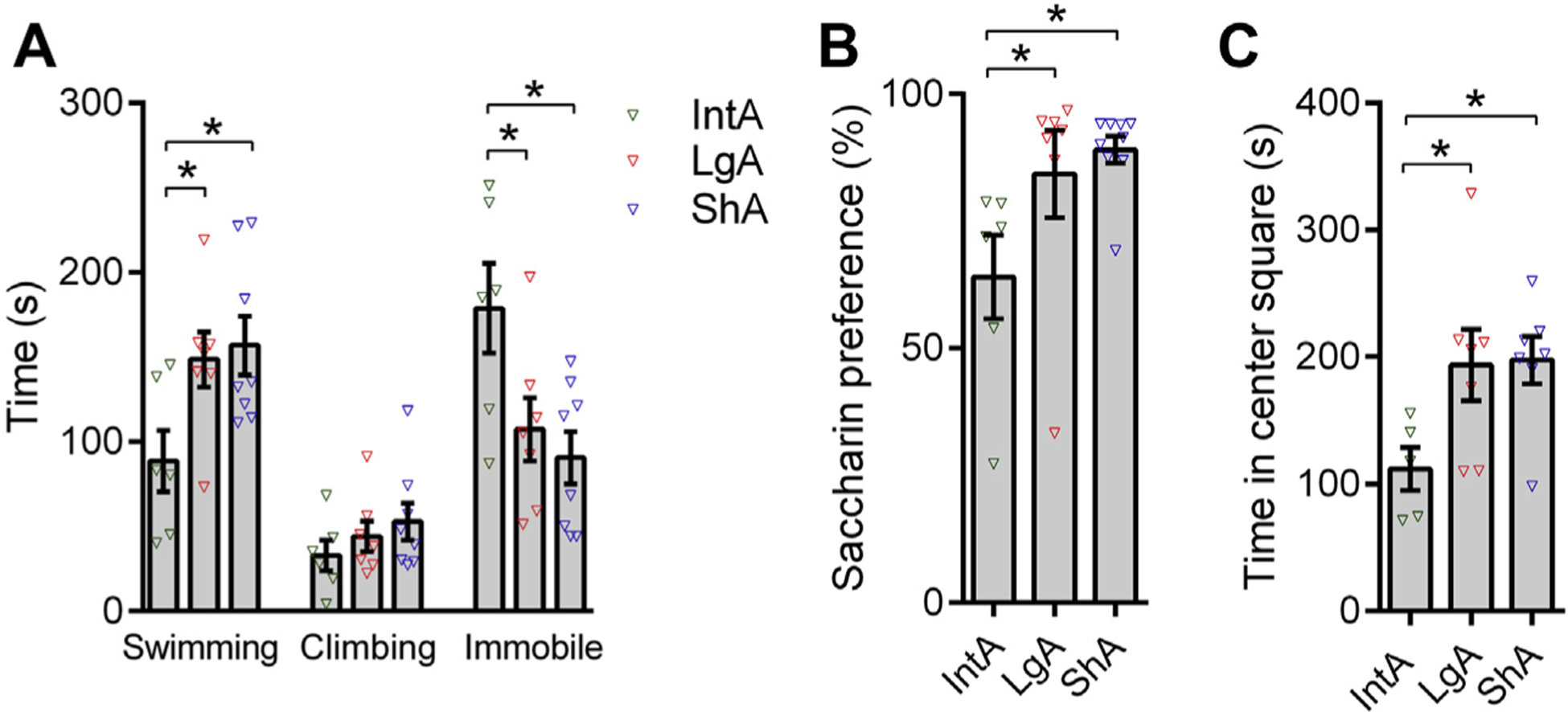
Intermittent access (IntA) is associated with the emergence of negative emotional states following 4 weeks of abstinence. (A) IntA animals spent less time swimming on the forced swim test (F2,18 = 4.265, p = .0305) compared with long access (LgA) (t18 = 2.348, p = .0305; Holm-Sidak post hoc test) or short access (ShA) (t18 = 3.132, p = .0263; Holm-Sidak post hoc test) control animals. On the same test, IntA animals spent more time immobile (F2,18 = 5.285, p = .0156) compared with LgA (t18 = 2.462, p = .0242; Holm-Sidak post hoc test) or ShA (t18 = 3.132, p = .0115; Holm-Sidak post hoc test) control rats. There was no significant difference between LgA and ShA animals in these measures (p > .05). IntA, n = 6; LgA, n = 7; ShA, n = 8. (B) IntA animals showed lower preference for saccharin (F2,19 = 4.027, p = .0348) compared with LgA (t19 = 2.121, p = .0473; Holm-Sidak post hoc test) or ShA (t19 = 2.757, p = .0249; Holm-Sidak post hoc test) rats, which may reflect anhedonia in IntA rats. There was no significant difference between LgA and ShA animals in this measure (p > .05). IntA, n = 6; LgA, n = 7; ShA, n = 7 (one ShA animal included in the forced swim test did not complete saccharin preference test or open field test). (C) IntA animals spent less time in the center square during a 15-minute open field test (F2,16 = 3.857, p = .0429) compared with LgA (t16 = 2.412, p = .0445; Holm-Sidak post hoc test) or ShA (t16 = 2.525, p = .0445; Holm-Sidak post hoc test) rats, indicating an enhanced anxiety-like state in these animals. There was no significant difference between LgA and ShA animals in this measure (p > .05). IntA, n = 5 (one animal excluded, see Statistical Analyses in Supplemental Methods and Materials); LgA, n = 7; ShA, n = 7. All analyses represent Holm-Sidak post hoc comparisons. Error bars are SEM. *p < .05.
Signaling at OxR1 Underlies Expression of an Addiction-like State Following IntA
The OxR1 antagonist SB reduces motivation for cocaine, particularly in animals with high motivation for drug (1,37). Thus, we tested effects of SB in animals that exhibit an addiction-like state following IntA. SB10 normalized α (Figure 3A, B) and Pmax (Supplemental Figure S3A) to pre-IntA values without affecting Q0 (Figure 3B); note that this dose is two to three times lower than the doses generally found to affect motivated behavior for cocaine. The behavioral effects of SB30 were also larger in IntA compared with LgA or ShA rats; in LgA rats, SB30 had no effect on α or Pmax (Figure 3C and Supplemental Figure S3B), but as in previous studies (32,37), SB30 significantly increased α (decreased motivation) and decreased Pmax in a subset of LgA rats with high motivation (Supplemental Figure S4). In ShA rats, neither SB10 nor SB30 significantly affected α (Figure 3D) or Pmax (Supplemental Figure S3C).
Figure 3.
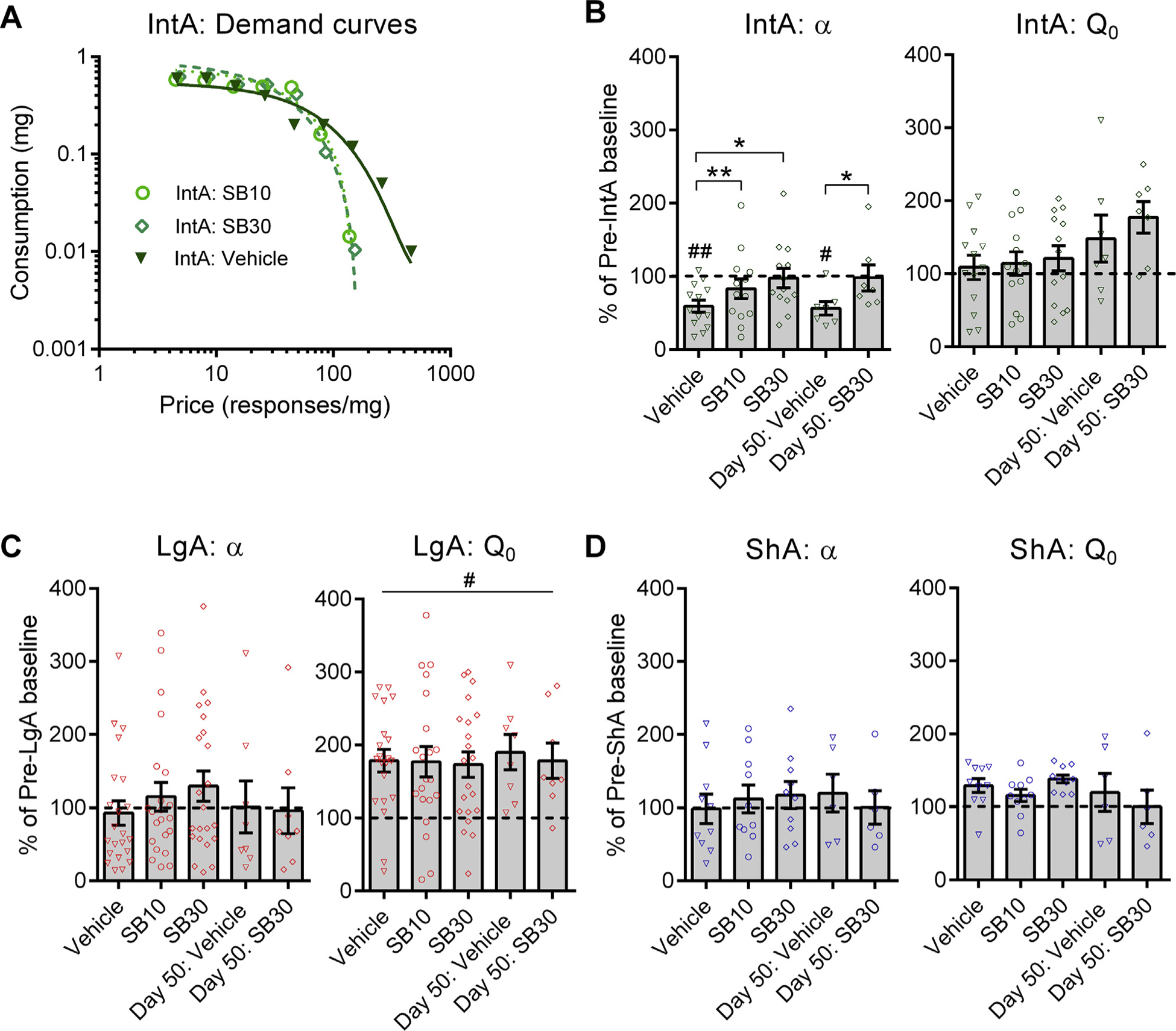
SB-334867 (SB), a selective orexin-1 receptor antagonist, reverses intermittent access (IntA)–induced changes in demand elasticity (α) without affecting cocaine consumption at null cost (Q0). (A) Sample demand curves from a representative IntA animal illustrating a significant increase in α (decrease in motivation) following SB30 treatment. (B) SB normalized α to baseline levels in IntA animals (F2,38 = 7.695, p = .0030) at both 10 mg/kg (t12 = 3.562, p = .0039) and 30 mg/kg (t12 = 2.545, p = .0438). SB30 remained effective at normalizing demand even when administered 50 days following IntA training (W = 28, p = .0156). SB had no effect on Q0 values in these subjects. Holm-Sidak/Wilcoxon post hoc comparisons with vehicle (Veh). Veh/SB10/SB30, n = 13; day 50 Veh/day 50 SB, n = 7. #p < .05 vs. pre-IntA values; ##p < .01 vs. pre-IntA values. (C) SB had no effect on α or Q0 overall in long access (LgA) animals (all p > .05). Veh/SB10/SB30, n = 21; day 50 Veh/day 50 SB, n = 8. #p < .05 vs. pre-LgA values. (D) SB had no effect on α or Q0 overall in short access (ShA) animals (all p > .05). Veh/SB10/SB30, n = 10; day 50 Veh/day 50 SB, n = 6. Error bars are SEM. *p < .05; **p < .01.
Because the IntA-induced addiction-like state was persistent for at least 50 days, we also tested the effect of SB on α and Pmax at this time point. Remarkably, SB30 normalized α and Pmax (Figure 3B and Supplemental Figure S3A) in IntA animals tested at 50 days but had no effect in LgA or ShA rats (Figure 3C, D; Supplemental Figure S3B, C).
SB at both doses was also most effective on cued reinstatement behavior in IntA animals: SB10 significantly attenuated cued reinstatement in IntA rats, whereas this dose in ShA or LgA rats was ineffective (Figure 4A–C) [note that previous studies indicate that 20 mg/kg is needed in ShA rats (38)]. Also, SB30 more strongly suppressed cued reinstatement responding in IntA subjects than in ShA or LgA animals (IntA vs. LgA or ShA; p = .0023, Holm-Sidak post hoc comparisons of Δ vehicle vs. SB30). Consistent with previous studies, SB had no effect on primed reinstatement (Figure 4D–F), indicating that the stimulus cueing properties of cocaine remained intact. SB also had no effect on nonrewarded responding during reinstatement testing (Supplemental Figure S5) or general locomotor activity (Figure 5), indicating that SB had no nonspecific effects on general activity. Together, these data indicate that rats that exhibit an addicted state following IntA are more susceptible to the antiaddiction properties of SB.
Figure 4.
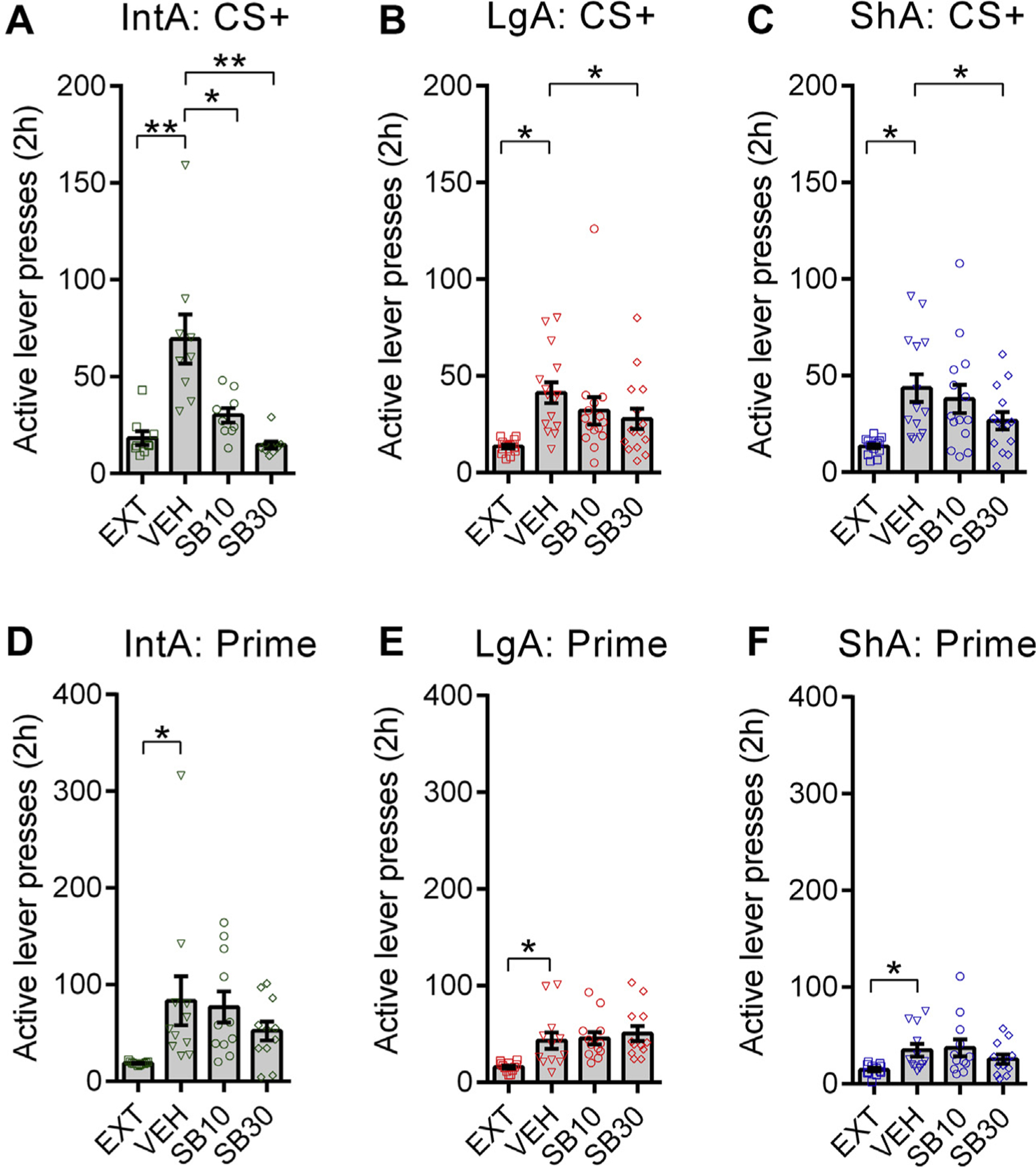
SB-334867 (SB) blocks cued reinstatement at a lower dose in intermittent access (IntA) animals. (A) IntA rats showed robust cued reinstatement behavior (F3,35 = 14.03, p = .0038, overall analysis of variance [ANOVA]). SB10 (t8 = 2.750, p = .0250; Holm-Sidak post hoc test) and SB30 (t8 = 4.128, p = .0066; Holm-Sidak post hoc test) significantly reduced cued reinstatement of active lever responding in IntA animals. n = 9 for all tests. (B) Long access (LgA) rats also showed cued reinstatement behavior (F3,59 = 5.772, p = .0068, overall ANOVA). Only SB30 was significantly effective at reducing reinstatement in LgA rats (t14 = 2.147, p = .0498; Holm-Sidak post hoc test). n = 15 for all tests. (C) Short access (ShA) animals showed cued reinstatement behavior (F3,55 = 8.510, p = .0008, overall ANOVA). Only SB30 significantly reduced reinstatement in ShA rats (t13 = 2.930, p = .0233; Holm-Sidak post hoc test). n = 14 for all tests. (D–F) A priming dose (10 mg/kg) of cocaine elicited reinstatement of active lever responding (relative to levels of pooled extinction values across all groups [EXT]) in IntA (F3,43 = 3.433, p = .0259, overall ANOVA; t10 = 2.887, p = .0186, Holm-Sidak post hoc comparison), LgA (F3,47 = 7.297, p = .0027, overall ANOVA; t11 = 3.152, p = .0274, Holm-Sidak post hoc comparison), and ShA (F3,47 = 4.013, p = .0291, overall ANOVA; t11 = 3.196, p = .0253, Holm-Sidak post hoc comparison) rats. Across all groups, SB had no effect on active lever responding during these tests. IntA, n = 11; LgA, n = 12; ShA, n = 12 for all tests. Holm-Sidak post hoc comparisons. Error bars are SEM. CS+, conditioned stimulus 1; VEH, vehicle. *p < .05; **p < .01.
Figure 5.
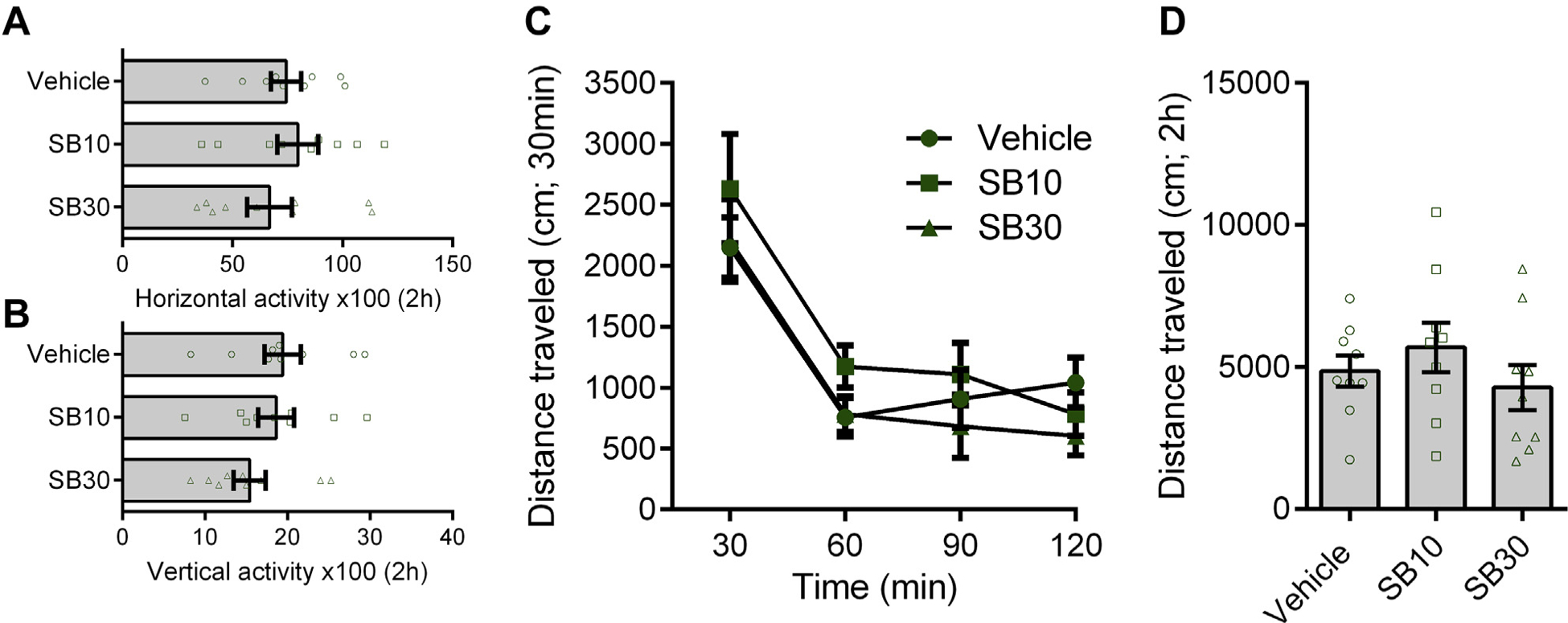
SB-334867 (SB) had no nonspecific effects on general locomotor activity in animals with a history of intermittent access. (A, B) In animals with a history of intermittent access to cocaine, SB had no effect on horizontal [panel (A) F2,26 = 0.6678, p = .4925] or vertical [panel (B) F2,26 = 1.551, p = .2450] activity over the 2-hour test period. (C, D) Similarly, SB did not affect the total distance traveled (in centimeters) across the 2-hour test period when assessed at 30-minute intervals [panel (C) F6,72 = 0.8465, p = .5384] or in sum [panel (D) F2,26 = 1.315, p = .2950]. n = 9 for all tests. Error bars are SEM.
IntA-Induced Addicted State Is Driven by Persistent Augmentation of LH Orexin Function
To test whether the IntA-induced addicted state is accompanied by enhanced orexin system function, we measured orexin cell number and activity following IntA in medial (DMH/PF) and lateral (LH) orexin cell populations. Rats were sacrificed 1 day or 150 days following IntA training and, in both cases, 90 minutes after being re-exposed to the cocaine self-administration environment. ShA rats were used as control subjects, as their total daily cocaine access (1 hour) was similar to that of IntA animals. Immediately after IntA training (day 1), we observed significantly more orexin-A immunoreactive (ir) cells in LH of IntA animals relative to ShA control subjects (Figure 6A, C). The self-administration environment also activated LH orexin neurons in IntA animals on day 1 to a greater extent than ShA animals, as indicated by a larger proportion of orexin-ir neurons that coexpressed the neuronal activity marker Fos (Figure 6B, D). Remarkably, the increased number and activity of LH orexin-ir neurons persisted at day 150 (Figure 6C, D). Moreover, activity of LH orexin-ir neurons was correlated with incubation of craving as assessed by reinstatement testing following 3 months of homecage abstinence (R2 = .870; p = .024; data not shown). Although we observed an increase in the number and activity of DMH/PF orexin-ir neurons immediately following IntA (day 1) compared with ShA control subjects, these differences did not persist into 150 days of withdrawal (Figure 6E, F).
Figure 6.
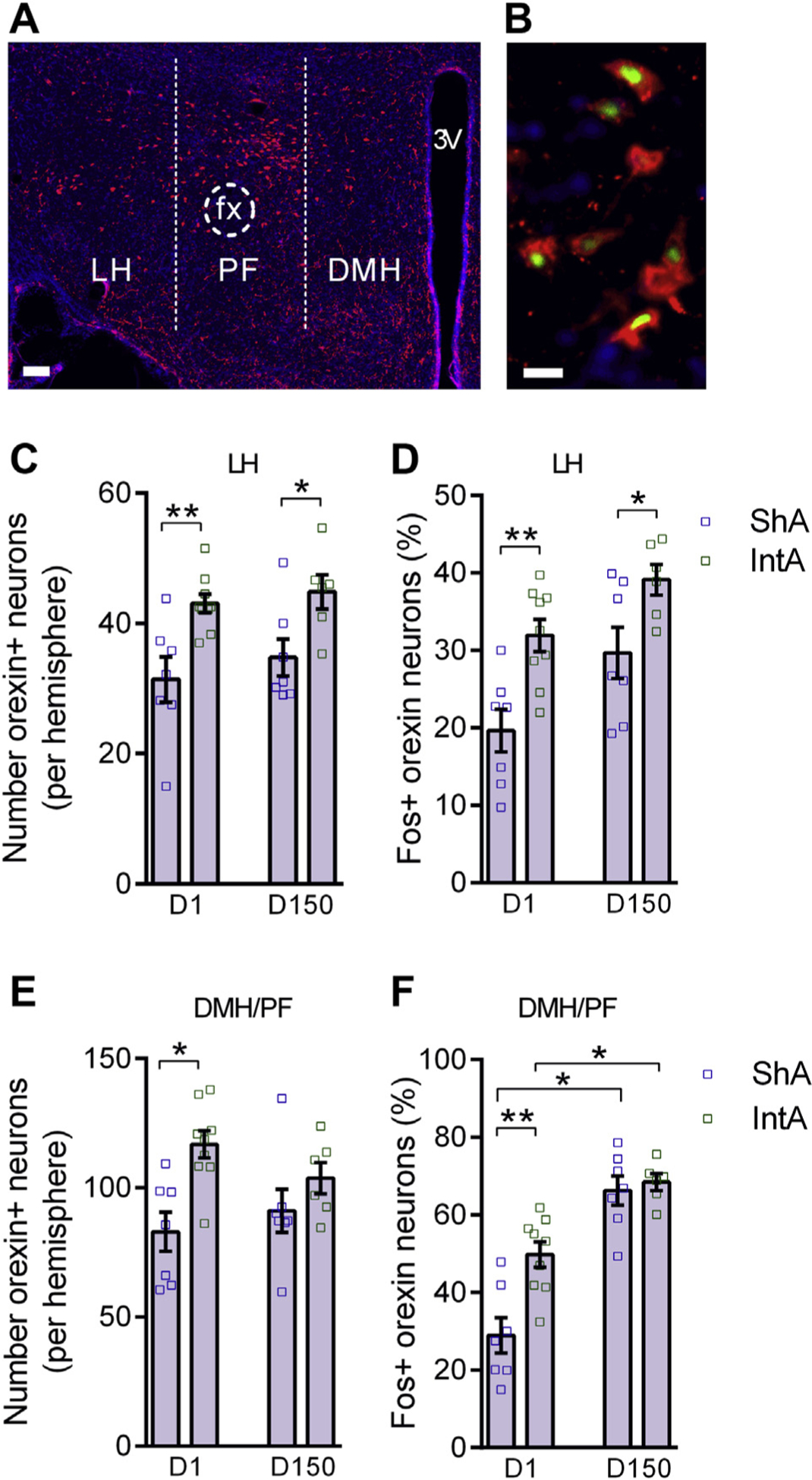
Intermittent access (IntA) phenotype is accompanied by a persistent increase in lateral hypothalamus (LH) orexin neuron number and activity. Low-magnification (A) and high-magnification (B) images of orexinimmunoreactive neurons in hypothalamus stained for orexin (red), Fos [green; panel (B) only], and 4,6-diamino-2-phenylindole (DAPI; blue), taken from an IntA rat. Scale bar = 100 μm (A) and 25 μm (B). (C) Compared with ShA control subjects, IntA animals had a significantly higher number of orexin-expressing neurons in LH 1 day (t14 = 3.384, p = .0045; independent samples t test) and 150 days (t11 = 2.570, p = .0261; independent samples t test) following IntA. (D) In LH, IntA animals had a significantly greater percentage of orexin-expressing cells that were activated in response to the drug environment on 1 day (t14 = 3.658, p = .0026; independent samples t test) and 150 days (t11 = 2.348, p = .0386; independent samples t test) following IntA. (E) IntA animals had a significantly higher number of dorsomedial (DMH)/perifornical (PF) orexin-expressing neurons on day 1 only (t14 = 2.320, p = .0359; independent samples t test). (F) In DMH/PF, IntA animals had a significantly greater percentage of orexin-expressing cells that were activated in response to the drug environment on day 1 only (t14 = 3.831, p = .0018; independent samples t test). Day 1 ShA, n = 7, IntA, n = 9; day 150 ShA, n = 7, IntA, n = 6. Error bars are SEM. *p < .05; **p < .01. fx, fornix; 3V, third ventricle.
In contrast to orexin, we observed no change in the number of neurons expressing melanin-concentrating hormone in either LH or DMH/PF in IntA rats at either day 1 or day 150 (Supplemental Figure S6). Together, these findings indicate that the addiction-like state induced by IntA is accompanied by a cell-specific and persistent augmentation of orexin cell number and function, particularly within LH.
Knockdown of LH Orexin Cells Attenuates Addiction Phenotype
To investigate the functional importance of augmented LH orexin signaling in the expression of an addiction-like state, we assessed the effect of knocking down orexin production in LH versus DMH/PF subregions on cocaine demand elasticity (α). We used OX-AS, which selectively reduces orexin protein expression without affecting other neuropeptides in interdigitated neurons (10,35). Microinjection of OX-AS into the LH orexin subfield (Supplemental Figure S7A) produced approximately 25% knockdown in LH, but not DMH/PF, orexin neurons (Figure 7A, B), which is roughly equivalent to the increase in cell numbers in this region following IntA. This knockdown of LH orexin was associated with a significant increase in α (decrease in motivation) (Figure 7C, D) and decrease in Pmax (Supplemental Figure S7B) but had no effect on Q0 (Supplemental Figure S7C). In contrast, knockdown of orexin expression in DMH/PF (Supplemental Figure S7D) had no effect on α, Pmax, or Q0 (Figure 7E, F and Supplemental Figure S7E, F). Together, these data show that LH, but not DMH/PF, orexin neurons are critically involved in motivation for cocaine and indicate that the persistent changes observed in this subpopulation following IntA may underlie the expression of an addicted state.
Figure 7.
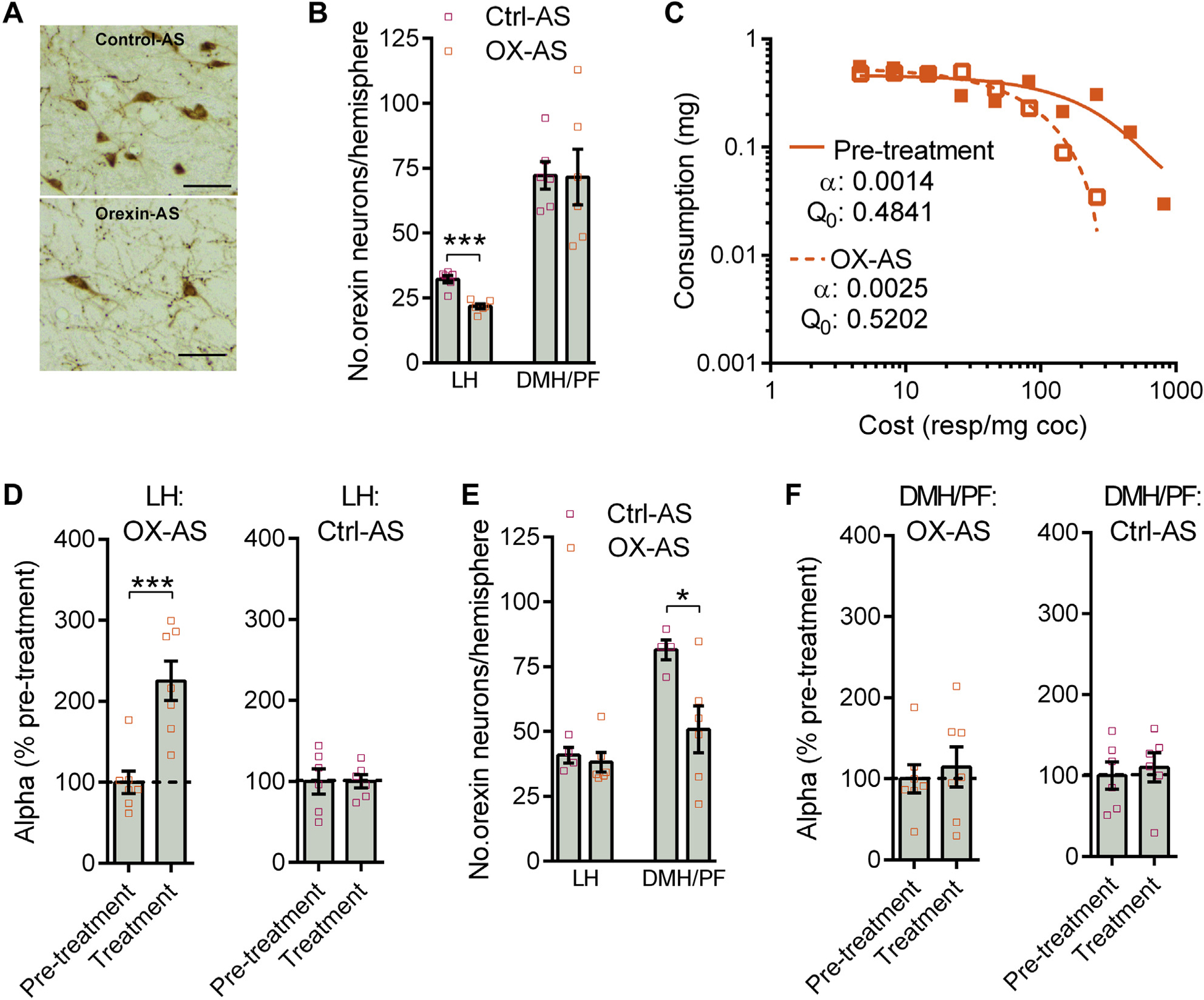
Knockdown of orexin in lateral hypothalamus (LH), but not in dorsomedial (DMH)/perifornical (PF), attenuates addiction phenotype. (A) Representative photomicrographs of LH orexin-A-immunoreactive (ir) cells 6 days after injection of control morpholino antisense (Ctrl-AS) (top panel) or orexin morpholino antisense (OX-AS) (bottom panel). Scale bars = 50 μm. (B) Site-specific delivery of OX-AS produced a knockdown in the number of orexin-ir neurons in LH (t10 = 6.140, p = .0001; independent samples t test) without affecting the number of orexin-ir neurons in DMH/PF (p > .05). (C) Sample demand curves of a representative animal before (pretreatment) and after OX-AS treatment in LH. (D) Overall, LH OX-AS treatment resulted in higher demand elasticity (α) values (lower motivation) (t12 = 4.455, p = .0008; paired-samples t test) (left panel). In contrast, LH Ctrl-AS treatment had no effect on α values (p > .05) (right panel). (E) Site-specific delivery of OX-AS decreased the number of DMH/PF orexin-ir neurons (t8 = 2.612, p = .0310; independent samples t test) without affecting LH orexin-expressing neurons (p > .05). (F) DMH/PF OX-AS treatment had no effect on α values (p > .05; paired samples t test) (left panel). Similarly, DMH/PF Ctrl-AS treatment did not affect α values (p > .05; paired samples t test) (right panel). For behavioral testing of both LH and DMH/PF injections, OX-AS, n = 7; Ctrl-AS, n = 6. Error bars are SEM. coc, cocaine; Q0, consumption at null cost; resp, response. *p < .05; ***p < .001.
DISCUSSION
In this study, we show that IntA to cocaine promotes a state characterized by the concomitant expression of multiple addiction-relevant behaviors. This addiction-like state persists for months and is more pronounced than that induced by LgA, the current gold standard model of addiction in laboratory animals. This IntA-induced addiction-like state is accompanied by augmented orexin system function in three ways: 1) increased number of orexin-expressing neurons, 2) heightened activity of orexin neurons in response to a drug-associated environment, and 3) enhanced effectiveness of an OxR1 antagonist in reducing cocaine seeking. These neuroadaptations persist long into withdrawal and are specific to orexin neurons in the LH orexin subfield. Moreover, we confirm that an increased number of orexin-expressing neurons in the LH orexin subfield is necessary for heightened motivation for cocaine. These data reveal a major role for LH orexin cells in the development and expression of multiple addiction behaviors, associated with persistent plasticity within this neuropeptide circuit.
IntA as a Model of Addiction in Rodents
DSM-5 defines addiction, or substance use disorder, as meeting two or more of 11 diagnostic criteria that focus on impaired control over drug use, including increased time and energy spent seeking and using the drug, intense craving to use the drug, inability to reduce drug use, and social impairment and compulsive drug use including drug use despite negative consequences (39). Several recent studies reported that IntA to cocaine was associated with escalation of cocaine intake, increased economic demand and motivation for cocaine, and increased compulsive responding for drug (24,27–29). In this study, we directly compared the IntA model with the current gold standard approach to modeling addiction in rats, the LgA model, with respect to the promotion of several DSM-5–relevant addiction endophenotypes. We show that IntA promoted stronger addiction-like behavior compared with LgA across all measures of drug-seeking, including escalation of intake, economic demand (motivation) for cocaine, compulsive (punished) responding for cocaine, and cued and primed reinstatement of drug seeking. Moreover, we show greater anxiety-like and depression-like behavior following abstinence in IntA rats compared with LgA rats; this is important, as the emergence of negative emotional states during withdrawal is thought to contribute to subsequent drug use via negative reinforcement (40). Strikingly, this IntA-induced addiction-like state persists for months, as enhanced motivation for drug measured by demand elasticity on a behavioral economics task was observed for at least 50 days following IntA, and heightened cued reinstatement behavior was observed for approximately 3 months of abstinence.
The term “hedonic set-point” was first used by Ahmed and Koob (21) to describe the level of cocaine needed to maintain hedonic homeostasis; an upward shift in this set-point was proposed to underlie enhanced addiction behaviors observed following LgA. The behavioral economics parameter Q0 is a measure of free cocaine consumption (or drug consumption under unrestricted conditions) and therefore can be taken as a proxy for an animal’s hedonic set-point (30,33). We observed a persistent (≥50 days) increase in Q0 among LgA animals; in contrast, changes in motivation for cocaine (α) were only transient in these animals (<1 week). Thus, our data indicate that, contrary to some views, pathological drug seeking in addiction is not necessarily a consequence of changes in the hedonic set-point for cocaine following extended use (20,21). Of note, several studies have reported an augmented addiction-like phenotype in LgA rats (21,41–47). However, in many of these studies, behavioral testing was carried out shortly after LgA training, a time point that may align with the transient increase in demand observed here.
Plasticity of LH Orexin System in Addiction
We found that the enduring behavioral phenotype induced by IntA is accompanied by a persistent (150 days) increase in the number of LH orexin-expressing neurons. Such increased expression was specific and not observed for all neuropeptides, as we found no increase in the number of melanin-concentrating hormone–expressing neurons in the same hypothalamic region. In IntA animals, orexin neurons were more responsive to drug-associated stimuli for up to 150 days, and the effects of SB were greatest in IntA animals at all doses. Indeed, in IntA rats, demand for cocaine and cued reinstatement of drug seeking was normalized by significantly lower doses of SB than have been reported previously (32,38,47,48); this may reflect a greater role for OxR1 signaling in addiction behaviors in these animals. Interestingly, although we previously showed that SB reduces demand for cocaine in restricted-access rats (32), we found here that SB had no effect on α in ShA animals. This may reflect greater engagement of the orexin system with longer (2 hours) self-administration sessions in our previous study compared to the 1-hour sessions used here.
Our results are consistent with previous findings that the orexin system undergoes substantial plasticity in response to several environmental changes. Both the number and the activity of orexin neurons are higher during wakefulness than during sleep (13,49,50), and acute food deprivation increases levels of orexin peptide and messenger RNA and the activity of orexin cells (51,52). In addition, prepro orexin levels are reduced following chronic social defeat stress (52). There is also prior evidence of enduring plasticity within the orexin system following exposure to drugs of abuse, as orexin messenger RNA is increased in LH following chronic alcohol exposure (18) and during withdrawal following cocaine (53). It is unclear from these reports, however, whether these changes reflect neural adaptations that confer a heightened propensity to addiction. Our findings indicate that functional consequences are likely for this orexinergic plasticity. Our findings also align with previous demonstrations that LH orexin cell activity is correlated with drug-seeking behaviors (4,54).
Plasticity in orexin system function persisted for months, consistent with the long-lasting behavioral addiction phenotype observed following IntA. IntA animals remained more sensitive to SB compared with LgA or ShA control subjects for at least 50 days following IntA training, and the number and reactivity of LH orexin-expressing neurons remained elevated in IntA animals following homecage abstinence (150 days after IntA). Greater numbers of LH orexin-expressing neurons were reactive to drug-associated stimuli following prolonged withdrawal in IntA subjects, indicating that plasticity in the LH orexin system may underlie protracted relapse vulnerability after the cessation of drug use. Indeed, changes in orexin neuron function during withdrawal were accompanied by incubation of craving and the development of negative emotional behaviors in the same animals. Mechanisms contributing to orexin system plasticity following IntA remain to be determined. A subpopulation of LH neurons may persistently increase orexin synthesis after IntA, as previously proposed for the active phase of the diurnal cycle (49). Other evidence indicates that glutamatergic input into orexin neurons is increased following repeated cocaine exposure (55,56), and this synaptic plasticity may be further enhanced by IntA. Additional work is required to examine these possibilities.
We confirm that plasticity in LH orexin neurons is critical for motivation for cocaine by showing that selective knockdown of orexin in this cell group reduces cocaine demand. This is the first functional evidence for a selective role of LH versus DMH/PF orexin neurons in drug self-administration. Together with our anatomical and activity mapping data, these results strongly support the proposed dichotomy in orexin cell function, whereby LH orexin neurons preferentially encode reward motivation, whereas DMH/PF orexin neurons process arousal and stress (1,15). Increased numbers and reactivity of DMH/PF orexin neurons immediately after IntA training (day 1) might reflect acute withdrawal-induced arousal and/or stress; however, our OX-AS results indicate that this does not contribute per se to the enhanced demand in these animals. In contrast, our results show that LH orexin neurons directly contribute to motivated responding for cocaine and that enhanced function of these neurons, both immediately following IntA and following protracted withdrawal, contributes to the development and expression of the addicted state following IntA. This may be due to enhanced LH orexin output onto key motivational sites, such as ventral tegmental area, following IntA (57–62).
In summary, we show that IntA is an effective model to promote a persistently addicted-like state in rats characterized by a comprehensive set of addiction-like behaviors that closely recapitulate key diagnostic criteria for addiction in humans. We show that this IntA-induced addicted state depends on enhanced orexin system function specifically within LH, such that this addicted state can be normalized via specific knockdown of LH orexin neurons or OxR1 pharmacological blockade. Thus, our results strongly implicate the orexin system in the addicted state and highlight this system as a promising target for addiction therapies.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Health and Medical Research Council of Australia C.J. Martin Fellowships (Grant No. 1072706 [to MHJ] and Grant No. 1128089 [to HEB]), National Institute of Drug Abuse U.S. Public Health Service award (Grant No. R01 DA006214 [to GA-J] and Grant No. F32 DA036995 [to BAZ]), and Charlotte and Murray Strongwater Endowment for Neuroscience and Brain Health (to GA-J).
We thank Ms. Shayna O’Connor for her invaluable assistance with carrying out behavioral experiments.
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.James MH, Mahler SV, Moorman DE, Aston-Jones G (2017): A decade of orexin/hypocretin and addiction: Where are we now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LC, Lawrence AJ (2017): The role of orexins/hypocretins in alcohol use and abuse. Curr Top Behav Neurosci 33:221–246. [DOI] [PubMed] [Google Scholar]
- 3.DiLeone RJ, Georgescu D, Nestler EJ (2003): Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768. [DOI] [PubMed] [Google Scholar]
- 4.Harris GC, Wimmer M, Aston-Jones G (2005): A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- 5.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. (2003): Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014): Motivational activation: A unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, et al. (2018): Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats [published online ahead of print April 6]. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. (1998): The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998): Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- 10.Sartor GC, Aston-Jones GS (2012): A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci 32:4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson KA, Aston-Jones G (2012): Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci 32:3809–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasheras MC, Laorden ML, Milanes MV, Nunez C (2015): Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology 95:168–180. [DOI] [PubMed] [Google Scholar]
- 13.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. (2001): Fos expression in orexin neurons varies with behavioral state. J Neurosci 21:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy JA, Deurveilher S, Semba K (2003): Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience 121:269–275. [DOI] [PubMed] [Google Scholar]
- 15.Harris GC, Aston-Jones G (2006): Arousal and reward: A dichotomy in orexin function. Trends Neurosci 29:571–577. [DOI] [PubMed] [Google Scholar]
- 16.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006): Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601. [DOI] [PubMed] [Google Scholar]
- 17.Zhang GC, Mao LM, Liu XY, Wang JQ (2007): Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem 103:400–407. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006): The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James MH, Aston-Jones G (2017): Orexin/hypocretin, central amygdala, and escalation of cocaine intake. Biol Psychiatry 81:552–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed SH (2012): The science of making drug-addicted animals. Neuroscience 211:107–125. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed SH, Koob GF (1998): Transition from moderate to excessive drug intake: Change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- 22.Allain F, Minogianis EA, Roberts DC, Samaha AN (2015): How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179. [DOI] [PubMed] [Google Scholar]
- 23.Beveridge TJR, Wray P, Brewer A, Shapiro B, Mahoney JJ, Newton TF, et al. (2012): Analyzing human cocaine use patterns to inform animal addiction model development Published abstract from College on Problems of Drug Dependence Annual Meeting, June 9–14, Palm Springs, California. [Google Scholar]
- 24.Zimmer BA, Oleson EB, Roberts DCS (2012): The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calipari ES, Siciliano CA, Zimmer BA, Jones SR (2015): Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology 40:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR (2013): Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38:2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawa AB, Bentzley BS, Robinson TE (2016): Less is more: Prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233:3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allain F, Bouayad-Gervais K, Samaha AN (2018): High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology 235:317–328. [DOI] [PubMed] [Google Scholar]
- 29.Singer BF, Fadanelli M, Kawa AB, Robinson TE (2018): Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? J Neurosci 38:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentzley BS, Fender KM, Aston-Jones G (2013): The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology 226:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oleson EB, Richardson JM, Roberts DC (2011): A novel IV cocaine self-administration procedure in rats: Differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology 214:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentzley BS, Aston-Jones G (2015): Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentzley BS, Jhou TC, Aston-Jones G (2014): Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hursh SR, Silberberg A (2008): Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- 35.Reissner KJ, Sartor GC, Vazey EM, Dunn TE, Aston-Jones G, Kalivas PW (2012): Use of vivo-morpholinos for control of protein expression in the adult rat brain. J Neurosci Methods 203:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koob GF, Le Moal M (1997): Drug abuse: Hedonic homeostatic dys-regulation. Science 278:52–58. [DOI] [PubMed] [Google Scholar]
- 37.Moorman DE, Aston-Jones G (2009): Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague-Dawley rats. Alcohol 43:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith RJ, See RE, Aston-Jones G (2009): Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 40.Koob GF, Le Moal M (2005): Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci 8:1442–1444. [DOI] [PubMed] [Google Scholar]
- 41.Vanderschuren LJ, Everitt BJ (2004): Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019. [DOI] [PubMed] [Google Scholar]
- 42.Kippin TE, Fuchs RA, See RE (2006): Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology 187: 60–67. [DOI] [PubMed] [Google Scholar]
- 43.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2004): Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology 175:26–36. [DOI] [PubMed] [Google Scholar]
- 44.Knackstedt LA, Kalivas PW (2007): Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322:1103–1109. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A (2008): Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry 32:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wee S, Mandyam CD, Lekic DM, Koob GF (2008): Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmeichel BE, Herman MA, Roberto M, Koob GF (2017): Hypocretin neurotransmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol Psychiatry 81:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. (2005): Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102:19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGregor R, Shan L, Wu MF, Siegel JM (2017): Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS One 12:e0178573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005): Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horvath TL, Gao XB (2005): Input organization and plasticity of hypocretin neurons: Possible clues to obesity’s association with insomnia. Cell Metab 1:279–286. [DOI] [PubMed] [Google Scholar]
- 52.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ (2008): Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci 28:3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, et al. (2008): Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience 153:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moorman DE, James MH, Kilroy EA, Aston-Jones G (2016): Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci 43:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV (2012): Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol 590:3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeoh JW, James MH, Adams CD, Bains JS, Sakurai T, Aston-Jones G, et al. (2018): Activation of lateral hypothalamic group III mGluRs suppresses drug-seeking following abstinence and cocaine-associated increases in excitatory drive to orexin/hypocretin cells [published online ahead of print Jan 1]. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baimel C, Lau BK, Qiao M, Borgland SL (2017): Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep 18:1346–1355. [DOI] [PubMed] [Google Scholar]
- 58.Mahler SV, Smith RJ, Aston-Jones G (2013): Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 226:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, et al. (2011): Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14:684–690. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein DL, Badve PS, Barson JR, Bass CE, Espana RA (2017): Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine [published online ahead of print Oct 2]. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espana RA, Melchior JR, Roberts DC, Jones SR (2011): Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology 214:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James MH, Yeoh JW, Graham BA, Dayas CV (2012): Insights for developing pharmacological treatments for psychostimulant relapse targeting hypothalamic peptide systems. J Addict Res Ther S4:008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


