Graphical abstract
Abbreviations: APAP, N-acetyl-p-aminophenol; CO, cinnamon oil; LPO, lipid peroxidation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GSH, glutathione; NAPQI, N-acetyl parabenzoquinoneimine; BHA, butylated hydroxyanisole; PMS, post mitochondrial supernatants; MDA, malondialdehyde; MEC, molar extinction coefficient; SOD, superoxide dismutase; GR, glutathione reductase; GPx, glutathione peroxidase; ANOVA, analysis of variance
Keywords: Acetaminophen, Cinnamon oil, Hepatotoxicity, Oxidative stress, DNA fragmentation
Abstract
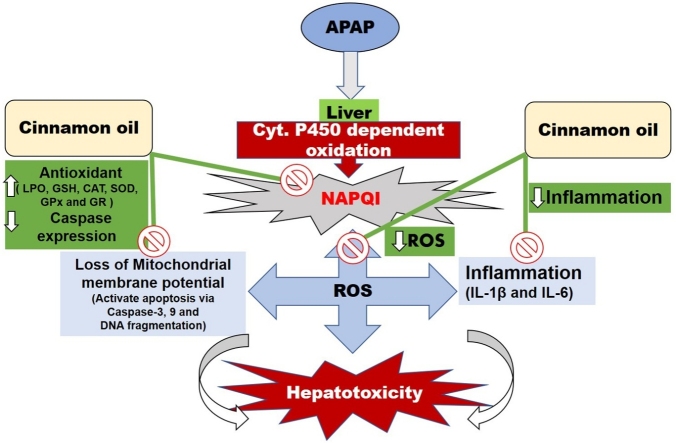
Acetaminophen (APAP) is used as a primary drug due to its antipyretic and analgesic activity. The mechanism of action of APAP toxicity in the liver is due to the depletion of glutathione which elicited free radicals generation. Therefore, the objective of our work is to investigate the APAP induced liver damage and its repair by free radical scavenging activity of cinnamon oil (CO) in male Wistar rats. To investigate the effects of CO at different doses (50, 100 and 200 mg/kg b.w.), animals were given a single oral dose of CO per day for 14 days between 12:00−1:00 PM. The biochemical changes, imbalance in oxidative markers, interleukins, caspases and histopathological studies were determined for quantifying the hepatoprotective effect of CO. One dose of APAP (2 g/kg b.w.) results in significant hepatotoxicity and marked increase the serum markers alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, albumin, total protein, content of lipid peroxidation (LPO), interleukins (IL-1β, IL-6), caspase-3, -9 expression, DNA fragmentation and histopathological changes were observed. Significant decrease in the levels of LPO, interleukins IL-1β, IL-6, caspase-3, -9 expressions, qualitative as well as quantitative determination of DNA fragments and histopathological changes were reversed by the administration of CO dose dependently. Furthermore, it also restores the depleted activity of antioxidative enzymes. Our study shows that an imbalance in the oxidative parameter in the liver by APAP is restored by treating the animals with CO.
1. Introduction
The metabolism of various endogenous as well as exogenous substances takes place in the liver, which is considered as a principal metabolizing organ. Liver is involved in the metabolism of various drugs, chemicals, xenobiotics and their elimination. These substances may induce liver damage by encouraging free radical generation [1], which leads to impaired liver function [2]. In Western countries, toxins and drugs are the main causes of liver related disorders [3]. Therefore, drugs and chemicals agents such as acetaminophen (APAP) and CCl4 are regular in practical for inducing experimental model in research studies. [4].
Antipyretic and mild to moderate discomfort easing APAP (N-acetyl- p-aminophenol) is widely accepted innocuous at the therapeutic quantity [5]. When APAP is uses as the dose of a physician, it is effective and nontoxic analgesic and antipyretic. Nevertheless, long term administration or overdoses of APAP can cause a deleterious effect on the liver [6]. APAP-induced liver injury was well recognized by numerous studies- [[7], [8], [9]].
Acetaminophen transformed into reactive metabolite, acetyl parabenzoquinoneimine (NAPQI) by liver enzymes (Cyp-P450) which bounds with cellular proteins and caused injury [10]. Formation of glutathione conjugates with NAPQI by glutathione form a non-toxic radical which is eliminated from the body [11]. However, the rapid generation of NAPQI due to the overdose of APAP depleted glutathione (GSH) level which plays a major role in hepatocellular damage [8]. Severity of liver injury is associated with the sum of radicals binding with protein [12]. APAP metabolites are also linked with lipid and nucleic acid leading to permeability perturbation and impaired cytoplasmic, serum indicators along with total protein. Serum levels of ALT, AST, ALP, serum bilirubin, albumin and total protein should serve as hepatotoxicity indexes [13,14]. The liver damaged by oxidative stress is also activated by reactive oxygen species which provoke inflammatory cytokines such as IL-1β and IL-6 which further worsens liver damage [14]. Oxidative stress and inflammation are well documented to have the foremost protagonist in APAP facilitated liver damage [15,16]. In a study, reported by Das et al. [17] confirms that APAP impairs mitochondrial membrane potential Δψm which encourage the discharges of cytochrome c that promote caspase-3 and -9 activation ended by hepatic cell death [15,17]. Apoptosis is also determined by the fragmentation of DNA by APAP [18]. Breakdown of a large DNA molecule into a number of smaller fragments is known as DNA fragmentation which is a maker of cell death by apoptosis and quantified by using the Agarose gel electrophoresis technique.
Cinnamon is a major constituent of flavoring and additives in spices used regularly in food industries. Its ingredients are of major parts of various food additives such as seasonings, chili sauces and baked goods. Cinnamon and its derivative possess antioxidant, antimicrobial, anti-inflammatory, anticancer, antidiabetic activities and safe for human beings [18,19]. The antioxidant properties of seven dessert spices (anise, cinnamon, ginger, licorice, mint, nutmeg, and vanilla) were compared with those of the common food antioxidants butylated hydroxyanisole (BHA) (E-320), butylated hydroxytoluene (BHT) (E-321), and propyl gallate (E-310) by the lipid peroxidation assay (LOO*). When the Trolox equivalent antioxidant capacity (TEAC) assay was used to provide a ranking order of antioxidant activity, the result in decreasing order of antioxidant capacity was cinnamon approximately equal to propyl gallate and higher than other spices [20]. The CO demonstrates an advanced proportion of inhibition of oxidation, as tested by the LPO assay [21].
Defensive action of Cinnamon that amended liver damage associated with APAP has not been investigated. Therefore, this study aimes to elaborate probable mechanisms of beneficial effects of cinnamon against acute APAP liver toxicity. Hepatotoxicity was evaluated by observation of serum markers, antioxidative enzymes activities, inflammatory cytokines, caspases, Qualitative as well as Quantitative determination of DNA fragmentation and a histopathological investigation was performed to approve the ameliorative action of Cinnamon oil.
2. Materials and methods
2.1. Chemicals
The highest analytical grade commercially available chemicals were used in the current study. Serum biomarkers and total protein were assessed by kit purchased from Randox Laboratories Ltd (Crumlin, UK). IL-1β, IL-6, caspase-3 and -9 kits were acquired from Abcam, UK. Cinnamon oil, acetaminophen and other reagents brought from Sigma Chemicals (St Louis, MO, USA). DNA extraction kit was purchased from Qiagen Science, Maryland, USA.
The Major constituent of cinnamon oil is cinnamaldehyde, cinnamate, cinnamic acid and a small amount of eugenol, among many other aromatic compounds that exhibits antioxidant, antimicrobial, anti-inflammatory, anticancer, antidiabetic activities and safe for human being [18,19].
2.2. Animals
Male Wistar rats matching around 170−220 g were obtained from Medical Research Center (MRC), Jazan University, Kingdom of Saudi Arabia. Rats were kept in solid bottom polycarbonated cage using rice husk as bedding in the animal house of Substance Abuse Research Center (SARC), College of Pharmacy, Jazan University, Kingdom of Saudi Arabia. They were keep for one week before the commencement of the experiment for acclimatization. The experimental procedure adopted in this study was approved by the Institutional Research Review and Ethics Committee (312/1509/1440, IRREC) of Faculty of Pharmacy, Jazan University,Kingdom of Saudi Arabia. The ideal laboratory conditions were maintained such as humidity-controlled room temperature (45–55 %, 25 ± 2 °C) with a 12-h light/dark cycle. Rats had free access to standard laboratory diet (autoclaved), and water ad-libitum.
2.3. Experimental design
APAP was dissolved in physiological saline (0.9 %, w/v). Randomly rats were distributed into six groups having six rats per group.
Group 1 served as the untreated control, received a single dose of the vehicle (saline) orally for 14 days.
Group 2 rats received only a single dose of CO at 200 mg/kg b.wt. only for 14 days,
Groups 3 rats treated with a single oral dose of APAP at 2 g/kg b.wt., on 12th days,
Groups 4 was treated daily with a single oral dose of CO at 50 mg/kg b.wt.,
Groups 5 was treated daily with a single oral dose of CO at 100 mg/kg b.wt.,
Groups 6 was treated daily with a single oral dose of CO at 200 mg/kg b.wt.,
A single oral dose of APAP was given in the groups 4, 5 and 6 rats on 12th days before 2 h administration of CO. The Group 2 rats were treated only with high dose of CO (200) to confirm any toxicity produced by it self. The doses APAP and CO were carefully preferred by previous study and literature reports [[22], [23], [24]].
2.4. Biochemical evaluation
Blood samples were collected from all group to separate serum by centrifuging blood sample at 1000−2000×g for 10 min and keep at -70 °C for further scrutiny of ALT, AST, ALP, serum bilirubin, albumin and total protein with commercially available test kits (Randox Laboratories Ltd, Crumlin, UK). Thereafter, animals were sacrificed and liver tissue was collected to make homogenate and post mitochondrial supernatants (PMS) in a sodium phosphate buffer for the biochemical analysis to check the relevance from serum results.
2.5. Estimation of LPO
Utley et al. [25] method with some modification was adopted for LPO assay in terms of malondialdehyde (MDA) which is indicators of LPO [25,26]. The mixture consisted of 10 % trichloroacetic acid and 0.67 % thiobarbituric acid. The supernatant was obtained by centrifuging at 3000×g for 15 min. After separation of supernatant, samples were kept for 10 min in boiling water. The sample reading was recorded at 535 nm after cooling. The content of LPO was determined as nmoles of MDA/g tissue by a molar extinction coefficient (MEC) of 1.56 × 105 M−1 cm−1.
2.6. Estimation of reduced glutathione (GSH)
GSH was assessed using the method of Jallow et al. 4% sulphosalicilic acid was used to precipitate sample PMS in a 1:1 ratio [27]. The mixture was incubated at 4 °C for 1 h and then separated supernatant by centrifuged at 3000 rpm. 3 mL assay sample included 2.2 mL of 0.1 M (molar) Na PB (pH 7.4), 0.4 mL of supernatant and 0.4 mL of dithibisnitrobenzoic acid. Absorbance of the resultant sample was taken instantly at 412 nm. 1.36 × 104 M−1 cm−1 MEC was used to determine GSH content in μmole/g tissue.
2.7. Estimation of activity of superoxide dismutase (SOD)
SOD activity was estimated as auto-oxidation of (-)-epinephrine in the liver PMS according to the assay of Stevens et al. [28]. 1 ml reaction mixture consists of glycine buffer (50 mM, pH 10.4), 0.2 ml of PMS and epinephrine. Activity of SOD expressed as nmole epinephrine protected from oxidation using 4.02 × 103 M−1 cm−1 molar extinction coefficient.
2.8. Estimation of catalase activity (CAT)
The method of Claiborne et al. [29] was followed to measure CAT activity. Reaction volume comprises of 50 μl of PMS, 1.95 ml of phosphate buffer and 6.0 mM H2O2. 43.6 × 103 M−1 cm−1 molar extinction coefficient used to determine the activity of CAT in nmol H2O2 consumed/min/mg protein. The absorbance of reaction was measured at 240 nm.
2.9. Assessment of glutathione reductase (GR) activity
The activity of GR was estimated by the procedure of Carlberg and Mannervik [30] with some modification. The 2 ml cocktail of sample consists of phosphate buffer 0.1 M, 7.4 pH, 1 mM GSSG, 0.5 mM EDTA, 0.1 mM NADPH and 10 % PMS. The absorbance of the sample was recorded at 340 nm and 6.22 × 103 M−1 cm−1 molar extinction coefficient used to express GR activity (nanomole NADPH oxidized/min/mg protein).
2.10. Assessment of glutathione peroxidase (GPx) activity
Assay protocol of Mohandas et al. [31] was pursued to estimate GPx activity in 2 ml volume of final sample include 0.05 M buffer, 1.0 mM sodium azide, 1.0 mM EDTA, 0.2 mM NADPH, 0.25 mM hydrogen peroxide, 0.1 ml GR, 1.0 mM glutathione and 0.1 ml PMS. The enzyme activity was taken at 340 nm and determined using molar extinction coefficient 6.22 × 10−3 M−1 cm−1 in terms of nanomole NADPH oxidized per minute per milligram protein.
2.11. Analysis of caspases and interleukin
ELISA kits (AB Cam, UK) were used to determine the interleukins (IL-1β and IL-6) and calorimetric estimation kits were used to determine the levels of Caspase-3 and -9. Assay procedures were following the instruction by the manufacturer. The duplicate sample was assayed to ensure the accuracy of the test. The absorbance was recorded at 450 nm via an ELISA microplate reader (BioTek ELx800 USA) for interleukins and absorbance was recorded at 405 nm for caspases-3 and -9.
2.12. Quantitative DNA fragmentation determination
Assay was done according to the previously reported method [18]. Liver samples stored at −70 °C were homogenized in pre-chilled 3 ml lysis buffer (10 mM Tris−HCl, 20 mM EDTA, 0.5 % Triton X, pH 8.0) using a Potter-Elvehjem homogenizer with a smooth-tipped Teflon pestle. After centrifuging the homogenates at 27,000 g (4−8 °C) to separate the fragmented DNA in the supernatant from the intact DNA in the pellet, the pellets were treated with 4 ml 0.5 N perchloric acid while the supernatants were treated with 1 ml 5.5 N perchloric acid. Lightly capped samples were then boiled for 20 min in a hot water bath followed by a sonication and a 2nd centrifugation at 10,000 g for 10 min to precipitate protein plus the debris. Aliquots (1 ml) of the supernatants were then transferred to clean, labeled test tubes. After adding 4 ml of freshly prepared Burton’s Reagent (diphenylamine), all the test tubes were then capped with Para film and kept in the dark for 16−24 hours at room temperature. Absorbances were recorded at 600 nm using a UV/VIS Beckman DU-640 spectrophotometer. DNA fragmentation in control samples was treated as 100 %. Percent DNA fragmentation in the treated samples was calculated by: (Fragmented DNA) / (Fragmented + Intact DNA) and the results were expressed as percent of control fragmentation.
2.13. Qualitative DNA fragmentation determination by agarose gel electrophoresis
Genomic DNA was isolated by a commercially available kit from Qiagen Science, Maryland, USA. The protocol for the DNA isolation is followed as described by the manufacturer. DNA samples (15 μg/mL) extracted from variously treated tissues were loaded onto 2% agarose gel containing 0.1 % of ethidium bromide and laddering pattern was determined by running the gel at 60 V using a Large Submarine (Hoeffer Instruments, San Francisco, CA). A Hind III digest of λ-DNA served as molecular weight standard. The gels were illuminated on a UV transilluminator and photographed (Polaroid film #667) [18].
2.14. Histopathological assessments
Morphological changes were evaluated by hematoxylin and eosin method. In this procedure, liver tissues keep in 10 % formalin solution for tissue fixation. Before sectioning, the formalin fixed tissue were processed through dehydration process by soaking up in alcohol and xylol, respectively. 5 μm thick section of liver tissue was collected using a microtome. Finally, the sections were stained in the staining media for histological evaluation. The stained sections were observed under a light microscope (magnification 40×).
2.15. Estimation of protein
The method of Lowry et al. was followed to estimate protein using BSA as standard [32]. Sample Protein reaction mixture contains 0.02 mL of PMS, 0.98 mL of H2O, 5 mL of alkaline copper reagent (ACR), and 0.5 mL of Folin-Ciocalteu reagent (FCR). After adding all the reagents, samples were incubated in the dark for 30 min, and after incubation, the absorbance of samples was recorded at 660 nm against a blank of 1 mL H2O, 5 mL ACR, and 0.5 mL FCR.
2.16. Statistical analysis
Statistical analysis of the data was performed by applying the analysis of variance (ANOVA), followed by Tukey-Kramer’s test for all experimental parameters. Results of the analysis were expressed as mean ± SEM of six rats. The p < 0.05 was considered statistically significant.
3. Results
3.1. Biochemical analysis in serum and tissue homogenate
APAP administration (2 g/kg b.wt.) produced significant elevations of all serum enzymes and biomarkers (ALT (p < 0.001), AST (p < 0.01), (p < 0.001), ALP (p < 0.001) and level of bilirubin (p < 0.01), (p < 0.001) in APAP group as compared to the control group. However, treatment with CO (50, 100 and 200 mg/kg b.wt.) significantly protected ALT (p < 0.001), AST (p < 0.01, p < 0.001), ALP (p < 0.01 p < 0.001) and bilirubin (p < 0.01, p < 0.001) when these serum biochemical indices were compared with the APAP treated group. While these biochemical changes (ALT, AST, ALP, and bilirubin) also reported in the liver homogenate which was significantly increased in the APAP group (p < 0.05; p < 0.01; p < 0.001) as compared to control. CO treatment significantly reverses these changes when compared with the APAP alone (p < 0.05; p < 0.01; p < 0.001). No significance changes were observed in serum and homogenate albumin and total protein (Table 1, Table 2).
Table 1.
Biochemical effects of cinnamon oil in serum of APAP induced hepatotoxicity in rats.
| Parameters | Control | Cinnamon oil (200 mg/kg b.w.) | APAP | APAP + Cinnamon oil (mg/kg b.w.) |
||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| ALT (IU/L) | 4.2 ± 0.41 | 6.42 ± 0.52 | 10.71 ± 0.38*** | 9.07 ± 0.81 | 8.67 ± 0.40 | 3.74 ± 0.31### |
| AST (IU/L) | 18.16 ± 2.71 | 20.98 ± 1.89 | 45.36 ± 1.21*** | 36.67 ± 0.96## | 26.5 ± 1.75## | 17.61 ± 0.51### |
| ALP (IU/L) | 209.79 ± 6.9 | 206.34 ± 13.11 | 481.73 ± 21.45*** | 383.32 ± 17.10## | 310.59 ± 21.09### | 282.57 ± 12.76### |
| Bilirubin | 0.16 ± 0.12 | 0.13 ± 0.09 | 0.39 ± 0.07*** | 0.21 ± 0.16## | 0.17 ± 0.08### | 0.14 ± 0.06### |
| Albumin | 4.4 ± 0.67 | 4.2 ± 0.46 | 4.2 ± 0.43ns | 4.2 ± 1.5ns | 4.72 ± 0.82ns | 3.96 ± 0.63ns |
| Total protein (g/dL) | 7.36 ± 1.41 | 7.44 ± 0.89 | 7.36 ± 0.64ns | 9.023 ± 1.84ns | 7.91 ± 0.52ns | 7.96 ± 0.73ns |
Estimation of serum biomarkers in APAP induces hepatotoxicity in rats. CO treatment significantly reveres the serum markers in APAP + CO group as compared to the APAP group. Values are expressed as mean ± S.E.M of n = 6 animals. Significance was determined by one-way ANOVA followed by Tukey-Kramer post-hoc test for multiple comparisons. (ns = non-significant).
***p < 0.001 APAP vs. control.
##p < 0.01, ###p < 0.001 APAP + CO vs. APAP.
Table 2.
Biochemical effects of cinnamon oil in liver homogenate of APAP induced hepatotoxicity in rats.
| Parameters | Control | Cinnamon oil (200 mg/kg b.w.) | APAP | APAP + Cinnamon oil (mg/kg b.w.) |
||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| ALT (IU/L) | 34.28 ± 2.34 | 24.64 ± 1.67 | 358.95 ± 24.11*** | 133 ± 19.81### | 130.43 ± 13.37### | 64 ± 10.09### |
| AST (IU/L) | 12.5 ± 2.13 | 12.67 ± 1.65 | 16.5 ± 3.21** | 15.25 ± 3.11 | 14 ± 0.98# | 13.5 ± 0.76## |
| ALP (IU/L) | 186.3 ± 24.45 | 178 ± 21.85 | 302.5 ± 22.37*** | 196 ± 19.65## | 163 ± 9.08### | 41.33 ± 7.59### |
| Bilirubin | 2.35 ± 0.76 | 2.28 ± 0.12 | 3.06 ± 1.2* | 2.13 ± 0.09# | 0.66 ± 0.17### | 0.18 ± 0.06### |
| Albumin | 0.80 ± 0.03 | 0.73 ± 0.12 | 0.86 ± 1.04ns | 0.83 ± 0.11ns | 0.81 ± 0.87ns | 0.78 ± 0.09ns |
| Total protein (g/dL) | 1.017 ± 0.19 | 0.98 ± 1.09 | 1.57 ± 0.98ns | 1.54 ± 0.46ns | 1.42 ± 1.76ns | 1.36 ± 1.18ns |
Estimation of tissue homogenate biomarkers in APAP induces hepatotoxicity in rats. CO treatment significantly reveres the markers in APAP + CO group as compared to the APAP group. Values are expressed as mean ± S.E.M of n = 6 animals. Significance was determined by one-way ANOVA followed by Tukey-Kramer post-hoc test for multiple comparisons. (ns = non-significant).
*p < 0.05; **p < 0.01; ***p < 0.001 APAP vs. control.
#p < 0.05; ##p < 0.01, ###p < 0.001 APAP + CO vs. APAP.
3.2. Effect of CO on LPO
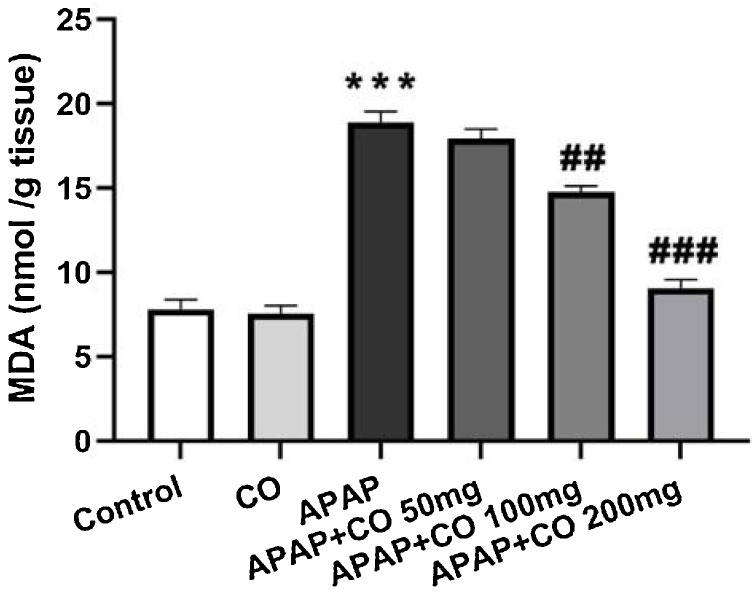
The level of lipid peroxidation in terms of MDA increased significantly (p < 0.001) after treatment with APAP in APAP group as compared with the control group rats only. Different doses of CO (50, 100 and 200 mg/kg) showed inhibition of increased content of MDA dose dependently and it was significant with 100 (p < 0.01) and 200 (p < 0.001) mg/kg of CO as shown in (Fig. 1).
Fig. 1.
Effect of CO on liver tissue level of LPO in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP with control group, ###p < 0.001 and ##p < 0.01 shows significant difference from APAP untreated group (VEH).
3.3. Effect of CO on GSH level
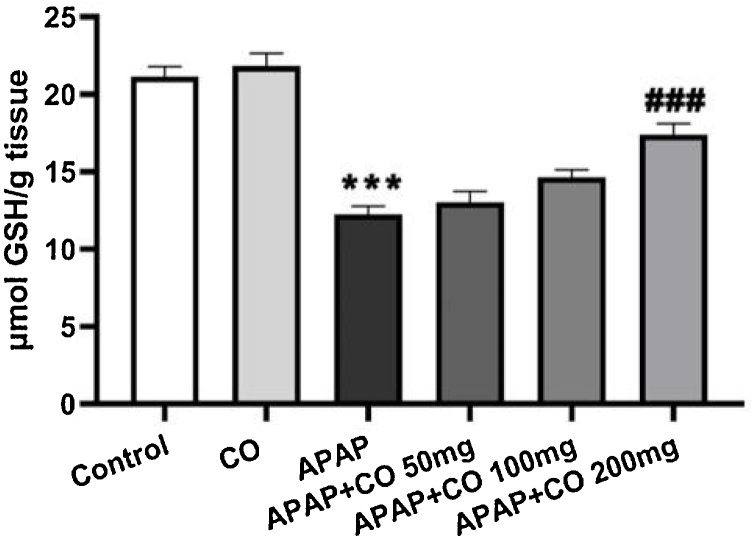
Level of GSH significantly depleted in the liver treated with APAP group when compared to control (p < 0.001) animals. On the other hand, different doses of CO (50, 100 and 200 mg/kg) significantly restored the depleted content of GSH dose dependently. It was significant with the dose of 200 (p < 0.001) mg/kg as compared with APAP group (Fig. 2).
Fig. 2.
Effect of CO on liver tissue level of GSH in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP from control group, ###p < 0.001 shows significant difference from APAP untreated group (VEH).
3.4. CO treatment protected the activities of antioxidant enzymes in liver
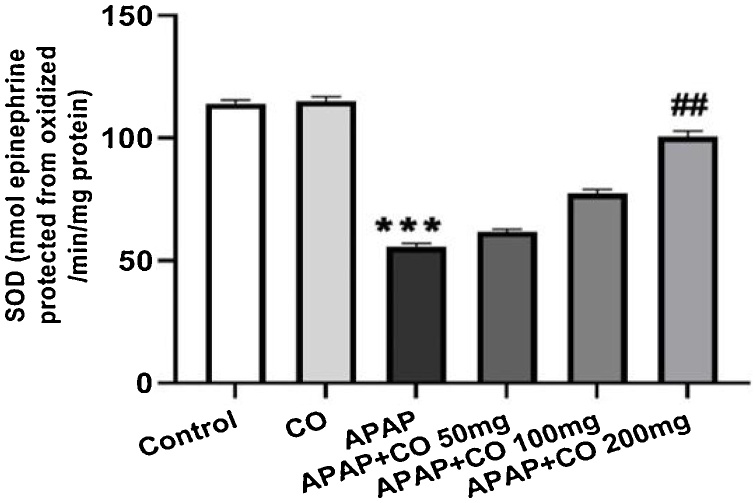
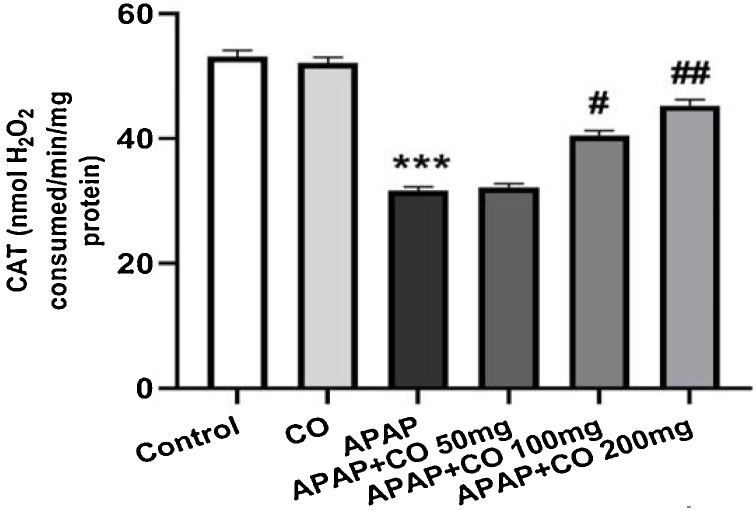
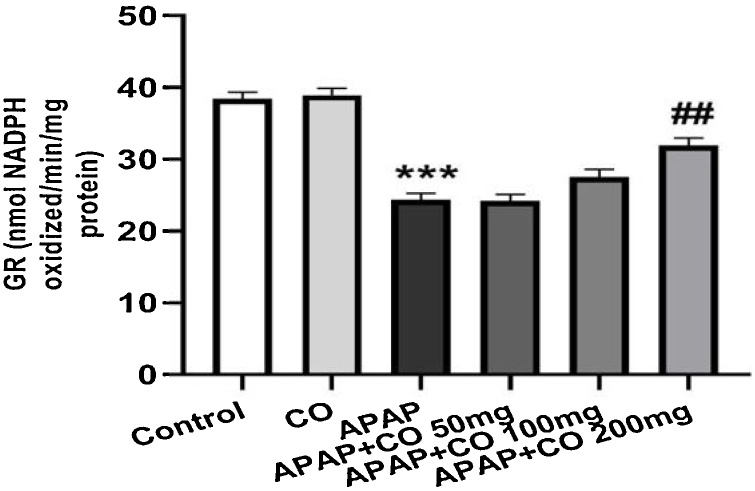
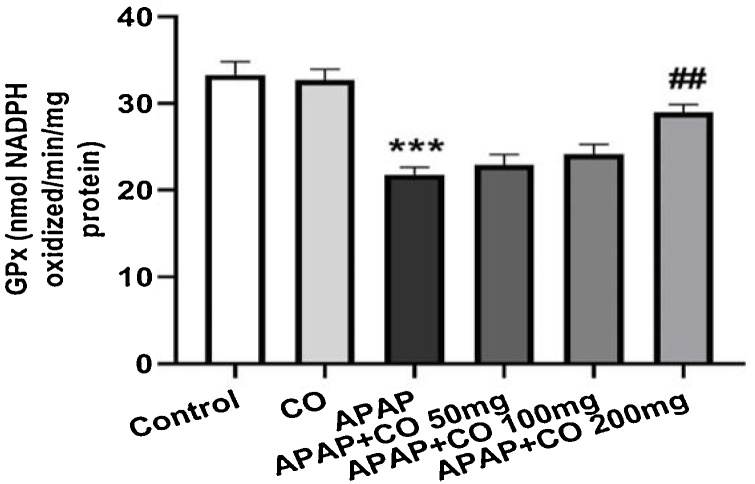
CO administration (only 200 mg/kg) significantly protected SOD (p < 0.01) in APAP treated rats as compared with APAP groups. CAT activity significantly (p < 0.05; p < 0.01) enhanced with the administration of CO (100 and 200 mg/kg) when compared to the APAP group rats. Antioxidant enzymes GR and GPx activity significantly restored (p < 0.01) when treated with CO (200 mg) in APAP treated group rats. Activity of all enzymes (SOD, CAT, GR and GPx) were diminishing in APAP group significantly (p < 0.001) as compared to the control group (Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Fig. 3.
Effect of CO on liver tissue levels of SOD in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP from control group, ##p < 0.01 shows significant difference from APAP untreated group (VEH).
Fig. 4.
Effect of CO on liver tissue level of catalase in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP from control group, ##p < 0.01 and #p < 0.05 shows significant difference from APAP untreated group (VEH).
Fig. 5.
Effect of CO on liver tissue level of glutathione reductase in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP from control group, ##p < 0.01 significant difference from APAP untreated group (VEH).
Fig. 6.
Effect of CO on liver tissue level of glutathione peroxidase in hepatotoxicity induced by APAP. Data presented as Mean ± SEM (n = 6). ***p < 0.001 designates significant difference between only APAP from control group, ##p < 0.01 significant difference from APAP untreated group (VEH).
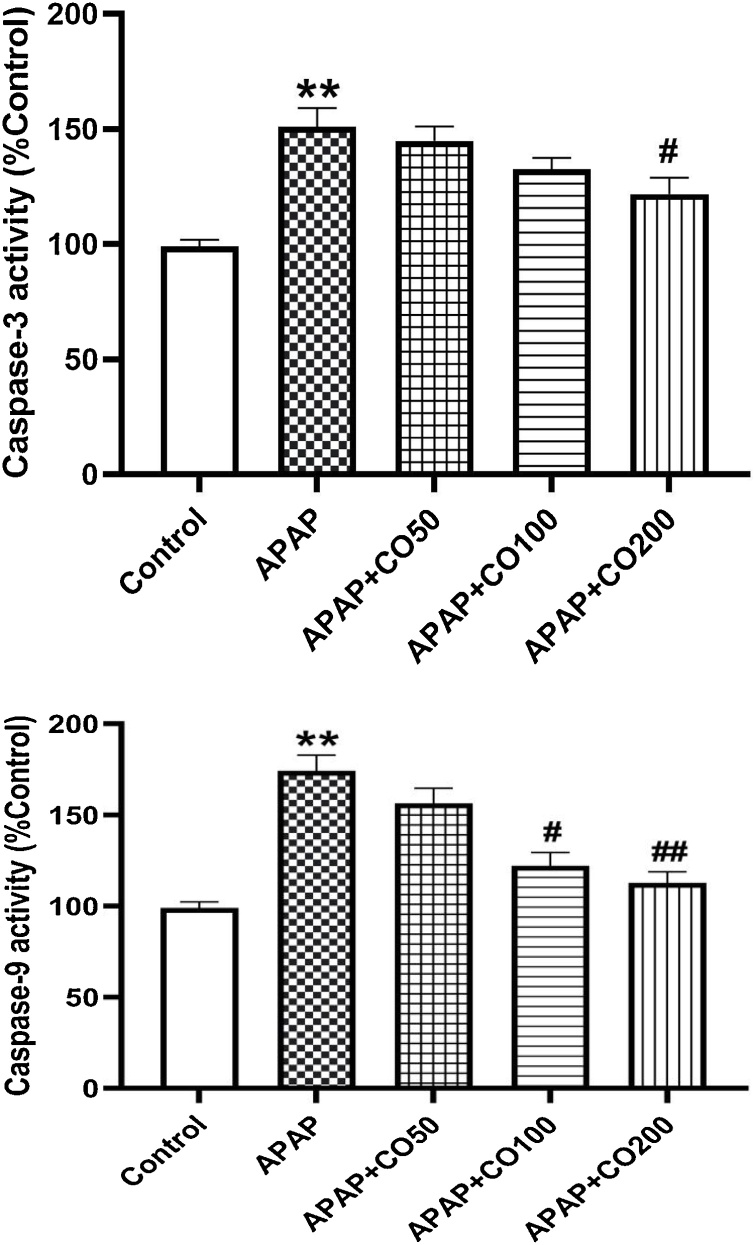
3.5. CO treatment suppress activated caspase-3, -9
The levels of caspase- 3 and -9 enhanced significantly (p < 0.01) in APAP treated group, when compared to the control. Caspase-3 and -9 levels were diminished significantly (p < 0.05; p < 0.01) when treated with CO in APAP + CO 100 and 200 groups as compared to APAP (Figs. 7 and 8 ).
Figs. 7 and 8.
CO attenuated activation of caspase-3 (7) and caspase-9 (8) in liver tissue of rats treated with APAP. Data presented as Mean ± SEM (n = 6). **p < 0.01 designates significant difference between only APAP from control group, #P<0.05 and ##p < 0.01 shows significant difference from APAP untreated group (VEH).
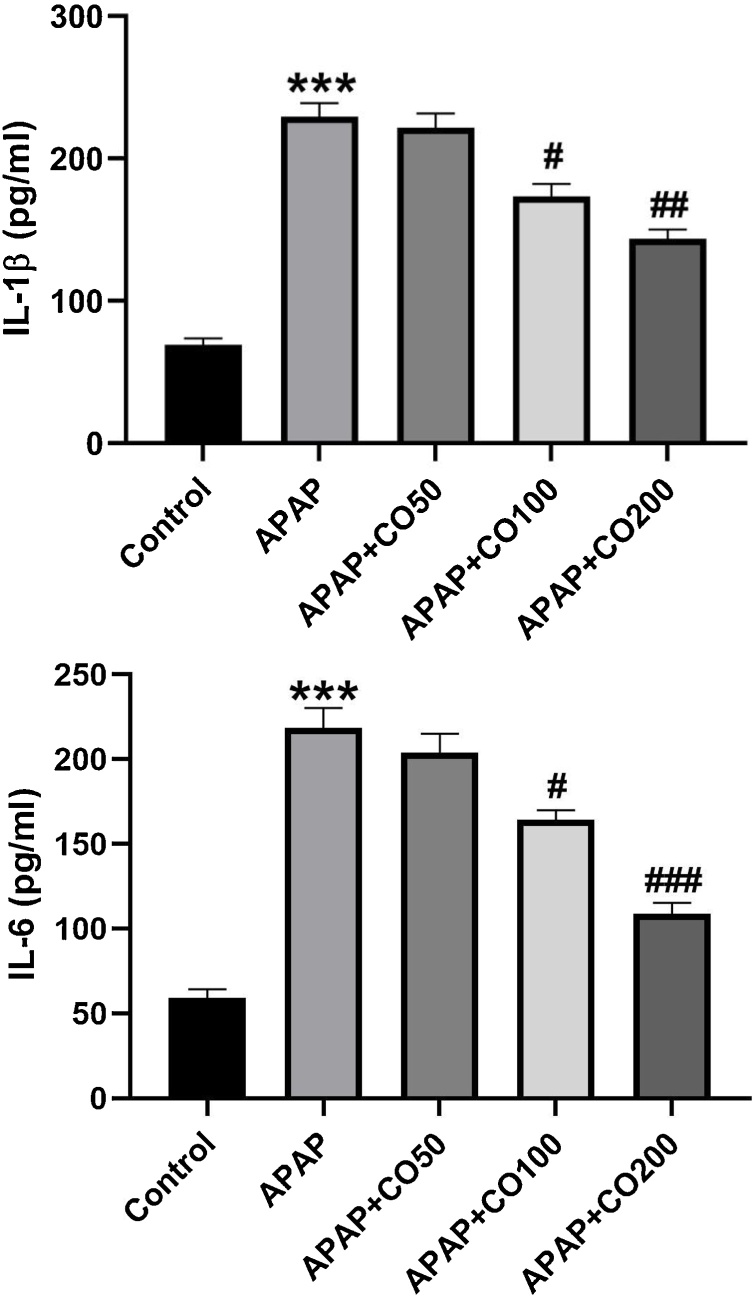
3.6. Effect of CO on cytokine
APAP induces elevation of interleukin IL-1β and IL-6 in APAP treated group as compared to the control (p < 0.001). Whereas CO treatment significantly (p < 0.05; p < 0.01; p < 0.001) attenuated the elevated level of interleukin in APAP + CO (100 and 200 mg) group when compared to the APAP (Figs. 9 and 10 ).
Figs. 9 and 10.
CO attenuates APAP induced activation of inflammatory mediators IL-1β (9) and IL-6 (10) in the liver of rats treated with APAP. ELISA results are presented as group Mean ± SEM (n = 6). ***p < 0.001 compared to control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 respectively, compared to the APAP group.
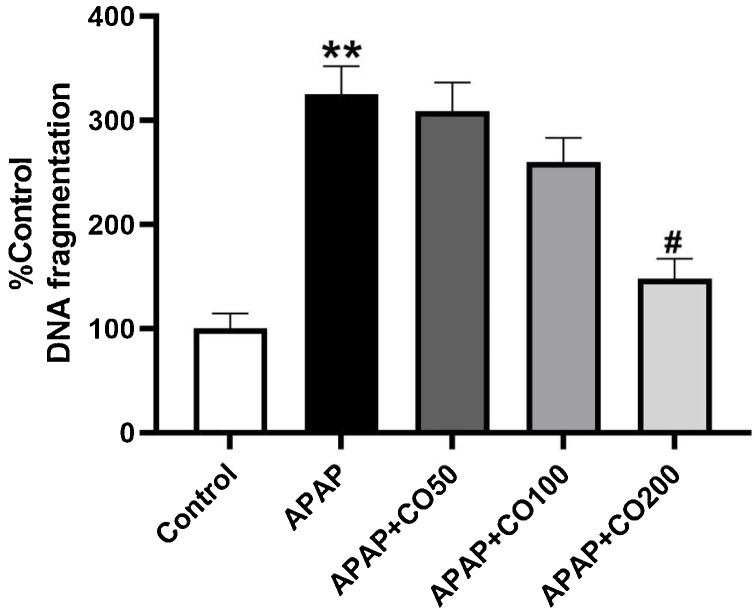
3.7. Quantitative DNA estimation
The preventive effect of CO pre-exposure on genomic integrity is showed in Fig. (11). In concurrence with various past reports [18], APAP toxicity causes a 325 % (p < 0.01) rise in DNA fragmentation as compared with control, however pretreatment CO with the dose of 50, 100 and 200 mg restrict this fragmentation to 309, 260, 148 % of control.
Fig. 11.
Effects of CO pre-exposure on minimization of APAP Induced DNA Fragmentation in the liver. Bars report the percent total DNA represented by nucleosomal fragments and show the degree of fragmentation induced by agents alone or in combination. Results are mean ± SEM with n = 4 mice per group. **Indicates control vs. APAP at p < 0.01 and *indicates APAP vs. APAP + CO (200 mg) at #p < 0.05.
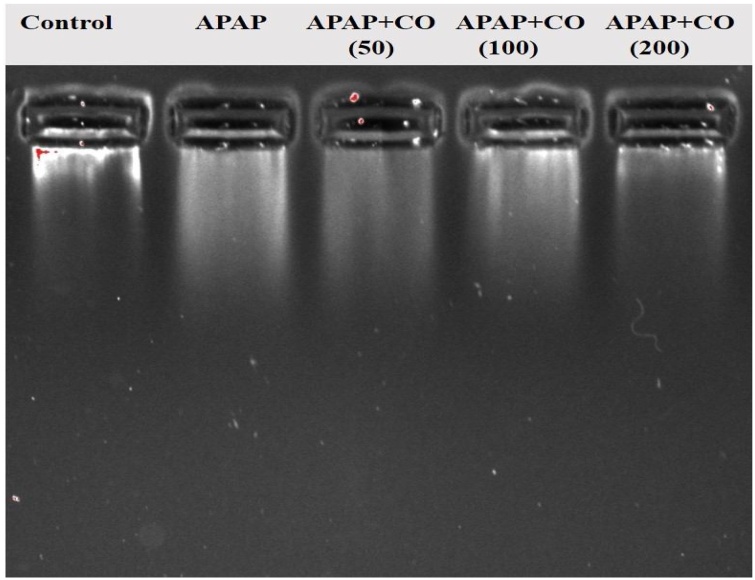
3.8. DNA fragmentation by agarose gel electrophoresis
It shows qualitative changes in the integrity of DNA Fig. (12). Lane 1, DNA is intact in the control group, Lane 2 from left, a smear-like pattern depicted extensive DNA damage by APAP. Furthermore, DNA extracted from the combined CO + APAP (200 mg) group (lane 5) shows the same results as of control, echoing the ability of CO to reverse the APAP-induced DNA damage.
Fig. 12.
Effects of CO on APAP-Induced genomic DNA fragmentation in the liver. Electrophoresis analysis demonstrates that APAP induced considerable cleavage of DNA into smaller fragments including a smear similar to ones seen during apoptotic cell death, and a substantial reduction in DNA damage with CO treatment. Each lane contains liver DNA (mitochondrial + nuclear: 100 ng/lane) extracted collectively from four treated or untreated livers. Lanes represent as follows: Lane-1: vehicle treated control; lane-2: APAP alone; lane-3 APAP + CO (50 mg); lane-4: APAP + CO (100 mg); lane-5: APAP + CO (200 mg).
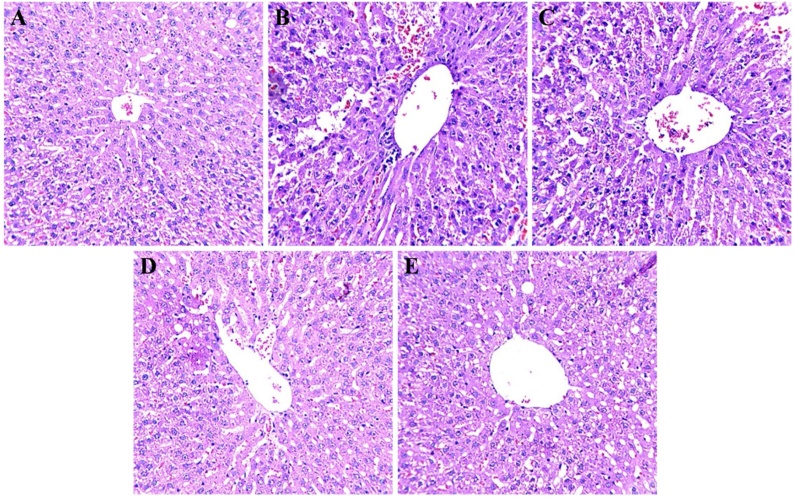
3.9. Effect of CO on histological changes
Histopathological changes in rat liver after APAP exposure and treatment with CO were investigated using H & E staining. Structural changes were seen in the liver of APAP treated group; distinguish by hepatocellular damage as compared with the control group. Cell morphology were irregular in shape along with increment in nuclear and cell size ratio. The corresponding area in the sections from the APAP + CO group (200 mg) showed a partial cellular damage as compared to APAP group. CO treatment protected hepatic cell damage by APAP. No observable changes were found in CO alone (second group) as compared to the control group (data not shown) (Fig. 13).
Fig. 13.
Effect of CO treatment using H&E staining in liver of control, APAP and APAP + CO groups. Liver from control-group rats showed normal portal hepatic tissue and hepatic cells (A). The APAP group showed an aggregate of inflammatory cells in the portal triads and hydropic degeneration of some hepatocytes, which had pyknotic nuclei (B). The APAP + CO (50, 100 and 200 mg) group showed partial degeneration of hepatic cells and the prevention of inflammatory cell aggregation in hepatic triads (C, D and E). Magnification, 40 × .
4. Discussion
In 2011, USA, FDA passed a regulation that the content material of APAP in prescribed drugs need to not be > 325 mg in keeping with one dose and restrained the maximum day by day advocated dose of APAP to < 4.0 g for an adult [33]. Chinese Pharmacopoeia also issued a regulation that the recommended dose of APAP should be not > 2 g in keeping with a single dose [34]. Currently, APAP is diagnosed for typically being used in vivo in animal models to result in acute hepatotoxicity to assess the hepatoprotective outcomes of herbal drugs [35].
This work is planned to explore the experimental model of liver damage by APAP overdose in rats. APAP treatment significantly increases the activity of serum enzymes such as AST, ALT, ALP and level of bilirubin. While CO treatment notably attenuated these biochemical indices as well as protected cellular changes in the liver. Reports of research studies suggested that APAP induces liver damage by enhancing free radical generation and diminishing antioxidant status of the liver [36,37]. Whereas CO treatment defenses liver injury by its scavenging property and improving antioxidant enzyme status in the liver. APAP mediated membrane damage is through the destruction of membrane lipids and oxidation of membrane proteins, collectively with APAP-induced mitochondrial disorder lead to impairment of tubular feature indicated by impaired uptake of albumin and proteins [38,39].
Liver damage provokes the release of ALT and AST in blood used as a hepatotoxicity biomarker along with ALP and total bilirubin [40]. In the serum of rats treated with APAP showed prominent elevation of liver damage indices which are in agreement with previous findings [41]. Increased activity of ALT, ALP, AST and level of bilirubin were normalizing in the serum of CO treated APAP group. The serum enzymes activities and bilirubin level decline may be due to the powerful antioxidant effects of CO. It also suppresses the level of bilirubin and activity of these enzymes in tissue homogenate, which supports our finding in serum.
Increased content of LPO and diminish enzymes activities such as SOD, CAT, GR, GPx and level of GSH were noticed in APAP group. In support of our data, previously reported work showed that APAP encourages oxidative stress and impairment of the antioxidant system of the liver [42,43]. While rats treated with CO reveal remarkable improvement in the activity of the antioxidant enzymes, GSH level and suppresses high level LPO in APAP group. Depletion of oxidative stress confirmed by increased activity of an endogenous antioxidant enzymes (SOD, CAT, GR and GPx) and GSH as well as decreased level of LPO in the liver is due to the potent antioxidant property of CO as reported earlier [44].
APAP-brought on liver toxicity is also related to inflammation due to the fact that activation of APAP metabolism results in inflammatory mobile infiltration and overexpression of inflammatory cytokines (including IL-1β and IL-6), which ultimately bring about inflammation [45] and liver damage, evidenced via an increase in liver function enzymes and pathological modifications to liver tissue. In addition, proinflammatory cytokines, such as IL-1β and IL-6 are concerned with the pathogenesis of liver toxicity by APAP [16]. Proinflammatory cytokines IL-1β and IL-6 have been released from 4 h to 24 h after overdose APAP [46]. Hence, stopping the release of IL-1β and IL-6 may additionally attenuate APAP triggered liver harm. In our study, CO significantly suppressed the discharge of these inflammatory cytokines because of APAP overdose, which indicated a capacity anti-inflammatory impact of CO in APAP-caused acute liver toxicity as this characteristic of CO is proven formerly [47].
Earlier reported, that excessive use of APAP leads to apoptosis liver cells of mice [48,49]. Apoptosis of cells plays a significant role in the APAP liver damage and controlling the apoptosis may decrease the development of its dysfunction [50]. A number of proteins are involved in apoptosis cascade of the liver cells such as caspase-3 and -9 [51] and our calorimetric estimation indicates an upregulation of caspase-3 and -9 in APAP-toxicity in rats, accordance with previous reports [17]. Pretreated the animals with CO markedly attenuate the expression of caspase-3 and -9. So, our results are also supported by earlier reported [52]. Furthermore, apoptosis results in the increase of serum enzymes activities lead to liver dysfunction, so if we can able to control the apoptosis then it ultimately limits the liver damage [53].
DNA fragmentation is also another oblique marker of oxidative harm and is assumed to be the biochemical hallmark of the programmed cell death. The present study, evaluated both quantitative and qualitative (DNA damage), which is able to give extra insight into the impact of CO on cellular death techniques orchestrated by using APAP. As shown in Fig. (11), APAP brought on such toxic injury that it leads to DNA fragmentation ranges to increase up to 325 % of control, while CO with the dose of 50, 100 and 200 mg pretreatment restrained those fragmentation values to 309 %, 260 %, 148 % of control, so our results are same as earlier reports [18].
Qualitative evaluation supports our findings through smear-like pattern formation in agarose gel electrophoresis (Fig. 12), it’s a hallmark of apoptosis. The effective antioxidant possession of CO are showed that there is no DNA fragmentation, which shows a compact band of DNA in APAP + CO (200 mg) organization is the same as in case of control, however, a smear-like formation in the APAP-treatment group represents huge apoptosis. So, our finding is further supported by quantitative assay (Fig. 11) because control confirmed very less fragmentation. So, we can say that our pattern of results is the same as mentioned formerly [18,54].
Histopathological results also provided evidence that the pretreated CO group attenuate the changes caused by APAP induces damage as centrilobular necrosis and infiltrating lymphocytes. Our examinations indicated that APAP exposure initiated hepatocellular damage or necrosis or cell death when observed in control section. However, CO shielded morphological alteration by its strong antioxidant property in our work. Natural derivatives have powerful antioxidant properties protected cellular damage in the liver also reported by others, which in conformity with present work [55,56].
Now, there was the right progress within the prevention and remedy of APAP-brought on liver damage. The protecting mechanisms concerned, include regulating oxidative pressure, an inflammatory reaction and apoptosis [57]. So, our finding suggests, that a marked hepato-protective effect of CO by decreasing serum markers, LPO, increasing antioxidative enzymes activities, regulating oxidative stress, inflammatory reaction and apoptosis. Therefore, we concluded that all the positive effects of CO are may be due to its antioxidative, anti-inflammatory and antiapoptotic possession. Furthermore, these results show that CO can be an alternative treatment for liver injury.
Author statements
| TYPE OF CONTRIBUTION | CONTRIBUTORS |
| Constructing an idea or hypothesis for research and/or manuscript | Prof. Fakhrul Islam |
| DESIGN Planning methodology to reach the conclusion | Dr. Sohail Hussain, Mohammad Ashafaq |
| Supervising the course of the project or the article and taking the responsibility | Dr. Sohail Hussain |
| DATA COLLECTION AND/OR PROCESSING Taking responsibility in execution of the experiments | Mohammad Ashafaq, Rahimullah Siddiqui |
| ANALYSIS AND/OR INTERPRETATION Taking responsibility in logical interpretation and presentation of the results | Saeed Alshahrani, Dr. Sohail Hussain |
| LITERATURE REVIEW Taking responsibility in this necessary function | Dr. Sohail Hussain, Gulrana Khuwaja |
| WRITER Taking responsibility in the construction of the whole or body of the manuscript | Dr. Sohail Hussain, Mohammad Ashafaq |
| CRITICAL REVIEW Reviewing the article, plagiarism | Saeed Alshahrani, Rayan A. Ahmed |
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
We are highly thankful to the Substance Abuse Research Centre (SARC), Jazan University for providing research facilities to do this work.
References
- 1.Gonzalez-Ponce H.A., Rincon-Sanchez A.R., Jaramillo-Juarez F., Moshage H. Natural dietary pigments: potential mediators against hepatic damage induced by over-the-Counter non-steroidal anti-inflammatory and analgesic drugs. Nutrients. 2018:10. doi: 10.3390/nu10020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro V.J., Senior J.R. Drug-related hepatotoxicity. N. Engl. J. Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 3.Fontana R.J. Acute liver failure including acetaminophen overdose. Med. Clin. North Am. 2008;92:761–794. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousar T., Kucera O., Krivakova P., Lotkova H., Kandar R., Muzakova V., Cervinkova Z. Evaluation of oxidative status in acetaminophen treated rat hepatocytes in culture. Physiol. Res. 2009;58:239–246. doi: 10.33549/physiolres.931437. [DOI] [PubMed] [Google Scholar]
- 5.James L.P., Mayeux P.R., Hinson J.A. Acetaminophen- induced hepatotoxicity. Drug Metab. Dispos. 2003;31(12):1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 6.Subramanya S.B., Venkataraman B., Meeran M.F.N., Goyal S.N., Patil C.R., Ojha S. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19123776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni H.M., Mitchell R., McGill M.R., Chao X., Du K., Williams A.J.A., Xie Y., Jaeschke H., Ding W.X. Removal of acetaminophen-protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 2016;65:354–362. doi: 10.1016/j.jhep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskovic A., Bukumirovic N., Paut Kusturica M., Milic N., Cabarkapa V., Borisev I., Capo I., Miljkovic D., Stilinovic N., Mikov M. Hepatoprotective and antioxidant potential of Pycnogenol® in acetaminophen-induced hepatotoxicity in rats. Phytother. Res. 2019;33:631–639. doi: 10.1002/ptr.6251. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z., Zhang J., Wei L., Yang X., Zhang Y. The protective effects of imperatorin on acetaminophen overdose-induced acute liver injury. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8026838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Hinson J.A., Roberts D.W., James L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Den Braver M.W., Vermeulen N.P.E., Commandeur J.N.M. Generic method for the absolute quantification of glutathione S-conjugates: application to the conjugates of acetaminophen, clozapine and diclofenac. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1046:185–194. doi: 10.1016/j.jchromb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Yan M., Huo Y., Yin S., Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–283. doi: 10.1016/j.redox.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A., Visschers R.G.J., Duan L., Akakpo J.Y., Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. Version 2. J. Clin. Transl. Res. 2018;4:75–100. doi: 10.18053/jctres.04.201801.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolbright B.L., Jaeschke H. Mechanisms of inflammatory liver injury and drug-induced hepatotoxicity. Curr. Pharmacol. Rep. 2018;4:346–357. doi: 10.1007/s40495-018-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C.T., Deng J.S., Huang W.C., Shieh P.C., Chung M.I., Huang G.J. Salvianolic acid C against acetaminophen-induced acute liver injury by attenuating inflammation, oxidative stress, and apoptosis through inhibition of the Keap1/Nrf2/HO-1 signaling. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/9056845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragomir A.C., Sun R., Mishin V., Hall L.B., Laskin J.D., Laskin D.L. Role of galectin-3 in acetaminophen-induced hepatotoxicity and inflammatory mediator production. Toxicol. Sci. 2012;127:609–619. doi: 10.1093/toxsci/kfs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das J., Ghosh J., Manna P., Sil P.C. Taurine protects acetaminopheninduced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269:24–34. doi: 10.1016/j.tox.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Bulku E., Stohs S.J., Cicero L., Brooks T., Halley H., Ray S.D. Curcumin exposure modulates multiple pro-apoptotic and Anti- apoptotic signaling pathways to antagonize acetaminophen-induced toxicity. Curr. Neurovasc. Res. 2012;9:58–71. doi: 10.2174/156720212799297083. [DOI] [PubMed] [Google Scholar]
- 19.Tuzcu Z., Orhan C., Sahin N., Juturu V., Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/1583098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murcia M.A., Egea I., Romojaro F., Parras P., Jimenez A.M., Martinez-Tome M. Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J. Agric. Food Chem. 2004;52:1872–1881. doi: 10.1021/jf0303114. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.S., Jeon S.M., Park E.M., Huh T.L., Kwon O.S., Lee M.K., Choi M.S. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J. Med. Food. 2003;6(3):183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- 22.Reshi M.S., Shrivastava S., Jaswal A., Sinha N., Uthra C., Shukla S. Gold nanoparticles ameliorate acetaminophen induced hepato-renal injury in rats. Exp. Toxicol. Pathol. 2017;69(4):231–240. doi: 10.1016/j.etp.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 23.El-Hadary A.E. Antioxidant and hepatoprotective effect of cinnamomum zeylanicumoil against paracetamol induced hepatotoxicity in male rate. J. Agric. Chem. Biotechnol. 2015;6:407–418. [Google Scholar]
- 24.Ashafaq M., Hussain S., Alshahrani S., Madkhali O., Siddiqui R., Khuwaja G., Alam M.I., Islam F. Role of cinnamon oil against acetaminophen overdose induced neurological aberrations through brain stress and cytokine upregulation in rat brain. Drug Chem. Toxicol. 2020;5:1–8. doi: 10.1080/01480545.2020.1747484. [DOI] [PubMed] [Google Scholar]
- 25.Utley H.G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- 26.Hussain S., Ashafaq M. Oxidative stress and anti-oxidants in pre and post-operative cases of breast carcinoma. Turk. J. Pharm. Sci. 2018;15:354–359. doi: 10.4274/tjps.93063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jollow D.J., Thorgeirsson S.S., Potter W.Z., Hashimoto M., Mitchell J.R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12:251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- 28.Stevens M., Obrosova I., Cao X., Huysen C.V., Green D.A. Effects of DLalpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 29.Claiborne A. Catalase activity. In: Green Wald R.A., editor. CRC Hand Book of Methods for Oxygen Radical Research. CRC Press; Boca Raton, FL: 1985. pp. 283–284. [Google Scholar]
- 30.Carlberg I., Mannerviek B. Glutathione reductase levels in rat brain. J. Biol. Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- 31.Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidneys: possible implication in analgesic neuropathy. Cancer Res. 1984;44:5086–5091. [Google Scholar]
- 32.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Mitka M. FDA asks physicians to stop prescribing high-dose acetaminophen products. JAMA. 2014;311:563. doi: 10.1001/jama.2014.716. [DOI] [PubMed] [Google Scholar]
- 34.Ghanem C.I., Perez M.J., Manautou J.E., Mottino A.D. Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016;109:119–131. doi: 10.1016/j.phrs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Wang X., Luo F., Tang X., Li K., Hu X., Bai J. Panaxatriol saponin ameliorated liver injury by acetaminophen via restoring thioredoxin-1 and pro-caspase-12. Liver Int. 2014;34:1068–1073. doi: 10.1111/liv.12329. [DOI] [PubMed] [Google Scholar]
- 36.Yoon M.Y., Kim S.J., Lee B.H., Chung J.H., Kim Y.C. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biol. Pharm. Bull. 2006;29:1618–1624. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- 37.Forouzandeh H., Azemi M.E., Rashidi I., Goudarzi M., Kalantari H. Study of the protective effect of teucrium polium l. Extract on acetaminophen-induced hepatotoxicity in mice. Iran. J. Pharm. Res. 2013;12:123–129. [PMC free article] [PubMed] [Google Scholar]
- 38.Abdeen A., Ghonim A., El-Shawarby R. Protective effect of cinnamon against cadmium-induced hepatorenal oxidative damage in rats. Int. J. Toxicol. 2017;5:17. [Google Scholar]
- 39.Abdel-Daim M.M., Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol. 2018;114:69–77. doi: 10.1016/j.fct.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 40.Hilal Ahmad M., Fatima M., Hossain M.M., Chandra Mondal A. Determination of potential oxidative damage, hepatotoxicity, and cytogenotoxicity in male Wistar rats: role of indomethacin. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22226. [DOI] [PubMed] [Google Scholar]
- 41.Kannan N., Sakthivel K.M., Guruvayoorappan C. Protective effect of Acacia nilotica (L.) against acetaminophen-induced hepatocellular damage in Wistar rats. Adv. Pharmacol. Sci. 2013 doi: 10.1155/2013/987692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govil N., Chaudhary S., Waseem M., Parvez S. Postnuclear supernatant: an in vitro model for assessing cadmium-induced neurotoxicity. Biol. Trace Elem. Res. 2012;146:402–409. doi: 10.1007/s12011-011-9263-y. [DOI] [PubMed] [Google Scholar]
- 43.Fan X., Wang L., Huang J., Lv H., Deng X., Ci X. Pterostilbene reduces acetaminophen-induced liver injury by activating the Nrf2 antioxidative defense system via the AMPK/Akt/GSK3β pathway. Cell. Physiol. Biochem. 2018;49:1943–1958. doi: 10.1159/000493655. [DOI] [PubMed] [Google Scholar]
- 44.Said S.M., Ali Husein. Hepatoprotective effect of Cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol. Res. 2009;42:93–98. [PubMed] [Google Scholar]
- 45.Wang L., Li X., Chen C. Inhibition of acetaminophen-induced hepatotoxicity in mice by exogenous thymosinbeta-4 treatment. Int. Immunopharmacol. 2018;61:20–28. doi: 10.1016/j.intimp.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Zhang F., Wang K., Liu G., Yang M., Luan Y., Zhao Z. Protective effect of allyl methyl disulfide on acetaminophen-induced hepatotoxicity in mice. Chem. Biol. Interact. 2016;249:71–77. doi: 10.1016/j.cbi.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Veilleux M.P., Grenier D. Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complement. Altern. Med. 2019;19(1):303. doi: 10.1186/s12906-019-2730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma S., Singh R.L., Kakkar P. Modulation of bax/bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem. Toxicol. 2011;49:770–779. doi: 10.1016/j.fct.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Hu J., Yan D., Gao J., Xu C., Yuan Y., Zhu R., Xiang D., Weng S., Han W., Zang G. Rhil-1ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab. Investig. 2010;90:1737–1746. doi: 10.1038/labinvest.2010.127. [DOI] [PubMed] [Google Scholar]
- 50.Dong D.L., Han Xu X. Effects of the total saponins from Rosa laevigata Michx fruit against acetaminophen induced liver damage in mice via induction of autophagy and suppression of inflammation and apoptosis. Molecules. 2014;19:7189–7206. doi: 10.3390/molecules19067189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanz A.B., Santamaría B., Ruiz-Ortega M., Egido J., Ortiz A. Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008;19(9):1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 53.Abdeen A., Abdelkader A., Abdo M., Wareth G., Aboubakr M., Aleya L., Abdel-Daim M. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. Res. - Int. 2019;26:240–249. doi: 10.1007/s11356-018-3553-2. [DOI] [PubMed] [Google Scholar]
- 54.Li G., Chen J.B., Wang C., Xu Z., Nie H., Qin X.Y., Chen X.M., Gong Q. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J. Gastroenterol. 2013;19:7440–7446. doi: 10.3748/wjg.v19.i42.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raskovic A., Bukumirovic N., Paut K.M., Milic N., Cabarkapa V., Borisev I., Capo I., Miljkovic D., Stilinovic N., Mikov M. Hepatoprotective and antioxidant potential of Pycnogenol in acetaminophen-induced hepatotoxicity in rats. Phytother. Res. 2018;33:631–639. doi: 10.1002/ptr.6251. [DOI] [PubMed] [Google Scholar]
- 56.Koca-Caliskan U., Yilmaz I., Taslidere A., Yalcin F.N., Aka C., Sekeroglu N. Cuscuta arvensis beyr “Dodder”: in vivo hepatoprotective effects against acetaminophen-induced hepatotoxicity in rats. J. Med. Food. 2018;21:625–631. doi: 10.1089/jmf.2017.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.K.W. Leung, A.S. Wong. Pharmacology of ginsenosides: a literature review. Chin Med, 5, 20. [DOI] [PMC free article] [PubMed]