Abstract
Informed consent can be defined as a freely-given decision or agreement following disclosure of relevant information. This review explores how legislation surrounding informed consent has impacted upon clinical research practices, with a focus on clinical trials involving individuals with the capacity to give consent in the non-emergency setting. We also highlight the challenges which remain with the informed consent process, including those which exist in the era of data protection legislation and genetic research.
Modern ethicists agree that informed consent encompasses three principal factors: disclosure of information, capacity for decision making, and voluntariness. In the context of clinical research, informed consent is now required by regulatory and ethical frameworks as well as by law, and various guidelines govern the practice of informed consent, including the Declaration of Helsinki and the Good Clinical Practice Guidelines. Historically, however, researchers acted paternalistically and included participants in research without their knowledge or consent. Following societal and political revolution, an autonomy model of consent became prevalent, and individuals became free to make individual choices about whether to participate. Despite this, it is also recognized that an individual's community has a role in supporting their decision making, and this may be a strong influence, particularly within some societies. Research scandals and controversies and whistle-blowers which exposed unethical practices in the area of informed consent also contributed to changes in societal attitudes and legislation changed as a result. Medical journals also have an established, although indirect, role in strengthening good practices surrounding informed consent.
Keywords: Informed consent, Clinical research, Research ethics
1. Introduction
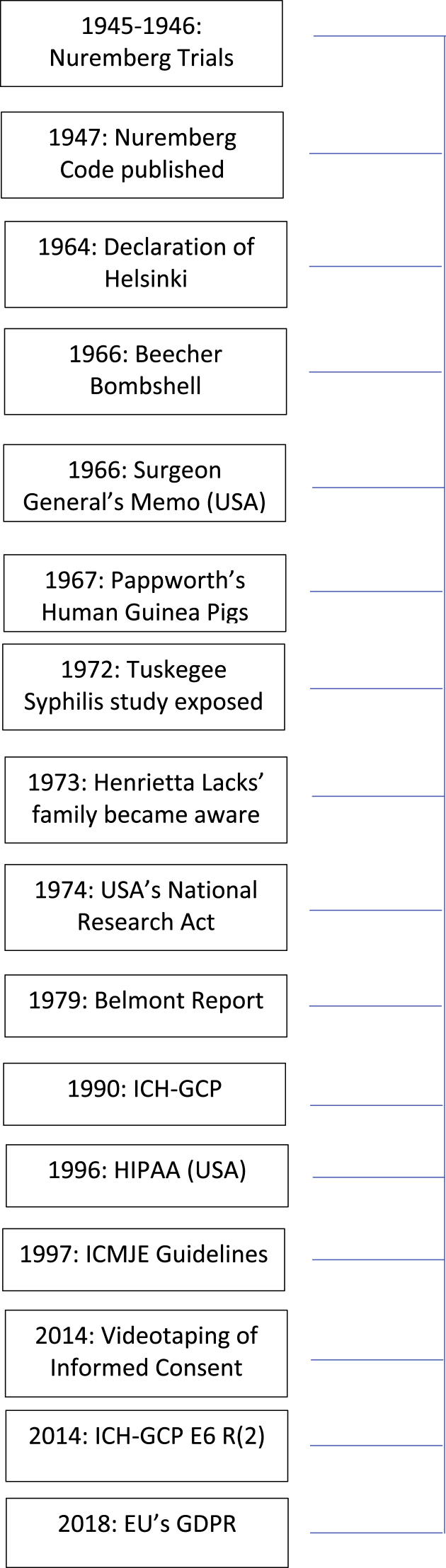
Informed consent (IC) can be defined as a freely-given decision or agreement following disclosure of all pertinent information. The International Council on Harmonisation-Good Clinical Practice (ICH-GCP) E6(R2) guidelines, the internationally recognized quality standard for the conduct of clinical research in humans, have been incorporated into law by the European Union (EU) Clinical Trial Directive 2001/20/EC and Regulation 12AD of the Therapeutic Goods Regulation 1990. The ICH-GCP E6(R2) guidelines further outline that a research participant should not be ‘coerced or unduly influenced’ [1]. Modern ethicists agree that IC depends on three factors: disclosure of information, capacity for decision making and voluntariness [2,3]. The Declaration of Helsinki (DOH) states that while family members or community leaders may support decision-making, the choice ultimately rests with the individual participant [4]. During the last 100 years, our understanding of IC has continued to evolve. Fig. 1 illustrates some of the factors which have affected IC over time, including the publication of legislation and guidance documents, and various research controversies. Recognizing the importance of IC and the evolution of the supporting frameworks, this review reflects on how societal changes have impacted on the legislation surrounding IC, and the resulting evolution in clinical research practices.
Fig. 1.
Timeline of the factors affecting informed consent in clinical research.
USA = United States of America.
ICH-GCP = International Council on Harmonisation, Good Clinical Practice.
HIPAA = Health Insurance Portability and Accountability Act.
ICMJE = International Committee of Medical Journal Editors.
EU = European Union.
GDPR = General Data Protection Regulation.
2. Paternalism
Medical paternalism can be defined as a healthcare professional making decisions on behalf of their patients, asserting that their superior knowledge allows them to act in their best interests [5]. The paternalistic model of consent assumes that the healthcare professional is acting with beneficence, promoting the patient's well-being, and non-maleficence, protecting the patient from harm. In the context of clinical research, however, there may be no direct benefit to the individual and the participant may be exposed to varying degrees of risk. Crucially, although well-intended, paternalism removes an individual's choice.
Paternalism thrived among generations who were reluctant to challenge their healthcare providers. However, the rise of consumerism and liberalism in the 1800s and 1900s prompted questioning of the established social and political authorities, including the medical establishment [6,7]. Increasingly informed and discerning patients wished to exert their autonomy, that is, to make their own healthcare choices [8], resulting in a conflict with the paternalistic mindset.
While paternalism is largely considered to be outmoded [[9], [10], [11]], some feel that the shift from beneficence and non-maleficence towards autonomy may place an undue burden on sick patients [12,13]. With the beneficence model, the onus to protect the patient's well-being lies solely with the clinician, while autonomy gives the patient responsibilities also. Jallinoja calls the patient autonomy model ‘consumer orientated’ and notes that patients may feel under pressure to understand complex information and make an ‘informed decision’ [12]. This is particularly relevant in clinical research, where participants often struggle to understand the relevant concepts and terminology [14,15]. In addition, patient's individual information needs can vary considerably [16], and the DOH affirms that these differences should be respected [4]. Drolet notes that these scenarios can be challenging for clinicians who have to understand the ‘nuances required for best practice of the profession’, to avoid being deemed negligent [17]. In the modern research setting, any attempt at coercion or not providing patients with the appropriate information is in clear violation of the ICH-GCP E6(R2) guidelines [1]. However, Deber draws a distinction between patients who may wish their clinician to carry out the ‘problem solving’ element’ (which requires scientific knowledge and clinical training), but want to be involved in the ‘decision making’ component [18].
3. Nazi experimentation
The horrific experiments perpetrated by Nazi doctors between 1933 and 1945 on concentration camps prisoners are well-known, including surgical and radiation sterilization as well as drug trials, often to test treatments for diseases with which the victims had been deliberately infected [19,20]. While consent was not sought from these victims, a distinction must be drawn between these atrocities, and paternalism. As mentioned previously, although misplaced, the basis of paternalism is to promote the well-being of patients. In contrast, the experimentation performed by Nazi doctors was solely for the advancement of science, without any regard for the rights or wishes of participants. The Nuremburg trials (1945–1946) included the conviction of 23 medical doctors in the so-called ‘Doctor's Trial’. The resulting Nuremberg Code, published in 1947, emphasizes that participation in human research must be voluntary, and must be performed with the aim of avoiding unnecessary adverse events. But as Jones points out, since the Nuremburg Code was not implemented into law, researchers were left to interpret and action the guidelines in their own way, or not at all [21].
The Nuremburg Trials also led to the publication of the DOH by the World Medical Association in 1964 [22], which was most recently updated in 2013 [4]. It describes among other important principles, that consent must be given, preferably in writing, by any human subject prior to participating in research. The DOH outlines that consent must be freely given and only after information has been provided about the ‘ … aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail … ’ [4].
However, Silverman commented in 1989 that despite the publication of the DOH and the resulting familiarity with IC, there did not appear to be much ‘meaningful implementation’ in practice [23]. This is reflected in the cynical tone of literature published at the time, in which clinicians questioned whether written consent was a ‘ritual’, without conferring any additional protection on participants [[23], [24], [25], [26]]. Herz reported a study of 106 patients who were educated by a neurosurgeon and specialist nurse prior to consenting for neurosurgery [24]. Despite significant efforts from the team, patients scored only 43.5% on a knowledge assessment, which dropped to 38.4% six weeks later [24]. Similarly, a consensus conference on controlled clinical trials in 1986 reported that the majority of clinicians felt that participants not understanding research studies hindered IC [27].
4. The ‘Bombshell’ and the ‘Whistleblower’: Beecher and Pappworth
In 1966 Henry Beecher, the head of Anesthesiology at Massachusetts General Hospital and a Harvard professor published a paper which subsequently became known as the ‘Beecher Bombshell’ [28]. The paper, which was published in the New England Journal of Medicine, cited 22 examples of unethical conduct towards clinical research participants – see Table 1. It was alleged that vulnerable or convenient groups of participants had been used: soldiers, ‘charity cases’, those described as ‘mental defectives’ or ‘juvenile delinquents’, and the elderly. Beecher was concerned by what he felt was a widespread trend towards unethical practices such as withholding proven, effective treatment, not explaining the risks of experimental treatment, and little emphasis on IC. Interestingly, Beecher felt that internal reform, rather than external regulation was needed, and that journals should exert pressure on investigators by not publishing even good quality data, if it was not obtained ethically.
Table 1.
Summary of Beecher's and Pappworth's findings.
| The ‘Beecher Bombshell’: Henry Beecher | Human Guinea Pigs: Maurice Pappworth |
|---|---|
| 22 examples of ethical misconduct to research participants including: | 14 examples of ethical misconduct to research participants including: |
|
|
| Effect: some participants developed adverse events (rheumatic fever, nephritis), some died | Effect: patient's heart stopped and patient went into shock. Patient recovered following resuscitation. |
| Alleged research misconduct: participants not fully informed | Alleged research misconduct: Vulnerable patient offered financial reward which incentivized him to take undue risk. |
|
|
| Effect: liver damage to some participants | Effect: participants exposed to unnecessary suffering (insulin withheld) and unnecessary risk (transabdominal biopsy) |
| Alleged research misconduct: participants not able to provide informed consent due to incapacity or being underage. | Alleged research misconduct: participants not fully informed. |
|
|
| Effect: not stated | Effect: not stated |
| Alleged research misconduct: participants not advised of the pertinent information | Alleged research misconduct: participants not advised of the pertinent information |
A contemporary of Beecher, Maurice Pappworth, was meanwhile pursuing a similar campaign in England. Pappworth, an outspoken clinician and medical tutor, published his accusations in the form of 14 letters and subsequently as a book, Human Guinea Pigs, in 1967 [29]. In particular, he denounced the lack of IC for clinical research participants in named British hospitals [30] – see Table 1. Pappworth's claims, including comparing British researchers to the Nazi clinicians, were widely criticized [31], but did increase awareness of these issues among the public. This exerted increased pressure on the medical establishment with regards to unethical clinical research, particularly concerning lack of IC. However, despite Pappworth's call for RECs to be established it took the publication of the Surgeon General's Memo in 1966, which required ethical review prior to receiving what is now known as the National Institutes of Health funding, to bring RECs into effect in England [32].
The Surgeon General's Memo of 1966 further changed the landscape of clinical research in the United States of America (USA) by mandating consent prior to participation and eliminating questionable practices such as only seeking participant consent if they were randomized to the intervention arm of a study [33]. The Memo was considered to be a political move, reacting to another scandal – verified reports of the injection of live cancer cells into 22 elderly patients without their consent, to test their immune systems' response [34]. Ultimately, scandals such as these provided the impetus for congressional hearings on human subject's research in the USA led by Senator Edward Kennedy [21,35]. These hearings led to the National Research Act of 1974, which outlined how IC should be sought in different clinical research settings [35]. The Act also mandated research ethics committee (REC) review for all human subject research. One role of the REC was to consider the appropriateness of the investigator's plan for IC of participants.
Silverman observed, 20 years after the Surgeon General's Memo, that written consent is fairly easy to implement and practice but queried whether a patient can ever fully understand a clinical trial, an opinion he felt was supported by contemporaneous letters to the editor, editorials etc. [23]. Silverman also queried whether it would ever be possible to carry out true IC to the satisfaction of all stakeholders and felt that the patient autonomy conflicted with the principles of beneficence and justice.
5. Research controversies
The exposure of a number of research controversies was a significant driver in the evolution of IC. One example is the Tuskegee syphilis scandal, where 600 African American men, some infected with syphilis, were enrolled onto an observational study in 1932 carried out by the American Public Health Service. Participants were told that the study would last 6 months and were promised free healthcare, food and burial in exchange for participation [36]. The study in fact lasted 40 years, participants were not informed that they had syphilis and the approved treatment, penicillin, was withheld after it became available [37,38]. This was a clear example of vulnerable participants not being fully informed prior to consent, and a dereliction of duty of the research team to safeguard their well-being, and the well-being of their partners and children. A review of the study by the Communicable Disease Centre concluded that IC was not possible in this cohort of patients due to their limited education and declining age and health. The study remained open until 1972, when it was exposed by a series of articles in the Associated Press.
Following the National Research Act of 1974 in the USA, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was established, re-enforcing the role of ethics committees in the USA. This was followed by the publication of the Belmont report in 1979, which focused on three principles: respect, beneficence and justice [39].
Another example is the case of Henrietta Lacks, whose aggressive cervical tumor cells were sampled and cultured for research purposes, without her consent [40]. Genetically modified variants of the cells were subsequently commercialized and sold in batches for $10,000 [41,42]. Henrietta's family only became aware when scientists from Johns Hopkins University Hospital requested familial blood samples in 1973 following contamination of the original cells. With the benefit of contemporary research integrity frameworks, it is easy to condemn these practices, but it was not customary at the time to obtain consent from patients to use their tissue for research purposes. An anonymous survey of 60 clinicians published in 1994 revealed that only 32% asked patients to provide written consent, and 5% did not seek consent at all [43]. Another study of 484 oncologists showed that 72% felt that IC hindered the physician-patient relationship and 87% of physicians stated IC regulations was a barrier to recruitment [44]. These data clearly show that despite cases such as that of Henrietta Lacks exposing deficiencies in research practice, there were still challenges to be overcome.
Another significant publication was the Good Clinical Practice Principles which arose from the International Council on Harmonisation (ICH) in 1990 [45] were original prepared to provide a unified standard for human research practice across the USA, Europe and Japan. The most recent update was as the E6(R2) [1] in 2017 although another amendment, E6(R3), is expected towards the end of 2021. The ICH-GCP E6(R2) guidelines, which are now legally enforced in the EU and Australia, are internationally recognized as the ethical and quality standard for research in humans, with a significant emphasis placed on IC. While mainly aimed at the investigational medicinal product trial, it is widely acknowledged that these standards should be applied to all clinical research [46,47].
Focus groups conducted in 1997 indicated a distrust among African Americans towards research [38,48], perhaps reflecting the lasting effects of the Tuskegee syphilis study. Indeed, many other disturbing examples of unethical conduct against African Americans are described in Harriet Washington's book ‘Medical Apartheid’, including those which took place early the 21st century [49]. Many of the focus group participants were concerned about the ethical conduct of clinical research, particularly among disadvantaged or minority groups [37]. A postal survey of 179 African and white Americans assessed the knowledge of respondents regarding the Tuskegee syphilis study and their level of trust in researchers [50]. The study found that 81% of African Americans and 28% of whites were aware of the Tuskegee study. Furthermore, 46% of African Americans and 34% of whites stated that this awareness would influence their participation in research in the future. Nelson also notes that while refusal rates for participation in research studies vary widely, they are highest among African Americans and minority groups [51]. This may explain why minority groups are unrepresented in research studies [52,53] and unfortunately may mean that the research and any resulting health policies are not generalizable to these groups.
6. Role of medical journals in promoting informed consent in clinical research
The DOH states that medical journals should not publish research which does not comply with the DOH guidelines for the conduct of research [4]. In 1997, the International Committee of Medical Journal Editors (ICMJE), a group of medical and biomedical journals produced a report, advocating for a transparent publication policy regarding IC and ethical approval, and clear reporting where these have taken place [54]. Olson's review of cardiopulmonary studies from the MEDLINE database in 1996 found that only 26% addressed the issue of IC, however practice improved in more recent studies [55]. A later report of 60 randomly selected clinical trial reports from six leading medical journals published before and after 1997, showed an increase from 64% to 82% in describing IC, suggesting the indirect power of medical journals to influence researcher's practices [56]. However, a recent study found that 42.9% of articles published in three leading otolaryngology journals did not specify if informed consent had taken place [57].
7. The role of the community in informed consent
Personal autonomy is enshrined in the principles of ICH-GCP E6(R2) [1]. In the context of clinical research, this means that while an individual may enlist support, the decision to participate in a research study is ultimately their own. However, Manda-Taylor suggests that an emphasis on the individual is a restrictive application of the ethical principles which govern IC [58]. Quinn similarly suggests that the current model of IC takes the atomistic view of an individual, which is perhaps not reflective of most people in society [59], since most individuals are part of a societal or family unit who support decision-making. Williams conducted 18 focus groups among two Native American tribes, Hispanic rural and urban communities and health care staff [60]. Participants were concerned that research to which individual members had consented could have negative consequences for the wider community, such as higher insurance premiums or employment discrimination. Since the original three Belmont Principles (respect, beneficence and justice) [39] focused solely on the individual, some authors suggest the addition of a fourth Belmont principle – that of respect for communities [61,62].
Levine cautions against assuming that the ‘Western’ model of consent, focusing on an individual's rights etc. can be applied to e.g. Central America, Central Africa and Asia. In fact, there may be a conflict between the Western concept of individual autonomy and the Eastern view of cooperation for the greater good [63,64]. For example in Japan, a technologically developed country, there is a societal emphasis on the responsibility to give and repay, which perhaps conflicts with the Western values of not placing undue obligation on a patient. Of 32 participants in a Phase I Japanese trial, >90% stated that they made the decision to participate with the input of family, friends or a healthcare professional [65] A similar result was found in Indian and Ugandan studies, where a reasonable proportion of respondents indicated that they would not make the decision to participate in research by themselves [66,67]. Corrigan notes that the Western IC process ignores the sociological context of the individual and points to a study done among a Tongan community where they rejected the individual consent model, pointing to their own sociological norm – i.e. consent in the family context [68]. Similarly, a proposed HIV seroprevalence study among mothers and newborns and in Tanzania had to be abandoned as the host REC (in the West) mandated individual consent and disclosure of any positive HIV results to participants [69]. However, the local authorities advised that due to the lack of medical care available for HIV, participants should not be informed about the nature of the testing nor should the results of the HIV tests be divulged.
Community Advisory Boards (CABs) have been proposed as a bridge between researchers and communities and are regarded as practice for research in developing countries, particularly to safeguard vulnerable research participants [59,70]. The concept of CABs closely aligns with the prevailing sentiment in western society that lay stakeholders should be involved with research design and conduct. This practice, often known as Patient-Public Involvement or Partnership, includes among other things, co-designing the research project, selecting meaningful endpoints, and preparation of patient-facing documents. However, it is important to note that while the DOH recognizes the role of the community in supporting an individual's decision to participate, the freely given decision rests with the participant [4].
8. Informed consent in the data protection era
The Health Insurance Portability and Accountability Act (HIPPA), published in the USA in 1996 put in place, among other things, additional protection of a patient's privacy. Similarly, the European General Data Protection legislation which came into force in May 2018 requires that consent must be ‘freely given, specific and informed’ and ‘unambiguous’ [71]. However, some researchers feel that the addition of even more complex information about data processing and storage presents an additional barrier to a participant's understanding [[72], [73], [74]]. A systematic review identified six studies exploring the relationship between the quantity of information provided to patients and on willingness to participate in clinical research. Four of the six studies indicated that increased information was linked to a lower rate of consent [75]. While this may mean a lower uptake rate in the post-paternalism era, the quality of consent may be higher, ultimately resulting in lower attrition rates and therefore less missing data. However, there does appear to be an inconsistency between the information which must be provided per legal requirements and what patients would like to know. Sand's semi-structured interviews found that half of the 22 oncology patients felt it was unnecessary for them to read information about data retention, confidentiality, and insurance [76]. The HIPPA Privacy Rule, published in the USA in 2003, aimed to protect individual's rights regarding their health information [77]. However, since researchers must gain consent prior to accessing each person's medical record, some research organizations warn that the Rule may introduce selection bias, and thus limiting the generalizability of the trial data [78].
Following an allegation raised by a non-governmental organization that participants were not adequately informed about the nature and risks of clinical trials videotaping of the consent discussion for all participants of clinical trials became a legal regulatory requirement in India in 2014 [79]. An amendment was published in 2015 which allows for an audio recording for leprosy and HIV trials, for privacy reasons. However, Chauhan found that 34% of 150 potential participants in rural India declined to participate because they did not wish to be videotaped [80]. Therefore, an effort to safeguard patients may have in fact resulted in diminished choice.
9. Informed consent for genomic research
Genomics research, undoubtedly continuing to give hope to both researchers and patients, challenges the autonomy model of consent. As previously mentioned, autonomous informed consent relies on the provision of the relevant information, participant capacity to give consent and voluntariness. However, the complexities and uncertainties of genetic research may challenge all three of these components [81]. The end results of exploratory genomic research can be uncertain and difficult to predict, making it difficult for researchers to disclose the full information to participants [82]. Some researchers also question the competence of most patients to fully understand the complexities of genomic research [81,83]. With whole genome or exome sequencing, there is also the possibility of incidental findings, which can lead to an ethical dilemma, especially if the significance of these findings is unclear [84]. In addition, given the results of such research may unavoidably have implications for biological family members of the research participant, so individuals who have not provided consent for genomic testing may also be affected [81]. Some authors have suggested a ‘tiered’ approach, where the individual consenting is offered a ‘menu’ of options to which they can agree or decline [85]. While this approach is considered by some to be best practice [86], there is some evidence that the additional choice, combined with already very complex information, may increase anxiety levels in participants [87].
10. Challenges which remain with the informed consent process
Despite progressive research regulations and guidelines, challenges remain with the IC process. Firstly, documentation of IC has traditionally relied on the patient information leaflet and consent form. However, there is evidence indicating that these documents are becoming longer and more complex [[88], [89], [90]], with Berger noting they have doubled in length in 20 years [91]. Tam's systematic review of studies during a 30 year period (up to 2013) concluded that participant's understanding of risks, side effects, placebo and the right to withdraw has not improved [92]. Participants most at risk of knowledge deficits are older, sicker patients, and those with a lower educational background, or residing in a low-income country [[92], [93], [94]]. It also appears that those facilitating the IC process receive limited or indeed no training [90,95,96], and it is likely that this impacts on the participant's experience.
Funders and publishers are increasingly encouraging the sharing of data for use in secondary analyses [97]. While this is a cost-effective use of valuable resources, it presents a challenge to explicit consent given by a participant for a specified purpose. While the trial consent form can allow individuals to provide consent to be contacted at a later stage or for their data to be anonymized and shared with other researchers, it is can difficult to predict the potential for secondary analysis at the beginning of the trial.
Various interventions have been proposed to optimize the IC process, including the use of an enhanced consent form or decision aids, extended consent discussions, and incorporating technologies [[98], [99], [100], [101], [102], [103], [104], [105], [106]]. Various tools have also been developed to evaluate how well patients have understood a research study [105,107,108]. However, there is no conclusive empirical evidence regarding whether the above interventions improve the quality of IC.
11. Conclusion
Clinicians formerly adopted a paternalistic mindset towards clinical research participants, but autonomous decision-making is now accepted. Society's perception of valid IC for clinical research has evolved significantly over the years and this is reflected in changes in global legislation. However, there is often a discrepancy between legislation and the implementation into clinical practice. While it has been established that individual IC is required for all clinical research participants, difficulties exist in achieving true informed consent, particularly given the ever increasing quantity and complexity of information, and the challenges posed by the movement towards data sharing. More empirical research is required to establish if alternative strategies can be employed to improve the quality of consent for research participants.
Funding statement
This work was supported by the Health Research Board Trials Methodology Research Network (HRB-TMRM) Ireland, as part of the HRB-TMRN-2017-1 grant.
Author contributions
Lydia O’Sullivan: Conceptualization, Methodology, Project administration, Writing – original draft and editing.
Rachel Crowley: Conceptualization, Writing – review and editing.
Éilish McAuliffe: Conceptualization, Writing – review and editing.
Peter Doran: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Declaration of competing interest
The authors have no competing interests.
Acknowledgements
The research team is grateful to the Health Research Board-Trials Methodology Research Network (HRB-TMRN) for their support.
References
- 1.International Council on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use, Good Clinical Practice Guidelines E(6) R(2) World Health Organization; 2016. [Google Scholar]
- 2.Cahana A., Hurst S.A. Voluntary informed consent in research and clinical care: an update. Pain Pract. 2008;8(6):446–451. doi: 10.1111/j.1533-2500.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta U.C. Informed consent in clinical research: revisiting few concepts and areas. Perspect. Clin. Res. 2013;4(1):26–32. doi: 10.4103/2229-3485.106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Declaration of Helsinki, World Medical Organisation, Helsinki, Finland. 2013. [Google Scholar]
- 5.Buchanan D.R. Autonomy, paternalism, and justice: ethical priorities in public health. Am. J. Publ. Health. 2008;98(1):15–21. doi: 10.2105/AJPH.2007.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Häyry H. Routledge; 2002. The Limits of Medical Paternalism. [Google Scholar]
- 7.Weiss G.B. Paternalism modernised. J. Med. Ethics. 1985;11(4):184–187. doi: 10.1136/jme.11.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Hara G. The complexities of ‘consumerism’: choice, collectivism and participation within Britain's National health Service, c.1961–c.1979. Soc. Hist. Med. 2012;26(2):288–304. doi: 10.1093/shm/hks062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman A. 1980. The Refutation of Medical Paternalism, the Moral Foundations of Professional Ethics. [Google Scholar]
- 10.Matthews E. Can paternalism be modernised? J. Med. Ethics. 1986;12(3):133. doi: 10.1136/jme.12.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cody W.K. Paternalism in nursing and healthcare: central issues and their relation to theory. Nurs. Sci. Q. 2003;16(4):288–296. doi: 10.1177/0894318403257170. [DOI] [PubMed] [Google Scholar]
- 12.Jallinoja P. Genetic screening in maternity care: preventive aims and voluntary choices. Sociol. Health Illness. 2001;23(3):286–307. [Google Scholar]
- 13.Levy N. Forced to be free? Increasing patient autonomy by constraining it. J. Med. Ethics. 2014;40(5):293. doi: 10.1136/medethics-2011-100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Featherstone K., Donovan J.L. Why don't they just tell me straight, why allocate it?" the struggle to make sense of participating in a randomised controlled trial. Soc. Sci. Med. 2002;55(5):709–719. doi: 10.1016/s0277-9536(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 15.Kelly C.M., Feighery R., McCaffrey J., Higgins M., Smith M., O'Reilly S., Murphy C., Horgan A., Walshe J., McDermott R., O'Donnell D., Morris P.G., Keane M., Martin M., Duffy K., Mihai A., Armstrong J., Mulroe E., Murphy V., Kelly C. 1465P_PRDo oncology patients understand clinical trials? A nationwide study by Cancer Trials Ireland. Ann. Oncol. 2017;28(suppl_5) [Google Scholar]
- 16.Kirkby H.M., Calvert M., Draper H., Keeley T., Wilson S. What potential research participants want to know about research: a systematic review. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drolet B.C., White C.L. Selective paternalism, AMA. J. Ethics. 2012;14(7):582–588. doi: 10.1001/virtualmentor.2012.14.7.oped2-1207. [DOI] [PubMed] [Google Scholar]
- 18.Deber R.B. Physicians in health care management: 8. The patient-physician partnership: decision making, problem solving and the desire to participate. CMAJ (Can. Med. Assoc. J.) 1994;151(4):423. [PMC free article] [PubMed] [Google Scholar]
- 19.Final Report: Advisory Committee on Human Radiation Experiments. ACHE: Advisory Committee on Human Radiation Experiments; Washington DC, USA: 1995. [Google Scholar]
- 20.Kolb S., Weindling P., Roelcke V., Seithe H. Apologising for Nazi medicine: a constructive starting point. Lancet. 2012;380(9843):722–723. doi: 10.1016/s0140-6736(12)61396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D.S., Grady C., Lederer S.E. “Ethics and clinical research” — the 50th anniversary of Beecher's Bombshell. N. Engl. J. Med. 2016;374(24):2393–2398. doi: 10.1056/NEJMms1603756. [DOI] [PubMed] [Google Scholar]
- 22.Declaration of Helsinki. World Medical Association; Helsinki, Finland: 1964. [Google Scholar]
- 23.Silverman W.A. The Myth of informed consent: in daily practice and in clinical trials. J. Med. Ethics. 1989;15(1):6–11. doi: 10.1136/jme.15.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herz D.A., Looman J.E., Lewis S.K. Informed consent: is it a Myth? Neurosurgery. 1992;30(3):453–458. doi: 10.1227/00006123-199203000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Verheggen F.W., Van Wijmen F.C. Myth and reality of informed consent in clinical trials. Med. Lab. 1997;16:53. [PubMed] [Google Scholar]
- 26.Dyckman J. The Myth of informed consent: an analysis of the Doctrine of informed consent and its (Mis) application in HIV experiments on pregnant women in developing countries. Columbia J. Gend. Law. 1999;9:91. [PubMed] [Google Scholar]
- 27.Blum A.L., Chalmers T.C., Deutsch E., Koch-Weser J., Rosen A., Tygstrup N., Zentgraf R. The Lugano statements on controlled clinical trials. J. Int. Med. Res. 1987;15(1):2–22. doi: 10.1177/030006058701500102. [DOI] [PubMed] [Google Scholar]
- 28.Beecher H.K. Ethics and clinical research. N. Engl. J. Med. 1966;274(24):1354–1360. doi: 10.1056/NEJM196606162742405. [DOI] [PubMed] [Google Scholar]
- 29.Pappworth M.H. Routledge & K. Paul; London: 1967. Human guinea Pigs: Experimentation on Man. [Google Scholar]
- 30.Hazelgrove J. The old faith and the new science: the Nuremberg Code and human experimentation ethics in Britain, 1946–73. Soc. Hist. Med. 2002;15(1):109–135. doi: 10.1093/shm/15.1.109. [DOI] [PubMed] [Google Scholar]
- 31.Seldon J. The University of Buckingham Press; Buckingham, UK: 2017. The Whisleblower: the Life of Maurice Pappworth:the Story of One Man's Battle against the Medical Establishment. [Google Scholar]
- 32.Hedgecoe A. A form of practical machinery": the origins of Research Ethics Committees in the UK. Med. Hist. 2009;53(3):331–350. doi: 10.1017/s0025727300000211. 1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart W.H. United States of America; 1966. Clinical Investigations Using Human Beings. [Google Scholar]
- 34.Mulford R.D. Experimentation on human beings. Stanford Law Rev. 1967;20(1):99–117. [Google Scholar]
- 35.Stark L. The unintended ethics of Henry K Beecher. Lancet. 2016;387(10036):2374–2375. doi: 10.1016/S0140-6736(16)30743-7. [DOI] [PubMed] [Google Scholar]
- 36.Brandt A.M. Racism and research: the case of the Tuskegee syphilis study. Hastings Cent. Rep. 1978;8(6):21–29. [PubMed] [Google Scholar]
- 37.Corbie-Smith G., Thomas S.B., Williams M.V., Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J. Gen. Intern. Med. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbie-Smith G. The continuing legacy of the Tuskegee syphilis study: considerations for clinical investigation. Am. J. Med. Sci. 1999;317(1):5–8. doi: 10.1097/00000441-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research, Report of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, United States. Department of Health, Education, and Welfare; 1979. [PubMed] [Google Scholar]
- 40.Skloot R. Pan Books; London: 2011. The Immortal Life of Henrietta Lacks. [Google Scholar]
- 41.Lucey B.P., Nelson-Rees W.A., Hutchins G.M. Henrietta lacks, HeLa cells, and cell culture contamination. Arch. Pathol. Lab Med. 2009;133(9):1463–1467. doi: 10.5858/133.9.1463. [DOI] [PubMed] [Google Scholar]
- 42.Truog R.D., Kesselheim A.S., Joffe S. Paying patients for their tissue: the legacy of Henrietta lacks. Science. 2012;337(6090):37. doi: 10.1126/science.1216888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams C.J., Zwitter M. Informed consent in European multicentre randomised clinical trials--are patients really informed? Eur. J. Canc. 1994;30a(7):907–910. doi: 10.1016/0959-8049(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 44.Taylor K.M., Kelner M. Interpreting physician participation in randomized clinical trials: the physician orientation profile. J. Health Soc. Behav. 1987;28(4):389–400. [PubMed] [Google Scholar]
- 45.International Council on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use, Good Clinical Practice Guidelines. World Health Organization; Brussels, Belgium: 1990. [Google Scholar]
- 46.Dixon J.R. The international conference on harmonisation: good clinical practice. Qual. Assur. 1999;6(2):65–74. doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 47.Verma K. Base of a research: good clinical practice in clinical trials. J. Clin. Trials. 2013;3(1):100–128. [Google Scholar]
- 48.Freimuth V.S., Quinn S.C., Thomas S.B., Cole G., Zook E., Duncan T. African Americans' views on research and the Tuskegee Syphilis study. Soc. Sci. Med. 2001;52(5):797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 49.H.A. Washington, Medical Apartheid: the Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present, Doubleday Books2006.
- 50.Shavers V.L., Lynch C.F., Burmeister L.F. Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. J. Natl. Med. Assoc. 2000;92(12):563–572. [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson R.M., Merz J.F. Voluntariness of consent for research: an empirical and conceptual review. Med. Care. 2002;40(9):V69–V80. doi: 10.1097/01.MLR.0000023958.28108.9C. [DOI] [PubMed] [Google Scholar]
- 52.Redwood S., Gill P.S. Under-representation of minority ethnic groups in research--call for action. Br. J. Gen. Pract. : J. Roy. Coll. Gen. Pract. 2013;63(612):342–343. doi: 10.3399/bjgp13X668456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H.-h., Coker A.D. Examining issues affecting African American participation in research studies. J. Black Stud. 2010;40(4):619–636. [Google Scholar]
- 54.Recommendations for the Conduct, reporting, editing, and publication of scholarly work in medical journals. www.icmje.org/index.html Accessed 10th January 2020. [PubMed]
- 55.Olson C.M., Jobe K.A. Reporting approval by research ethics committees and subjects' consent in human resuscitation research. Resuscitation. 1996;31(3):255–263. doi: 10.1016/0300-9572(95)00928-0. [DOI] [PubMed] [Google Scholar]
- 56.Yank V., Rennie D. Reporting of informed consent and ethics committee approval in clinical trials. J. Am. Med. Assoc. 2002;287(21):2835–2838. doi: 10.1001/jama.287.21.2835. [DOI] [PubMed] [Google Scholar]
- 57.Murphy S., Nolan C., O'Rourke C., Fenton J.E. The reporting of research ethics committee approval and informed consent in otolaryngology journals. Clin. Otolaryngol. 2015;40(1):36–40. doi: 10.1111/coa.12320. [DOI] [PubMed] [Google Scholar]
- 58.Manda-Taylor L. Establishing community advisory boards for clinical trial research in Malawi: engendering ethical conduct in research. Malawi Med. J. : J. Med. Assoc. Malawi. 2013;25(4):96–100. [PMC free article] [PubMed] [Google Scholar]
- 59.Quinn S.C. Protecting human subjects: the role of community advisory boards. Am. J. Publ. Health. 2004;94(6):918. doi: 10.2105/ajph.94.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams R.L., Willging C.E.P., Quintero G.P., Kalishman S.P., Sussman A.L.P., Freeman W.L.M.D., On behalf of RIOS Net Members Ethics of health research in communities: perspectives from the Southwestern United States. Ann. Fam. Med. 2010;8(5):433–439. doi: 10.1370/afm.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gostin L. Ethical principles for the conduct of human subject research: population-based research and ethics. Law Med. Health Care. 1991;19(3–4):191–201. doi: 10.1111/j.1748-720x.1991.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 62.Weijer C. Protecting communities in research: philosophical and pragmatic challenges. Camb. Q. Healthc. Ethics. 1999;8(4):501–513. doi: 10.1017/s0963180199004120. [DOI] [PubMed] [Google Scholar]
- 63.Hofstede G., Hofstede G.J. 2005. Cultures and Organizations: Software of the Mind (Revised and Expanded 2nd ed.), New York. [Google Scholar]
- 64.Nijhawan L.P., Janodia M.D., Muddukrishna B.S., Bhat K.M., Bairy K.L., Udupa N., Musmade P.B. Informed consent: issues and challenges. J. Adv. Pharm. Technol. Res. (JAPTR) 2013;4(3):134–140. doi: 10.4103/2231-4040.116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itoh K., Sasaki Y., Fujii H., Ohtsu T., Wakita H., Igarashi T., Abe K. Patients in phase I trials of anti-cancer agents in Japan: motivation, comprehension and expectations. Br. J. Canc. 1997;76(1):107–113. doi: 10.1038/bjc.1997.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loue S., Okello D., Kawuma M. Research bioethics in the Ugandan context: a program summary. J. Law Med. Ethics. 1996;24(1):47–53. doi: 10.1111/j.1748-720x.1996.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 67.DeCosta A., D'Souza N., Krishnan S., Chhabra M.S., Shihaam I., Goswami K. Community based trials and informed consent in rural north India. J. Med. Ethics. 2004;30(3):318–323. doi: 10.1136/jme.2002.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corrigan O. Empty ethics: the problem with informed consent. Sociol. Health Illness. 2003;25(7):768–792. doi: 10.1046/j.1467-9566.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 69.Christakis N.A. Ethics are local: engaging cross-cultural variation in the ethics for clinical research. Soc. Sci. Med. 1992;35(9):1079–1091. doi: 10.1016/0277-9536(92)90220-k. [DOI] [PubMed] [Google Scholar]
- 70.Strauss R.P., Sengupta S., Quinn S.C., Goeppinger J., Spaulding C., Kegeles S.M., Millett G. The role of community advisory boards: involving communities in the informed consent process. Am. J. Publ. Health. 2001;91(12):1938–1943. doi: 10.2105/ajph.91.12.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clarke N., Vale G., Reeves E.P., Kirwan M., Smith D., Farrell M., Hurl G., McElvaney N.G. GDPR: an impediment to research? Ir. J. Med. Sci. 2019:72–74. doi: 10.1007/s11845-019-01980-2. [DOI] [PubMed] [Google Scholar]
- 72.Kulynych J., Korn D. The new HIPAA (health insurance portability and accountability act of 1996) medical privacy Rule. Circulation. 2003;108(8):912–914. doi: 10.1161/01.CIR.0000080642.35380.50. [DOI] [PubMed] [Google Scholar]
- 73.Shalowitz D., Wendler D. Informed consent for research and authorization under the health insurance portability and accountability act privacy Rule: an integrated Approach HIPAA Authorization and informed consent. Ann. Intern. Med. 2006;144(9):685–688. doi: 10.7326/0003-4819-144-9-200605020-00012. [DOI] [PubMed] [Google Scholar]
- 74.Cohen E.P. HIPAA threatens clinical research. Ann. Diagn. Pathol. 2008;12(5):311–312. doi: 10.1016/j.anndiagpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edwards S.J., Lilford R.J., Braunholtz D.A., Jackson J.C., Hewison J., Thornton J. Ethical issues in the design and conduct of randomised controlled trials. Health Technol. Assess. 1998;2(15) i-vi, 1-132. [PubMed] [Google Scholar]
- 76.Sand K., Loge J.H., Berger O., Gronberg B.H., Kaasa S. Lung cancer patients' perceptions of informed consent documents. Patient Educ. Counsel. 2008;73(2):313–317. doi: 10.1016/j.pec.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Health Portability and Accountability Act Privacy Rule. United States of America; 2003. [Google Scholar]
- 78.Nass S. The value, importance, and oversight of health research. In: Nass S.J., Levit L.A., Gostin L.O., editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health through Research. Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: the HIPAA Privacy Rule. National Academies Press (US); Washington (DC): 2009. [PubMed] [Google Scholar]
- 79.Gowri S., Kannan S. Audio-visual recording of obtaining informed consent: mandatory for clinical trials. Indian J. Dent. Res. 2015;26(3):333. doi: 10.4103/0970-9290.162887. [DOI] [PubMed] [Google Scholar]
- 80.Chauhan R.C., Purty A.J., Singh N. Consent for audio-video recording of informed consent process in rural South India. Perspect. Clin. Res. 2015;6(3):159. doi: 10.4103/2229-3485.159941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horton R., Lucassen A. Consent and autonomy in the genomics era. Curr. Gene. Med. Rep. 2019;7(2):85–91. doi: 10.1007/s40142-019-00164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiss K.M. Genetic pointillism versus physiological form. Perspect. Biol. Med. 2018;61(4):503–516. doi: 10.1353/pbm.2018.0060. [DOI] [PubMed] [Google Scholar]
- 83.Reiff M., Bernhardt B.A., Mulchandani S., Soucier D., Cornell D., Pyeritz R.E., Spinner N.B. What does it mean?": uncertainties in understanding results of chromosomal microarray testing. Genet. Med. 2012;14(2):250–258. doi: 10.1038/gim.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Courbier S., Dimond R., Bros-Facer V. Share and protect our health data: an evidence based approach to rare disease patients' perspectives on data sharing and data protection - quantitative survey and recommendations. Orphanet J. Rare Dis. 2019;14(1):175. doi: 10.1186/s13023-019-1123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ram N. Tiered consent and the Tyranny of choice. Jurimetrics. 2007;(3):253–284. [Google Scholar]
- 86.Eiseman E., Bloom G., Brower J., Clancy N., Olmsted S.S. Rand Corporation; 2003. Case Studies of Existing Human Tissue Repositories:" Best Practices" for a Biospecimen Resource for the Genomic and Proteomic Era. [Google Scholar]
- 87.Schwartz B., Ward A. 2004. Doing Better but Feeling Worse: the Paradox of Choice, Positive Psychology in Practice; pp. 86–104. [Google Scholar]
- 88.Hammerschmidt D., Keane M. Institutional review board (IRB) review lacks impact on the readability of consent forms for research. Am. J. Med. Sci. 1992;304(6):348–351. doi: 10.1097/00000441-199212000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Beardsley E., Jefford M., Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. United States. 2007:e13–e14. doi: 10.1200/JCO.2006.10.3341. [DOI] [PubMed] [Google Scholar]
- 90.Grady C. Enduring and emerging challenges of informed consent. N. Engl. J. Med. 2015;372(9):855–862. doi: 10.1056/NEJMra1411250. [DOI] [PubMed] [Google Scholar]
- 91.Berger O., Grønberg B.H., Sand K., Kaasa S., Loge J.H. The length of consent documents in oncological trials is doubled in twenty years. Ann. Oncol. 2008;20(2):379–385. doi: 10.1093/annonc/mdn623. [DOI] [PubMed] [Google Scholar]
- 92.Tam N.T., Huy N.T., Thoa le T.B., Long N.P., Trang N.T., Hirayama K., Karbwang J. Participants' understanding of informed consent in clinical trials over three decades: systematic review and meta-analysis. Bull. World Health Organ. 2015;93(3) doi: 10.2471/BLT.14.141390. 186-98h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schaeffer M.H., Krantz D.S., Wichman A., Masur H., Reed E., Vinicky J.K. The impact of disease severity on the informed consent process in clinical research. Am. J. Med. 1996;100(3):261–268. doi: 10.1016/S0002-9343(97)89483-1. [DOI] [PubMed] [Google Scholar]
- 94.Sustersic M., Gauchet A., Foote A., Bosson J.L. How best to use and evaluate Patient Information Leaflets given during a consultation: a systematic review of literature reviews. Health Expect. 2017;20(4):531–542. doi: 10.1111/hex.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boden-Albala B., Carman H., Southwick L., Parikh Nina S., Roberts E., Waddy S., Edwards D. Examining barriers and practices to recruitment and retention in stroke clinical trials. Stroke. 2015;46(8):2232–2237. doi: 10.1161/STROKEAHA.114.008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nusbaum L., Douglas B., Damus K., Paasche-Orlow M., Estrella-Luna N. Communicating risks and benefits in informed consent for research: a qualitative study. Glob. Qual. Nurs. Res. 2017;4 doi: 10.1177/2333393617732017. 2333393617732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taichman D.B., Sahni P., Pinborg A., Peiperl L., Laine C., James A., Hong S.-T., Haileamlak A., Gollogly L., Godlee F. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Jama. 2017;317(24):2491–2492. doi: 10.1001/jama.2017.6514. [DOI] [PubMed] [Google Scholar]
- 98.Edwards S.J., Lilford R.J., Thornton J., Hewison J. Informed consent for clinical trials: in search of the "best" method. Soc. Sci. Med. 1998;47(11):1825–1840. doi: 10.1016/s0277-9536(98)00235-4. [DOI] [PubMed] [Google Scholar]
- 99.Flory J., Emanuel E. Interventions to improve research participants' understanding in informed consent for ResearchA systematic review. J. Am. Med. Assoc. 2004;292(13):1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 100.Ryan R.E., Prictor M.J., McLaughlin K.J., Hill S.J. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst. Rev. 2008;1 doi: 10.1002/14651858.CD003717.pub2. [DOI] [PubMed] [Google Scholar]
- 101.Nishimura A., Carey J., Erwin P.J., Tilburt J.C., Murad M.H., McCormick J.B. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med. Ethics. 2013;14(1):28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinnersley P., Phillips K., Savage K., Kelly M.J., Farrell E., Morgan B., Whistance R., Lewis V., Mann M.K., Stephens B.L., Blazeby J., Elwyn G., Edwards A.G. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst. Rev. 2013;7 doi: 10.1002/14651858.CD009445.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Synnot A., Ryan R., Prictor M., Fetherstonhaugh D., Parker B. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst. Rev. 2014;5 doi: 10.1002/14651858.CD003717.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farrell E.H., Whistance R.N., Phillips K., Morgan B., Savage K., Lewis V., Kelly M., Blazeby J.M., Kinnersley P., Edwards A. Systematic review and meta-analysis of audio-visual information aids for informed consent for invasive healthcare procedures in clinical practice. Patient Educ. Counsel. 2014;94(1):20–32. doi: 10.1016/j.pec.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Gillies K., Cotton S.C., Brehaut J.C., Politi M.C., Skea Z. Decision aids for people considering taking part in clinical trials. Cochrane Database Syst. Rev. 2015;11 doi: 10.1002/14651858.CD009736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McWhirter R.E., Eckstein L. Moving forward on consent practices in Australia. J. bioeth. Inq. 2018;15(2):243–257. doi: 10.1007/s11673-018-9843-z. [DOI] [PubMed] [Google Scholar]
- 107.Joffe S., Cook E.F., Cleary P.D., Clark J.W., Weeks J.C. Quality of informed consent: a new measure of understanding among research subjects. J. Natl. Cancer Inst. 2001;93(2):139–147. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 108.Prentice K.J., Appelbaum P.S., Conley R.R., Carpenter W.T. Maintaining informed consent validity during lengthy research protocols. Irb. 2007;29(6):1–6. [PubMed] [Google Scholar]