Abstract
Studies provide evidence that practicing meditation enhances neural plasticity in reward processing areas of brain. No studies till date, provide evidence of such changes in Rajyoga meditation (RM) practitioners. The present study aimed to identify grey matter volume (GMV) changes in reward processing areas of brain and its association with happiness scores in RM practitioners compared to non-meditators. Structural MRI of selected participants matched for age, gender and handedness (n = 40/group) were analyzed using voxel-based morphometric method and Oxford Happiness Questionnaire (OHQ) scores were correlated. Significant increase in OHQ happiness scores were observed in RM practitioners compared to non-meditators. Whereas, a trend towards significance was observed in more experienced RM practitioners, on correlating OHQ scores with hours of meditation experience. Additionally, in RM practitioners, higher GMV were observed in reward processing centers—right superior frontal gyrus, left inferior orbitofrontal cortex (OFC) and bilateral precuneus. Multiple regression analysis showed significant association between OHQ scores of RM practitioners and reward processing regions right superior frontal gyrus, left middle OFC, right insula and left anterior cingulate cortex. Further, with increasing hours of RM practice, a significant positive association was observed in bilateral ventral pallidum. These findings indicate that RM practice enhances GMV in reward processing regions associated with happiness.
Subject terms: Cognitive neuroscience, Reward, Social behaviour
Introduction
An important aspect of human life is the subjective experiences of happiness1,2. Happiness necessarily does not merely depend on wealth and material possessions3,4, but a kind of subjective feeling of positive emotions linked to pleasure and pleasant feelings. It is challenging to define happiness because of its subjective nature. It can be conceptualized by two general approaches, hedonic and eudaimonic. Hedonic approach importantly focusses on affective components such as positive emotions and pleasure, whereas the eudaimonic approach mainly focuses on personal well-being that includes concepts of personal growth, purpose in life and sense of autonomy. Individual happiness depends on the coherence functions of both approaches5,6. Happiness plays a crucial role in positive psychology and the fundamental goal of many individuals7, which can be achieved by practicing meditation regularly. Meditation awakens the individual’s inner consciousness and allows them to remain in conscious attention and positive state of mind despite fluctuating experiences of the external environment at any given moment. This helps to achieve happiness.
Studies on happiness by psychologists have helped to identify various features of happiness, whereas investigations by neuroscientists have identified the functional neuroanatomy of reward and pleasure together, providing a comprehensive insight into happiness8. Happiness is most relevant to reward and pleasure that are multifaceted psychological concepts. The process within the phenomenon of reward consists of motivation (wanting), learning and affect (happiness—pleasure liking)5. Physiological, pharmacological, and behavioral studies confirm that dopamine secreting regions in the nucleus accumbens (NAcc) located in the ventral striatum (VS) and ventral tegmental area (VTA) of the brain play a central role in reward9. The NAcc is known as ‘‘pleasure center’’ in the brain and is known to generate positive hedonic states regardless of any source of pleasure-inducing stimuli such as monetary reward, social reward, food, water, sex, drugs, etc. It involves the regulation of subjective and behavioral aspects of pleasure and reward. Further, activation of NAcc helps one to be in joyful states, achieved by repeated contemplative practices10. The dopamine secreting neurons in these regions play an essential role in positive emotions by mediating pleasure and positive reinforcement11,12. Whereas in a study by Pecina et al. 2006, opioid secreting neurons have been reported to be responsible for regulating pleasure in animals13. Though NAcc plays an important role in reward processing, it is also involved in modulating behavior to aversive and painful stimuli14.
Recent studies on reward centers emphasize that there are more extensive brain areas involved in the reward process apart from VTA and NAcc. Research studies on both animals and humans show that VTA is activated when reward is obtained. This center further innervates the NAcc, and the orbitofrontal cortex (OFC). Activation in medial OFC is associated with subjective reports of pleasure for musical, olfactory, and gustatory stimuli, at the time of a reward15. By using state of the art of neuro-imaging technologies like positron emission tomography and functional magnetic resonance imaging (MRI), various other regions, including cortical and subcortical regions, were identified to be involved in reward processing in the human brain. The cortical regions include OFC, cingulate cortex, medial prefrontal cortex (mPFC), and insula. The subcortical regions consist of the NAcc, ventral pallidum (VP), substantia nigra (SN), and VTA. These areas are also called “hedonic hotspots” which form reward-related circuitry in the brain5,8. These identifications reveal that the cortico-basal ganglia network is an integral part in reward circuit which is a central component for developing and monitoring motivated behavior. Besides, the limbic structure such as the hippocampus and amygdala send inputs to the VS and takes part in reward processing. VP plays a vital role in integrating the GABA, glutamate, opioid, and dopamine signals from NAcc, striatum, amygdala, and prefrontal cortex9. VP involves in learning, motivation, and reward through mesocorticolimbic circuits16. A study on SOHAM meditation suggests that higher grey matter density in VP is associated with reward processing and positive motivation17. Apart from these cortical and sub-cortical regions that involve in reward process, the precuneus also engages in different other processing such as reward-based decision-making task. It is a key structure in the default mode network that exhibits task-dependent connectivity within this network and right frontoparietal network18,19. Several studies reported the importance of precuneus, which has a role in the subjective experience of happiness, including emotional and cognitive components and subjective well-being1,2,20,21.
The term Raja yoga has been described in many scripts and texts including the Bhagavat Gita, and books rewritten by Yoga Sutra of Patanjali, Raja Yoga by Swami Vivekananda etc. In this manuscript Rajyoga meditation (RM) refers to Brahmakumaris school of thought which describes the basis for this type of meditation. RM is different from other types of meditation as it is an open-eye meditation technique. Most of the mediation techniques are closed-eye meditation practice focusing on their thoughts or count breathing, or chanting mantra, or remaining silent, etc. In RM practice, a person is instructed to realize himself/herself as self/soul (an eternal form of point of light) situated between the eyebrows while gazing on a meaningful external symbol (a point of light that is considered as symbolic representation of Supreme Soul/God)22. Also they are instructed to think positively about the innate qualities such as peace, love, bliss, etc. which are latent within the self23,24. There are very few studies published on RM, particularly in the field of neurocognitive research. A study by Sharma, K 2018, using electroencephalography (EEG), observed alpha and theta wave pattern in frontal and parietal areas of the brain that involves in the processes of regulation of selective and sustained attention for emotional and cognitive processing in long-term RM practitioners during meditation23. In another EEG study, enhanced lower alpha power in EEG recording was observed in the long-term as well as a short-term meditation practice. It suggests that the RM practitioners can move into a stable state of meditation within a few minutes irrespective of their meditation training. However, long-term practitioners can attain this state quicker than short-term meditators under varying external conditions. Hence RM practice, being an open-eye meditation technique, has potential applications in daily life25. A combined EEG and functional MRI study by Panda 2016, has provided the first evidence on the mental state during rest and meditative state of RM practitioners by analyzing on spatial extents and temporal dynamics of the default mode network26. These studies in RM practitioners suggest that the open-eye meditation technique plays a potential role in daily life and influences the brain regions that are mentioned in various other types of meditation techniques.
There are no studies have reported the impact of RM practice on inducing changes in reward processing areas of brain that are associated with subjective states of happiness. Therefore, we hypothesize that RM practice will induce grey matter volume (GMV) changes in brain regions that process reward and happiness and more years of RM experience will induce more GMV changes in brain regions that process reward. Thus, the aim of the present study was to identify RM induced neural changes in the brain regions that are involved in reward processing and its association with individual happiness scores measured by using Oxford happiness questionnaire (OHQ). To test our hypothesis, ROI masks created were used for the brain regions involved in reward process and happiness by applying threshold free cluster enhancement (TFCE) approach.
Results
Analysis of OHQ
RM practitioners showed a significantly higher OHQ score (mean ± SD: 131.88 ± 16.74), (t = 3.33, df = 78, p = 0.001) than non-meditators (NM) (mean ± SD, 118 ± 18.17). Further on analysis, a trend towards significant positive association was observed (Spearman’s correlation coefficient = 0.276, p = 0.084) when OHQ scores were correlated with normalized hours of meditation experience (Fig. 1).
Figure 1.
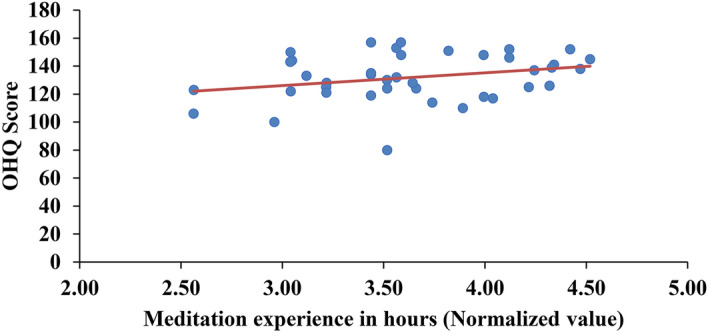
Correlation of OHQ scores with normalized hours of meditation experience showing a trend towards significance (p = 0.084).
Comparison of GMV of reward centers of brain between RM practitioners and NM
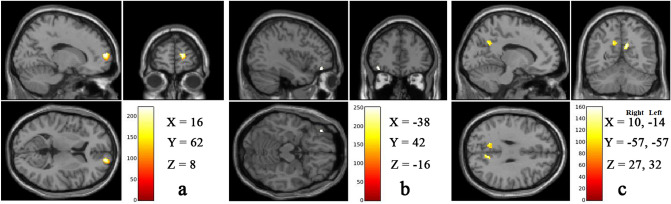
In RM practitioners, a significant increase in GMV in the right superior frontal gyrus (SFG), left inferior OFC, and bilateral precuneus were observed as shown in Fig. 2. The details of peak voxel coordinates in MNI space, numbers of voxels in a cluster and p values are given in Table 1.
Figure 2.
Representative structrual MRI images, showing significant difference (p < 0.05 FWE TFCE corrected) in (a) right superior frontal gyrus, (b) inferior orbitofrontal gyrus and (c) bilateral precuneus in RM practitioners than NM. Color bar represents TFCE-value. These representative structural MRI images were compiled and MNI coordinates were inserted into this compiled image using Adobe Photoshop version CS3 (https://www.adobe.com/in/).
Table 1.
GMV increase in brain regions that process reward and happiness of RM practitioners.
| Brain regions | Side | MNI coordinates | k | p-value* | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Superior frontal gyrus | Right | 16 | 62 | 8 | 280 | 0.005 |
| Inferior orbito frontal cortex | Left | − 38 | 42 | − 16 | 45 | 0.037 |
| Precuneus | Right | 10 | − 57 | 27 | 264 | 0.011 |
| Left | − 14 | − 57 | 32 | 112 | 0.019 | |
*p < 0.05 FWE TFCE corrected, k = cluster size (number of voxels).
Association of GMV of brain regions that process reward and happiness with their OHQ scores in RM practitioners
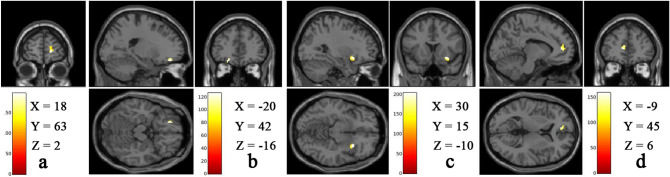
The result of multiple regression analysis showed a correlation of OHQ scores with brain regions related to reward processing in RM practitioners and not in NM. A significant positive correlation was found in the right SFG, left middle OFC, right insula and left ACC (Fig. 3) in RM practitioners. No significant negative correlations in brain regions that process reward and happiness were observed in RM practitioners. Table 2 shows details of cluster size of the identified brain regions, x, y, z coordinates in MNI space and p-values.
Figure 3.
Representative structual MRI image, showing significant (p < 0.05, FWE TFCE corrected) positive correlation with GMV of (a) right superior frontal gyrus, (b) left middle orbitofrontal gyrus, (c) right insula and (d) left anterior cingulate cortex in RM practitioners. Color bar represents TFCE value. These representative structural MRI images were compiled and MNI coordinates were inserted into this compiled image using Adobe Photoshop version CS3 (https://www.adobe.com/in/).
Table 2.
Positive association of OHQ scores with reward processing areas of the brain in RM practitioners.
| Brain regions | Side | MNI coordinates | k | p-value* | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Superior frontal gyrus | Right | 18 | 63 | 2 | 357 | 0/008 |
| Middle orbitofrontal cortex | Left | − 20 | 42 | − 16 | 58 | 0.002 |
| Insula | Right | 30 | 15 | − 10 | 155 | 0.001 |
| Anterior cingulate cortex | Left | − 9 | 45 | 6 | 195 | 0.027 |
*FWE TFCE corrected p value, k = cluster size (number of voxels).
GMV of brain regions that process reward and happiness associated with hours of meditation experience
A significant positive association was found in bilateral VP of brain regions related to reward processing in RM practitioners on performing multiple regression analysis to correlate meditation experience, as shown in Fig. 4. There were no significant negative correlations observed in RM practitioners. Table 3 shows details of cluster size of the identified brain regions, x, y, z coordinates in MNI space and p-values.
Figure 4.
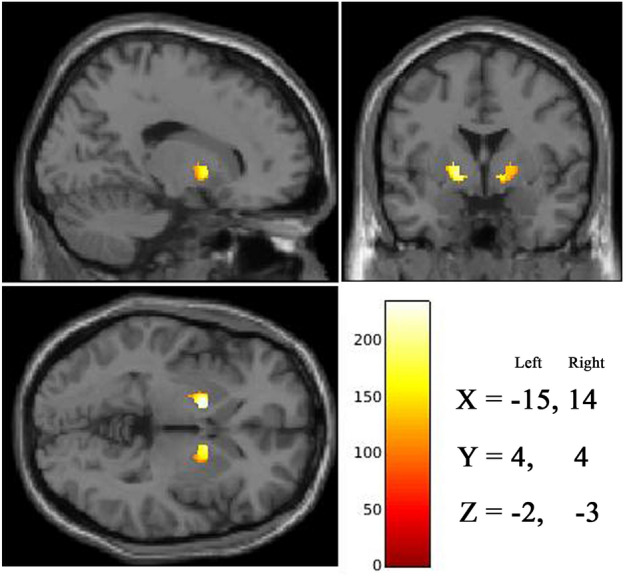
Representative structural MRI image, showing significant (p < 0.05 FWE TFCE corrected) positive association of GMV of bilateral ventral pallidum in RM practitioners with hours of meditation practice. Color bar represents TFCE value. These representative structural MRI images were compiled and MNI coordinates were inserted into this compiled image using Adobe Photoshop version CS3 (https://www.adobe.com/in/).
Table 3.
Correlation of meditation experience associated with GMV change in ventral pallidum in RM practitioners.
| Brain regions | Side | MNI Coordinates | k | p-value* | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Ventral Pallidum | Left | − 15 | 4 | −2 | 220 | 0.009 |
| Right | 14 | 4 | − 3 | 203 | 0.016 | |
*FWE TFCE corrected p-value, k = cluster size (number of voxels).
Discussion
In the present study, RM practitioners had significantly higher OHQ scores than NM. Additionally, a trend towards significance in OHQ scores was observed in RM practitioners with more hours of RM experience. An earlier study on mindfulness meditation reported that happiness, self-compassion and higher levels of dispositional mindfulness are positively associated with frequency of meditation practice27. In contrary to this report, the present study shows that the association between happiness scores and RM experience was not significantly correlated, although there was a trend towards significance. This suggests that RM practice can improve happiness levels, irrespective of hours of RM experience. The higher happiness scores observed in experienced RM practitioners maybe because of emphasizing on a fundamental spiritual understanding of self and rediscovering the latent positive qualities within an individual22,28.
Further, group comparison between RM and NM showed that RM practitioners had higher GMV in the right precuneus. Studies show that this region along with the posterior cingulate cortex, is of primary importance since it receives the highest blood flow and cortical glucose metabolism at rest, as it is a part of the default mode network. Neuroimaging studies in humans reveal its essential role in subjective consciousness29 and subjective experience30 for integrating internal and external information31. Thus, the precuneus region appears to be involved in gathering information from different modes at a conscious level and is responsible for the subjective experience of happiness. A study on healthy volunteers using subjective happiness measure, purpose in life, positive and negative emotional intensity scales by Sato W 2015, reported a positive association between GMV of precuneus with subjective measures of happiness in healthy volunteers. This study suggests that the precuneus plays a vital role in mediating subjective experience of happiness by combining emotional and cognitive components of happiness2. Neuroimaging studies using VBM analytical technique on meditators have also shown findings of higher GMV in the precuneus region. In a pilot study by Kurth F 2014, observed higher GMV in the right precuneus after mindfulness meditation intervention and suggested that this region is essential for meditation practice32. This region has extensive communication with resting-state and external task regions. It processes self-relevant information which helps to engage in meditation33,34. Thus, in the present study, observation of increased GMV in precuneus in RM practitioners when compared with NM indicates that these changes in the precuneus may be due to higher subjective experiences of happiness in RM practitioners (indicated by their higher OHQ scores).
Moreover, the present study also shows significantly higher GMV in right SFG (part of mPFC) and left ACC that is significantly positively associated with OHQ scores in RM practitioners than those in NM. Both SFG and ACC are part of reward centers35,36. Studies on long-term Sahaja yoga meditation and others suggest that mPFC is responsible for maintaining meditation, attention, emotional regulation, behavior and cognitive control37–39. The interoceptive functioning of this region contributes to value-based decision-making. Such higher interoceptive abilities are associated with mindfulness meditation experience40. Positive thinking and happiness support brain growth, as well as the generation and reinforcement of new synapses, particularly in PFC, which serves as the integration center of all brain-mind functions41. The dorsolateral and medial PFC areas are involved in the choice of reward that varies over time and also important in normative decision-making related to monetary reward42.
Further, a study also shows that insight meditation experience is associated with cognitive control and self-regulation by dorsolateral PFC and dorsal ACC43. Greater activation in rostral ACC and dorsal mPFC in mindfulness meditators provides evidence that meditators can keep away from distractions and can control their emotions. The activity in the ACC decreases in long-term adept and Buddhist meditators, which suggests that, as these meditators become more efficient on focused attention, emotional regulation becomes unnecessary44,45. Integrative body-mind training, 30 min/day for five days, improves the cerebral blood flow to the ventral ACC, mPFC and insula which are the critical brain regions for self-regulation46. Research on transcendental meditation suggests that mPFC and ACC are essential in the mechanism of regulating mental state47. ACC is the complex structure that has an extensive connection with other reward regions such as VS and VP9. A positive hedonic environment attenuates the ACC activity and is responsible for behavioral adaptation48. Studies on ACC and amygdala in mindfulness meditators suggest that ACC helps to maintain the attention to a stimulus and the amygdala is responsible for generating emotions. Through these brain regions, meditation regulates negative thoughts and decreases emotional reactivity that helps to overcome psychiatric disorders such as depression and borderline personality disorders49,50. The higher GMV in right SFG and left ACC in the present study indicates that practicing RM may help to gain voluntary control over attention, behavior and emotional regulation.
Additionally, in the present study, a significant increase in GMV in left inferior OFC, right insular cortex and a smaller left middle OFC that was positively associated with happiness scores were observed in RM practitioners. Anatomically, insular region has bidirectional connections with the structures that are related to reward and decision making which includes the OFC, ACC, NAcc, and the amygdala51. Insular cortex plays a key role in integrating information related to emotions and introspection. These information are then forwarded to the OFC and ACC, which influence decision making52. Functional MRI study in several Buddhist traditions including Shamatha/breath-focus, Vipasana/insight, compassion, Tong-Len and other Tibetan style meditations, suggests that the individuals with more meditation experience may have an enhanced awareness of present moment experience53. Insula, along with ACC and striatum, are involved in the maintenance of meditative states with less effort and attentional control54. OFC regulates the process of reward and punishment55. Increased activation of this region has been observed during reward or loss incurred56. A study by Estela C et al. 2009, reported a graded increase in the activation of this region in relation to reward52. Greater grey matter concentration in the medial OFC is dependent on mindfulness meditation training might reflect an improved ability to modify emotional responses57. Thus, these supporting findings indicate that long-term RM practice may enhance awareness of present moment experience, attentional control and helps the practitioner to maintain the meditative states with less effort.
Finally, the interesting finding in the present study is the significant correlation of GMV in bilateral VP with hours of RM experience. VP is part of the basal ganglia and is well known to play an important role in the planning and regulation of motor activity. Modern-day high-resolution human brain imaging technology validates and clarifies the role of the VP in human emotional response and psychiatric disorders58. Accumulating evidence from recent studies reveals that apart from its role in motor activity, the VP also plays an essential role in hedonic response to a pleasant stimulus. Also, VP produces this response in the background activity of ACC5,59. The dopaminergic fibers regulate the activity of glutamatergic neurons from the frontal cortex to striatum and project back to the frontal cortex via pallidum and thalamus. It is reported that increased dopamine release during meditation, which suppresses the cortico-striatal glutamatergic transmission, is associated with motor control. The increase of dopamine associated with meditation training may contribute to dopaminergic neurogenesis37. VP is one of the central components of the reward circuit which sub-serve the functions of positive motivation and reward9. The VP receives a reward signal from NAcc and other forebrain limbic structures to execute it60,61. Studies on volunteers who had chronic pain and sadness correlate with the deactivation of m-opioid receptors in the VP. This is identified that they had behavioral problems associated with negative affect which suggests that the m-opioid receptors in the VP regulate the positive affect. It regulates the affective/motivational process at the conscious or unconscious level58,62,63. A study on SOHAM meditators suggests that higher grey matter density in VP is associated with reward and positive motivation17.
Additionally, an earlier study shows that reward-centers down-regulate hedonic effects themselves as a result of repeated exposure of worldly rewards corresponding to the conditioned suppression of dopamine64. Everyday thought patterns that are repetitive, predictable and rewarded behaviors initially support their development but later may lose their ability to process the rewarded behavior which has become repetitive. This may be due to a continuous suppression of dopamine-related activity in NAcc10 which is the key region in reward processing. This may explain the reason for not findings a significant increase in GMV or any correlation in VS and NAcc, in the present study. Moreover, the interesting finding in the present study is the significant positive association of GMV in bilateral VP with RM experience, which may indicate that RM practice may contribute to the automatic mind states of developing positive motivation and experience of reward.
The potential limitation in the present study is the education levels which were not matched between groups. Education plays an important role in brain modulation based on the individual’s education levels and may introduce a confounding effect if not matched65–67. The hippocampus and amygdala regions play an important role in the reward processing9 are directly or indirectly influenced by levels of education of the participants. In this study, no significant changes were observed in the hippocampus and amygdala either in t-test or in correlation analysis even after adding education levels as a covariate. In the future, repeating a similar type of study with larger sample size and matching years or levels of education may help to overcome this confounding effect.
Conclusion
This study provides the first evidence for the underlying neural substrate of higher GMV changes in reward processing areas of RM practitioners including right SFG, left ACC, left OFC, right insula and bilateral VP as well as precuneus an important structure that mediates happiness. This was observed to be associated with higher happiness scores in RM practitioners compared to the happiness levels in NM. Besides, years of RM experience was observed to enhance GMV in these reward processing regions to greater extents. However, the happiness scores were not positively associated with hours of meditation experience, indicating that RM practice provides happiness, irrespective of the time span of RM experience, and RM practice may contribute to the automatic mind states of developing positive motivation and experience of reward.
Methods
Participants
The total number of RM practitioners and non-meditators (NM) in both the groups were eighty (n = 40/group). They were matched for age, gender and handedness. Subjective happiness levels were measured using OHQ and MRI obtained was used to study structural changes in the brain regions that process reward and happiness. The social-demographic details of all participants are given in Table 4. The participants who had the following exclusion criteria for the study, such as metabolic disorders, addiction to drugs or alcohol, mental disorders, physical illness, and claustrophobia, were not selected. Participants who were practicing only RM and regularly attending spiritual and meditation classes were recruited from Brahma Kumaris Rajyoga Meditation Centre, Manipal, Udupi, and Mangalore cities of Karnataka, India. The hours of meditation experience were calculated by their years of meditation experience, hour/s spent in meditation per day and hours spent in meditation retreat. NM participants were volunteers for this study and were selected from Manipal, Udupi regions of Karnataka, India. After obtaining approval from the institutional ethical committee (IEC 566/2013) Kasturba Hospital, MAHE, Manipal for human studies, this study was conducted. Standard ethical guidelines laid down by Declaration of Helsinki-World Health Organization was followed for all methods in this study. All the participants were informed about the study well in advance and MRI safety precautions were explained to all. Before the study, a signed-in informed consent form was obtained from each participant.
Table 4.
Socio-demographic details of participants.
| Meditators (n-40) | Non-meditators (n = 40) | P value | |
|---|---|---|---|
| Age (mean ± SD) (Range: minimum–maximum) | 41.28 ± 8.05 (26.4–52.85) | 40.06 ± 7.21 (26.32–55.16) | 0.48 (t-test) |
| Gender (n) | |||
| Male | 20 | 20 | 1.0 (X2 test) |
| Female | 20 | 20 | |
| Handedness (n) | |||
| Right | 39 | 39 | 1.0 (Fisher’s exact test) |
| Left | 1 | 1 | |
| Educational levels | |||
| School | 20 | 21 | 0.001 (Fisher’s exact test) |
| Degree | 3 | 14 | |
| Postgraduate | 13 | 5 | |
| PhD/PDF | 4 | 0 | |
| Meditation Experience (in hours) | 8022.24 ± 8767.24 | NA | NA |
NA not applicable.
Psychological test tool
OHQ, developed by Peter Hills in 200268, a self-reported cognitive scale that measures subjective happiness level was used for this study. Psychologists and sociologists widely use it as a tool for assessing happiness status69–73. OHQ is a unidimensional scale with 29-items, designed to measure happiness status, which is a reliable and valid questionnaire with reliability alpha coefficient of 0.91. It employs a 6-point Likert-type format of the response, from ‘strongly disagree’ to ‘strongly agree, to measure self-reported happiness score. Higher the score more will be the happiness level. The filled-in OHQs by all participants were collected and the total score for the 29 items were calculated. In this study, the overall score of the OHQ was considered.
MRI acquisition
Structural MRI data were acquired for all participants with Philips Acheiva Medical Imaging 1.5 T scanner with 8 SENSE head-coil. High-resolution three-dimensional sets of data of the whole brain were collected using T1 weighted, TFE—turbo field echo sequence. It consists of sagittal partitions with slice thickness 1 mm, TE/TR (echo/repetition time) 3.703 ms /7 ms, flip angle 8°, matrix 256 × 256, 1 × 1 voxel dimension, and field of view 256 mm which yielded 175 slices encompassing the whole brain.
Image analysis
Voxel based morphometric analysis is a method for investigating neuroanatomical alterations in the brain in an unbiased and objective way using T1-weighted structural MRI scans. VBM analysis involves the measurement of voxels in the brain MRI, and regional GMV can be obtained74. Statistical comparison of GMV between two or more different experimental groups can be analysed and the statistical significant differences in the volume of brain regions can be established. In recent years, this technique has become very popular and it is also used to detect stimuli-induced morphological changes in the brain where functional MRI is not suitable for study75. In the present study to detect GMV changes in brain, VBM method was applied by using the CAT12 toolbox version 12.6 (https://www.neuro.uni-jena.de/cat/) installed in the SPM12 software toolbox (ftp://ftp.fil.ion.ucl.ac.uk/spm), and the common platform for this software was MATLAB software version R2019a.
Initially, all the DICOM images were converted into single file NIFTY format by using MRI Convert version 2.1.0 (https://lcni.uoregon.edu/downloads/mriconvert). Then all the converted images were manually reoriented and fixed with the anterior commissure as origin which matches the canonical image template provided in SPM12. All the preprocessing steps for VBM analysis were carried out using the CAT12 tool as mentioned in the VBM manual (https://www.neuro.uni-jena.de/cat12/CAT12-Manual.pdf). For initial segmentation, a sensitive segmentation method than the normal VBM method known as DARTEL (Diffeomorphic anatomic registration through exponentiated Lie algebra algorithm) segmentation method was applied to segment the MRI images. By this method, we obtained affine transformed images of grey and white matter. TIV estimate option in CAT12 tool generates total intracranial volume (TIV) for each subject and was used for homogeneity check and as nuisance covariate. After the segmentation process, all images were inspected manually by displaying one slice for all images. Moreover, quality of data such as resolution, noise and bias were also obtained. Using this information, the weighted average quality of all images were B or higher, which represents very good quality. Therefore, none of the images were excluded from the analysis. A study-specific template was created representing the sample of study using affine registered grey and white matter images. Further, this template was used to generate normalized, modulated, non-linear DARTEL warped segmented images of grey and white matter in Montreal Neurological Institute (MNI) Space. A FWHM (full width at half maximum) isotropic Gaussian kernel with 8 mm size was used for VBM statistical analysis.
Creating Region of Interest (ROI) Masks
A 3D ROI sphere mask was created for the regions that represent the reward processing regions using WFU-Pickatlas (Wake Forest University) atlas tool (https://fmri.wfubmc.edu/software/pickatlas) to test the study hypothesis. Bilateral union masks for cortical and sub-cortical centers using “x, y, z” coordinates in MNI space mentioned in the studies related to reward and meditation are as follows: for cortical centers 8 mm masks created for OFC (± 27, 42, − 14)15,76,77, ACC (± 6, 51, 10)78, insula (± 30, 10 -15)38, and precuneus (± 6, 54, 22)77, regions. For sub-cortical structures, 4 mm masks were created for NAcc (± 10, 9, − 4)15, VP (± 14, − 10, − 8)59, basolateral amygdala (± 32, 0, -26)79, and hippocampus (± 18, − 37, − 11)80 For mid-brain regions that involve in reward processing, 2 mm masks were created including SN (± 12, − 12, − 12 ), and VTA (0, − 15, − 12) regions15,81. Totally 11 bilateral union masks for reward centers were created. Since this tool creates masks with voxel dimensions of 2 mm in x, y, z directions by default, using a co-register (reslice) option in SPM12, all the masks were co-registered with a VBM output image to get the same voxel dimensions for all ROI masks created.
Statistical analysis
SPSS version 22, was used to perform t-test and find out the difference in mean OHQ score between groups. A correlation analysis was performed to correlate hours of meditation experience with OHQ score in RM. Since hours of meditation experience were not normally distributed (mean ± SD: 8022.24 ± 8767.69), a normalized value for hours of meditation experience (mean ± SD: 3.64 ± 0.52) and Spearman correlation coefficient was considered in the correlation analysis. Correlation results were reported significant if the p-value < 0.05.
For VBM analysis, to compare between 2 groups, the basic statistical model was applied to conduct a t-test to find out voxel-wise gray matter (GM), and white matter (WM) volume changes between NM and RM using the CAT12 toolbox. ANCOVA was performed with a flexible factorial design to compare group differences and adjusted for TIV, age, and education levels which were entered as covariates to remove confounding effect due to variable head/brain size, aging process and influence of education. An absolute threshold masking 0.1 was applied to all the smoothed images to avoid edge effect around the borders of GM and WM. The t-contrasts [-1 1] for NM > RM and [-1 1] for RM > NM were used to measure group differences. A multiple regression analysis was performed to correlate the reward center of the brain with hours of meditation experience and OHQ by entering these variables as independent variables. Since there was collinearity (cos θ = 0.7) between these two variables which was identified from the SPM design orthogonality output map. Hence, multiple regression analysis was performed separately for these two independent variables to avoid multi collinearity issues. For both multiple regression analyses TIV, age, and education level were entered as nuisance covariates. The t-contrasts [1] for positive correlation and [− 1] for negative correlation were used. All other parameters were kept the same as for the t-test. TFCE, a non-parametric methods, with 5000 permutations82 was applied by using the TFCE tool version 185 (https://dbm.neuro.uni-jena.de/tfce/). This approach combines focal effects with large voxel height and size of the cluster in VBM data. It is more reliable and effective for group-level analysis with or without the requirement of high and uniform spatial smoothness83. The 3D ROI sphere masks were used for small volume correction in the TFCE approach. The resultant voxels were considered significant, if it is above the threshold value p < 0.05, after correcting for family wise error (FWE) using multiple comparison with TFCE in the ROIs. The final output, SPM statistical map image, was overlaid on the single subject representative image from SPM canonical template.
Acknowledgements
We sincerely thank Rajyogini BK. Sowrabha, and BK. Sujatha, from the Rajyoga Meditation Centre, Manipal, for facilitating the meditators to participate in this study. Our sincere thanks to all the participants for volunteering in this study. We are grateful to the staff, especially the radiographers from Department of Radiodiagnosis and Imaging, at the Kasturba Hospital, Manipal, for their support. Our sincere thanks to the faculties of Centre for Imaging, Philips, Manipal for their technical support.
Author contributions
Authors R.B.M.G., R.K., and K.S.R. contributed to the conceptualization of this manuscript. Data analysis and interpretation were carried out by authors R.B.M.G., R.K., B.S. and K.S.R. All authors have contributed for reviewing and editing this manuscript.
Funding
We gratefully acknowledge the support and infrastructure provided by MAHE, Manipal for this study. We are also thankful to the Headquarters of Brahmakumaris Rajyoga Meditation Centre and Rajyoga Education & Research Foundation (RERF), Mount Abu, Rajasthan for permission to recruit local RM practitioners and provide partial financial support for this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lyubomirsky S. Why are some people happier than others? The role of cognitive and motivational processes in well-being. Am. Psychol. 2001;56:239–249. [PubMed] [Google Scholar]
- 2.Sato W, et al. The structural neural substrate of subjective happiness. Sci. Rep. 2015;5:16891. doi: 10.1038/srep16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas-Diener R, Diener E. Making the best of a bad situation: Satisfaction in the slums of Calcutta. Soc. Indic. Res. 2001;55:329–352. [Google Scholar]
- 4.Diener E, Biswas-Diener R. Will money increase subjective well-being? A literature review and guide to needed research. Soc. Indic. Res. 2002;57:119–169. [Google Scholar]
- 5.Kringelbach ML, Berridge KC. The functional neuroanatomy of pleasure and happiness. Discov. Med. 2010;9:579–587. [PMC free article] [PubMed] [Google Scholar]
- 6.Campos D, et al. Meditation and happiness: Mindfulness and self-compassion may mediate the meditation-happiness relationship. Pers. Individ. Dif. 2016;93:80–85. [Google Scholar]
- 7.Cohn MA, Fredrickson BL, Brown SL, Mikels JA, Conway AM. Happiness unpacked: Positive emotions increase life satisfaction by building resilience. Emotion. 2009;9:361–368. doi: 10.1037/a0015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funahashi S. Brain mechanisms of happiness. Psychologia. 2011;54:222–233. [Google Scholar]
- 9.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp PE. Meditation-induced bliss viewed as release from conditioned neural (thought) patterns that block reward signals in the brain pleasure center. Relig. Brain Behav. 2014;4:202–229. [Google Scholar]
- 11.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 12.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 14.Levita L, et al. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagerty MR, et al. Case study of ecstatic meditation: FMRI and EEG evidence of self-stimulating a reward system. Neural Plast. 2013;2013:1–12. doi: 10.1155/2013/653572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J. Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar U, Guleria A, Kishan SSK, Khetrapal CL. Effect of SOHAM meditation on human brain: a voxel-based morphometry study. J. Neuroimaging. 2014;24:187–190. doi: 10.1111/jon.12040. [DOI] [PubMed] [Google Scholar]
- 18.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk U, Brown KW, Downar J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc. Cogn. Affect. Neurosci. 2015;10:752–759. doi: 10.1093/scan/nsu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyubomirsky S, Lepper HS. A measure of subjective happiness: preliminary reliability and construct validation. Soc. Indic. Res. 1999;46:137–155. [Google Scholar]
- 21.Shi L, et al. Brain networks of happiness: dynamic functional connectivity among the default, cognitive and salience networks relates to subjective well-being. Soc. Cogn. Affect. Neurosci. 2018;13:851–862. doi: 10.1093/scan/nsy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay T, Manderson L, Smith W. Changing a mountain into A mustard seed: spiritual practices and responses to disaster among New York Brahma Kumaris. J. Contemp. Relig. 2010;25:89–105. [Google Scholar]
- 23.Sharma K, Chandra S, Dubey AK. Exploration of lower frequency EEG dynamics and cortical alpha asymmetry in long-term rajyoga meditators. Int. J. Yoga. 2018;11:30–36. doi: 10.4103/ijoy.IJOY_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telles S, Desiraju T. Automatic changes in Brahmakumaris Raja yoga meditation. Int. J. Psychophysiol. 1993;15:147–152. doi: 10.1016/0167-8760(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 25.Nair AK, Sasidharan A, John JP, Mehrotra S, Kutty BM. Just a minute meditation: rapid voluntary conscious state shifts in long term meditators. Conscious. Cogn. 2017;53:176–184. doi: 10.1016/j.concog.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Panda R, et al. Temporal dynamics of the default mode network characterize meditation-induced alterations in consciousness. Front. Hum. Neurosci. 2016;10:372. doi: 10.3389/fnhum.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos D, et al. Exploring the role of meditation and dispositional mindfulness on social cognition domains: a controlled study. Front. Psychol. 2019;10:809. doi: 10.3389/fpsyg.2019.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telles S, Hanumanthaiah B, Nagarathna R, Nagendra HR. Improvement in static motor performance following yogic training of school children. Percept Mot Ski. 1993;76:1264–1266. doi: 10.2466/pms.1993.76.3c.1264. [DOI] [PubMed] [Google Scholar]
- 29.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lou HC, Nowak M, Kjaer TW. The mental self. Prog. Brain Res. 2005;150:197–204. doi: 10.1016/S0079-6123(05)50014-1. [DOI] [PubMed] [Google Scholar]
- 31.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 32.Kurth F, Luders E, Wu B, Black DS. Brain gray matter changes associated with mindfulness meditation in older adults: an exploratory pilot study using voxel-based morphometry. Neuro. 2014;1:23–26. doi: 10.17140/NOJ-1-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurth F, MacKenzie-Graham A, Toga AW, Luders E. Shifting brain asymmetry: The link between meditation and structural lateralization. Soc. Cogn. Affect. Neurosci. 2015;10:55–61. doi: 10.1093/scan/nsu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boccia M, Piccardi L, Guariglia P. The meditative mind: A comprehensive meta-Analysis of mri studies. Biomed Res. Int. 2015;2015:1–11. doi: 10.1155/2015/419808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong G, Lin X, Hu Y, Lu Q. Brain activity in advantageous and disadvantageous situations: Implications for reward/punishment sensitivity in different situations. PLoS ONE. 2013;8:e80232. doi: 10.1371/journal.pone.0080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bush G, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang DH, et al. The effect of meditation on brain structure: Cortical thickness mapping and diffusion tensor imaging. Soc. Cogn. Affect. Neurosci. 2013;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández SE, Suero J, Barros A, González-Mora JL, Rubia K. Increased grey matter associated with long-term Sahaja yoga meditation: A voxel-based morphometry study. PLoS ONE. 2016;11:e0150757. doi: 10.1371/journal.pone.0150757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirk U, Montague PR. Mindfulness meditation modulates reward prediction errors in a passive conditioning task. Front. Psychol. 2015;6:1–8. doi: 10.3389/fpsyg.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susan, R. Happy brain, happy life. Positive Psychology (2011). https://www.psychologytoday.com/us/blog/prime-your-gray-cells/201108/happy-brain-happy-life. Accessed 27 Sept 2019.
- 42.Cho SS, et al. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013;26:479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox KCR, et al. Meditation experience predicts introspective accuracy. PLoS ONE. 2012;7:e45370. doi: 10.1371/journal.pone.0045370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holzel BK, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci. Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 45.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl. Acad. Sci. USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan K, et al. Reduced Fractional Anisotropy of Corpus Callosum Modulates Inter-Hemispheric Resting State Functional Connectivity in Migraine Patients without Aura. PLoS ONE. 2012;7:30–32. doi: 10.1371/journal.pone.0045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto S, Kitamura Y, Yamada N, Nakashima Y, Kuroda S. Medial prefrontal cortex and anterior cingulate cortex in the generation of alpha activity induced by transcendental meditation: a magnetoencephalographic study. Acta Med. Okayama. 2006;60:51–58. doi: 10.18926/AMO/30752. [DOI] [PubMed] [Google Scholar]
- 48.Horga G, et al. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. J. Neurosci. 2011;31:16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silbersweig D, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am. J. Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- 50.Abercrombie HC, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J. Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camara E, Rodriguez-Fornells A, Münte TF. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2009;2:19. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 2012;6:1–14. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn. Sci. 2012;16:330–337. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 56.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 57.Holzel BK, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Napier TC, Mickiewicz AL. The role of the ventral pallidum in psychiatric disorders. Neuropsychopharmacology. 2010;35:337. doi: 10.1038/npp.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Steenbergen H, Band GPH, Hommel B, Rombouts SARB, Nieuwenhuis S. Hedonic hotspots regulate cingulate-driven adaptation to cognitive demands. Cereb. Cortex. 2015;25:1746–1756. doi: 10.1093/cercor/bht416. [DOI] [PubMed] [Google Scholar]
- 60.Churchill L, Dilts RP, Kalivas PW. Changes in γ-aminobutyric acid, μ-opioid and neurotensin receptors in the accumbens-pallidal projection after discrete quinolinic acid lesions in the nucleus accumbens. Brain Res. 1990;511:41–54. doi: 10.1016/0006-8993(90)90223-x. [DOI] [PubMed] [Google Scholar]
- 61.Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J. Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zubieta JK, et al. μ-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zubieta JK, et al. Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Arch. Gen. Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 64.Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Tang X, Varma VR, Miller MI, Carlson MC. Education is associated with sub-regions of the hippocampus and the amygdala vulnerable to neuropathologies of Alzheimer’s disease. Brain Struct. Funct. 2017;222:1469–1479. doi: 10.1007/s00429-016-1287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang DW, Lim HK, Joo SH, Lee NR, Lee CU. The association between hippocampal subfield volumes and education in cognitively normal older adults and amnestic mild cognitive impairment patients. Neuropsychiatr. Dis. Treat. 2018;14:143–152. doi: 10.2147/NDT.S151659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Hum. Brain Mapp. 2011;32:282–289. doi: 10.1002/hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hills P, Argyle M. The Oxford happiness questionnaire: a compact scale for the measurement of psychological well-being. Pers. Individ. Dif. 2002;33:1073–1082. [Google Scholar]
- 69.Kamthan S, et al. Happiness among second year MBBS students and its correlates using Oxford Happiness Questionnaire. J. Oral Biol. Craniofacial Res. 2019;9:190–192. doi: 10.1016/j.jobcr.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malmir M, Khanahmadi M, Farhud D. Dogmatism and happiness. Iran. J. Public Health. 2017;46:326. [PMC free article] [PubMed] [Google Scholar]
- 71.Moghadam M, Rezaei F, Ghaderi E, Rostamian N. Relationship between attachment styles and happiness in medical students. J. Fam. Med. Prim. Care. 2016;5:593. doi: 10.4103/2249-4863.197314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramesh MG, Sathian B, Sinu E, Rai KS. Efficacy of Rajayoga meditation on positive thinking: an index for self-satisfaction and happiness in life. J. Clin. Diagn. Res. 2013;7:2265–2267. doi: 10.7860/JCDR/2013/5889.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medvedev ON, Landhuis CE. Exploring constructs of well-being, happiness and quality of life. PeerJ. 2018;6:e4903. doi: 10.7717/peerj.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 75.Naegel S, et al. Short latency gray matter changes in voxel-based morphometry following high frequent visual stimulation. Neural Plast. 2017 doi: 10.1155/2017/1397801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitayama S, et al. Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proc. Natl. Acad. Sci. USA. 2017;114:7969–7974. doi: 10.1073/pnas.1704831114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirk U, Skov M, Christensen MS, Nygaard N. Brain correlates of aesthetic expertise: a parametric fMRI study. Brain Cogn. 2009;69:306–315. doi: 10.1016/j.bandc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Hernández SE, Barros-Loscertales A, Xiao Y, González-Mora JL, Rubia K. Gray matter and functional connectivity in anterior cingulate cortex are associated with the state of mental silence during Sahaja Yoga meditation. Neuroscience. 2018;371:395–406. doi: 10.1016/j.neuroscience.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Hölzel BK, et al. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luders E, Kurth F, Toga AW, Narr KL, Gaser C. Meditation effects within the hippocampal complex revealed by voxel-based morphometry and cytoarchitectonic probabilistic mapping. Front. Psychol. 2013;4:398. doi: 10.3389/fpsyg.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb. Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Nickerson LD, Nichols TE, Gao J-H. Comparison of a non-stationary voxelation-corrected cluster-size test with TFCE for group-Level MRI inference. Hum. Brain Mapp. 2017;38:1269–1280. doi: 10.1002/hbm.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]