Highlights
-
•
The α-Glucosidase was successfully entrapment into p(HEMA) cryogels at −18 °C.
-
•
Higher stability for α-Glucosidase@p(HEMA) than free one at wide range of T and pHs.
-
•
∼1.5-fold increase in Vmax value for the entrapped α-Glucosidase was accomplished.
-
•
Entrapped enzyme, α-Glucosidase maintained >50 % activity after 10 repeated use.
-
•
Entrapped α-Glucosidase can be stored at 25 °C for 10 days with >50 % activity.
Keywords: Enzyme, Super porous cryogel, Enzyme immobilization/entrapment, α-Glucosidase, Enzymatic reaction
Abstract
Here, poly(2-hydroxyethyl methacrylate) (p(HEMA)) cryogel were prepared in the presence 0.48, 0.96, and 1.92 mL of α-Glucosidase enzyme (0.06 Units/mL) solutions to obtain enzyme entrapped superporous p(HEMA) cryogels, donated as α-Glucosidase@p(HEMA)-1, α-Glucosidase@p(HEMA)-2, and α-Glucosidase@p(HEMA)-3, respectively. The enzyme entrapped p(HEMA) cryogels revealed no interruption for hemolysis and coagulation of blood rendering viable biomedical application in blood contacting applications. The α-Glucosidase@p(HEMA)-1 was found to preserve its’ activity% 92.3 ± 1.4 % and higher activity% against free α-Glucosidase enzymes in 15–60℃ temperature, and 4–9 pH range. The Km and Vmax values of α-Glucosidase@p(HEMA)-1 cryogel was calculated as 3.22 mM, and 0.0048 mM/min, respectively versus 1.97 mM, and 0.0032 mM/min, for free enzymes. The α-Glucosidase@p(HEMA)-1 cryogel was found to maintained enzymatic activity more than 50 % after 10 consecutive uses, and also preserved their activity more than 50 % after 10 days of storage at 25 ℃, whereas free α-Glucosidase enzyme maintained only 1.9 ± 0.9 % activity under the same conditions.
1. Introduction
The biggest reason for the use of enzymes in diverse areas is their unique properties such as high biological and chemical reaction efficiency and specificity, high reaction rates, stereo and enantio-selectivity, high product purity, environmental friendliness and mild reaction conditions [[1], [2], [3]]. In addition, major obstacles such as the high cost of enzyme production and purification due to their complex structure, low stability and activity in unnatural or harsh environments, partial or total inhibition in high substrates and/or product concentrations are the major drawbacks to their industrial applications [4,5]. Various methods have been developed to eliminate these disadvantages, such as the use of stabilizing additives [6], chemical modification [7], selection of enzymes from thermophilic organisms [8] and immobilization techniques [[9], [10], [11]]. Amongst them, enzyme immobilization as one of most frequently investigated methods which aims to develop continuous processes that are long-lasting, afford high enzymatic activity under harsh operating conditions, economically viable and environmentally friendly and so on [5,12]. Many immobilization techniques have been used to enhance the stability, lifetime, and industrial applicability of enzymes to perform at harsher conditions. In general, enzyme immobilization is accomplished by means of 5 basic methods of adsorption [13], covalent bonding [14], cross-linking [15], encapsulation [16] and entrapping [17]. To choose the most appropriate immobilization method, very important factors such as enzyme tolerance to immobilization conditions (pH, temperature, ionic strength, etc.), surface functional groups on enzymes, size of the enzyme, polarity of the enzyme and substrate/product transport need be taken into account [5,18]. The advantages of immobilized enzymes are easy removal from the reaction medium, possible reusability, attaining a clean product, boosting enzyme stability against pH, temperature, solvents, contaminants and impurities, and so on [19]. The adsorption technique has some disadvantages such as loose adsorption of enzyme onto surfaces, or covalent bonding of enzymes to solid surfaces and crosslinking of enzymes to each other may result in loss or inactivation of some active sides due to the compromise of active sides. Also, the encapsulation technique has also some disadvantages one of which is the easy decomposition of encapsulated materials [1]. On the other hand, the entrapment techniques depending on the reaction conditions may not show significant disadvantages as other techniques.
Cryogels are a special type of hydrogels with interconnected superporous structure which provide attractive features such as high mechanical stability and flexibility properties in comparison to common hydrogels [20,21]. Cryogels are the materials that are synthesized at temperatures below the freezing point of the solvent of gel forming materials. An aqueous solution containing cryogel precursors, monomer, crosslinker, initiator, and accelerator are brought into below the freezing point of solvent (water) where the ice crystals are formed under reaction conditions leading to interconnected super porous network formation. The pore sizes, range from a few micrometers to several hundred micrometer, and the network connectivity also related to the extent and the amount of ice crystal amongst other parameters can be readily designed using appropriate amount of material components [22,23]. Compared to normal hydrogels, cryogels offer superior properties in terms of high elasticity, mechanical strength and rapid response [24,25]. The synthesis conditions of cryogels in cryogenic conditions make them exceptionally promising materials for enzyme immobilization. In recent years, only a few reports about enzyme immobilized cryogels for various types of enzymes and reactions were published in the literature [[26], [27], [28], [29]]. Also, the synthesis of p(HEMA) cryogel was already reported in literature [21,30,31]. P(HEMA) as one of the most widely used synthetic polymers in many applications including biomedical fields e.g., contact lenses [32], wound dressing materials [33], and controlled drug release systems [34] and even reported as a blood-compatible surface [35]. Moreover, p(HEMA) and its composites were used as support material for enzyme immobilization employing different methods [36,37].
The enzyme, α-Glucosidase, catalyzes the hydrolysis of α-1,4-linked glucose residues from aryl (or alkyl)-glycosides, disaccharides or oligosaccharides [38], and is found on the brushy surface of the small and middle intestine [38,39]. Many systems have been reported in the literature with immobilized α-glucosidase enzymes [[40], [41], [42], [43], [44], [45], [46]]. Prodanovic et al. reported the stability and activities of immobilized α-Glucosidase enzymes in the cosolvent systems and compared their activities [44]. Additionally, the screening of various α-Glucosidase enzyme inhibitors was also investigated by using various immobilized α-Glucosidase enzyme systems [[40], [41], [42],45,46]. Furthermore, co-immobilized α-Glucosidase and pyranose oxidase enzymes were utilized as a biosensor for maltose molecules [43]. Moreover, the enzyme inhibition plays a significant role in many biological events. For example, the inhibition of enzymes involved in the digestion of carbohydrates is very important in the controlled management of hyperglycemia and type II diabetes [47]. According to the proposed hypothesis, postprandial hyperglycemia can be alleviated and controlled management of type II diabetes can be achieved as the increase in blood sugar levels can be significantly reduced after a mixed carbohydrate diet with inhibition of enzymes such as ɑ-Glucosidase and amylase [47].
In this study, α-Glucosidase enzymes were entrapped within p(HEMA) cryogels during synthesis of the polymeric network. The characterization of prepared α-Glucosidase enzymes entrapped p(HEMA) cryogels were carried out in terms of swelling %, porosity %, pore volume %, gel yield %, and also by using Scanning Electron Microscopy (SEM), Fourier Transform Infrared (FT-IR) spectrometer, and Thermogravimetric Analyses (TGA). Moreover, the blood compatibility of α-Glucosidase@p(HEMA) cryogels were done via hemolysis and blood clotting tests. The activity% of α-Glucosidase@p(HEMA) cryogels was compared with free α-Glucosidase in a wide temperature range, 15–60 ℃, and pH range, 4−9. The kinetic parameters of α-Glucosidase@p(HEMA) cryogels and free ɑ-Glucosidase were assessed by using the Lineweaver-Burk plotting method. Moreover, the reusability and storage capacity of α-Glucosidase@p(HEMA) cryogels were investigated.
2. Experimental
2.1. Materials
The chemicals such as 2-hydroxy ethyl methacrylate (HEMA, 99 %, Sigma- Aldrich), N,N′-Methylenebisacrylamide (MBA, 99 %, Acros), potassium persulfate (KPS, 99 %, Sigma-Aldrich), and N,N,N′,N′-tetramethylethylenediamine (TEMED, 98 % Acros) were used for the corresponding cryogel synthesis. The enzyme, α-Glucosidase from Saccharomyces cerevisiae (100 Unit/mg, Sigma Aldrich), was entrapped within p(HEMA) cryogel and p-nitrophenyl-α-d-glucopyranoside (p-NPG, 99 %, Acros) was used as substrate for the enzymatic reactions. Rosmarinic acid (RA, 96 %, Aldrich) was used as inhibitor. Potassium phosphate monobasic (98–100.5 %, Sigma Aldrich), sodium hydroxide (NaOH, 99.9 %, VWR chemicals), and hydrochloric acid (HCl, 36.5 %, Sigma Aldrich) were used for the preparation of buffer solutions.
2.2. Synthesis of p(HEMA), and ɑ-Glucosidase@p(HEMA) cryogels
Synthesis of p(HEMA) cryogels was carried out by following the earlier reported literature [21]. In brief, 0.48 mL of HEMA monomer was placed in a vial with MBA at 10 % mole of HEMA monomer. Then, the volume was completed to 6.5 mL by adding 6.02 mL water. TEMED, 50 μL, as accelerator was added into the mixture after MBA crosslinker was dissolved, and then the mixture was placed in a deep freezer at -18 ℃ for chilling. Next, 1 mL of cooled initiator solution of KPS at 0.015 g/mL concentration was added into the cryogel precursor mixture and quickly vortexed and mixed before placing into plastic straws (0.8 cm in diameter). Then, the plastic straws were then kept in a deep freezer (-18 ℃) for 16 h to attain simultaneous polymerization and crosslinking of HEMA monomers under cryogenic conditions to deliver macroporous p(HEMA) cryogels.
The ɑ-Glucosidase enzyme entrapped in p(HEMA) cryogels (α-Glucosidase@p(HEMA)) was also prepared similarly as mentioned above. In short, various volumes of prepared α-Glucosidase-l-Glutathione solutions, 0.48, 0.96, and 1.92 mL, were added to the vial containing HEMA and MBA before completing the total volume of the solution to 6.5 mL. Then, 50 μL of TEMED was added into this mixture after dissolving the MBA crosslinker, and the mixture was placed in a deep freezer at -18 ℃ for cooling purposes. Next, 1 mL of KPS solution (0.015 g/mL) was added into this mixture and vortexed. Finally, the mixture was placed in plastic straws (0.8 cm diameter) and kept in a deep-freezer (-18 ℃) for 16 h to obtain the enzyme-entrapped p(HEMA) cryogels, α-Glucosidase@p(HEMA) cryogels.
The bare and enzyme-entrapped cryogels then were cut to similar shapes and sizes and washed with double distilled water 3 times and dried in a freeze-dryer. Then, the cryogels were stored in zip-lock bags at -18 ℃ in a deep-freezer for further usage. The prepared α-Glucosidase@p(HEMA) cryogels using 0.48, 0.96, and 1.92 mL enzyme solutions were donated as α-Glucosidase@p(HEMA)-1, α-Glucosidase@p(HEMA)-2, and α-Glucosidase@p(HEMA)-3, respectively.
2.3. Characterization
The swelling %, porosity %, pore volume %, and gel yield % of super porous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were calculated by using the follow equations [48,49];
| Swelling % = (mswollen – mdry) / mdry x 100 | (1) |
| Porosity % = (mswollen – mdry) / (mswollen – msqueezed) x 100 | (2) |
| Pore volume % = (mcyclohexane – mdry) / mdry x 100 | (3) |
| Gel yield % = (mproduct / mreactant) x 100 | (4) |
where is “mswollen” is the weight of water saturated cryogel pieces, “mdry” is the weight of washed and dried cryogel pieces, “msqueezed” is the weight of squeezed cryogel pieces after water saturation, “mcyclohexane” is the weight of cryogel pieces which were left in cyclohexane for 1 h, “mproduct” is the weight of prepared, washed and dried cryogel, and “mreactant” is the weight of cryogel precursors, respectively.
The super porous p(HEMA), and ɑ-Glucosidase@p(HEMA) cryogels were dried by using a freeze-dryer (Christ, Alpha 2-4 LSC). For this purpose, cryogel pieces were placed into a deep-freezer to freeze them and then these frozen cryogel pieces were placed into a freeze dryer (-86 ℃, 0.011 mbar pressure) and left for 1 one day to obtain dried cryogels.
The morphological properties of super porous p(HEMA) and α-Glucosidase@p(HEMA) cryogels were probed by using a scanning electron microscope (SEM, Hitachi, Regulus 8230). The SEM images of cryogels were acquired using cryogel pieces on SEM stabs that were coated with gold (∼few nanometers thickness) in low vacuum and 1 kV operating voltage.
The Fourier transform Infrared (FT-IR, Thermo, Nicolet iS10) spectra of p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were taken by using attenuated total reflection (ATR) between 4000−650 cm−1.
Thermal stabilities of p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were determined with a thermo gravimetric analyzer (TGA, Exstar, SII TG/DTA 6300). The analysis was carried out under continuous purging of nitrogen gas with 200 mL/min flow and the heating rate of 10 °C/min up to 800 °C.
Blood compatibility tests for p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were also determined via hemolysis %, and blood clotting indexes in accordance with the Human Research Ethics Committee of Canakkale Onsekiz Mart University (2011-KAEK-27/2020) by following the literature [50].
The ɑ-Glucosidase@p(HEMA), and free ɑ-Glucosidase enzyme used in reactions were monitored via ultraviolet-visible (UV–vis, PG Instruments, T80Plus) spectrometer at 400 nm wavelength.
2.4. Enzyme assay
The enzyme assays for free and entrapped ɑ-Glucosidase were determined by using p-NPG as substrate and following p-NP as product, according to the technical information provided by the manufacturer. The l-glutathione solution used for free and entrapped ɑ-Glucosidase enzyme assays as it serves as a cofactor for the enzymes. The observed absorbance values at 400 nm were presumed to be the enzyme activity [51].
In short, 5 mL of 67 mM potassium phosphate buffer solutions (PBS) at pH 6.8 were placed in a vial, and then certain volumes of 0.2 mM reduced glutathione solution containing enzyme:glutathione (1:1) was added into the buffer solution and incubated for 10 min at 37 ℃. Then, 0.5 mL of 10 mM p-NPG solution in water as substrate was added into the buffer solution containing the enzyme and reacted for 20 min at 37 ℃. Double distilled water, 0.4 mL, was added into the buffer solution instead of enzyme solution for the preparation of a control solution. The reaction was stopped by adding of 2 mL of test and control solutions into 8 mL of 100 mM Na2CO3 solutions separately. Finally, the absorbance values were recorded at 400 nm as Atest, and Acontrol. The activity of the enzyme in Units/mL enzyme was calculated by using Eq. (5) [51];
| Units/mL enzyme=((Atest–Acontrol)x(10)x(5.9)x(df))/((18.3)x(20)x(2)x(enzyme solution)) | (5) |
Where, “10” is the final solution volume (mL), “5.9” is volume of the reaction mixture (mL), “df” is dilution factor, “18.3” is mM extinction coefficient of p-NP at 400 nm, “20” is time for the assay (min), and “2” is the volume of reaction mixture added to the final solution (mL) of enzyme solution added into reaction, respectively. The definition of “Unit” is the liberated μmole of d-glucose from p-NPG per minute at pH 6.8 at 37 ℃.
The same assay medium was used for the determination of the activity of the entrapped enzyme. The one piece of ɑ-Glucosidase@p(HEMA) cryogel in 20−30 mg range was placed into 5 mL of buffer solution at pH 6.8 and incubated at 37 ℃ for 10 min, and then 0.5 mL 10 mM of p-NPG was added into the buffer solution containing ɑ-Glucosidase@p(HEMA) cryogels. The reaction was terminated with the removal of the enzyme-entrapped p(HEMA) cryogels and absorbance values were measured at 400 nm. All measurements were done in triplicate.
2.5. Effect of various parameters on enzyme activity
The effect of the amount of enzyme solutions, incubation time, pH, and temperature on reactions catalyzed by free and entrapped enzymes was investigated. The activity assays for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels and also for the system containing the equivalent amount of free enzyme were carried out by applying various incubation times, between 0−60 min, and also at different pHs, range 4.0–9.0, and temperature, range 15–60 ℃. The enzyme activity dependence on the amount of enzyme, incubation time in pH 6.8 at 37 ℃ before adding substrate, pH, and temperature was investigated in accordance with the system containing an equivalent amount of free enzyme being assigned the value of 100 % activity.
2.6. Kinetic and inhibition study
The kinetic and inhibition studies of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels and the system containing the equivalent amount of free enzyme were performed according to enzyme assays mentioned above. In the determination of kinetic parameters of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels with different amounts of enzyme, and the matching equivalent amounts of free enzyme, the well-known Lineweaver-Burk plotting method was used according to Eq. (6) [52];
| 1/v = Km/vmax x 1/[S] + 1/vmax | (6) |
Where “v” is initial and “vmax” is maximum reaction rate (mM/min), “[S]” is the substrate concentration (mM), and Km is the Michaelis-Menten constant, respectively.
For the comparison of inhibition capability of ɑ-Glucosidase@p(HEMA) cryogels containing different amounts of enzyme with matching equivalent amount of free enzyme, rosmarinic acid was used as a model inhibitor [53]. For this purpose, rosmarinic acid stock solutions at 3.6 mg/mL concentration in pH 6.8 buffer solution were used for the preparation of various rosmarinic acid solutions at different concentrations of 1.8, 1.2, 0.9, 0.45, 0.22, and 0.11 mg/mL. Then, 1 mL of each rosmarinic acid solution was added into the mixture of solution containing enzymes. The observed absorbance values for ɑ-Glucosidase@p(HEMA) cryogels as Atest, and absorbance values from the reaction involving the inhibitor as Ainh were used in the calculation of inhibition%. The inhibition% of free and ɑ-Glucosidase@p(HEMA) cryogels was calculated using Eq. (7) [54];
| Inhibition % = (Atest – Ainh) / Atest x100 | (7) |
2.7. Reusability, and Stability of ɑ-Glucosidase@p(HEMA) cryogels
The reusability of ɑ-Glucosidase@p(HEMA) cryogels was tested by using the same enzyme assay sequentially. One piece of ɑ-Glucosidase@p(HEMA) cryogels containing different amounts of enzyme were washed with 10 mL buffer solution at pH 6.8 for 30 min, and then were used again 10 times consecutively. The decrease in the activity% of prepared ɑ-Glucosidase@p(HEMA) cryogels was calculated assuming the activity% of the first usage of each set was 100 % activity.
The stability of ɑ-Glucosidase@p(HEMA) cryogels was examined by comparing with the equivalent amounts of free enzymes after storing them at room temperature (25 ℃) for 10 days. For this purpose, one piece of ɑ-Glucosidase@p(HEMA) cryogels, and the matching equivalent amount of free enzyme was used for the reaction, and the decrease in activity% of each of the enzyme systems were calculated. The activity% on first day for each set was considered to be 100 % activity.
3. Result and discussions
3.1. Synthesis and characterization of ɑ-Glucosidase entrapped p(HEMA) cryogels
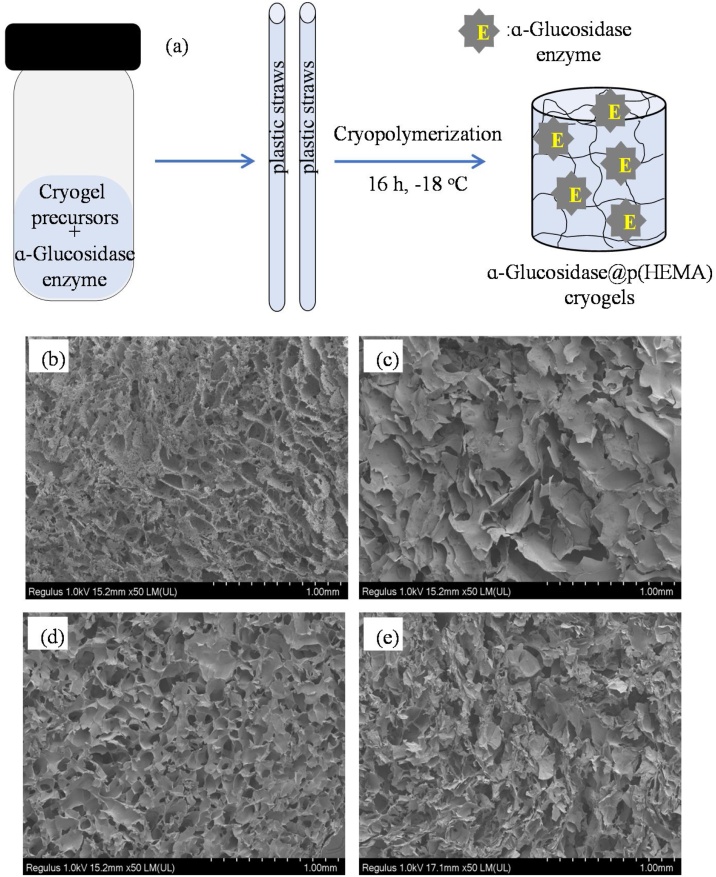
However, p(HEMA) cryogels used in this study were not a support material for enzyme immobilization, rather they were constructed around ɑ-Glucosidase to engulf or entrap them within the pores of the cryogel networks. For this aim, during the synthesis of p(HEMA) cryogel, the aqueous solutions of enzymes at 0.48, 0.96 and 1.92 mL were added to the enzyme + reduced glutathione solution and mixed with cryogel precursors. Then, the common cryopolymerization procedure was followed to achieve enzyme entrapment within p(HEMA) cryogels, ɑ-Glucosidase@p(HEMA). It was presumed that the low temperature synthesis conditions (-18 ℃ typically) do not affect the enzyme stability and/or enzyme structure. The schematic presentation of synthesis of ɑ-Glucosidase@p(HEMA) cryogels is illustrated in Fig. 1(a). As seen, the solution of cryogel precursors and enzyme (at varying amounts) was placed into plastic straws and cryo-entrapped at −18 ℃ for 16 h.
Fig. 1.
(a) The schematic presentation of preparation of ɑ-Glucosidase@p(HEMA) cryogels, and SEM images of (b) p(HEMA), (c) ɑ-Glucosidase@p(HEMA)-1, (d) ɑ-Glucosidase@p(HEMA)-2, and (e) ɑ-Glucosidase@p(HEMA)-3 cryogels.
The frozen ɑ-Glucosidase@p(HEMA) cryogels were cut to similar shapes and sizes and washed with double distilled water 3 times and stored in zip-lock bags at -18 ℃ after freeze-drying. In this system, the polymerization and simultaneous crosslinking of HEMA monomers occurred around the ice crystals, and ɑ-Glucosidase enzymes were entrapped within pores under cryogenic conditions. The SEM images of p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels with various ratios of HEMA:enzyme according to HEMA monomer of 1:1, 1:2, and 1:4 are given in Fig. 1(b), (c), (d), and (e), respectively. The SEM images clearly reveal that the pores and pore wall structures of p(HEMA) cryogels are slightly different then the pores and pore wall structures of ɑ-Glucosidase@p(HEMA) cryogels. However, the pore sizes of p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels are very similar and range from 10−200 μm in size. Therefore, the presence of ɑ-Glucosidase does not alter the macroporous structure of p(HEMA) cryogels.
The FT-IR spectra of superporous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels are compared in Fig. 2(a). The characteristic peaks for p(HEMA) cryogels such as C = O peaks at about 1710 cm−1, C-O-H peaks at about 1450 cm−1, and -C-O peaks between 1320−1210 cm−1 were observed for all the FT-IR spectra of p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels prepared with different amounts of enzyme. The disappearance of the peak at about 902 cm−1 with the increase in the amount of ɑ-Glucosidase enzyme can be attributed to the more discernible feature of enzymes with higher concentration in the cryogel pores.
Fig. 2.
(a) FT-IR spectra, (b) TGA thermogram of prepared ɑ-Glucosidase@p(HEMA) cryogels, and (c) schematic presentation of enzymatic reaction.
The thermal stability of superporous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were also compared as demonstrated in Fig. 2(b). It was observed that p(HEMA) cryogels have one distinct thermal degradation step between 250–450 ℃ with 95.2 % weight loss, and then 97 % cumulative weight loss was observed at 800 ℃. On the other hand, ɑ-Glucosidase@p(HEMA) cryogels had two thermal degradation steps in all of the thermograms of ɑ-Glucosidase@p(HEMA) cryogels, with 2.1, 3.3, and 7.5 % weight losses observed up to 100 ℃ heating which can be assigned to denaturation of enzymes. Moreover, the degradation steps which started between 125–360 ℃ had 69.7, 63.8, and 51.7 % weight loss, and another degradation interval occurred between 370–440 ℃ with 82.9, 85.9, and 76.6 % weight loss for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively. Also 95.3, 90.5, and 89.6 % weight loss was observed at 800 ℃ for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively. Therefore, it is apparent that the thermal stability of p(HEMA) cryogels was decreased with increase in the amount of ɑ-Glucosidase enzyme entrapped, due to lower thermal stability of the enzymes.
The change in swelling %, porosity %, pore volume %, and gel yield % of the prepared superporous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels were calculated by using Eq. (1),(2),(3), and (4), respectively, and are compared in Table 1. The swelling %, porosity %, pore volume %, and gel yield % of superporous p(HEMA) cryogels were determined as 960 ± 59 %, 58.1 ± 3.2 %, 90.2 ± 1.3 %, and 94.4 ± 0.8 %, respectively.
Table 1.
Comparison of swelling, porosity, pore volume, and gel yield% values of superporous p(HEMA), and ɑ-Glucosidase@p(HEMA) cryogels.
| Cryogel | Swelling % | Porosity % | Pore volume % | Gel yield % |
|---|---|---|---|---|
| P(HEMA) | 960 ± 59 | 58.1 ± 3.2 | 90.2 ± 1.3 | 94.4 ± 0.8 |
| ɑ-Glucosidase@p(HEMA)-1 | 942 ± 24 | 50.5 ± 1.9 | 87.2 ± 2.1 | 107.2 ± 3.1 |
| ɑ-Glucosidase@p(HEMA)-2 | 927 ± 41 | 44.6 ± 4.7 | 86.1 ± 1.5 | 111.1 ± 2.8 |
| ɑ-Glucosidase@p(HEMA)-3 | 902 ± 29 | 41.6 ± 3.4 | 83.4 ± 2.6 | 119.7 ± 3.3 |
It is apparent that these values were slightly decreased with the presence of ɑ-Glucosidase within cryogels and this decrease was augmented by the increase in the amounts of enzyme within p(HEMA). The swelling % values were calculated as 942 ± 24, 927 ± 41, and 902 ± 29 %, porosity % values were determined as 50.8 ± 1.9, 44.6 ± 4.7 %, and 41.6 ± 3.4 %, and the pore volume % values computed as 87.2 ± 2.1, 86.1 ± 1.5, and 83.4 ± 2.6 %. Interestingly, the gel yield % values were found to increase with the increase in the enzyme feed amount during synthesis as 107.2 ± 3.1, 111.1 ± 2.8, and 119.7 ± 3.3 % gel yields were calculated for ɑ-Glucosidase@p(HEMA) cryogels by using 0.48, 0.96, and 1.92 mL enzyme solutions, respectively, during synthesis.
One of the key parameters for materials in biomedical fields is their blood compatibility. Therefore, hematolytic tests were completed for superporous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels. The comparison of hemolysis % values for p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels is illustrated in Fig. S1 (a). Hemolysis, also known as the destruction of erythrocyte cells, is required to be less than 5% for blood contact materials to be considered blood compatible [55]. Here, the hemolysis% of p(HEMA) cryogels was found to be 0.14 ± 0.08 %, whereas the hemolysis% of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels was observed as 1.10 ± 0.7, 1.3 ± 0.05, and 1.05 ± 0.3 %, respectively. Although there is a slightly increase with the amount of enzyme used, cryogels are well below the 5% threshold for blood compatibility proving that ɑ-Glucosidase@p(HEMA) cryogels are non-hemolytic and can be safely deployed in blood contacting applications.
Another parameter for blood compatibility is blood clotting index which is also known as the anti-thrombogenic activity of materials upon contact with blood [50]. The comparison of blood clotting indexes for p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels with different enzyme content is illustrated in Fig. S1 (b). It was observed that the blood clotting index of p(HEMA) cryogels is 93.1 ± 0.6 and it changed slightly by entrapping different amounts of enzyme solutions, e.g., 88.7 ± 1.3, 97.7 ± 0.6, and 87.4 ± 2.4 % for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively. These results thus show that all superporous p(HEMA) and ɑ-Glucosidase@p(HEMA) cryogels have similar anti-thrombogenic properties that will not cause blood clotting in contact with blood.
3.2. Activity of ɑ-Glucosidase enzyme
The activity of ɑ-Glucosidase enzyme was calculated according to the technical information provided by the manufacturer [51]. The measured absorbance values were measured at 400 nm due to liberation of p-NP from p-NPG in the presence of ɑ-Glucosidase enzyme and p-NPG substrate in 67 mM Potassium phosphate buffer solution at pH 6.8, 37 ℃ in 20 min reaction time. The activity of free ɑ-Glucosidase solution was calculated as 0.065 ± 0.003, 0.031 ± 0.002, and 0.015 ± 0.003 U/mL enzyme for 20, 40, and 80 μL enzyme solutions, respectively.
3.2.1. Activities of free and entrapped ɑ-Glucosidase enzymes
The activity of free and entrapped ɑ-Glucosidase enzymes was measure by following of p-NP as a byproduct of enzyme-p-NPG reaction. Schematic presentation of reaction in given in Fig. 2 (c). The ɑ-Glucosidase@p(HEMA) cryogels prepared by adding 0.48, 0.96, and 1.92 mL of enzyme stock solutions by replacing the equivalent amount of water in the synthesis of p(HEMA) cryogels, were cut to about the similar shapes and sizes, with each piece weighing in 20−30 mg range. The activity of ɑ-Glucosidase@p(HEMA) cryogels with different amounts of enzymes were calculated and compared with the equivalent amount of free enzyme, and the corresponding graph is given in Fig. 3(a). It is obvious that with the increase in the amounts of entrapped enzyme, there is a decrease in the activities of both free and entrapped ɑ-Glucosidase.
Fig. 3.
The comparison of (a) activities, (b) activity % of ɑ-Glucosidase@p(HEMA) cryogels with equivalent amounts of free enzyme, and (c) the effect of incubation time on activity % of ɑ-Glucosidase@p(HEMA) cryogels. [Reaction conditions: 5 mL of pH 6.8 buffer solution, 0.5 mL of 10 mM substrate, 37 ℃].
The activity of ɑ-Glucosidase@p(HEMA)-1 cryogels was measured as 0.06 U/mL enzyme, whereas the activity values were found to decrease with the increase in volume of enzyme solutions, as 0.028 U/mL enzyme, and 0.012 U/mL enzyme were measured for ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively. The decrease in both free and entrapped enzyme activity can be explained by the enzyme inclination to self-associate in such a way that the active site can be blocked. Further increases in the amounts of entrapped enzymes can lead to enzyme aggregation due to the overcrowded enzymes in a confined space. This would reduce the active sites for the substrate [56]. The decrease in activity% of free and entrapped enzymes was also compared in Fig. 3(b), and as seen the activity% of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels maintained their activity% as 92.3 ± 1.4, 84.8 ± 1.9, and 85.7 ± 2.1 % in comparison to the equivalent amount of free enzymes, respectively. The activity% of prepared enzyme stock solutions were calculated as 0.065 ± 0.003, 0.031 ± 0.002, and 0.015 ± 0.003 U/mL enzyme for 20, 40, and 80 μL enzyme solutions by using Eq. 5. After that, the certain amounts of stock enzyme solutions such as 0.48 mL, 0.96 mL, and 1.92 mL, were added into cryogel precursor solutions. Then, the activity% of prepared and equivalent amount α-Glucosidase enzyme containing α-Glucosidase@p(HEMA)-1, α-Glucosidase@p(HEMA)-2, and α-Glucosidase@p(HEMA)-3 cryogel pieces in 0.25 mg of each cryogel were calculated as 0.06 ± 0.001, 0.028 ± 0.002, and 0.012 ± 0.0004 U/mL enzyme, respectively.
After the α-Glucosidase@p(HEMA) cryogels were synthesized, the washing solutions were reacted with p-NPG substrate according to the enzyme assay and no absorbance values was observed at a wavelength of 400 nm. Therefore, the amount of enzyme entrapped with cryogel was accepted as 100 %.
Equivalent amount of free and entrapped enzyme was calculated gravimetrically. Therefore, approximately 0.25 g of cryogels of α-Glucosidase@p(HEMA)-1, α-Glucosidase@p(HEMA)-2, and α-Glucosidase@p(HEMA)-3 were weighed to contain 20, 40, and 80 μL of enzymes and used in the enzymatic reactions. Moreover, the effect of incubation time, pH and temperature on activity% of ɑ-Glucosidase@p(HEMA) cryogels were investigated and compared with free enzyme.
3.2.1.1. Effect of incubation time on activity% of ɑ-Glucosidase@p(HEMA) cryogels
In enzymatic reactions, whether free or immobilized or entrapped enzyme systems need to be tested for the optimum conditions for their particular reactions. Therefore, the effect of incubation time on activity of ɑ-Glucosidase@p(HEMA) cryogels was investigated and compared with free enzyme and illustrated in Fig. 3(c). Free enzymes were incubated in 5 mL of potassium phosphate buffer solution at pH 6.8, 37 ℃ before adding the substrate solution for the reaction times of 5, 10, 20, 30, 45, and 60 min. It was observed that the activity% of free enzymes was increased up to 10 min incubation and decreased with the increase of incubation time. Similarly, the ɑ-Glucosidase@p(HEMA) cryogels were incubated in 5 mL of potassium phosphate buffer solution at pH 6.8 and 37 ℃ before adding the substrate solution for the reaction times of 5, 10, 20, 30, 45, and 60 min. As can be seen in Fig. 3(c), for all ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, the maximum activity was observed for 10 min incubation times. Therefore, the optimum incubation time for ɑ-Glucosidase@p(HEMA) cryogels was 10 min at 37 ℃. As the common conditions required to store enzymes are generally at 5 °C and below, the enzymes can lose their activity even when they remain too much time in reaction conditions [57].
3.2.1.2. Effect of reaction temperature on activity% of ɑ-Glucosidase@p(HEMA) cryogels
As the reaction temperature is very important for any kind of reactions including enzymatic reactions, the effect of reaction temperature for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels were investigated and the results were compared with systems containing the equivalent amount free enzyme in 67 mM potassium phosphate buffer at pH 6.8, at medium temperature 15, 30, 37, 45, and 60 ℃. It is clear from Fig. 4(a), (b) and (c) that the maximum activities% for both free and ɑ-Glucosidase enzymes entrapped cryogels was found at 37 ℃. These results corroborate that the technical information provided by the manufacturer is correct. Also, the activities% of both free and entrapped ɑ-Glucosidase enzymes were found to decrease with reaction temperature below or above 37 ℃ with similar decrease rates in activity% regardless of their amount. For example, the ɑ-Glucosidase@p(HEMA)-1 cryogels preserved their activity% as 40 ± 2.5 % at 30 ℃, whereas this value reduced to 17 ± 1.3 % at 15 ℃ as shown in Fig. 4(a). On the other hand, the free ɑ-Glucosidase enzymes preserved their activity% as 26 ± 2.1 and 8 ± 0.7 % at 30 and 15 ℃, respectively. Also, with the increase in reaction temperature e.g., 45 and 60 ℃, the activity% of ɑ-Glucosidase@p(HEMA)-1 cryogels prepared were 51 ± 2.2, and 20 ± 1.8 %, respectively, whereas the free ɑ-Glucosidase enzymes had lower activity%, e.g., 32 ± 2.6, and 6 ± 0.6 %, respectively at the same reaction conditions.
Fig. 4.
The effect of reaction temperature on activity% of (a) ɑ-Glucosidase@p(HEMA)-1, (b) ɑ-Glucosidase@p(HEMA)-2, and (c) ɑ-Glucosidase@p(HEMA)-3 cryogels and their comparison with equivalent amounts of free enzyme. [Reaction condition: 5 mL of pH 6.8 buffer solution, 0.5 mL of 10 mM substrate].
The effect of reaction temperature on activity% of ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels also showed similar results as demonstrated in Fig. 4(b), and (c), respectively.
In summary, there is no shift for activity% from optimal temperature, 37 ℃, for the free or entrapped enzyme to higher values as is a common case for regular synthetic catalysts. Also, it was clearly seen that the entrapment of ɑ-Glucosidase enzymes into p(HEMA) cryogels slightly increased the thermal stability of the entrapped enzyme than free ɑ-Glucosidase enzymes. This can be explained by the fact that ɑ-Glucosidase enzymes entrapped within pores and pore walls of p(HEMA) cryogels can become be protected against harsh conditions, e.g., temperature, maintaining the active structure of the ɑ-Glucosidase enzymes firmly becoming more stable [58,59].
3.2.1.3. Effect of reaction pH on activity% of ɑ-Glucosidase@p(HEMA) cryogels
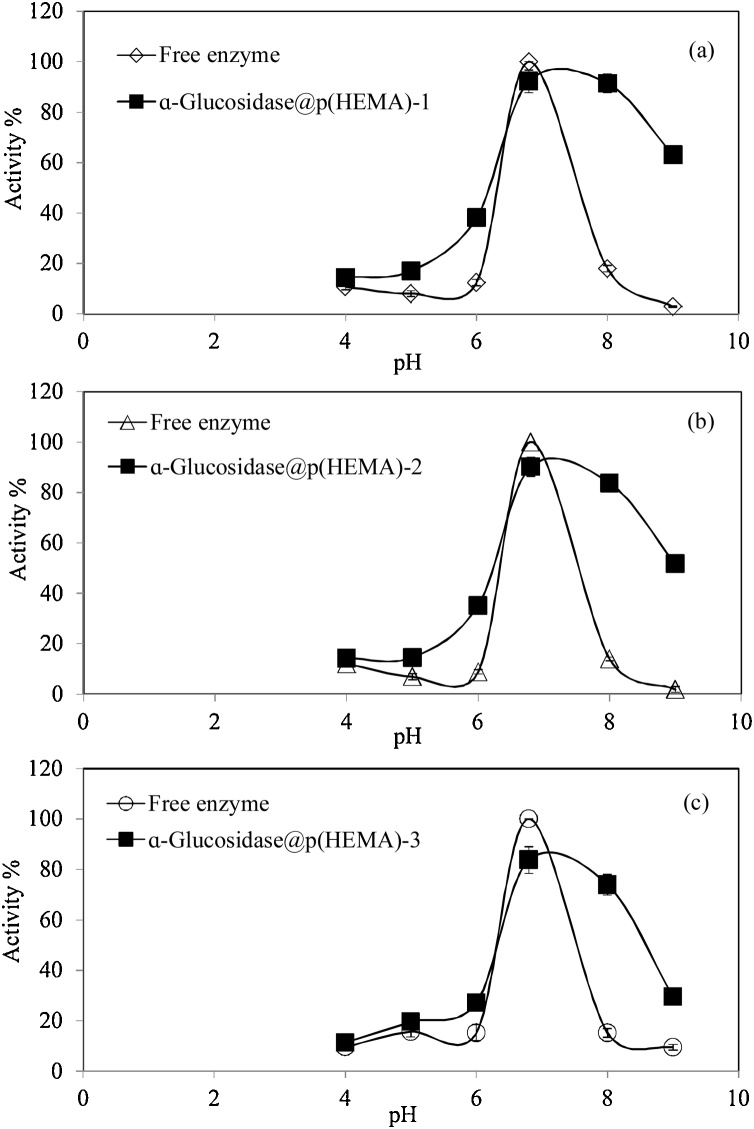
In enzymatic reactions, the medium pH is also very important as well as incubation time and temperature. Therefore, the effect of reaction pH on activity% of ɑ-Glucosidase@p(HEMA) cryogels and free ɑ-Glucosidase enzymes was compared and given in Fig. 5. The studies were carried out in 5 mL of 67 mM potassium phosphate buffer solution at 37 ℃ in the pH range of 4−9. Fig. 5(a), (b), and (c) presents the effect of pH medium for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively, along with matching amounts of free enzymes. Similar activity% profiles were observed for all entrapped and free enzymes and the pH 6.8 value was found to be the optimum solution pH for all ɑ-Glucosidase@p(HEMA) cryogels and free ɑ-Glucosidase enzymes with highest activity% values. Similarly, it was also observed that the activity% of free ɑ-Glucosidase and entrapped ɑ-Glucosidase have lower activity% values at solution pHs of 4, 5 and 6 compared to the higher pH values. Interesting, at higher solution pHs, the ɑ-Glucosidase@p(HEMA) cryogels revealed much higher activity% than free enzymes at pH 8 and 9, as shown in Fig. 5(a), (b), and (c). Also, there is little change in activity% values of entrapped enzymes between pH 7 and 8. For example, ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels preserved their activity% as 91 ± 3.8, 84 ± 2.8, and 74 ± 4.1 % activities at pH 8 in comparison to their activity% values of 92 ± 4.5, 90 ± 3.8, and 84 ± 5.3 % activities at pH 7, respectively.
Fig. 5.
The effect of reaction pH on activity% of (a) ɑ-Glucosidase@p(HEMA)-1, (b) ɑ-Glucosidase@p(HEMA)-2, and (c) ɑ-Glucosidase@p(HEMA)-3 cryogels and their equaivalent amounts of free enzyme. [Reaction condition: 5 mL of buffer solution, 0.5 mL of 10 mM substrate, 37 ℃].
To sum up, the entrapped ɑ-Glucosidase in p(HEMA) cryogel can enable ɑ-Glucosidase enzyme to react in a higher pH range as higher enzyme activity% were observed compared to the free ɑ-Glucosidase enzyme at pH 8 and 9. This can be attributed to the existence of some functional group such as −OH and carbonyl groups around the ɑ-Glucosidase within the pores of cryogels that render it resistant to pH changes. It was also reported that the immobilized enzymes show high activity in a broader working pH range [42,60], due to the transformation of enzymes into more stable structures by immobilization [42]. In addition, the ability of the support material to act as an ionic heat exchanger plays an important role in acting as a "solid" buffer that produces a pH that can be substantially different from the pH in the reaction medium where enzymes can operate at high activity [61].
3.3. Comparison of kinetic parameters
Kinetic parameters of the enzymatic reaction, such as Km and vmax values for each ɑ-Glucosidase@p(HEMA) cryogels and free ɑ-Glucosidase enzyme, can be estimated by the direct linear method of the Lineweaver–Burk plot for the initial p-NPG degradation rates at various p-NPG substrate concentrations in 5 mL 67 mM potassium phosphate buffer solution at 37 ℃, and corresponding graphs are given in Fig. 2S. The maximum velocity of product formation is Vmax and the affinity of the enzyme toward its substrate is presented by Km. The comparisons of results are summarized in Table 2. The Km values were calculated as 3.22, 1.04, and 0.69 mM for ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, respectively and their Km values for the equivalent amounts of free enzymes were estimated as 1.97, 1.17, and 0.68 mM. The Km value of ɑ-Glucosidase@p(HEMA)-1 cryogels is almost 1.5-fold higher than equivalent amounts of free enzymes, whereas Km values were almost the same for ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels and equivalent amounts of free enzymes.
Table 2.
Kinetic parameters of ɑ-Glucosidase@p(HEMA) cryogels, and equivalent amount of free enzyme.
| Material | Enzyme | Km (mM) | Vmax (mM/min) |
|---|---|---|---|
| ɑ-Glucosidase@p(HEMA)-1 | 0.48 mL | 3.22 | 0.0048 |
| ɑ-Glucosidase@p(HEMA)-2 | 0.96 mL | 1.04 | 0.0021 |
| ɑ-Glucosidase@p(HEMA)-3 | 1.92 mL | 0.69 | 0.0016 |
| Equaivalent amount of free enzyme | 20 μL | 1.97 | 0.0032 |
| 40 μL | 1.17 | 0.0024 | |
| 80 μL | 0.68 | 0.0018 |
The fact that the ɑ-Glucosidase@p(HEMA) cryogels have higher Km value than the free ɑ-Glucosidase enzyme can be attributed to the change in the substrate affinity of the enzyme. This may be due to structural changes in the entrapped ɑ-Glucosidase enzyme as it can be more difficult to access the substrate to reach the active site of the ɑ-Glucosidase than for free ɑ-Glucosidase enzyme due to free mean path within p(HEMA) cryogel network [[62], [63], [64]].
Moreover, the Vmax value of ɑ-Glucosidase@p(HEMA)-1 cryogels was calculated as 1.5-fold higher than equivalent amounts of free enzymes with Vmax values of 0.0048 mM/min, and 0.0032 mM/min, respectively. On the other hand, Vmax values of ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels were determined as 0.0021 mM/min, and 0.0016 mM/min, respectively, which are almost the same as Vmax values of equivalent amounts of free enzymes, 0.0024, and 0.0018 mM/min, respectively. The Vmax value of ɑ-Glucosidase@p(HEMA) cryogels prepared by using 0.48 mL enzyme solution is higher than both the ɑ-Glucosidase@p(HEMA) cryogels prepared by using 0.96, and 1.92 mL enzyme solutions and all the compared free ɑ-Glucosidase enzyme, revealing the highest product formation rate [62] which suggests this amount is an optimum concentration. The high or low stabilization of the transition complex between enzyme and substrate is probably a crucial event related to the achieved higher Vmax even higher Km values. Also, higher Vmax value for entrapped enzyme than free enzyme suggest that immobilized enzyme is more efficient than free enzyme [65].
3.4. Stability and reusability of ɑ-Glucosidase@p(HEMA) cryogels
The stability and reusability of enzymes are also the most important parameters to consider in industrial biotechnological applications. Therefore, the stability and reusability of prepared ɑ-Glucosidase@p(HEMA) cryogels were investigated and corresponding graphs are illustrated in Fig. 6. In reusability studies, one piece of cryogel containing enzymes, e.g., ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, were used in enzyme assays as mentioned earlier in 67 mM potassium phosphate buffer at pH 6.8 and 37 ℃, and washed in 10 mL of 67 mM potassium phosphate buffer at pH 6.8 to remove the product before reuse again. This cycle was repeated ten times, consecutively. The decrease in the activity% of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels in 10 repeated uses is compared in Fig. 6(a). It is clear that the activity% of ɑ-Glucosidase@p(HEMA) cryogels decreased with the increasing number of reuses. Amongst all the different amounts of cryogels containing enzyme, ɑ-Glucosidase@p(HEMA)-1 cryogels, were able to retain their activity% more than 50 % after 10 consecutive uses.
Fig. 6.
The comparisons of (a) the reusability of ɑ-Glucosidase@p(HEMA)-1, ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, (b) the storage stability of ɑ-Glucosidase@p(HEMA)-1 cryogels with its equivalent amount of free enzyme. [Reaction condition: 5 mL of pH 6.8 buffer solution, 0.5 mL of 10 mM substrate, 37 ℃].
The activity% of ɑ-Glucosidase@p(HEMA)-2, and ɑ-Glucosidase@p(HEMA)-3 cryogels, decreased under 50 % after 7 and 6 consecutively uses and further decreased to 30 ± 2.8, and 15 ± 1.3 % after 10 consecutive uses, respectively. Again, it seems ɑ-Glucosidase@p(HEMA)-1 cryogels could afford better operational cost with better economic efficiency in practical applications.
In the stability studies, the ɑ-Glucosidase@p(HEMA)-1 cryogels, and the corresponding free enzyme solution (containing the same amount of enzyme) were stored at room temperature, 25 ℃ for ten days. Then, the activity % of ɑ-Glucosidase@p(HEMA) cryogel and equivalent amounts of free enzymes were calculated for each 10 days by using new pieces of ɑ-Glucosidase@p(HEMA) cryogel, and equivalent amounts of free enzymes, and the corresponding graph is shown in Fig. 6(b). It is apparent that the activity% of free ɑ-Glucosidase decreased to less than 50 % after the 5th day, and almost completely lost its activity linearly until the 10th day. On the other hand, the ɑ-Glucosidase@p(HEMA) cryogel maintained 50 % activity even after the 10th day. Therefore, the stability of ɑ-Glucosidase at room temperature increased with the entrapment in p(HEMA) cryogel and ɑ-Glucosidase@p(HEMA)-1 cryogels can offer economic viability for industrial biotechnological applications.
3.5. Inhibition profile of ɑ-Glucosidase@p(HEMA) cryogel
Therefore, the inhibition profile of ɑ-Glucosidase@p(HEMA)-1 cryogel was also investigated by using rosmarinic as inhibitor and compared with free ɑ-Glucosidase enzyme.
As Fig. 7 clear shows, both entrapped and free ɑ-Glucosidase enzymes had similar inhibition profiles. As 0.45 mg/mL concentration of rosmarinic acid can inhibit almost 50 % activity of both entrapped and free ɑ-Glucosidase enzymes, and almost 100 % inhibition was observed in the presence of 1.2 mg/mL concentration of rosmarinic acid, any inhibitor can affect both enzymes in the same manner suggesting that the enzyme entrapment does not cause any inhibitory effect for entrapped enzymes.
Fig. 7.
The inhibition % profile of α-glucosidase@p(HEMA)-1 cryogels, and its equivalent amount of free enzyme. [Reaction condition: 4 mL of pH 6.8 buffer solution, 0.5 mL of 10 mM substrate, 1 mL of inhibitor solution in pH 6.8 buffer 37 ℃].
4. Conclusion
Here, we reported the use of a biocompatible superporous p(HEMA) cryogel for ɑ-Glucosidase enzyme entrapment during generation of a superporous network formation. The amount of entrapped ɑ-Glucosidase enzyme within p(HEMA) cryogels can be readily increased with the increase in the amount of enzyme solution during synthesis of p(HEMA) cryogel. The activity% of ɑ-Glucosidase@p(HEMA) cryogels decreased with the increase in the amount of enzyme, attributed to aggregation due to overcrowding of enzymes within the pores of p(HEMA) cryogels. The stability of ɑ-Glucosidase enzyme was increased after entrapping in p(HEMA) cryogels resulting in higher activity% values at a wide range of temperature (≥37 ℃) and pH (≥6.8–9) values. The reuse studies of ɑ-Glucosidase enzyme entrapped within p(HEMA) cryogel provided 10 consecutive enzymatic reactions with more than 50 % activity, and 10 days of storage at room temperature that is superior to the free ɑ-Glucosidase enzyme. Because of the interconnected superporous structure of the cryogels, it is inferred that p(HEMA) cryogels can be successfully employed for different enzymatic reactions, e.g., the hydrolysis of reactions of α-1,4-linked glucose residues from aryl (or alkyl) -glycosides, disaccharides or oligosaccharides. Therefore, the use of cryogel networks with interconnected pore sizes may enable entrapment of other enzymes for novel biotechnological use in real industrial applications.
CRediT authorship contribution statement
Sahin Demirci: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Mehtap Sahiner: Formal analysis, Investigation, Methodology, Writing - original draft. Selehattin Yilmaz: Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing - review & editing. Erdener Karadag: Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing - review & editing. Nurettin Sahiner: Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
A scholarship for Sahin Demirci from Council of Higher Education System (YOK-100/2000) is gratefully acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00534.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Homaei A.A., Sariri R., Vianello F., Stevanato R. Enzyme immobilization: an update. J. Chem. Biol. 2013;6:185–205. doi: 10.1007/s12154-013-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illanes A., Cauerhff A., Wilson L., Castro G.R. Recent trends in biocatalysis engineering. Bioresour. Technol. 2012;115:48–57. doi: 10.1016/j.biortech.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Pera L.M., Baigori M.D., Pandey A., Castro G.R. Elsevier B.V.; 2015. Biocatalysis, Industrial Biorefineries and White Biotechnology. [DOI] [Google Scholar]
- 4.Cacicedo M.L., Manzo R.M., Municoy S., Bonazza H.L., Islan G.A., Desimone M., Bellino M., Mammarella E.J., Castro G.R. Biomass, Biofuels, Biochemicals: Advances in Enzyme Technology. 2019. Immobilized enzymes and their applications. [DOI] [Google Scholar]
- 5.Moehlenbrock M.J., Minteer S.D. Enzyme Stabilization and Immobilization. 2017. Introduction to the Field of enzyme immobilization and stabilization; pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Pooni J., Giustozzi F., Robert D., Setunge S., O’Donnell B. Durability of enzyme stabilized expansive soil in road pavements subjected to moisture degradation. Transp. Geotech. 2019;21 doi: 10.1016/j.trgeo.2019.100255. [DOI] [Google Scholar]
- 7.Krishnamurthy A., Mundra S., Belur P.D. Improving the catalytic efficiency of Fibrinolytic enzyme from Serratia marcescens subsp. Sakuensis by chemical modification. Process Biochem. 2018;72:79–85. doi: 10.1016/j.procbio.2018.06.015. [DOI] [Google Scholar]
- 8.Ribeiro A.L., Sánchez M., Hidalgo A., Berenguer J. Stabilization of enzymes by using thermophiles. In: Barredo J.-L., Herráiz I., editors. Microbial Steroids, Methods in Molecular Biology. Springer; New York, New York, NY: 2017. pp. 297–312. [DOI] [Google Scholar]
- 9.Kurayama F., Mohammed Bahadur N., Furusawa T., Sato M., Suzuki N. Facile preparation of aminosilane-alginate hybrid beads for enzyme immobilization: kinetics and equilibrium studies. Int. J. Biol. Macromol. 2020;150:1203–1212. doi: 10.1016/j.ijbiomac.2019.10.130. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi M., Sohrabi M.J., Hosseinali S.H., Hasan A., Kani P.H., Talaei A.J., Karim A.Y., Nanakali N.M.Q., Salihi A., Aziz F.M., Yan B., Khan R.H., Saboury A.A., Falahati M. Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy. Int. J. Biol. Macromol. 2020;143:665–676. doi: 10.1016/j.ijbiomac.2019.12.064. [DOI] [PubMed] [Google Scholar]
- 11.Tang W., Chen C., Sun W., Wang P., Wei D. Low-cost mussel inspired poly(Catechol/Polyamine) modified magnetic nanoparticles as a versatile platform for enhanced activity of immobilized enzyme. Int. J. Biol. Macromol. 2019;128:814–824. doi: 10.1016/j.ijbiomac.2019.01.161. [DOI] [PubMed] [Google Scholar]
- 12.Tran D.N., Balkus K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011;1:956–968. doi: 10.1021/cs200124a. [DOI] [Google Scholar]
- 13.Askaripour H., Vossoughi M., Khajeh K., Alemzadeh I. Examination of chondroitinase ABC I immobilization onto dextran-coated Fe3O4 nanoparticles and its in-vitro release. J. Biotechnol. 2020;309:131–141. doi: 10.1016/j.jbiotec.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Girelli A.M., Quattrocchi L., Scuto F.R. Silica-chitosan hybrid support for laccase immobilization. J. Biotechnol. 2020;318:45–50. doi: 10.1016/j.jbiotec.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Lopez L., Pedrero S.G., Lopez-Carrobles N., Virgen-Ortíz J.J., Gorines B.C., Otero C., Fernandez-Lafuente R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers: avoiding enzyme release from the support. Process Biochem. 2017;54:81–88. doi: 10.1016/j.procbio.2016.12.018. [DOI] [Google Scholar]
- 16.Song J., He W., Shen H., Zhou Z., Li M., Su P., Yang Y. Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability. Chem. Commun. 2019;55:2449–2452. doi: 10.1039/c8cc09717h. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Haq I., Prakash J., Raj A. Improved enzyme properties upon glutaraldehyde cross-linking of alginate entrapped xylanase from Bacillus licheniformis. Int. J. Biol. Macromol. 2017;98:24–33. doi: 10.1016/j.ijbiomac.2017.01.104. [DOI] [PubMed] [Google Scholar]
- 18.Hanefeld U., Gardossi L., Magner E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009;38:453–468. doi: 10.1039/b711564b. [DOI] [PubMed] [Google Scholar]
- 19.Kharisov B.I., Eldin M.S.M. Enzyme immobilization: nanopolymers for enzyme immobilization applications. CRC Concise Encycl. Nanotechnol. 2018:220–228. doi: 10.1201/b19457-22. [DOI] [Google Scholar]
- 20.Lozinsky V.I., Okay O. Advances in Polymer Science. 2014. Basic principles of cryotropic gelation; pp. 49–101. [DOI] [Google Scholar]
- 21.Seven F., Sahiner N. Modified macroporous P(2-hydroxyethyl methacrylate) P(HEMA) cryogel composites for H2 production from hydrolysis of NaBH4. Fuel Process. Technol. 2014;128:394–401. doi: 10.1016/j.fuproc.2014.08.008. [DOI] [Google Scholar]
- 22.Karacan P., Okay O. Ethidium bromide binding to DNA cryogels. React. Funct. Polym. 2013;73:442–450. doi: 10.1016/j.reactfunctpolym.2012.11.014. [DOI] [Google Scholar]
- 23.Lozinsky V.I. Polymeric cryogels as a new family of macroporous and supermacroporous materials for biotechnological purposes. Russ. Chem. Bull. 2008;57:1015–1032. doi: 10.1007/s11172-008-0131-7. [DOI] [Google Scholar]
- 24.Bereli N., Şener G., Altintaş E.B., Yavuz H., Denizli A. Poly(glycidyl methacrylate) beads embedded cryogels for pseudo-specific affinity depletion of albumin and immunoglobulin G. Mater. Sci. Eng. C. 2010;30:323–329. doi: 10.1016/j.msec.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Sahiner N., Yildiz S. Preparation of superporous poly(4-vinyl pyridine) cryogel and their templated metal nanoparticle composites for H2 production via hydrolysis reactions. Fuel Process. Technol. 2014;126:324–331. doi: 10.1016/j.fuproc.2014.05.025. [DOI] [Google Scholar]
- 26.Busto M.D., Meza V., Ortega N., Perez-Mateos M. Immobilization of naringinase from Aspergillus niger CECT 2088 in poly(vinyl alcohol) cryogels for the debittering of juices. Food Chem. 2007;104:1177–1182. doi: 10.1016/j.foodchem.2007.01.033. [DOI] [Google Scholar]
- 27.Das-Bradoo S., Svensson I., Santos J., Plieva F., Mattiasson B., Hatti-Kaul R. Synthesis of alkylgalactosides using whole cells of Bacillus pseudofirmus species as catalysts. J. Biotechnol. 2004;110:273–286. doi: 10.1016/j.jbiotec.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Efremenko E.N., Lyagin I.V., Lozinsky V.I. Enzymatic biocatalysts immobilized on/in the cryogel-type carriers. Supermacroporous Cryogels Biomed. Biotechnol. Appl. 2016:309–332. doi: 10.1201/b19676-15. [DOI] [Google Scholar]
- 29.Hedström M., Plieva F., Galaev I.Y., Mattiasson B. Monolithic macroporous albumin/chitosan cryogel structure: a new matrix for enzyme immobilization. Anal. Bioanal. Chem. 2008;390:907–912. doi: 10.1007/s00216-007-1745-6. [DOI] [PubMed] [Google Scholar]
- 30.Derazshamshir A., Baydemir G., Andac M., Say R., Galaev I.Y., Denizli A. Molecularly imprinted PHEMA-based cryogel for depletion of hemoglobin from human blood. Macromol. Chem. Phys. 2010;211:657–668. doi: 10.1002/macp.200900425. [DOI] [Google Scholar]
- 31.Savina I.N., Cnudde V., D’Hollander S., Van Hoorebeke L., Mattiasson B., Galaev I.Y., Du Prez F. Cryogels from poly(2-hydroxyethyl methacrylate): macroporous, interconnected materials with potential as cell scaffolds. Soft Matter. 2007;3:1176–1184. doi: 10.1039/b706654f. [DOI] [PubMed] [Google Scholar]
- 32.Opdahl A., Kim S.H., Koffas T.S., Marmo C., Somorjai G.A. Surface mechanical properties of pHEMA contact lenses: Viscoelastic and adhesive property changes on exposure to controlled humidity. J. Biomed. Mater. Res. - Part A. 2003;67:350–356. doi: 10.1002/jbm.a.10054. [DOI] [PubMed] [Google Scholar]
- 33.Tsou T.L., Tang S.T., Huang Y.C., Wu J.R., Young J.J., Wang H.J. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J. Mater. Sci. Mater. Med. 2005;16:95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 34.Lou X., Munro S., Wang S. Drug release characteristics of phase separation pHEMA sponge materials. Biomaterials. 2004;25:5071–5080. doi: 10.1016/j.biomaterials.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M., Motomura T., Kawada M., Anzai T., Kasori Yuu, Shiroya T., Shimura K., Onishi M., Mochizuki Akira. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)-relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21:1471–1481. doi: 10.1016/S0142-9612(00)00031-4. [DOI] [PubMed] [Google Scholar]
- 36.Perçin I., Sener G., Demirçelik A.H., Bereli N., Denizli A. Comparison of two different reactive dye immobilized poly(Hydroxyethyl methacrylate) cryogel discs for purification of lysozyme. Appl. Biochem. Biotechnol. 2015;175:2795–2805. doi: 10.1007/s12010-014-1454-5. [DOI] [PubMed] [Google Scholar]
- 37.Soomro R., Perçin I., Memon N., Iqbal Bhanger M., Denizli A. Gelatin-loaded p(HEMA-GMA) cryogel for high-capacity immobilization of horseradish peroxidase. Artif. Cells Nanomed. Biotechnol. 2016;44:1708–1713. doi: 10.3109/21691401.2015.1089252. [DOI] [PubMed] [Google Scholar]
- 38.Terra W.R., Ferreira C. Biochemistry of digestion. Compr. Mol. Insect Sci. 2005;4–6:171–224. doi: 10.1016/B0-44-451924-6/00053-3. [DOI] [Google Scholar]
- 39.Cristofoletti P.T., Ribeiro A.F., Deraison C., Rahbé Y., Terra W.R. Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acyrthosiphon pisum. J. Insect Physiol. 2003;49:11–24. doi: 10.1016/S0022-1910(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G., Xing J., Pi Z., Liu S., Liu Z., Song F. α-Glucosidase immobilization on functionalized Fe 3 O 4 magnetic nanoparticles for screening of enzyme inhibitors. Chin. Chem. Lett. 2019;30:656–659. doi: 10.1016/j.cclet.2018.12.003. [DOI] [Google Scholar]
- 41.Liu D.M., Chen J., Shi Y.P. α-Glucosidase immobilization on chitosan-modified cellulose filter paper: preparation, property and application. Int. J. Biol. Macromol. 2019;122:298–305. doi: 10.1016/j.ijbiomac.2018.10.177. [DOI] [PubMed] [Google Scholar]
- 42.Liu D.M., Chen J., Shi Y.P. α-Glucosidase immobilization on chitosan-enriched magnetic composites for enzyme inhibitors screening. Int. J. Biol. Macromol. 2017;105:308–316. doi: 10.1016/j.ijbiomac.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Odaci D., Telefoncu A., Timur S. Maltose biosensing based on co-immobilization of α-glucosidase and pyranose oxidase. Bioelectrochemistry. 2010;79:108–113. doi: 10.1016/j.bioelechem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Prodanović R., Milosavić N., Jovanoví S., Prodanović O., Ćirković Veličkoví T., Vujčić Z., Jankov R.M. Activity and stability of soluble and immobilized α-glucosidase from baker’s yeast in cosolvent systems. Biocatal. Biotransformation. 2006;24:195–200. doi: 10.1080/10242420600655903. [DOI] [Google Scholar]
- 45.Qiu B., Shi Y., Yan L., Wu X., Zhu J., Zhao D., Khan M.Z.H., Liu X. Development of an on-line immobilized α-glucosidase microreactor coupled to liquid chromatography for screening of α-glucosidase inhibitors. J. Pharm. Biomed. Anal. 2020;180 doi: 10.1016/j.jpba.2019.113047. [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y., Liu Q., Yin X. Synthesis of α-glucosidase-immobilized nanoparticles and their application in screening for α-glucosidase inhibitors. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1022:75–80. doi: 10.1016/j.jchromb.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Kwon Y.I.I., Vattem D.A., Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006;15:107–118. [PubMed] [Google Scholar]
- 48.Plieva F.M., Karlsson M., Aguilar M.R., Gomez D., Mikhalovsky S., Galaev’ I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter. 2005;1:303–309. doi: 10.1039/b510010k. [DOI] [PubMed] [Google Scholar]
- 49.Tripathi A., Melo J.S. Preparation of a sponge-like biocomposite agarose-chitosan scaffold with primary hepatocytes for establishing an in vitro 3D liver tissue model. RSC Adv. 2015;5:30701–30710. doi: 10.1039/c5ra04153h. [DOI] [Google Scholar]
- 50.Suner S.S., Demirci S., Yetiskin B., Fakhrullin R., Naumenko E., Okay O., Ayyala R.S., Sahiner N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019;130:627–635. doi: 10.1016/j.ijbiomac.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Sigma-Aldrich Enzymatic Assay of α-Glucosidase (EC 3.2.1.20) p-Nitrophenyl α-D-Glucoside as Substrate. Sigma Qual. Control Test Proced. 1996:1–3. [Google Scholar]
- 52.Engelking L.R., Engelking L.R. Chapter 6 – enzyme kinetics. Textb. Vet. Physiol. Chem. 2015:32–38. doi: 10.1016/B978-0-12-391909-0.50006-2. [DOI] [Google Scholar]
- 53.Sahiner M., Blake D.A., Fullerton M.L., Suner S.S., Sunol A.K., Sahiner N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019;137:836–843. doi: 10.1016/j.ijbiomac.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 54.Zhu F., Asada T., Sato A., Koi Y., Nishiwaki H., Tamura H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014;62:885–892. doi: 10.1021/jf404318j. [DOI] [PubMed] [Google Scholar]
- 55.Li R., Wu G., Ye Y. In vitro hemocompatibility of sulfonated polypropylene non-woven fabric prepared via a facile γ-ray pre-irradiation grafting method. Appl. Surf. Sci. 2015;356:1221–1228. doi: 10.1016/j.apsusc.2015.08.052. [DOI] [Google Scholar]
- 56.Wang C.Y., Yang C.H., Huang K.S., Yeh C.S., Wang A.H.J., Chen C.H. Electrostatic droplets assisted in situ synthesis of superparamagnetic chitosan microparticles for magnetic-responsive controlled drug release and copper ion removal. J. Mater. Chem. B. 2013;1:2205–2212. doi: 10.1039/c3tb00467h. [DOI] [PubMed] [Google Scholar]
- 57.Worthington C.C., Wortington V., Worthington A. 2016. Introduction to Enzymes 1–14. [Google Scholar]
- 58.Fan C., Li K., Wang Y., Qian X., Jia J. The stability of magnetic chitosan beads in the adsorption of Cu2+ RSC Adv. 2016;6:2678–2686. doi: 10.1039/c5ra20943a. [DOI] [Google Scholar]
- 59.Pan C., Hu B., Li W., Sun Y., Ye H., Zeng X. Novel and efficient method for immobilization and stabilization of β-d-galactosidase by covalent attachment onto magnetic Fe3O4-chitosan nanoparticles. J. Mol. Catal. B Enzym. 2009;61:208–215. doi: 10.1016/j.molcatb.2009.07.003. [DOI] [Google Scholar]
- 60.Deng H., Li X., Peng Q., Wang X., Chen J., Li Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chemie. 2005;117:2842–2845. doi: 10.1002/ange.200462551. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues R.C., Ortiz C., Berenguer-Murcia Á., Torres R., Fernández-Lafuente R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013;42:6290–6307. doi: 10.1039/c2cs35231a. [DOI] [PubMed] [Google Scholar]
- 62.Sahoo B., Sahu S.K., Bhattacharya D., Dhara D., Pramanik P. A novel approach for efficient immobilization and stabilization of papain on magnetic gold nanocomposites. Colloids Surf. B Biointerfaces. 2013;101:280–289. doi: 10.1016/j.colsurfb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Sun L., Liang H., Yuan Q., Wang T., Zhang H. Study on a carboxyl-activated carrier and its properties for papain immobilization. J. Chem. Technol. Biotechnol. 2012;87:1083–1088. doi: 10.1002/jctb.3714. [DOI] [Google Scholar]
- 64.Zhang B., Li P., Zhang H., Fan L., Wang H., Li X., Tian L., Ali N., Ali Z., Zhang Q. Papain/Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme. RSC Adv. 2016;6:46702–46710. doi: 10.1039/c6ra05308d. [DOI] [Google Scholar]
- 65.Handayani N., Miletic N., Loos K., Achmad S., Wahyuningrum D. Properties of immobilized Candida antarctica lipase B on highly macroporous copolymer. Sains Malays. 2011;40:965–972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.