Abstract
Antimicrobials are used to support livestock health and productivity, but might pose a risk for the development of antimicrobial resistance; in particular, when multiple livestock species are raised together in production systems. On integrated chicken-fish farms, chickens are raised over fish ponds and poultry faeces is excreted into the ponds. We investigated antimicrobial usage and the antimicrobial susceptibility of Escherichia coli cultured from poultry faeces on 301 integrated farms in Ayeyarwady Delta of Myanmar. Antimicrobials were used by 92.4% of farmers for chickens, but they were not applied to fish. The most common antimicrobials used were Octamix (amoxicillin and colistin sulfate) on 28.4%, enrofloxacin on 21.0% and amoxicillin on 16% of farms. Overall, 83.1% (152/183) of the E. coli were resistant to at least one antimicrobial. The highest level of resistance was to amoxicillin (54.6%), tetracycline (39.9%), sulfamethoxazole/trimethoprim (35.5%) and enrofloxacin (34.4%). Multidrug resistance was identified in 42.4% of isolates. In general, we found similar levels of antimicrobial resistance in non-users of antimicrobials as in users of antimicrobials for more commonly applied antimicrobials. Overall, antimicrobial resistance was lower in chickens on these integrated farms in Myanmar, compared to poultry farms in other countries of South East and East Asia.
Subject terms: Microbiology, Antimicrobials, Bacteria, Medical research, Epidemiology
Introduction
Antimicrobial agents such as beta-lactams, aminoglycosides, macrolides, and quinolones are used for growth promotion, prophylaxis and therapy in intensive poultry production systems in Southeast Asia1. This is of concern as the use of antimicrobials in food producing animals can select for antimicrobial resistant bacteria and resistance genes which can be transmitted from animals to humans via the food chain, direct contact and via the environment2. The World Health Organization (WHO) has recently highlighted the major public health risks posed by antimicrobial resistant bacteria and created a global action plan to combat antimicrobial resistance3.
The use of antimicrobial agents is of particular concern in systems where multiple livestock species are raised together and in systems, that are open to the surrounding environment. Such a production system is integrated chicken-fish farming, where chickens are raised over fishponds. Poultry faeces excreted into the ponds support photosynthetic organisms. Fish consume these organisms along with spilled chicken feed and faeces. The fish are often not fed any additional feed4 and water from the ponds is used to fertilise crops5. Thus integrated chicken-fish production provides an economical usage of land, labour and water5, but also raises a number of public health concerns because, antimicrobials, their residues, and antimicrobial-resistant pathogens or commensal organisms may accumulate in the environment promoting the development of antimicrobial-resistant bacteria4. Integrated chicken-fish farming plays a significant role in Myanmar’s economy, but the usage of antimicrobials and resistance to common bacteria is not known.
This study assessed the current practices of antimicrobial usage and antimicrobial susceptibility of Escherichia coli cultured from poultry faeces from integrated chicken-fish farms.
Results
Study population
A total of 304 integrated chicken-fish farmers were interviewed between February and July 2017 in the Maubin and Naungdon Townships of AD of Myanmar. Three farms did not have any poultry at the time of the interview and were excluded from the analysis. Of the 301 integrated chicken-fish farmers, 74.1% (N = 223) raised broilers, 23.9% (N = 72) raised layers, and 2.0% (N = 6) raised both broilers and layers. The four most common fish breeds on the farms were Rohu (79.1%, N = 238), Mrigal (59.1%, N = 178), Pacu (39.9, N = 120%), and Catla (35.2%, N = 106).
Antimicrobial use in integrated poultry production
On broiler farms no antimicrobials were used on 10.8% (N = 24) farms, 33.2% (N = 74) used one antimicrobial and 56.0% (N = 125) used two or more antimicrobials. On layer farms no antimicrobials were used on 4.2% (N = 3) farms, 11.1% (N = 8) used one antimicrobial and 84.7% (N = 61) used two or more antimicrobials. Antimicrobials were used by all farms raising both broilers and layers (N = 6). Antimicrobials were more frequently used on layer compared to broiler farms (p < 0.001). All antimicrobials were administered in water.
The most common antimicrobials used were Octamix (amoxicillin and colistin sulfate) in 42.2% and 44.4% of broiler and layer flocks, respectively; enrofloxacin in 27.4% and 50.0%, and amoxicillin in 18.4% and 43.1%. Amoxicillin, enrofloxacin and tylosin were used more frequently (P < 0.05) in layer flocks compared to broilers flocks (Table 1), while some antimicrobials (neomycin, doxycycline, oxytetraclycine, lincomycin) were only used in broiler, but not in layer flocks.
Table 1.
Cross-tabulation of types of antimicrobials used in broilers and layers on integrated chicken-fish farming systems in the Ayeyarwady Delta of Myanmar.
| Antimicrobials | Overall (N = 295) | Broiler (N = 223) | Layer (N = 72) | P value | |||
|---|---|---|---|---|---|---|---|
| N users | Percentage | N users | Percentage | N users | Percentage | ||
| Amoxicillin and colistin products | |||||||
| Amoxicillin | 72 | 24.4 | 41 | 18.4 | 31 | 43.1 | P < 0.001 |
| Colistin | 15 | 5.1 | 14 | 6.3 | 1 | 1.4 | P = 0.128 |
| Octamix (amoxicillin and colistin) | 126 | 42.7 | 94 | 42.2 | 32 | 44.4 | P = 0.785 |
| Fluoroquinolones (ciprofloxacin, enrofloxacin and ofloxacin) | |||||||
| Fluoroquinolone | 7 | 2.4 | 4 | 1.8 | 3 | 4.2 | P = 0.367 |
| Ciprofloxacin | 11 | 3.7 | 11 | 4.9 | 0 | 0.0 | P = 0.071 |
| Enrofloxacin | 97 | 32.9 | 61 | 27.4 | 36 | 50.0 | P = 0.001 |
| Ofloxacin | 24 | 8.1 | 21 | 9.4 | 3 | 4.2 | P = 0.216 |
| Aminoglycosides | |||||||
| Neomycin | 5 | 1.7 | 5 | 2.2 | 0 | 0.0 | P = 0.340 |
| Streptomycin | 6 | 2.0 | 3 | 1.3 | 3 | 4.2 | P = 0.158 |
| Tetracyclines | |||||||
| Doxycycline | 11 | 3.7 | 11 | 4.9 | 0 | 0.0 | P = 0.071 |
| Oxytetracycline | 1 | 0.3 | 1 | 0.4 | 0 | 0.0 | P = 1.000 |
| Macrolide | |||||||
| Tylosin | 56 | 19.0 | 28 | 12.6 | 28 | 38.9 | P < 0.001 |
| Lincosamide | |||||||
| Lincomycin | 7 | 2.4 | 7 | 3.1 | 0 | 0.0 | P = 0.201 |
| Folic acid inhibitors | |||||||
| Sulfamethoxazole/ trimethoprim | 14 | 4.7 | 13 | 5.8 | 1 | 1.4 | P = 0.200 |
| Sulfadiazine | 6 | 2.0 | 5 | 2.2 | 1 | 1.4 | P = 1.000 |
Ocatmix, amoxicillin and tylosin were the antimicrobials most commonly used for treatments, while also being used prophylactically by other farmers. Streptomycin and sulfadiazine were predominately used for treatments only. Of all the farmers that provided octamix (42.7%, N = 126), about 43.7% (N = 55) administered it daily (Supplementary Table S1 online). In 99.3% of cases antimicrobials were obtained by integrated chicken-fish farmers from feed shops, and only on 0.7% of cases antimicrobials were supplied to farmers through veterinarians.
Antimicrobial susceptibility testing
Escherichia coli was cultured from the faecal samples in 183 of 301 (60.8%) farms. Overall, 83.1% (152/183) of the E. coli were resistant to at least one antimicrobial (Table 2). The highest level of resistance was to ampicillin (54.6%), tetracycline (39.9%), sulfamethoxazole/trimethoprim (35.5%) and enrofloxacin (34.4%). Lower levels of resistance were identified to gentamicin (13.1%), cephalothin (10.2%), neomycin (6.6%), colistin (6.0%), amoxicillin clavulanic acid (3.8%) and cefoxitin (2.2%). No isolates were resistant to chloramphenicol. Multidrug resistance was identified in 65 isolates (42.4%). Many antimicrobial resistance patterns were detected (Supplementary Table S2 online).
Table 2.
Antimicrobial susceptibility of 183 E. coli cultured from chicken faecal samples on integrated chicken-fish farming systems in the Ayeyarwady Delta of Myanmar.
| Antimicrobial | Abbreviation | N farms (Percentage) | ||
|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||
| Ampicillin/amoxicillin | AMP | 58 (31.7) | 25(13.7) | 100 (54.6) |
| Amoxicillin/clavulanic acid | AMC | 142 (77.6) | 34 (18.6) | 7 (3.8) |
| Cephalothin | KF | 121 (66.1) | 43 (23.5) | 19 (10.2) |
| Cefoxitin | FOX | 178 (97.3) | 1 (0.5) | 4 (2.2) |
| Ceftiofur | EFT | 175 (95.6) | 8 (4.4%) | 0 (0) |
| Chloramphenicol | CHL | 177 (96.7) | 6 (3.3) | 0 (0) |
| Colistin | Ct | 172 (94.0) | a | 11 (6.0) |
| Enrofloxacin | ENR | 85 (46.4) | 35 (19.1) | 63 (34.4) |
| Gentamicin | GEN | 154 (84.2) | 5 (2.7) | 24 (13.1) |
| Neomycin | N | 177 (93.4) | a | 12 (6.6) |
| Sulfamethoxazole/trimethoprim | SXT | 112 (61.2) | 6 (3.3) | 65 (35.5) |
| Tetracycline | TET | 107 (58.5) | 3 (1.6) | 73 (39.9) |
aColistin and neomycin only have susceptible and resistant clinical breakpoints.
Relationship between antimicrobial use and antimicrobial resistance
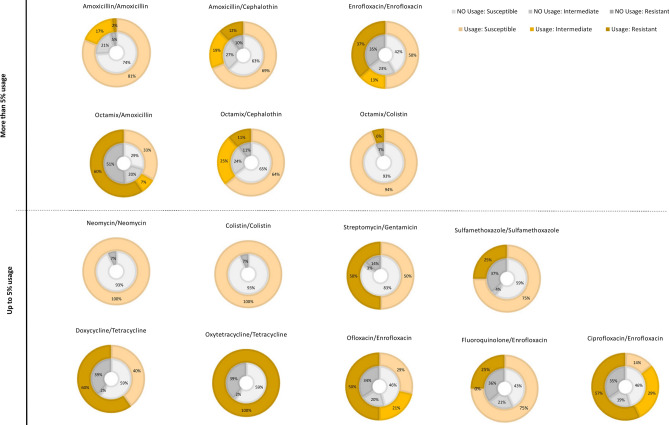
For 172 integrated chicken-fish farms the relationship between antimicrobial usage and antimicrobial resistance was explored. Focusing on antimicrobials with less than 95% susceptibility, we explored the relationship between usage and non-usage of antimicrobials in poultry on the integrated chicken-fish farms and the relevant resistance patterns for these antimicrobials. For more commonly applied antimicrobials (usage on more than 5% of farms), a similar resistance pattern was observed for users and non-users of this specific antimicrobial (Fig. 1), for example, amoxicillin resistance was similar for non-users and users of Octamix. For less commonly applied antimicrobials (usage in less than 5% of farms), more variability in antimicrobial resistance was observed between users and non-users.
Figure 1.
Resistance patterns (susceptible, intermediate, resistant) for antimicrobials that were used or not used on 172 integrated chicken-fish farms in Myanmar. Antimicrobials were separated in two groups, representing frequent application (> 5%) and infrequent application (< = 5%) across all integrated chicken-fish farms.
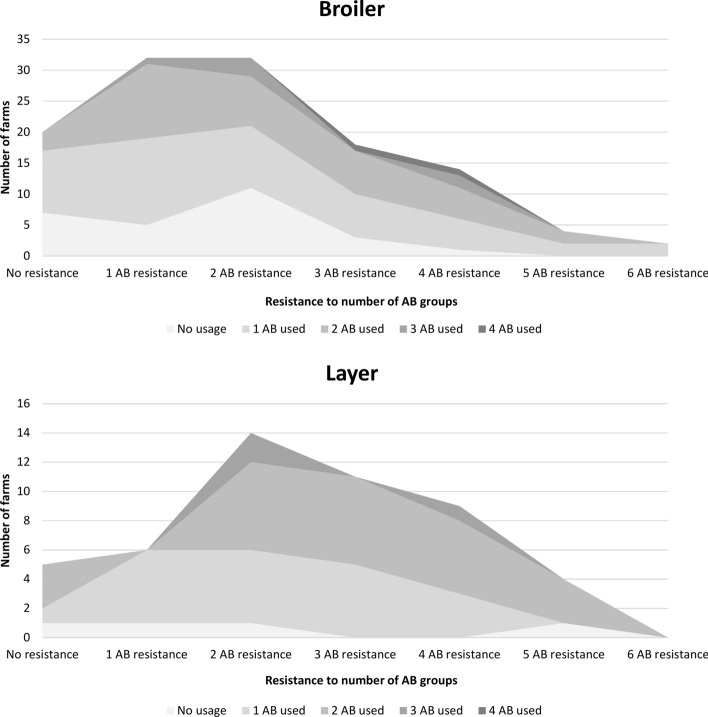
About 16.4% of broiler and 10.2% of layer on the integrated chicken-fish farms showed no antimicrobial resistance, while resistance to one group or one antimicrobial category6 was 26.2% and 12.2% and for two or more antimicrobial categories was 57.4% and 77.6% for integrated broiler and layer farms, respectively (Fig. 2). Resistance to multiple antimicrobial categories was more prevalent on integrated layer compared to broiler farms (p = 0.048).
Figure 2.
Relationship between the number of antimicrobials used on farms and the resistance to number of antimicrobial groups on these farms. Data is presented separately for layer and broilers raised on integrated chicken-fish farms in Myanmar.
However, the correlation of the number of antimicrobials used on integrated chicken-fish farms did not show a linear relationship with the number of antimicrobial resistance groups found on these farms, thus a sequential larger number of antimicrobials used on integrated chicken-fish farms was not necessarily associated with a proportional increased resistance to the number of antimicrobial categories (Supplementary Fig. S1 online).
Discussion
Antimicrobial resistance was found on integrated chicken-fish farms in Myanmar, but in general, we found lower levels of antimicrobial resistance in E. coli from healthy chickens compared with other countries in Southeast Asia1,7–9 and China10,11. In this study, the highest level of resistance was detected to older generation antimicrobials; ampicillin (54.6%), tetracycline (39.9%), and sulfamethoxazole/trimethoprim (35.5%).
Of most concern is the identification of resistance to the highest priority critically important antimicrobials, fluoroquinolones (e.g. enrofloxacin) (34.4%) and colistin (6%). These drugs are recognized as critically important for human therapy12 and the World Health Organisation recommends these drugs should not be used for treatment of food-producing animals”13. Fluoroquinolone resistance in this study was lower than that detected in faecal E. coli from chickens in Vietnam (59.6–70.2%)8 and Bangladesh (83–100%)9 and similar to a study from Indonesia (35.9%)7 and across studies from Southeast Asia > 20 to 50%1. Resistance to colistin in Southeast Asia in previous studies was reported as > 20 to 50%1.
Resistance to critically important third generation cephalosporins was low, cefoxitin (2.2%) similar to previous studies in Southeast Asia where levels < 10% were reported1,8. No third generation cephalosporins were reported as administered to chickens in this study. The macrolide, tylosin was also administered commonly to poultry in this study. Macrolides are generally administered to prevent or treat chronic respiratory disease (CRD) associated with Mycoplasma gallisepticum. Macrolides are also identified as highest priority critically important to human health12. Macrolides are generally not used to treat colibacillosis in poultry and no clinical breakpoints are available, therefore the faecal E. coli were not screened for susceptibility to macrolides. Future studies could involve culturing a gram-positive indicator organism e.g. Enterococcus spp. and screening to detect macrolide resistance.
A recent study on salmonella from poultry meat, from the Yangon Region Myanmar identified higher levels of resistance to sulfamethoxazole/trimethoprim (70.3%), tetracycline (54.3%), chloramphenicol (29.7%), amoxicillin clavulanic acid (17.4%) and multidrug resistance (52.2%) and lower levels of resistance to ampicillin (47.1%), ciprofloxacin (9.4%) and gentamicin (8%) than the current study14. Other studies in Myanmar have focused on MDR bacteria, extended spectrum beta-lactamase and carbapenemase producing Enterobacteriaceae from human clinical isolates15–17, or isolates cultured from the environmental16 or foods18.
In our study we did not test for carbapenems, however, we had no isolates resistant to ceftiofur, and only four isolates resistant to cefoxitin, three of which were MDR and MDR was identified in 42.4% of isolates.
Interestingly, we were able to show that for frequently used antimicrobials, similar antimicrobial resistance patterns were observed, even on those farms that did not use these antimicrobials. This suggests that a possible spread of antimicrobial resistance genes between farms for commonly used antimicrobials, including to farms that did not use specific antimicrobials. Our field data support and highlight the concerning relationship that antimicrobial resistance is a global public health concern as it did not stop at farm boundaries involving even farms that abstain of using specific antimicrobials.
However, our study also was not able to identify a clear linear correlation between antimicrobial use and resistance. A study investigating development of antimicrobial resistance in bacteria at integrated pig-fish farms in Vietnam described an increase in prevalence of naladixic acid and enrofloxacin resistance in faecal E.coli with enrofloxacin in feed, however the association between development of resistance and provision of feed containing enrofloxacin was not clear19. Tetracycline resistance was high prior to the study, and no association with provision of tetracycline in feed and increase in tetracycline resistance was identified19. However, the antimicrobial relationship between usage and resistance and spread of antimicrobial resistance genes is complex and is difficult to describe with field data, therefore modelling approaches and molecular studies are needed to illustrate the development of antimicrobial resistance over time.
We only used E. coli as an indicator pathogen for chicken faeces and did not sample the water in the ponds. Two types of indicator organisms for antimicrobial resistance in aquatic environments were used in another studies: Acinetobacter spp. and Enterococcus spp.4. In future studies E. coli and Enterococcus spp. could be used as indicator organisms in poultry and pigs while Acinetobacter spp. and Enterococcus spp. could be used for detection of resistance in the aquatic environment.
Overall, antimicrobial resistance was lower in broilers and layers on the studied integrated chicken-fish farms in Myanmar, compared to poultry farms in other countries of South East and East Asia. Although all famers used antimicrobials for poultry, none of the farmers used them for fish. Thus, chicken-fish farming in the study region of Myanmar seems to be a well-placed with respect to antimicrobial resistance, but adequate training of farmers is required to reduce the development and dissemination of antimicrobial resistance.
Materials and methods
Study design
A cross-sectional study was used to interview integrated chicken-fish farmers in the Ayeyarwady Delta (AD) of Myanmar. As no 'lists' or directories of integrated chicken-fish farmers existed, integrated chicken-fish farmers had to be located through participatory approaches. Thus, fishery officers from the Myanmar Livestock and Fisheries Department and veterinary officers from the Myanmar Livestock, Breeding and Veterinary Department ‘identified’ integrated chicken-fish farmers in selected townships by discussing with individual chicken or fish farmers where integrated chicken-fish farms were located. Integrated chicken-fish farmers were then visited and invited to participate in the interviews. Additional chicken-fish farmers were identified by snowball sampling, i.e. interviewed chicken-fish farmers provided information on other integrated chicken-fish farmers in the same township.
As the proportion of farms where E. coli is resistant to at least one antimicrobial was unknown, we assumed a prevalence of resistance 0.5, which would generate the largest sample size. A sample size of 302 was required to estimate a confidence interval up to 0.10 wide if the observed proportion is between 0.3 and 0.7 (Confidence level = 95%, Acceptable difference = 0.05, Assumed proportion = 0.5, Size of population = 1200). Sample size calculations were conducted in WinPepi version 11.6520.
Questionnaire and data collection
A questionnaire was developed in English and digitized using the mobile data collection platform CommCare (Dimagi, Inc, https://www.commcarehq.org/home/). CommCare is an open source platform that allows the construction of specific mobile apps tailored for collection data. Digital tablets and smartphones were used to record data on the administration of antimicrobials (type, amount, type of administration, source), to collect spatial information on the farm location and to take photographs of labels of antimicrobials and supplements used by the farmers. Questionnaire data were collected by two veterinarians (who are co-authors of this paper) that were enrolled in master’s program at the University of Veterinary Science, Yezin, Myanmar at the time of the interviews.
Human Ethics Approval was obtained for this survey from the UQ Research and Innovation Ethical Review Committee (#2016001804). All research was performed in accordance with relevant guidelines/regulations of the Australian National Health and Medical Research Council Act. Informed consent was obtained from all interviewees (none of the interviewees were under 18, in which case, consent from a parent and/or legal guardian would have been required).
Sample collection
Pooled chicken faecal samples were collected from the 301 integrated broiler and layer farms. Samples were collected from the slatted floor of the poultry sheds on each farm. Samples were transferred to a sterile container containing sterile saline (0.85% NaCl) and transported to the Department of Pharmacology and Parasitology, University of Veterinary Science (UVS), Yezin at 4 °C. The samples were stored at − 80 °C at UVS and sample processing commenced after the field data collection was completed in January 2018.
Bacterial isolation
Samples were thawed at room temperature, vortexed and 100 µL spread onto MacConkey agar (MCA) plates (Oxoid, Basingstoke, United Kingdom). Plates were incubated aerobically at 37 °C for 24 h. One colony from each sample was selected on the basis of colony morphology and subcultured onto nutrient agar plates (Oxoid). Colonies were confirmed to be E. coli via API 20E testing (bioMerieux, Helios, Singapore). If the organism selected was not E. coli, another colony was selected. E. coli ATCC 25922 was used as a quality control organism.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by disc diffusion as per Clinical and Laboratory Standards Institute guidelines (CLSI) for 12 antimicrobials of veterinary and/or human health importance: amoxicillin/clavulanic acid (30 μg); ampicillin (10 μg); cefoxitin (30 μg); ceftiofur (30 μg); cephalothin (30 μg); chloramphenicol (30 μg); colisitn (10 μg); enrofloxacin (5 μg); gentamicin (10 μg); neomycin (30 μg); sulfamethoxazole/trimethoprim (1.25/23.75 μg) and tetracycline (30 μg)21 between March 2017 and January 2018. All antimicrobial discs were obtained from Oxoid. The quality control organism used was E. coli ATCC 25922. Only enrofloxacin has a veterinary specific breakpoint for poultry21, therefore, human breakpoints were used for other antimicrobials22. For neomycin breakpoint information was obtained directly from the manufacturer (Zoetis, West Ryde, NSW, 2114, Australia). Isolates were considered multidrug resistant (MDR) if they acquired non-susceptibility to at least one agent in three or more antimicrobial categories6.
Data analysis
Frequency and contingency tables and descriptive statistics were produced focusing on the usage of antimicrobials. The relationship between antimicrobial usage and resistance was visualized using a range of graphical displays and compared between integrated layer and broiler farms using the Fisher’s Exact test. Data analysis was conducted using Stata 14.0 (StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA).
Ethical statement
Institutional approval for conducting interviews was obtained from The University of Queensland, Human Research Ethics Committee (No. 2016001804).
Supplementary information
Acknowledgements
We would like to thank all integrated chicken-fish farmers for their time to conduct the interviews with them and for allowing us to collect samples on their farms. We are grateful for the help provided by the fishery officers from the Myanmar Livestock and Fisheries Department and veterinary officers from the Myanmar Livestock, Breeding and Veterinary Department in identifying the integrated chicken-fish farmers in selected townships. We also thank the University of Veterinary Science, Yezin, Myanmar for providing the facilities for the diagnostic testing.
Author contributions
J.G. and J.H. designed the research study, led and supervised the research team, drafted the research protocol and developed the analytical plan. JH prepared the figures. H.W., S.S.M.L., E.M.M.H., ZML conducted the farm visits and data collection. H.W., S.S.M.L., E.M.M.H. performed the laboratory diagnoses. SSW and LLH provided advice on the research design and supported the laboratory diagnoses. HPL designed the questionnaire, conducted the data management and the initial data analysis. All authors have read, contributed to, and approved the final version of the manuscript.
Funding
The research was financially supported by funding received from Australian Centre for International Agricultural Research (Grant number C2016/015). The funder was not involved in the data collection, data analysis and decision relating to the publication of the results.
Data availability
The dataset analysed in this study is available from the https://doi.org/ 10.6084/m9.figshare.11971995.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73076-2.
References
- 1.Nhung NT, Cuong NV, Thwaites G, Carrique-Mas J. Antimicrobial usage and antimicrobial resistance in animal production in southeast Asia: a review. Antibiotics (Basel) 2016 doi: 10.3390/antibiotics5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Costa PM, Loureiro L, Matos AJF. Transfer of multidrug-resistant bacteria between intermingled ecological niches: the interface between humans, animals and the environment. Int. J. Environ. Res. Public Health. 2013;10:278–294. doi: 10.3390/ijerph10010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global action plan on antimicrobial resistance (2015). https://www.who.int/drugresistance/global_action_plan/en/. [DOI] [PubMed]
- 4.Petersen A, Andersen JS, Kaewmak T, Somsiri T, Dalsgaard A. Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl. Environ. Microbiol. 2002;68:6036–6042. doi: 10.1128/aem.68.12.6036-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little, D. & Satapornvanit, K. Poultry and fish production a framework for their integration in Asia. In: Second Food and Agriculture Organization of the United Nations Electronic Conference on Tropical Feeds 425–453.
- 6.Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 7.Usui M, et al. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia and Thailand) J. Vet. Med. Sci. 2014;76:685–692. doi: 10.1292/jvms.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vounba P, Arsenault J, Bada-Alambédji R, Fairbrother JM. Pathogenic potential and the role of clones and plasmids in beta-lactamase-producing E. coli from chicken faeces in Vietnam. BMC Vet. Res. 2019;15:106. doi: 10.1186/s12917-019-1849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad M, et al. Susceptibility and multidrug resistance patterns of Escherichia coli isolated from cloacal swabs of live broiler chickens in Bangladesh. Pathogens. 2019 doi: 10.3390/pathogens8030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassin AK, et al. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE. 2017;12:e0185326. doi: 10.1371/journal.pone.0185326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zhao M, Liu J, Zhou Y, Miao Z. Prevalence and antibiotic resistance profiles of extended-spectrum beta-lactamase-producing Escherichia coli isolated from healthy broilers in Shandong Province, China. J. Food Prot. 2016;79:1169–1173. doi: 10.4315/0362-028x.Jfp-16-025. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organisation. Critically important antimicrobials for human medicine (2017). https://www.who.int/foodsafety/publications/cia2017.pdf?ua=1.
- 13.World Health Organisation. WHO guidelines on use of medically important antimicrobials in food-producing animals (2017). [PubMed]
- 14.Moe AZ, et al. Prevalence and antimicrobial resistance of salmonella isolates from chicken carcasses in retail markets in Yangon, Myanmar. J. Food Prot. 2017;80:947–951. doi: 10.4315/0362-028x.Jfp-16-407. [DOI] [PubMed] [Google Scholar]
- 15.Mon M, et al. Study of multi-drug resitant gram-negative bacteria isolates obtained from hospitalised patiens in Yangon, Myanmar. Myanmar Health Sci. Res. J. 2017;29:151–157. [Google Scholar]
- 16.Sugawara Y, et al. Spreading patterns of NDM-producing Enterobacteriaceae in clinical and environmental settings in Yangon, Myanmar. Antimicrob. Agents Chemother. 2019 doi: 10.1128/AAC.01924-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myat TO, et al. ESBL- and carbapenemase-producing Enterobacteriaceae in patients with bacteremia, Yangon, Myanmar, 2014. Emerg. Infect. Dis. 2017;23:857–859. doi: 10.3201/eid2305.161100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara Y, et al. Dissemination of carbapenemase-producing Enterobacteriaceae harbouring blaNDM or blaIMI in local market foods of Yangon, Myanmar. Sci. Rep. 2019;9:14455. doi: 10.1038/s41598-019-51002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang STT, Petersen A, Van Truong D, Chu HTT, Dalsgaard A. Impact of medicated feed on the development of antimicrobial resistance in bacteria at integrated pig-fish farms in Vietnam. Appl. Environ. Microbiol. 2011;77:4494. doi: 10.1128/AEM.02975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Insitute (CLSI). Performance Standards for Antimicrobial Disc and Dilution Susceptiblity Testing for Bateria Isoalted from Animals. 5th edn. CLSI standard VET01 edn. (Clinical and Laboratory Standards Institute, Wayne, 2018).
- 22.Clinical and Laboratory Standards Insitute (CLSI) Performance Standards for Antimicrobial Suceptibility Testing. 29. Wayne: Clinical and Laboratory Standards Institute; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed in this study is available from the https://doi.org/ 10.6084/m9.figshare.11971995.