Abstract
This systematic review identified 45 original published research articles related to oil and gas extraction activities and human reproductive endpoints. Reproductive outcomes were categorized as [1] birth outcomes associated with maternal exposure, [2] semen quality, fertility, and birth outcomes associated with adult paternal exposure, [3] reproductive cancers, and [4] disruption of human sex steroid hormone receptors. The results indicate there is moderate evidence for an increased risk of preterm birth, miscarriage, birth defects, decreased semen quality, and prostate cancer. The quality of the evidence is low and/or inadequate for stillbirth, sex ratio, and birth outcomes associated with paternal exposure, and testicular cancer, female reproductive tract cancers, and breast cancer, and the evidence is inconsistent for an increased risk of low birth weight; therefore, no conclusions can be drawn for these health effects. There is ample evidence for disruption of the estrogen, androgen, and progesterone receptors by oil and gas chemicals, which provides a mechanistic rationale for how exposure to oil and gas activities may increase the health risks we have outlined. The results from this systematic review suggest there is a negative impact on human reproduction from exposure to oil and gas activities. Many of the 45 studies reviewed identified potential human health effects. Most of these studies focused on conventional oil and gas activities. Few studies have been conducted to evaluate the impact of unconventional oil and gas operations on human health. The impact of unconventional oil and gas activities may be greater than that of conventional activity, given that unconventional activities employ many of the same approaches and use dozens of known endocrine-disrupting chemicals in hydraulic fracturing.
Keywords: Birth defects, cancer, oil and natural gas, human reproduction, hydraulic fracturing, endocrine disrupting chemicals, environmental pollution, fracking, hormonal activity, prenatal exposure, preterm birth, semen quality
The world’s need for energy is met substantially by natural gas and oil. In February 2016 alone, the United States produced 9 million barrels of oil and 92 billion cubic feet of natural gas per day (1, 2). As of 2014 there were 514,786 producing gas wells, and natural gas has been forecast to be the leading source of energy by 2040 (3). Increased production of oil and gas has been facilitated by the use of unconventional oil and gas extraction, which involves directional drilling and hydraulic fracturing to access previously unreached sources of oil and gas (tight gas, coal bed methane, and shale gas). Unconventional oil extraction is projected to increase from 35% of the U.S. oil production in 2008 to 50% by the year 2019 (2). With this increased production, there is the potential for increased exposure to chemicals and products from oil and gas extraction, processing, and wastewater, which may increase the risk for adverse human health effects.
Conventional and unconventional oil and natural gas extraction activities, including drilling, hydraulic fracturing, extraction, processing, transportation and disposal of wastewater, lead to many opportunities for environmental contamination with the chemicals used in and produced during these processes. Potential routes of human exposure to these chemicals and products include inhalation, dermal, and oral exposure. Chemicals can be volatilized or aerosolized during extraction and by active evaporating pits, flares, surface spills, processing, and transportation (4–6). Oil and gas industry activities can contaminate surface, ground, and drinking water through the drilling process, hydraulic fracturing, failure of well casings, wastewater spills, and structural failure in abandoned wells (5–10). This has led to concerns about health risks to those exposed to the chemicals used and produced in the oil and gas industry either via residential proximity or occupational exposure (11–13).
One health effect of particular concern is the impact on human reproduction. Human reproduction is controlled by hormones in the hypothalamic, pituitary, and gonadal axis. We have shown that many chemicals used in oil and gas extraction processes can disrupt hormone receptors, the endocrine system, and development (5, 14–16). We previously reviewed the literature on a limited number of chemicals associated with unconventional oil and gas extraction and found that many are associated with adverse reproductive outcomes (17). More recently, Elliott et al. (18) performed a systematic evaluation of chemicals used in hydraulic fracturing fluids for reproductive or developmental toxicity in the ReproTox database. Out of 1,021 chemicals identified in hydraulic fracturing fluids, 240 chemicals had toxicity information. Of this subset, 43% were suggestive of reproductive toxicity. Although individual oil and gas chemicals have been linked to reproductive toxicity, few studies have assessed human exposure to individual chemicals. The objective of this review was to systematically evaluate original published research examining potential human reproductive health effects associated with exposure to both conventional and unconventional oil and gas extraction operations.
MATERIALS AND METHODS
Literature Search
Four literature search strategies were used to comprehensively identify published literature on this topic.
Search 1.
A literature search was conducted in PubMed. The search was limited to search terms listed in Table 1 that were found within the title or abstract. Search terms were built from PubMed MeSH terms. To refine the search, irrelevant terms plant oil, essential oil, lipid oil, and fish oil were excluded from the search. With the PubMed filter “full text” we searched only articles available through PubMed. The final search for inclusion was conducted on April 25, 2016.
TABLE 1.
| Oil and gas terms | ||||
| Coal bed methane | Crude oil | Hydrofracking | Petroleum | Unconventional gas |
| Coal seam gas | Fracking | Natural gas | Shale | Unconventional oil |
| Coal bed methane | Hydraulic fracturing | Oil and gas | Tight oil | |
| Reproduction terms | ||||
| Abortion | Epidydymis | Luteinization | Parturition | Sertoli |
| Acrosome | Estrogen | Luteolysis | Paternal age | Sertoli cell-only syndrome |
| Adnexa | Estrous | Lymphnagiogenesis | Penile | Sex |
| Adrenarch | Estrus | Maternal | Penile erection | Sex characteristics |
| Androgen | Fecundity | Maternal age | Penis | Sex determination |
| Andropause | Fertile | Maternal-fetal exchange | Perimenopause | Sex differentiation |
| Anestrus | Fertility | Menarche | Peripartum | Sexual development |
| Anogenital | Fertilization | Menopause | Placenta | Sexual maturation |
| Anovulation | Fetal development | Menstrual | Placentation | Sperm |
| Aspermia | Fetal movement | Menstruation | Postmenopause | Sperm capacitation |
| Asthenozoospermia | Fetal organ | Metestrus | Postpartum | Sperm maturation |
| Birth | Fetal organ maturity | Miscarriage | Pregnancy | Sperm transport |
| Birth rate | Fetal viability | Multiple pregnancy | Pregnancy loss | Sperm-ovum interaction |
| Breech | Fetal viability | Musculoskeletal development Pregnancy maintenance | Spermatazoa | |
| Bulbourethral glands | Fetal weight | Neurogenesis | Pregnancy outcome | Spermatic cord |
| Cementogenesis | Follicular | Neurulation | Pregnancy rate | Spermatogenesis |
| Cervical | Follicular | Obstetric | Premenopause | Still birth |
| Cervix | Folliculara atresia | Odontogenesis | Prenatal nutritional physiological phenomena | Subfertility |
| Climacteric | Gametogenesis | Oligospermia | Proestrus | Superfetation |
| Coitus | Gastrulation | Oogenesis | Progesterone | Superovulation |
| Contraception | Genitalia | Organogenesis | Prostate | Term birth |
| Contraception behavior | Germ cell | Orgasm | Psuedopregnancy | Testes |
| Corpus luteum | Gestational age | Ovarian reserve | Puberty | Testis |
| Dentinogenesis | Gonads | Ovary | Puberty | Trimesters |
| Diestrus | Gravidity | Oviparity | Reproduction | Uterus |
| Ectogenesis | Infertility | Oviposition | Reproductive | Vagina |
| Ejaculation | Insemination | Ovoviviparity | Reproductive behavior | Vas deferens |
| Ejaculatory ducts | Lactation | Ovulation | Reproductive physiologic processes | Vitellogenesis |
| Embryonic and fetal development | Lacteal elimination | Ovulation inhibition | Reproductive physiological phenomena | Viviparity |
| Embryonic development | Live birth | Ovum | Scrotum | Vulva |
| Embryonic induction | Luteal | Parity | Seminal vesicles | |
Search 2.
Another search was conducted in Scopus with the same term inclusion and exclusion criteria as in search 1. The terms were again limited to the title or abstract, and only included articles and reviews (reviews included in original searches for contextual background). Medline was excluded as it was covered within the PubMed search. The final search for inclusion was conducted on April 25, 2016.
Search 3.
On May 3, 2016, we conducted the following search in PubMed: (“Fossil Fuels”[MeSH] NOT “Coal”[MeSH]) OR (“Petroleum”[MeSH] OR “Natural Gas”[MeSH]) AND (“Reproductive Physiological Phenomena”[MeSH] OR “endocrine disruptors”[MeSH]) AND “humans”[MeSH Terms]. Only journal articles, reviews (included in original searches for contextual background), and full text were included in the search.
Search 4.
Relevant references cited within the identified articles were also included for analysis.
Screening
The inclusion strategy (Fig. 1) was based on relevance through a three-tier system by two or more authors. Conflicts were resolved through discussion. Relevance was determined a priori as follows. The first tier of relevance was to include all articles related to oil and gas industry processes: extraction, processing, and transportation. The second tier narrowed these to include only articles related to reproduction and development. The third tier narrowed this further to include only publications related specifically to humans, human receptors, and/or human cell lines (Fig. 1). In addition, the articles had to be available in English, had to be original research, and had to be available as open access or through the University of Missouri, Duke University, or interlibrary loan.
FIGURE 1.
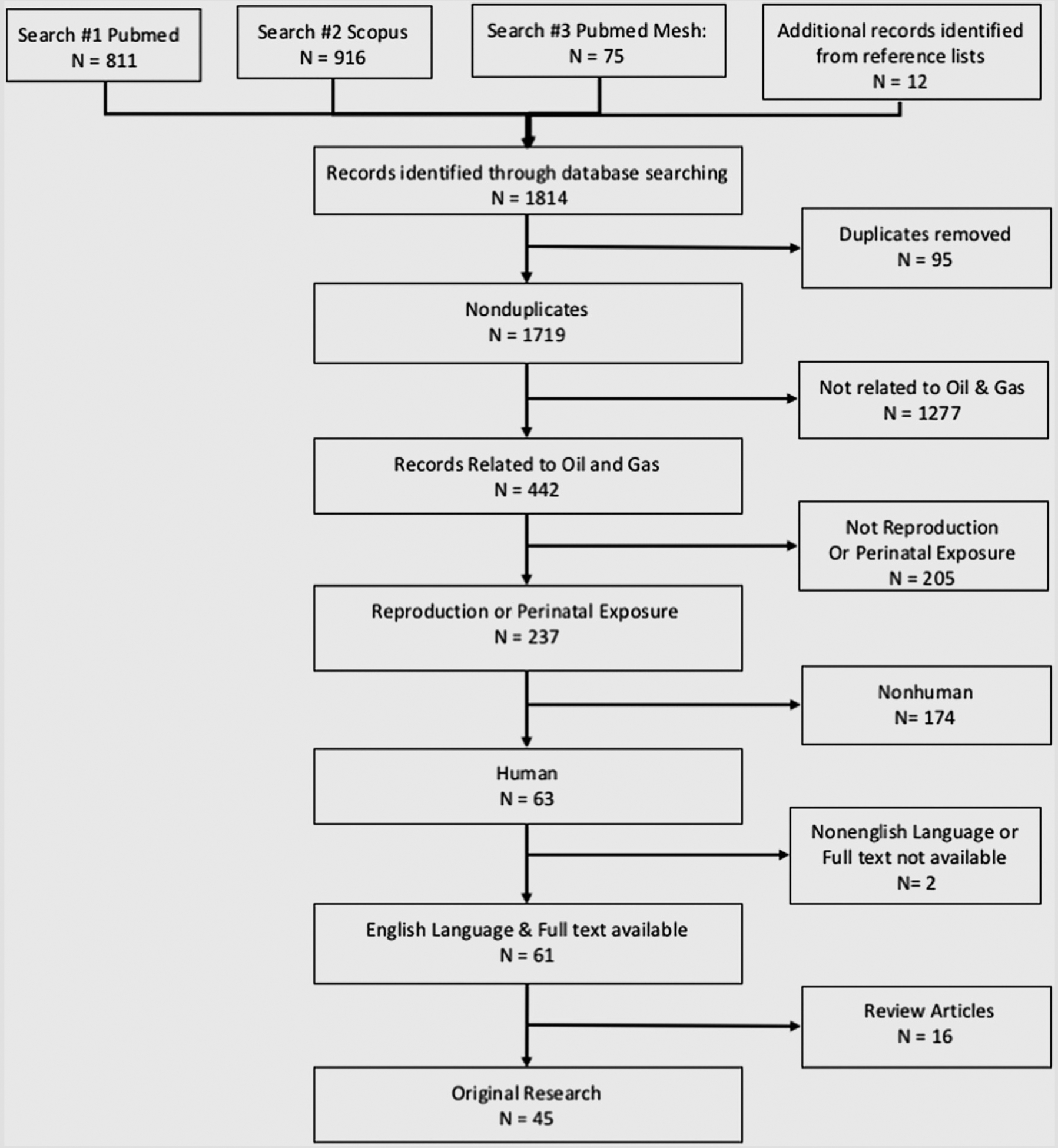
Inclusion flow chart. Summary of literature search strategies, levels of screening, and final inclusion.
Assessing Quality of Evidence
The included articles were assessed by modified Office of Health Assessment and Translation(OHAT) guidelines (19)asdescribed herein. The level of evidence and each preceding step was rated individually for each reproductive endpoint presented within each original research publication. The risk of bias was rated by answering questions (Table 2) (20) as either “yes” or “no,” assigning each “no” as one point. If a parameter was not specifically stated within the article, it was designated as a “no.” The investigators were not contacted to clarify questions because the range of publishing dates would likely bias this toward recently published research. Total points were based on the total number of applicable bias questions. The final risk of bias was determined by the percentage of “no” points to total points with the following rankings: 0 to 25%, definitely low; 26% to 50%, probably low; 51% to 75%, probably high; and 76% to 100%, definitely high. Confidence and level of evidence were determined as outlined by Rooney et al. (19). Initial confidence was given based on key features, including controlled exposure, exposure before development of the outcome, outcome assessed on individual level, and the presence of a comparison or control group resulting in a “high,” “moderate,” “low,” or “very low” level of confidence. Confidence was down-gradedfor factors including riskof bias,inconsistency,indirectness, imprecision, and publication bias. Confidence was upgraded for factors including large magnitude of effect, dose response, and consideration of residual confounding variables.
TABLE 2.
Risk of bias questions answered as part of level of evidence rating.
| Type of bias | Questions | Applicable articles |
|---|---|---|
| Selection bias | Was administered dose or exposure level adequately randomized? | Experimental animal, human controlled trial |
| Was allocation to study groups adequately concealed? | Experimental animal, human controlled trial | |
| Were the comparison groups appropriate? | Cohort, case control, cross-sectional | |
| Confounding bias | Did the study design or analysis account for important confounding and modifying variables? | All |
| Performance bias | Were experimental conditions identical across study groups? | Experimental animal |
| Were the research personnel and human subjects blinded to the study group during the study? | Experimental animal, human controlled trial | |
| Attrition/exclusion bias | Were outcome data complete without attrition or exclusion from analysis? | Experimental animal, human controlled trial, cohort, case control, cross-sectional |
| Detection bias | Can we be confident in the exposure characterization? | All |
| Can we be confident in the outcome assessment? | All | |
| Selective reporting bias Other bias | Were all measured outcomes reported? | All |
| Were there no other potential threats to internal validity (e.g., statistical methods were appropriate)? | All |
Note: Questions from OHAT handbook (20).
Direction for each endpoint was given as no effect or effect. Endpoints with a very low confidence did not proceed to level of evidence. Level of evidence was rated by considering both confidence and direction. Effects with a high, moderate, or low level of confidence were given high, moderate, or low levels of evidence ratings, respectively. No effect with “high” was given “no evidence of health effect.” No effect with either “moderate” or “low” was given an “inadequate” level of evidence (20). The details for determining a level of evidence for each article and endpoint are documented in Supplemental Table 1 (available online).
Integration of Evidence
Evidence was integrated for each endpoint based on each article’s level of evidence and level of confidence. Any endpoint that could not be rated for level of evidence (very low confidence) was not included in the integration of evidence. High, moderate, and low level of confidence findings were weighted by multiplying the number of articles by 3 for high, 2 for moderate, and 1 for low. This method was applied to all studies with the following exceptions. For the endpoints of prostate and testicular cancers, two articles by Gun et al. (21, 22) reported findings on the same cohort, so only the most recent data were considered for assessing the overall integration of evidence. For preterm birth, four studies originated from the same research group and used similar methods within the Taiwanese population; because these were similar to replicate studies or repeated measures, they were multiplied by one instead of two (moderate evidence) (23–26).
In Vitro Studies
Consistent with the OHAT guidelines, in vitro studies were not assessed for risk of bias (40). In vitro studies were integrated by assessing separate receptors with either effect or no effect.We did not apply this to Mandani et al. (43) as it was considered ex vivo.
RESULTS
Forty-five original articles were identified that pertained to oil and gas industry processes and human reproduction (Fig. 1) (14–16, 21–62). These studies were divided into four categories: [1] birth outcomes associated with maternal exposure; [2] semen quality, fertility, and birth outcomes associated with adult paternal exposure; [3] reproductive cancers; and [4] disruption of human sex steroid hormone receptors. The results for human studies are presented by endpoint in order of the direction of evidence, and the studies receiving very low confidence are presented separately (Supplemental Table 1). Table 3 summarizes the endpoints assessed, exposure type, results, and level of evidence. Further details, including descriptions of exposure for all endpoints, can be found in Supplemental Table 2 (available online). In vitro studies are summarized in Table 4, including cell line and experimental treatment.
TABLE 3.
Endpoints assessed.
| Endpoint | Exposure type | Publication year | First author | Study location | Health effect (effect/no effect) | Results | Level of confidence | Level of evidence |
|---|---|---|---|---|---|---|---|---|
| Birth outcomes associated with maternal exposure | ||||||||
| Preterm birth | ||||||||
| Preterm birth | Residential | 2002a | Yang (30) | Taiwan | No effect | OR 1.03(95% CI, 0.94–1.13) | Moderate | Inadequate |
| Preterm birth | Residential | 2015 | Stacy (35) | USA | No effect | OR ≈ 1.0(95% CI, crossing 1) (data presented in figure, no actual number) for fourth quartile vs. first quartile exposure | Moderate | Inadequate |
| Preterm birth | Residential | 2016 | Casey (29) | USA | Effect | OR 1.9(95% CI, 1.2–2.9) for fourth quartile vs. first quartile exposure | High | High |
| Preterm birth | Residential | 2001a | Lin (25) | Taiwan | Effect | OR 1.41 (95% CI, 1.08–1.82) | Moderate | Moderate |
| Preterm birth | Residential | 2003 | Tsai (23) | Taiwan | Effect | OR 1.11 (95% CI, 1.02–1.21) | Moderate | Moderate |
| Preterm birth | Residential | 2002b | Yang (24) | Taiwan | Effect | OR 1.18 (95% CI, 1.04–1.34) | Moderate | Moderate |
| Preterm birth | Residential | 2004 | Yang (26) | Taiwan | Effect | OR 1.14(95% CI, 1.01–1.28) | Moderate | Moderate |
| Preterm birth | Residential | 2014 | McKenzie (32) | USA | Effect | OR 0.91 (95% CI, 0.85–0.98) | High | High |
| Miscarriage | ||||||||
| Miscarriage | Residential | 1988 | Axelsson (27) | Sweden | No effect | OR 1.15(95% CI, 0.75–1.76) | Moderate | Inadequate |
| Miscarriage | Residential | 2002 | Sebastian (34) | Ecuador | Effect | OR 2.47 (95% CI, 1.61–3.79) | High | High |
| Miscarriage | Occupational | 1998 | Xu (36) | China | Effect | OR 2.7 (95% CI, 1.8–3.9) | High | High |
| Miscarriage | Occupational | 1989 | Axelsson (28) | Sweden | Effect | Observed/expected = 3, P<.05 | Very low | NA |
| Stillbirth | ||||||||
| Stillbirth | Residential | 2002 | Sebastian (34) | Ecuador | No effect | OR 0.85 (95% CI, 0.35–2.05) | Low | Inadequate |
| Stillbirth | Residential | 2002 | Oliveira (33) | Brazil | No effect | OR 0.78 (95% CI, 0.22–2.72) P=.659 | Low | Inadequate |
| Birth weight | ||||||||
| Low birth weight (term) | Residential | 2002a | Yang (39) | Taiwan | No effect | OR 1.07(95% CI, 0.95–1.22) | Moderate | Inadequate |
| Low birth weight | Residential | 2002 | Oliveira (33) | Brazil | No effect | OR 1.42 (95% CI, 0.87–2.31) | Moderate | Inadequate |
| Birth weight (term) | Residential | 2016 | Casey (29) | USA | No effect | Difference in mean: −20 g (95% CI, −56–16) for fourth quartile vs. first quartile exposure | Moderate | Inadequate |
| Birth weight | Residential | 1988 | Axelsson (27) | Sweden | No effect | Mean ± SD: 3,464 ±507 vs. 3,405 ± 581; difference in mean: 59 g, P>.05 for first baby | Moderate | Inadequate |
| Low birth weight (term) | Residential | 2001b | Lin (31) | Taiwan | Effect | OR 1.77(95% CI, 1.0–3.1) | Moderate | Moderate |
| Low birth weight (term) | Residential | 2014 | McKenzie (32) | USA | Effect | OR 0.9 (95% CI, 0.8–1.0) for high exposure vs. reference | Moderate | Moderate |
| Low birth weight | Residential | 1988 | Axelsson (27) | Sweden | Effect | exposed area: observed/expected = 0.66 (95% CI, 0.44, 0.94) | Moderate | Moderate |
| Birth weight | Residential | 2015 | Stacy (35) | USA | Effect | 3,323.1 ± 558.2 vs. 3,343.9 ± 543.9 (difference in mean: −20.8g) P=.02 for fourth quartile vs. first quartile exposure | Moderate | Moderate |
| Birth weight (small for gestational age) | Residential | 2015 | Stacy (35) | USA | Effect | OR 1.34(95% CI, 1.10–1.63) for fourth quartile vs. first quartile exposure | Moderate | Moderate |
| Birth weight | Occupational | 1989 | Axelsson (28) | Sweden | Effect | No statistical conclusion | Very low | NA |
| Birth Defects | ||||||||
| Birth defects (not specified) | Residential | 2002 | Oliveira (33) | Brazil | No effect | OR 1.08 (95% CI, 0.30–3.88) | Low | Inadequate |
| Birth defects (oral clefts) | Residential | 2014 | McKenzie (32) | USA | No effect | OR 0.82 (95% CI, 0.55–1.2) for high exposure vs. reference | Moderate | Inadequate |
| Birth defects (congenital heart defect) | Residential | 2014 | McKenzie (32) | USA | Effect | OR 1.3 (95% CI, 1.2–1.5) for high exposure vs. reference | Moderate | Moderate |
| Birth defects (neural tube defects) | Residential | 2014 | McKenzie (32) | USA | Effect | OR 2.0 (95% CI, 1.0–3.9) for high exposure vs. reference | Moderate | Moderate |
| Birth defects (oral clefts) | 2006 | Chevrier(30) | France | Effect | OR 3.64 (95% CI, 1.5–8.8) | Moderate | NA | |
| Birth defects (not specified) | Residential | 1988 | Axelsson (27) | Sweden | Effect | Observed/expected = 0.68 based on registry of congenital malformation, observed/expected = 0.79 based on medical birth registry | Very low | NA |
| Sex ratio | ||||||||
| Sex ratio | Residential | 2000a | Yang (37) | Taiwan | No effect | Not significant (Z scores for individual year of a total of 26 y were listed in table) | Moderate | Inadequate |
| Sex ratio | Residential | 2000b | Yang (24) | Taiwan | Effect | Male/female = 109.3 (Z=2.96, P=.003) | Moderate | Moderate |
| Semen quality, fertility, and birth outcomes associated with adult paternal exposures | ||||||||
| Sperm and Fertility | ||||||||
| Sperm concentration | Occupational | 1985 | Rosenberg (40) | Unknown | No effect | Unexposed = 80.8 mill/mL; exposed = 66.9 mill/mL: P=.16 | Low | Inadequate |
| Sperm concentration | Occupational | 1997 | Khalifa (42) | Saudi Arabia | Effect | During employment = 0.02B mill/mL to 3.2 mill/mL; after employment = 12 mill/mL to 20.6 mill/mL | Very low | NA |
| Sperm concentration | Occupational | 2001 | Wang (41) | China | Effect | Control = 60.07 mill/mL, exposed = 52.52 mill/mL | Moderate | Moderate |
| Sperm count | Occupational | 2001 | Wang (41) | China | Effect | Control = 152 mill/ejac, exposed = 127.02 mill/ejac | Moderate | Moderate |
| Sperm motility | Occupational | 1997 | Khalifa (42) | Saudi Arabia | Effect | During employment: rapid = 0–15, sluggish = 0–15%; after employment: rapid = 30–40, sluggish = 5–15% | Very low | NA |
| Sperm motility | Occupational | 2001 | Wang (41) | China | Effect | Control = 2.41, scale, exposed = 2.02 (P<.05) | Moderate | Moderate |
| Sperm motility | Experimental | 2013 | Mandani (43) | Unknown | Effect | Control = 76%, 0.3 ppm = 2.2%(P<.001) | High | High |
| Sperm motility | Experimental | 2013 | Mandani (43) | Unknown | Effect | Control = 76%, 0.05 ppm = 52%(P<.01) | High | High |
| Sperm viability | Occupational | 2001 | Wang (41) | China | No effect | Control = 61.44%, Exposed = 63.41 % (P>.05) | Moderate | Inadequate |
| Sperm viability | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 59%, 0.05 ppm = 49% | High | High |
| Sperm viability | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 60%, 0.05 ppm = 52% (P<.01) | High | High |
| DNA damage, intact DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 86.5%, phenol-hydroquinone = 47.9% (P<.001) | High | High |
| DNA damage, denatured SS DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 14.5%, phenol-hydroquinone = 63.3% (P<.001) | High | High |
| DNA damage, effective DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 73.5%, phenol-hydroquinone = 40.5% (P<.001) | High | High |
| DNA damage, intact DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 86.5%, catechol = 55.3% (P<.001) | High | High |
| DNA damage, denatured SS DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 14.5%, catechol = 44.7% (P<.001) | High | High |
| DNA damage, effective DNA | Occupational | 2013 | Mandani (43) | Unknown | Effect | Control = 73.5%, catechol = 47% (P<.001) | High | High |
| Sperm morphology | Occupational | 1985 | Rosenberg (40) | Unknown | No effect | Unexposed = 49.1%; exposed = 44.5% (P=.94) | Low | Inadequate |
| Sperm morphology | Occupational | 1997 | Khalifa (42) | Saudi Arabia | Effect | During employment = 10–90%; after employment = 10–35% | Very low | NA |
| Fertility | Occupational | 1999 | Bull (44) | Unknown | No effect | FR 0.89 (95% CI, 0.61–1.29) | Low | Inadequate |
| Paternal birth outcomes | ||||||||
| Miscarriages | Occupational | 1999 | Bull (44) | Unknown | No effect | OR 1.1 (95% CI, 0.4–3.1) | Low | Inadequate |
| OR 1.4 (95% CI, 0.6–3.2) | ||||||||
| Birth defects | Occupational | 2012 | Desrosiers (45) | USA | Effect | OR 2 (95% CI, 0.8–5.1) | Low | Low |
| Birth defects | Occupational | 2012 | Desrosiers (45) | USA | Effect | OR 2.8 (95% CI, 0.9–9.1) | Low | Low |
| Birth defects | Occupational | 2012 | Desrosiers (45) | USA | Effect | OR 2.6 (95% CI, 1.1–6.5) | Low | Low |
| Birth defects | Occupational | 2012 | Desrosiers (45) | USA | Effect | OR 1.6(95% CI, 1.0–2.4) | Low | Low |
| Childhood cancer | Occupational | 1987 | Johnson (46) | USA | No effect | OR 2.7 (95% CI, 0.9–7.8) | Very low | NA |
| Reproductive cancer | ||||||||
| Prostate cancer | ||||||||
| Prostate cancer | Residential | 1984 | Kaldor (49) | USA | Effect | Trend (P=.002) for increasing prostate cancer incidence rates with areas of increasing exposure | Moderate | Moderate |
| Prostate cancer | Residential | 1989 | Schechter(52) | Canada | No effect | SIR 1.76 (95% CI, 0.84–4.38) | Very low | NA |
| Prostate cancer | Occupational | 2006 | Rybicki (48) | USA | No effect | OR 1.12 (95% CI, 0.73–1.73); P=.61 | Low | Inadequate |
| Prostate cancer | Occupational | 2006 | Rybicki (48) | USA | No effect | OR 0.74 (95% CI, 0.48–1.13); P=.16 | Low | Inadequate |
| Prostate cancer | Occupational | 1991 | Christie (47) | Australia | No effect | SIR 1.0 (95% CI, 0.4–1.9) | Low | Inadequate |
| Prostate cancer | Occupational | 2004 | Gun (22) | Australia | Effect | SIR 1.19(95% CI, 1.00–1.40) | Moderate | Moderate |
| Prostate cancer | Occupational | 2006 | Gun (21) | Australia | Effect | SIR 1.18 (95% CI, 1.04–1.34) | Moderate | Moderate |
| Prostate cancer | Occupational | 1997 | Jarvholm (50) | Sweden | No effect | SIR 1.1 (90% CI, 0.78–1.5) | Very low | NA |
| Prostate cancer | Occupational | 2003 | Lewis (51) | Canada | No effect | SIR 0.67 (95% CI, 0.41–1.03) | Very low | NA |
| Testicular cancer | ||||||||
| Testicular cancer | Residential | 1984 | Kaldor (49) | USA | No effect | No trend (P>.1) | Low | Inadequate |
| Testicular cancer | Occupational | 2004 | Gun (22) | Australia | No effect | SIR 1.24 (95% CI, 0.68–2.08) | Low | Inadequate |
| Testicular cancer | Occupational | 2006 | Gun (21) | Australia | No effect | SIR 1.33 (95% CI, 0.80–2.08) | Low | Inadequate |
| Testicular cancer | Occupational | 1991 | Christie (47) | Australia | No effect | SIR 1.0(95% CI, 0.2–2.8) | Very low | NA |
| Testicular cancer | Occupational | 2003 | Lewis (51) | Canada | No effect | SIR 0.82 (95% CI, 0.45–1.37) | Very low | NA |
| Reproductive tract cancer | ||||||||
| Cervical cancer | Residential | 1984 | Kaldor (49) | USA | No effect | No trend (P>.1) | Low | Inadequate |
| Uterine cancer | Residential | 1984 | Kaldor (49) | USA | No effect | No trend (P>.1) | Low | Inadequate |
| Gynecologic cancer | Residential | 1989 | Schechter (52) | Canada | No effect | 1 case out of 1,038 residents (no statistical analysis) | Very low | NA |
| Cervical cancer | Occupational | 2004 | Gun (22) | Australia | No effect | SIR 1.61 (95% CI, 0.33–4.71) | Very low | NA |
| Cervical cancer | Occupational | 2003 | Lewis (51) | Canada | Effect | SIR 0.42 (95% CI, 0.17–0.86) | Very low | NA |
| Uterine cancer | Occupational | 2003 | Lewis (51) | Canada | Effect | SIR 0.31 (95% CI, 0.06–0.89) | Very low | NA |
| Ovarian, fallopian tube, and broad ligament cancer | Occupational | 2003 | Lewis (51) | Canada | No effect | SIR 1.40 (95% CI, 0.78–2.30) | Very low | NA |
| Breast cancer | ||||||||
| Breast cancer | Residential | 1984 | Kaldor (49) | USA | No effect | No trend (P>.1) | Low | Inadequate |
| Breast cancer | Residential | 1989 | Schechter (52) | Canada | No effect | 9 cases out of 1,038 residents (no statistical analysis) | Very low | NA |
| Breast cancer | Occupational | 2004 | Gun (22) | Australia | No effect | SIR 1.02 (95% CI, 0.53–1.79) | Low | Inadequate |
| Breast cancer | Occupational | 2003 | Lewis (51) | Canada | No effect | SIR 1.02 (95% CI, 080–1.28) | Very low | NA |
Note: Results are limited to highest dose group or most relevant exposure group. Complete results in more descriptive detail can be found in Supplemental Table 2. China = People’s Republic of China; CI = confidence interval; FR = fecundity ratio; OR = odds ratio; ppm = parts per million; SD = standard deviation; SIR = standard incidence ratio; NA = not applicable.
TABLE 4.
Experimental in vitro studies with human cells and/or human steroid hormone receptors.
| Receptor | Year | First author | Cell line | Effect | Experimental treatment |
|---|---|---|---|---|---|
| Androgen | 2004 | Thomas (59) | Yeast with human AR | No effect | Five produced water effluents |
| 2015 | Tang (57) | U2OS osteosarcoma | No effect | Groundwater samples from gas coal seam | |
| 2000 | Kizu (62) | LNCaP human prostate | Effect | Crude extracts of C-heavy oils | |
| 2007 | Tollefsen (61) | Yeast with human AR | Effect | Produced water samples in North Sea | |
| 2009 | Thomas (60) | Yeast with human AR | Effect | Produced water samples in North Sea | |
| 2010 | Vrabie (55) | Yeast with human AR | Effect | Seven refined oil products and four crude oils | |
| 2011 | He (58) | MDA-kb2 | Effect | Oil sands wastewater | |
| 2014 | Kassotis (16) | Ishikawa human endometrial | Effect | Hydraulic fracturing chemicals; water from spill sites | |
| 2015 | Kassotis (15) | Ishikawa human endometrial | Effect | Hydraulic fracturing chemicals and mixtures | |
| 2016 | Kassotis (14) | Ishikawa human endometrial | Effect | Surface water associated with wastewater injection facility | |
| Estrogen | 1999 | Arcaro (53) | MCF7 human breast | Effect | PAHs, extracts from water and sediment cores |
| 2001 | Arcaro (54) | MCF7 human breast | Effect | Clarified slurry, heavy crude, and light crude oils | |
| 2004 | Thomas (59) | Yeast with human ER | Effect | Produced water | |
| 2007 | Tollefsen (61) | Yeast with human ER | Effect | Produced water samples in | |
| 2009 | Thomas (60) | Yeast with human ER | Effect | North Sea Produced water samples in North Sea | |
| 2010 | Vrabie (55) | Yeast with human ER | Effect | Seven refined oil products and four crude oils | |
| 2011 | He (58) | T47D-kbluc cells | Effect | Oil sands wastewater | |
| 2011 | Vrabie (56) | MCF7 human breast and U2OS | Effect | Distillate marine grade A | |
| 2014 | Kassotis (16) | Ishikawa human endometrial | Effect | Hydraulic fracturing chemicals; water from spill sites | |
| 2015 | Tang (57) | U2OS human osteosarcoma | Effect | Groundwater samples from coal seam gas mining region | |
| 2015 | Kassotis (15) | Ishikawa human endometrial | Effect | Hydraulic fracturing chemicals and mixtures | |
| 2016 | Kassotis (14) | Ishikawa human endometrial | Effect | Surface water associated with wastewater injection facility | |
| Progesterone | 2015 | Tang (57) | U2OS human osteosarcoma | No effect | Groundwater samples from coal seam gas mining region |
| 2015 | Kassotis (15) | Ishikawa human endometrial | Effect | Hydraulic fracturing chemicals and mixtures | |
| 2016 | Kassotis (14) | Ishikawa human endometrial | Effect | Surface water associated with wastewater injection facility |
Note: AR = androgen receptor; ER = estrogen receptor; PAH = polycyclic aromatic hydrocarbons.
Birth Outcomes Associated with Maternal Exposure
Seventeen studies specific to maternal exposure were identified for inclusion in this review (Table 3) (23–39). The reproductive endpoints from these studies were separated into six categories: preterm birth, miscarriage, stillbirth, birth weight, birth defects, and sex ratio.
Preterm birth.
A total of eight retrospective cohort studies investigated whether maternal residential exposure to oil and gas industry activities was associated with preterm birth (Table 3) (23–26,29,32,35,39). Two of these studies found no evidence of a health effect and six found evidence of a health effect (increased risk: n = 5; decreased risk: n = 1) (23–26,29,32,35,39). The preterm birth records used in these studies were all retrieved either from hospital records or government birth registration (23–26,29,31,32,35,39). The mother’s address during pregnancy and at the time of delivery was assumed to be the same (23–26,29,32,35,39). There was no information on maternal occupation for any of the studies (23–26,29,32,35,39).
Studies reporting no health effect.
Yang et al. (39) studied the prevalence of preterm birth in women from 16 petrochemical industrial municipalities compared with women from 16 reference municipalities in Taiwan. The exposed area was defined as an individual municipality where more than 2% of the total population worked in petroleum or petrochemical plants (39). The reference area was characterized as an individual municipality in which less than 2% of the total population was employed in petroleum or petrochemical plants (39). Among the live singleton infants born by nonparous women, the rate of preterm deliveries for women (n = 20,077) living in the exposed municipalities was 4.72%, and 4.58% for women (n = 19,673) living in the reference municipalities (OR 1.03; 95% CI, 0.94–1.13) (39).
Stacy et al. (35) conducted a study in southwest Pennsylvania examining the correlation between maternal residential proximity to unconventional natural gas operations and perinatal outcomes. This study included 15,451 live births during the period of 2007 to 2010 (35). Exposure quartiles were divided according to the inverse distance weighted unconventional natural gas well count (the first quartile with 0.9 wells within 1 mile, and the fourth quartile with six wells within 1 mile), and there was no significant association between first and fourth quartiles of well density and preterm birth (35).
Studies reporting a health effect.
Casey et al. (29) employed an inverse-distance squared model incorporating distance to maternal address; unconventional natural gas well pad development dates and time span, drilling, and hydraulic fracturing; and volume of production to examine the association of maternal residential exposure with preterm birth. This study included 9,384 mothers who delivered 10,496 singleton neonates (29). They found that preterm birth increased across quartiles of increasing exposure (29). Relative to the first quartile, odds ratios were 1.3 (95% CI = 1.0–1.8), 1.6 (95% CI = 1.1–2.4), and 1.9 (95% CI = 1.2–2.9) for the second, third, and fourth quartile, respectively (Table 3) (29).
Four studies with similar designs examined whether preterm birth was associated with maternal residence in Taiwan municipalities where petrochemical complexes or oil refinery plants were located (23–26). All four studies had overlapping study populations and were restricted to maternal first-parity and singleton live births (23–26). The control groups in all four studies were random samples of all births in nonexposed Taiwan municipalities (23–26). All studies reported a greater prevalence of first-parity preterm birth in mothers living in exposed areas relative to reference areas with an adjusted OR of 1.41 (95% CI, 1.08–1.82) by Lin et al. (25), 1.11 (95% CI, 1.02–1.21) by Tsai et al. (23), 1.18 (95% CI, 1.04–1.34) by Yang et al. (24), and 1.14 (95% CI, 1.01–1.28) by Yang et al. (26) (Table 3) (23–26). The sample sizes in exposed versus reference areas were 2,027 versus 49,673; 14,545 versus 49,670; 5,338 versus 51,789; and 7,095 versus 50,388, respectively (23–26).
McKenzie et al. (32) investigated whether maternal exposure to natural gas development activity was associated with preterm birth in rural Colorado, United States. By calculating inverse distance weighted well counts, they divided the residential exposure levels into tertiles (low, medium, and high exposure) that were compared with the reference group (zero gas wells within 10 mile radius of maternal residence) (32). The distributions of live births obtained from the city birth registry were 18,884 (low), 18,854 (medium), 19,384 (high), and 65,506 (reference) (32). A monotonic decrease was observed in preterm birth occurrence associated with mother’s proximity to natural gas development with an adjusted OR of 0.96 (95% CI, 0.89–1.0), 0.93 (95% CI, 0.87–1.0), and 0.91 (95% CI, 0.85–0.98), respectively (trend analysis, P<.0001) (Table 3) (32).
Miscarriage or stillbirth.
Miscarriage is defined as natural death of an embryo or fetus before 20 weeks of gestation, and stillbirth refers to fetal death after 20 weeks of gestation. Four studies evaluated the possible association between miscarriage and exposure to oil and gas industry activity (27, 28, 34, 36). Two studies evaluated maternal residential, one study evaluated occupational, and one evaluated both occupational and residential exposures (Table 3) (27, 28, 34, 36). The study that evaluated both exposures reported no health effect with residential exposure but reported a health effect with occupational exposure; however, this exposure received a very low confidence rating (27). Two studies reported a large magnitude effect, and one reporting an effect was rated as having very low confidence (28, 34, 36). Two studies examined whether stillbirth was associated with maternal proximity to oil fields or petrochemical plants and found no association, but they were rated as having inadequate evidence due to imprecision (insufficient sample size) (Supplemental Table 1) (33, 34).
Studies reporting no health effect.
Axelsson and Molin (27) evaluated whether maternal proximity to petrochemical industries was associated with miscarriage in Sweden. This study evaluated the pregnancies that occurred in 607 women living near petrochemical industries versus 705 women living in a reference area (27). Reports of miscarriage were verified by hospital records (27). No association was found between miscarriage and residential proximity (OR 1.15; 95% CI, 0.75–1.76) (27).
Sebasti an et al. (34) conducted a cross-sectional survey study in communities of the Amazon basin of Ecuador to examine the association between stillbirth and residential proximity to oil fields (34). Exposed or reference areas were defined by whether an area was within 5 km downstream (exposed) or beyond 30 km upstream of an oil field (reference) (34). Water analysis of total petroleum hydrocarbons confirmed the presence of pollution in the exposed area and the absence of pollution in the unexposed area (34). No association was reported between stillbirth and hydrocarbon exposure for 791 reported pregnancies from exposed areas compared with 586 pregnancies from reference areas (OR 0.85; 95% CI, 0.35–2.05; P = .83) (34).
Oliveira et al. (33) performed a case control study to evaluate whether maternal residential proximity to petrochemical industry at the time of delivery was associated with stillbirth in Brazil (33). Exposure was categorized based on maternal residential distance from the plant (33). In this study, 230 stillbirths were identified out of the 17,113 hospital-recorded births, and a control was assigned to match each case (33). There were no significant differences in expected cases of stillbirth that occurred in mothers living close to the petrochemical plant (OR 0.78; 95% CI, 0.22–2.72) or living in an area with preferential wind direction (OR 0.98; 95% CI, 0.38–2.54) when compared with the reference regions, although the study had a small sample size (33).
Studies reporting a health effect.
Sebasti an et al. (34), as previously described, reported a greater rate of miscarriage in women with residential exposure to oil fields (OR 2.47; 95% CI, 1.61–3.79). Xu et al. (36) also reported an elevated miscarriage risk in Chinese women with occupational exposure to petrochemical plants. During the first trimester of their pregnancies, exposed women (1,620 women based on employment history recorded exposure; 1,232 women based on self-reported exposure) compared with those without exposure (1,233 based on employment history recorded expo sure; 1,621 women based on self-reported exposure) had an increase in miscarriage risk (OR 2.7; 95% CI, 1.8–3.9 based on employment history recorded exposure; and OR 2.9; 95% CI, 2.0–4.0 based on self-reported exposure) (Table 3) (36).
Studies with very low confidence rating.
Axelsson and Molin (27) reported a greater rate of miscarriage (OR 6.6; 95% CI, 2.3–19.2) in pregnant women with occupational exposure to petrochemicals (n = 15) when compared with women without occupational exposure (n = 1,549). However, in the same study, when comparing women living near petrochemical industries (n = 607) with the reference group (n = 705), there was no difference in miscarriage rate (27). Following this study, Axelsson and Rylander (28) conducted another cross-sectional study in Sweden and found a significant increase in expected miscarriage rate among 23 first-trimester women with occupational exposure to petrochemicals. However, there was no difference when an analysis was performed for three other study years, and their study did not include an appropriate comparison group (28). Both studies investigated maternal occupational exposure, but the total number of pregnancies in the exposed groups was very low (n = 15 and 23) (Supplemental Table 1) (27, 28).
Birth weight.
A total of eight studies assessed the possible association between maternal exposure to oil and gas industry activities and birth weight (Table 3) (27–29, 31–33, 35, 39). Seven of these studies were based on maternal residential exposure, and one study evaluated maternal occupational exposure (27–29, 31–33, 35, 39). One study found no health effect for birth weight and a health effect of decreased risk for low birth weight (27). Three studies reported no evidence of a health effect; three studies found evidence of a health effect (increased risk for low birth weight); and one study, which was rated as having very low confidence, reported a decreased risk of low birth weight (27–29, 31–33, 35, 39).
Studies reporting no health effect.
Yang et al. (39), as previously described, reported no increase in low birth weight among 39,750 live babies born by nonparous mothers living in a petrochemical municipality compared with the controls with an adjusted OR 1.07 (95% CI, 0.95–1.22).
Oliveira et al. (33), as previously described, reported no increase in low birth weight among residents living near a petrochemical plant compared with unexposed controls, after adjustment for confounders. In their raw analysis, low birth weight was found to be associated with residential proximity to the petrochemical plant (OR 1.66; 95% CI, 1.01–2.72) or residence with preferential wind direction (OR 1.62; 95% CI, 1.03–2.56) (33). However, when potential risk factors including mother’s age, chronic diseases, and smoking status were included in the analysis, the association was no longer significant (maternal residence in a region adjacent to the plant: OR 1.50; 95% CI, 0.90–2.50; maternal residence in a region with preferential wind direction: OR 1.42; 95% CI, 0.87–2.31) (Table 3) (33).
In the cohort study conducted by Casey et al. (29), as previously described, no difference was reported after adjustment for several covariates; the fourth quartile of the natural gas development activity index was associated with decreased term birth weight (20 g reduction in the mean weight), but not after further adjustment for year, a factor highly correlated with unconventional natural gas development (Table 3).
In the study conducted by Axelsson and Molin (27) as previously described, the pregnancies were stratified by parity. The mean infant birth weight was slightly but not significantly higher for each group (difference in mean weight: 59 g, 64 g, and 60 g for the first, second, and third births, respectively) in the exposed area (n = 185, 186, and 99 for the first, second, and third births, respectively) than in the unexposed area (n = 221, 198, and 112 for the first, second, and third births, respectively) (Table 3) (27).
Studies reporting a health effect.
In Taiwan, Lin et al. (31), as previously described, reported a greater risk of term low birth weight in the petrochemical municipalities than in control municipalities (3.22% in 1,677 singleton newborns vs. 1.84% in 868 singleton newborns born by nonparous women; adjusted OR 1.77; 95% CI, 1.0–3.1) (Table 3). Although there was no information on maternal occupational exposure, the study retrieved air pollution records to define the exposed municipality and found consistently higher levels of sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10), sulfate ion (SO42−), ammonium cation (NH4+), and nitrate ion (NO3−) than the reference area (31).
Stacy et al. (35), as previously described, found that compared with the reference group (n = 3,604) the infants in the highest (fourth) exposure quartile (n = 4,151) had lower birth weights (difference in mean: −20.8 g, P = .02) a higher incidence of small for gestational age and (OR 1.34; 95% CI, 1.10–1.63) (Table 3).
McKenzie et al. (32), as previously described, reported that term low birth weight was negatively associated with maternal residential proximity to natural gas development at birth (OR 0.79, 0.70, and 0.62 in low, medium, and high exposure areas, respectively). After possible confounders were adjusted, a weak nonlinear tendency remained: low exposure, OR 1.0 (95% CI, 0.9–1.1); medium exposure, OR 0.86 (95% CI, 0.77–0.95); and high exposure, OR 0.9 (95% CI, 0.8–1.0) (Table 3) (32). The numbers of full-term live births in the individual exposure tertiles and reference group were 17,525, 17,565, 18,104, and 60,650, respectively (32). The decreased prevalence of low term birth weight was consistent with the result of mean birth weight (5–24 g heavier in the higher exposure tertiles, calculated by inverse distance weighted well count, than the reference group) (32).
Axelsson and Molin (27), as previously described, reviewed all single births of mothers living in the area near petrochemical industries (n = 1,255) versus the unexposed area with no petrochemical plants (n = 1,527), and found fewer infants than expected in the exposed area had a birth weight lower than 2,500 g (30 observed vs. 45.5 expected; observed/expected = 0.66; 95% CI, 0.44–0.94) (Table 3).
Studies with very low confidence rating.
Axelsson and Rylander (28), as previously described, studied the association between birth weight and maternal occupational exposure. They reported that the mean birth weight of the 40 live born infants whose mothers worked in laboratories at a petrochemical plant during pregnancy were slightly higher than the birth weights of infants born to mothers living in the same general area (n = 1,238) (Table 3) (28). This study was rated very low confidence because of the low number of 40 live births and the lack of an appropriate reference group (28).
Birth defects.
Four studies explored the relationship between birth defects and maternal residential exposure to oil and gas industry activities (Table 3) (27, 30, 32, 33). Three studies were based on residential exposure, and one study evaluated occupational exposure (27, 30, 32, 33). Out of these studies, one found no evidence of health effect, two studies found evidence of a health effect, and one study found no health effect and was rated as having very low confidence (Supplemental Table 1) (27, 30, 32, 33).
Studies reporting no health effect.
In the case control study conducted by Oliveira et al. (33), as previously described, 159 malformed newborns were compared with 158 matched controls for maternal place of residence relevant to a petrochemical plant. There were no significant differences in expected cases of malformed infants born to mothers living in areas near a petrochemical plant (OR 0.30; 95% CI, 0.70–1.27; P=.103) and in areas with preferential wind direction (OR 1.08; 95% CI, 0.30–3.88; P=.907) when compared with mothers living in the reference areas (Table 3) (33).
Studies reporting a health effect.
McKenzie et al. (32) examined whether maternal residential proximity to natural gas wells was associated with neonatal malformation in a retrospective study, as previously described. This study reported that compared with the area that had zero gas wells within a 10-mile radius, the risk for congenital heart defects increased across tertiles (medium exposure tertile: OR 1.2; 95% CI, 1.0–1.3; high exposure tertile: OR 1.3; 95% CI, 1.2–1.5) (Table 3) (32). They also found that the prevalence of neural tube defects increased in the highest exposure tertile (OR 2.0; 95% CI, 1.0–3.9) (32). The evidence of these effects is moderate, however; they did not account for two potential confounders: maternal folate intake and genetic anomaly (32). In this study, oral clefts were not found to be related to exposure (32).
Chevrier et al. (30) conducted a case control study in French children diagnosed with cleft lip and/or cleft palate (n = 17) and matched control children who were admitted for treatment of some disorders but without any birth defect, cancer, or genetic disease (n = 10). After controlling for potential confounders including folate intake, they found that maternal occupational exposure to petroleum increased the risk of oral clefts (OR 3.64; 95% CI, 1.5–8.8) (30).
Studies with very low confidence rating.
Axelsson and Molin (27), as previously described, examined the possible association between exposure to emissions from petrochemical industries and malformed infants. Based on the birth registry records in the exposed area and unexposed area, the numbers of malformed infants in the exposed area were lower than expected (observed/expected = 0.68 based on registry of congenital malformation; observed/expected = 0.79 based on medical birth registry), but no statistical analyses were performed (27). This study was rated as having very low confidence because of the risk of bias and imprecision resulting from low sample size (Supplemental Table 1) (27).
Sex ratio.
Two studies explored the relationship between sex ratio and oil and gas industry activities. Both studies were based on residential exposure (Table 3) (37, 38). Out of these studies, one found no evidence of health effect, and one found evidence of a health effect (37, 38).
Studies reporting no health effect.
Yang et al. (37) examined the period between 1971 and 1996 in two municipalities near a petroleum refinery plant in Taiwan to explore whether maternal residential exposure was associated with altered sex ratio. In this study, the sex ratio was assessed by calculating the Z statistic, taking the following into account: number of boys and girls in the municipality, the proportion of boys in the municipality, and the proportion of boys or girls in all of Taiwan (37). The Z statistic was considered statistically significant when it exceeded 1.96 (37). The data were stratified by year. No significant association was found between sex ratios and exposure to air pollution from petroleum plant in individual year (Table 3) (37). The proportions of all Taiwanese boys and girls were incorporated to calculate the Z statistic. (Supplemental Table 1) (37).
Studies reporting a health effect.
Yang et al. (38) also conducted a similar study employing the same Z-statistic method in 16 municipalities of Taiwan with exposure to oil and gas activities (defined as ≥ 2% of the municipality’s total population worked in the petroleum and petrochemical industry) during the years of 1987–1996. This study reported a sex ratio (male vs female) of 109.3 (108,889 males vs. 99,612 females) in petrochemical industrial municipalities, which is significantly higher than the average live-birth sex ratios at a national level. The Z statistic in this study was 2.96 (P = 0.003) (Supplemental Table 1) (38).
Semen Quality, Fertility, and Birth Outcomes Associated with Adult Paternal Exposures
Seven studies specific to oil and gas industry activities and adult male reproduction were identified for inclusion in this review (40–46). The reproductive endpoints from these studies were separated into two categories: semen quality and fertility, and birth outcomes arising from paternal exposure.
Semen Quality and Fertility
Semen quality (sperm concentration, motility, viability, and morphology) is a critical parameter in adult male reproduction, and several studies have examined semen quality in relation to occupational exposure in oil and gas industry activities and associated chemicals such as benzene (and its metabolites) and crude oil (40–44).
Sperm concentration.
Three studies investigated occupational exposure to oil and gas operations and sperm concentration (40–42). One found no health effect, one found a health effect, and one found a health effect with very low confidence (40–42).
Studies reporting no health effect.
Rosenberg et al. (40) evaluated the sperm concentration of 42 employees working in a petroleum refinery’s wastewater facility (exposed) compared with 74 employees in other positions at the refinery (unexposed) to determine whether sperm concentration was associated with occupational exposure. The sperm concentration of exposed individuals was approximately 17% lower than that of unexposed individuals (unexposed = 80.8 million/mL; exposed = 66.9 million/mL; P=.16) (40). This reduction in sperm concentration was not significant (40). The authors note that this study only had 80% power to detect a 39% decrease in sperm concentration; as a result, they conclude it was underpowered for this endpoint, which resulted in this study receiving an inadequate level of evidence rating (Supplemental Table 1) (40). This study also found no significant correlation between hours worked in the previous 6 months and sperm concentration (40).
Studies reporting a health effect.
Wang et al. (41) examined sperm count and concentration in employees at a petrochemical complex in the People’s Republic of China (41). This complex had eight major plants that processed petroleum and other chemicals (41). The workers were divided into four categories: [1] 45 exposed workers, smokers; [2] 23 exposed workers, nonsmokers; [3] 81 unexposed workers, smokers; and [4] 49 unexposed workers, nonsmokers (41). This study found an approximate decrease of 24% to 29% in the sperm counts of exposed smokers compared with the unexposed groups (unexposed/nonsmokers = 152 million/ejaculate; unexposed/smokers = 141.86 million/ejaculate; exposed/nonsmokers = 127.02 million/ejaculate; and exposed/smokers = 108.48 million/ejaculate [P<.05]) (Table 3) (41). Because the sperm counts were only decreased in the exposed smokers, Wang et al. (41) suggested that the health effect from petrochemical occupational exposure may be exacerbated by smoking (41). Wang et al. (41) also found a 31% decrease in the sperm concentration of the exposed smoker group (41.49 million/mL) compared with the unexposed nonsmoker group (60.07 million/mL, P<.01), but no difference was seen in relation to the exposed/nonsmoker group (52.52 million/mL) or the nonexposed/smoker group (55.32 million/mL) (Table 3) (41). This study also found that sperm concentration was negatively correlated to years of smoking and years of exposure combined (r = −0.28; P<.05) (41).
Studies with very low confidence rating.
Khalifa et al. (42) presented a case study of a petroleum field worker in Saudi Arabia evaluated at time points both during and after the subject’s employment. This study observed a low sperm concentration on four separate occasions from 1992 to 1995 during the subject’s employment in the petroleum fields (0.025 million/mL to 3.2 million/mL) (42). The subject’s sperm concentration began to improve 1 month after taking an office job (12 million/mL to 20.6 million/mL in two separate measurements) and continued to increase after 5 months (16 million/mL to 18 million/mL in two separate measurements, both at 5 months) (Table 3) (42). This report was assigned a very low confidence rating because it was case study of a single individual who had been previously diagnosed with primary infertility due to oligospermia before becoming employed by the petroleum industry (Supplemental Table 1) (42).
Sperm motility.
Three studies investigated exposure to oil and gas operations and associated chemicals and sperm motility (41–43). Two studies evaluated occupational exposure to oil and gas operations, and one study evaluated ex vivo experimental exposure to chemicals used in oil and gas operations (41–43). Two found a health effect, and one found a health effect with very low confidence (41–43).
Studies reporting no health effect.
No studies reported oil and gas industry activities as having no health effect on sperm motility.
Studies reporting a health effect.
Wang et al. (41), as previously described, found that occupational exposure to petrochemicals was associated with 16% lower sperm motility for nonsmokers (P<.05) and 18% lower for smokers (P<.01) (Table 3) (41). Mandani et al. (43) exposed healthy human sperm samples (sperm count 75–110 million/mL) ex vivo to the benzene metabolites phenol-hydroquinone or catechol at concentrations ranging from 0.05 to 0.3 parts per million (ppm). Exposure at all concentrations reduced sperm motility, and there was a dose-dependent decrease from 76% in control sperm to 2.2% in the 0.3 ppm phenol-hydroquinone and 24% in the 0.3 ppm catechol groups (Table 3) (43).
Studies with very low confidence rating.
The case study by Khalifa et al. (42), as previously described, observed poor sperm motility (rapid sperm = 0 to 15%) at the time of employment as a petroleum field worker, which improved within 1 to 5 months after the subject began to work in an office job (rapid sperm = 30% to 40%) (42).
Sperm viability.
Two studies investigated exposure to oil and gas operations and sperm viability (41, 43). One investigated occupational exposure, and the other was an ex vivo experimental exposure (41, 43). One study found no health effect, and the other study found a health effect (41, 43).
Studies reporting no health effect.
Wang et al. (41), as previously described, found no difference in sperm viability between petrochemical-exposed workers (nonsmokers = 63.41%, smokers = 60.78%) and unexposed workers (nonsmokers = 61.44%, smokers = 60.28%).
Studies reporting a health effect.
Mandani et al. (43), as previously described, found that concentrations of benzene metabolites ranging from 0.05 ppm to 0.3 ppm reduced sperm viability in a dose-dependent decrease from 59% in control sperm to 13% in the 0.3 ppm phenol-hydroquinone, and 24% in the 0.3 ppm catechol exposed sperm (Table 3).
Sperm morphology.
Two studies investigated occupational exposure to oil and gas operations and sperm morphology; one reported no health effect, and one found a health effect with very low confidence (40, 42).
Studies reporting no health effect.
Rosenberg et al. (40), as previously described, reported no difference in the percent abnormal sperm of workers in the wastewater facility of a petroleum refinery (44.5%) compared with that of employees in other positions at the refinery (49.1%; P=.94). This study also reported no correlation with hours worked in the past 6 months and sperm morphology (Table 3) (40).
Studies reporting a health effect.
No studies reported oil and gas industry activities as having a health effect on sperm morphology.
Studies with very low confidence rating.
The case study by Khalifa et al. (42), as previously described, reported that the percentage of abnormal sperm in a patient with occupational exposure to petroleum was elevated during employment in the petroleum fields (10% to 90%). This value appeared to improve after leaving the petroleum field (10% to 35%) (42).
Sperm DNA damage.
One study investigated ex vivo experimental exposure to chemicals associated with oil and gas operations and sperm DNA damage. This study found a health effect (43).
Studies reporting no health effect.
No studies reported oil and gas industry activities as having no health effect on sperm DNA damage.
Studies reporting a health effect.
Mandani et al. (43), as previously described, reported that upon exposure to benzene metabolites, healthy human sperm showed DNA damage. There was a decrease in intact double-stranded DNA from 86.5% for the control sperm to 47.9% (P<.001) after treatment with 30 μL of phenol-hydroquinone, and to 55.3% (P<.001) after treatment with 30 μL of catechol (43). There was also an increase in denatured single-stranded DNA from 14.5% for the control sperm to 63.3% (P<.001) after treatment with 30 mL of phenol-hydroquinone and 44.7% (P<.001) after treatment with 30 μL of catechol (43). A decrease in effective DNA was also observed from 73.5% for the control sperm to 40.5% (P<.001) after treatment with 30 mL of phenol-hydroquinone and 47% (P<.001) after treatment with 30 μL of catechol (Table 3) (43).
Male fertility.
One study investigated occupational exposure to oil and gas operations and fertility. This study found no health effects (44).
Studies reporting no health effect.
Bull et al. (44) reported paternal occupational exposure to offshore oil processes had no effect on fecundity ratios (ratio defined as the probability of getting pregnant during the next cycle for exposed/control). The exposed workers were 30 offshore mechanics (fecundity ratio 0.8; 95% CI, 0.49–1.32) and 95 drilling personnel (fecundity ratio 0.89; 95% CI, 0.61–1.29), and the control group was 51 offshore operators (Table 3) (44). The power analysis supplied by Bull et al. (44) indicates that they would require 55 subjects in each category to detect a 50% decline in conception with 80% power (44). This indicates this study was underpowered for this endpoint; as a result, this study was given an inadequate level of evidence rating (Supplemental Table 1) (44). The fecundity ratio calculated in this study excluded cases where no pregnancy resulted after 12 months of unprotected intercourse (44). These rates were reported, but with no analysis or discussion (control: 5.7%, offshore mechanics: 25.0%, drilling personnel: 12.6%) (44). Due to the lack of statistical analysis, it is difficult to make conclusions about these data; however, they suggest there was an increase in the rate of infertility in offshore mechanics and drilling personnel (44).
Studies reporting a health effect.
No studies reported oil and gas industry activities as having a health effect on male fertility.
Birth Outcomes Arising from Paternal Exposure
Much of the research investigating developmental outcomes focuses on maternal exposure, but there has been evidence to suggest paternal exposure is also important (63). Male-mediated effects on the birth outcomes of offspring have been hypothesized to occur via genetic or epigenetic changes and/or the transmission of chemicals to the mother via seminal fluid transfer (63). Therefore, it is important to evaluate paternal occupational exposures in this systematic review (63).
Miscarriage.
One study investigated paternal occupational exposure to oil and gas operations and miscarriages (44). This study found no health effect (44).
Studies reporting no health effect.
Bull et al. (44), as previously described, found no significant increase in the incidence of miscarriages associated with paternal occupational exposure to offshore drilling processes (offshore mechanics, OR 1.1; 95% CI, 0.4–3.1; offshore drilling personnel, OR 1.4; 95% CI, 0.6–3.2) (44). This study reported that 112 pregnancies in each category would be necessary to detect a twofold increase in miscarriages with 80% power as the offshore drilling personnel category was the only category that had a sufficient sample size to meet this criterion (44). This study was underpowered for this endpoint (Supplemental Table 2) (44).
Studies reporting a health effect.
No studies reported paternal exposure to oil and gas industry activities as having a health effect on miscarriages.
Birth defects and childhood cancers.
Two studies investigated paternal occupational exposure to oil and gas operations and birth defects and childhood cancer (45, 46). One found a health effect, and one found no health effect with very low confidence (45, 46).
Studies reporting no health effect.
No studies reported oil and gas industry activities as having no health effect on birth defects and childhood cancers.
Studies reporting a health effect.
Desrosiers et al. (45) evaluated 9,998 known cases of birth defects in the United States for any possible correlation to paternal occupation. In this study, 4,066 fathers of children without birth defects were used as the control group (45). Upon evaluation it was determined that there was a positive correlation between multiple birth defects and paternal occupational exposure to petroleum and gas operations (45). These defects were glaucoma/anterior chamber defects (OR 2; 95% CI, 0.8–5.1), colonic atresia/stenosis (OR 2.8; 95% CI, 0.9–9.1), intercalary limb deficiency (OR 2.6; 95% CI, 1.1–6.5), and atrial septal defect (ASD), secundum, or not otherwise specified (OR 1.6; 95% CI, 1.0–2.4) (45).
Studies with very low confidence rating.
A cohort study by Johnson et al. (46) analyzed birth certificates in Texas to determine whether there was any association between child hood nervous system cancers and paternal occupations related to hydrocarbons. This study included subjects in the following three categories: 30 controls and 20 cases from employees of chemical, petroleum, and rubber industries; 6 controls and 8 cases from petroleum-refining industry employees; and 6 controls and 6 cases from petroleum refinery employees (46). Each group was then evaluated to determine whether there was any association between paternal occupational exposure and childhood nervous system cancers (46). There were no detectable changes in incidence found (chemical, petroleum, and rubber employees, OR 1.3; 95% CI, 0.8–2.4; petroleum refining industry employees, OR 2.7; 95% CI, 0.9–7.8; and petroleum refinery employees, OR 2.0; 95% CI, 0.6–6.2) (46). According to power analysis performed by the investigators this study was powered to detect a twofold increase with a 3% prevalence; none of these groups met that criterion (46). This indicates that this study was underpowered to detect a change in cancer prevalence for these particular groups (46). This study by Johnson et al. (1987) received a very low confidence rating due to residual confounding variables and lack of power to detect a health effect (Supplemental Table 1) (46).
Reproductive Cancers
Chemicals used in and produced by the oil and gas industry include known carcinogens such as benzene and polycyclic aromatic hydrocarbons (PAHs). This systematic review evaluated a total of eight studies that assessed four reproductive cancers: prostate, testicular, female reproductive tract (cervix, uterus, broad ligament, ovary, and “gynecologic”), and breast. Cancer incidence was selected over mortality to avoid both the exclusion of cases that did not result in death and an overlap between morbidity and mortality instances.
Prostate cancer.
A total of eight studies evaluated the potential association between exposure to oil and gas industry activities and prostate cancer (21, 22, 47–52). Two of these studies found no evidence of health effect, three reported evidence of a health effect, and three found no health effect with very low confidence (21, 22, 47–52).
Studies reporting no a health effect.
Two occupational exposure studies found no evidence of an effect on prostate cancer incidence (47, 48). Christie et al. (47) reported no difference in prostate cancer morbidity (standard incidence ratio [SIR] 1.0; 95% CI 0.4–1.9) between a cohort of over 15,000 Australian petroleum industry employees and the national rates. Rybicki et al. (48) used a case control design in which 637 men with prostate cancer (cases) and 244 without cancer (controls) were both occupationally exposed to PAHs from petroleum. Levels of PAH exposure were estimated based on job title by industrial hygienists, and potential correlations between occupational PAH exposure, mutations of glutathione S-transferase (GSTP1; a gene expressed in normal prostate cells that is responsible for detoxifying the body of mutagenic metabolites of PAH), and prostate cancer were evaluated (48). No difference was discerned between respiratory, or cutaneous PAH exposure and prostate cancer (OR 1.12; 95% CI, 0.73–1.73; P=.61; and OR 0.74; 95% CI, 0.48–1.13; P=.16, respectively) (Table 3) (48).
Studies reporting a health effect.
Two of the three articles that reported evidence of a health effect were based on occupational exposure to oil and gas industry activities (21, 22, 49). Gun et al. (21, 22) conducted two analyses on updated results from the Christie et al. (47) cohort (as previously described). The first of the two reports found an increased rate of prostate cancer (SIR 1.19; 95% CI, 1.00–1.40) for male employees compared with the national rates (22). The second study, which followed the cohort for a longer period of time, detected similar results (SIR 1.18; 95% CI, 1.04–1.34) (21). No correlation was discovered between prostate cancer and type of work, decade of hire, employment duration, time since hire, or hydrocarbon exposure ranking. Hydrocarbon exposure was determined by industrial hygienists, who estimated exposure levels based on job codes (21).
In a residential-exposure cohort study, Kaldor et al. (49) evaluated the risk of prostate cancer in Contra Costa County, California, a core of oil refining and chemical production industries in northern California for almost a century before the study’s initiation. The county was subdivided into areas of high (n = 486,691), medium (n = 300,494), low (n = 411,180) estimated exposure to air and emissions from nearby petroleum and chemical plants via a pollution dispersion model derived from point-of-emission measurements, data on chemicals used and produced by refineries/plants in the area, and topographic and meteorologic data (49). The low exposure area was used as the reference group (49). A positive trend (P=.002) was found for increasing risk of prostate cancer with increasing exposure (incidences per 100,000 = 46.3, 48.4, and 62.2 for the low, medium, and high exposure groups, respectively) (Table 3) (49).
Studies with very low confidence rating.
There were three studies rated with very low confidence, and were therefore not evaluated for level of evidence of health effect (50–52). J€arvholm et al. (50) calculated the SIR of prostate cancer for 4,128 occupationally exposed men employed in the Swedish petroleum industry relative to historical data from the general population. This study found no increased risk of cancer in the exposed group compared with the general population (SIR 1.1; 90% CI, 0.78–1.5), though it received a very low confidence rating due to a high risk of bias (Supplemental Table 1) (50).
Lewis et al. (51) used a similar design when evaluating the potential association between prostate cancer and occupational exposure in a group of 17,230 Canadian petroleum company employees. No difference was found in industry workers compared with national rates (SIR = 0.67; 95% CI, 0.41–1.03). This study was given a very low confidence rating because the authors report that this study had limited statistical power because of very low incidence of prostate cancer in this young cohort (average age = 29 years) (Supplemental Table 1) (51). Schechter et al. (52) evaluated risk of all-site cancers in 1,126 individuals living downwind of two Alberta, Canada, natural gas refineries compared with three reference populations. The exposure area was defined by plume pat terns of refinery emissions (52). No difference was found between the exposed and reference populations for prostate cancer (SIR 1.76; 95% CI, 0.84–4.38). This study received a very low confidence rating as the study was powered to detect all-site cancers and was underpowered to detect specific cancers (Supplemental Table 1) (52).
Testicular cancer.
A total of five studies reported on the incidence of testicular cancer associated with oil and gas industry activities (21, 22, 47, 49, 51). Three articles reported no health effect, and two found no health effect with very low confidence (21, 22, 47, 49, 51).
Studies reporting no health effect.
Gun et al. (22), as previously described, reported no difference in testicular cancer in occupationally exposed petroleum industry employees compared with expected rates in the general population (SIR 1.24; 95% CI, 0.68–2.08). A later analysis of the same cohort by Gun et al. (21), as previously described, found similar results (SIR 1.33; 95% CI, 0.80–2.08). Kaldor et al. (49), as previously described, found no disparity in risk of testicular cancer between men in “medium” or “high” estimated residential exposure areas from petroleum refinery and chemical plant emissions and those in the “low” exposure reference group (incidences per 100,000 = 4.0, 3.7, and 4.1 for the low, medium, and high exposure areas, respectively; P>.1) (Table 3).
Studies reporting a health effect.
No studies reported oil and gas industry activities as having an effect on testicular cancer incidence.
Studies with very low confidence rating.
Christie et al. (47), as previously described, reported no difference in testicular cancer in occupationally exposed petroleum industry employees compared with the national rates (SIR 1.0; 95% CI, 0.2–2.8). Lewis et al. (51), as previously described, also found no disparity in cases of testicular cancer between petroleum company workers and the general population (SIR 0.82; 95% CI, 0.45–1.37). Both of these studies were rated as having very low confidence due to lack of statistical power (Supplemental Table 1) (47, 51).
Female reproductive tract cancers.
A total of four articles presented findings on female reproductive tract cancer endpoints (cervical, uterine, ovarian, fallopian tube, broad ligament, and “gynecologic” cancers), with multiple endpoints assessed in two of the articles (22, 49, 51, 52). One study found no evidence of a health effect, two found a health effect with very low confidence, and one found a health effect and no health effect in different cancers with very low confidence (22, 49, 51, 52).
Studies reporting no health effect.
Kaldor et al. (49), as previously described, reported no differences in the incidence of cervical and uterine cancers between women residentially exposed to air emissions from area petroleum and chemical industries in the medium (n = 303,397) or high exposure (n = 506,191) cohorts relative to the low exposure reference group of 421,995 (incidences per 100,000 = 15.2/36.1, 14.7/34.8, and 18.0/35.3 for cervical/uterine cancers in the low, medium, and high exposure areas, respectively; P>.1 for both types of cancer) (Table 3).
Studies reporting a health effect.
No studies reported oil and gas industry activities as having an effect on reproductive tract cancers.
Studies with very low confidence rating.
Schechter et al. (52), as previously described, reported one incident of “gynecologic” cancer (not defined) out of 1,038 female residents exposed to natural gas refinery emissions. Gun et al. (22), as previously described, reported no difference in cervical cancer risk of 867 female petroleum employees compared with the general population (SIR 1.61; 95% CI, 0.33–4.71). Lewis et al. (51), as previously described, found decreased rates of cervical and uterine cancers in occupationally exposed female petroleum employees compared with national rates (SIR 0.42; 95% CI, 0.17–0.86; and 0.31; 95% CI, 0.06–0.89, respectively) (Table 3). This study found no difference in ovarian, fallopian tube, or broad ligament cancer incidence (grouped) between exposed female employees and the general population (SIR 1.40; 95% CI, 0.78–2.30) (51). Each of these articles received a very low confidence rating due to lack of statistical power for these endpoints (Supplemental Table 1) (22, 51, 52).
Breast cancer.
Four studies assessed the incidence of breast cancer, with two reporting no evidence of a health effect, and two studies showing no health effect with very low confidence (22, 49, 51, 52).
Studies reporting no health effect.
Kaldor et al. (49), as previously described, found no difference in breast cancer rates between high and low residential exposure groups (incidence rates per 100,000 = 81.2, 83.2, and 83.2 for low, medium, and high exposure areas, respectively; P>.1) (Table 3). Gun et al. (22), as previously described, reported no disparities in breast cancer incidence for occupationally exposed petroleum employees compared with the general population (SIR 1.02; 95% CI, 0.53–1.79).
Studies reporting a health effect.
No studies reported oil and gas industry activities as having an effect on breast cancer incidence.
Studies with very low confidence rating.
Schechter et al. (52), as previously described, reported nine cases of breast cancer in 1,038 women residentially exposed to natural gas refinery emissions, though a statistical analysis was not performed. Lewis et al. (51), as previously described, found no difference in breast cancer for petroleum employees when compared with expected general population rates (SIR 1.02; 95% CI, 0.80–1.28). These articles both received a very low confidence rating due to lack of statistical power (Supplemental Table 1) (51, 52).
Disruption of Human Sex Steroid Hormone Receptors
Numerous publications have assessed the mechanistic endocrine impacts of specific chemicals used in and/or produced by oil and gas operations (17, 64), though studies of individual chemicals are beyond the scope of this review. However, due to the chemical complexity of oil and gas samples and wastewater, and the variety of impacted environmental matrices, containing thousands of chemicals (65, 66), few studies have attempted to assess oil and gas mixtures either through direct testing or effect-directed analysis. Additional work is necessary in this area, and new tools must be developed to better isolate and identify the ligands responsible for the receptor activities.
Estrogen receptor disruption.
Our search identified 12 studies that evaluated the impacts of oil and gas operation products on estrogen receptor (ERa and/or β) disruption in vitro (14–16, 53–61). These studies assessed agonist and antagonist activities via reporter gene assays, relative binding affinity assays, cell development assays, and mechanistic hormone metabolism assays (14–16, 53–61).
Studies reporting no association.
None of the 12 studies assessing an effect on ER activity reported no effect on some measure of ER disruption (Table 4) (14–16, 53–61). Six studies did not find specific agonist or antagonist activity for specific endpoints (53–58).
Two studies by Arcaro et al. (53, 54) reported no estrogenic activity associated with polycyclic aromatic hydrocarbons (PAHs) and oil-influenced sediment samples (53) or with several crude oils (54) using the cell focus assay, which measures estrogen-mediated multicellular nodule or foci production in MCF-7 cells.
Vrabie et al. (55, 56) found no ERβ antagonism for refined oil products using reporter gene assays in two studies. Two out of nine studies found no ERa antagonism (14–16, 53–58). Tang et al. (57) found no ER antagonism in groundwater samples from an active coal seam gas mining region, and He et al. (58) found no antagonism in oil sands process-affected water from two settling ponds. It is possible that these differing results are due to the diversity of matrices assessed (i.e., surface/groundwater from a variety of regions, wastewater and byproducts, a range of crude and refined oil and gas products) rather than any differences in study integrity.
Studies reporting an association.
All 12 of the identified studies reported some type of ER disruption (Table 4) (14–16, 53–61).
Three studies assessed crude and/or refined oil products directly (Table 4) (54–56). Arcaro et al. (54) found that crude oils inhibited estradiol-mediated foci formation in MCF-7 cells through direct receptor binding, and that this inhibition was correlated with PAH concentration. Vrabie et al. (55, 56) found that a range of crude and refined oil products elicited agonist activity for ERα and ERβ and antagonist activity for ERα in reporter gene assays, with crude oil exhibiting higher ERα agonist activity and refined oil exhibiting higher ERα antagonism and higher ERβ agonist activity.
Environmental studies have assessed ER activity in a diversity of sample types (Table 4). Tang et al. (57) assessed groundwater from a coal seam gas-mining region and reported ER agonist activity (0.05–0.10 ng estradiol equivalence per liter water [E2-EQ/L]). Three studies assessed produced water effluents from North Sea oil platforms for ER agonist activity (59–61). Thomas et al. (59) reported %28 ng E2-EQ/L, Thomas et al. (60) reported 42 ng E2-EQ/L, and Tollefsen et al. (61) reported <4 ng E2-EQ/L. Two of these studies suggested alkyl-substituted phenols were likely causative (59, 61).
We have assessed surface and/or groundwater associated with unconventional oil and gas (UOG) operations (Table 4). Estrogenic and antiestrogenic activity was exhibited by 89% and 41% of water sample extracts concentrated 40-fold from a dense-drilling region, with significantly greater estrogenic activity near dense-drilling spill sites and greater antiestrogenic activity in two surface water spill sites (16). Surface water on and downstream of an UOG wastewater injection disposal site exhibited <200 ng ICI 182,780 EQ/L water, whereas upstream and reference samples exhibited no antagonism (14). Collaborative work provided geochemical evidence that these samples were specifically contaminated by UOG wastewater (67).
Our laboratory has previously found that 21 of 24 commonly used UOG chemicals exhibited significant ER antagonism, with eight chemicals reaching an IC50 (concentration required to inhibit half of the positive control) and apparent synergistic antagonism observed in a mixture of 24 oil and gas chemicals (15), low estrogen agonist activity for a naphthenic acid mixture (58), antiestrogenic activities for a range of PAHs (53).
Androgen receptor disruption.
Our review identified 10 studies that have evaluated the impact of oil and gas operations on androgen receptor (AR) disruption in vitro (Table 4) (14–16, 55, 57–62). Disruption of the AR, typically via antagonism, has been assessed in a variety of oil and gas production matrices via reporter gene assays, relative binding affinity assays, and gene expression analyses.
Studies reporting no association.
Two of the 10 studies reported no androgen agonist or antagonist activities from oil and gas operations (Table 4) (14–16, 55, 57–62). Tang et al. (57) reported no AR activity in groundwater from an active coal seam gas mining region, though evidence of contamination in these samples was sparse and hydraulic fracturing had not been performed in these wells. Thomas et al. (59) reported no androgenic activity in produced water effluents from North Sea oil production platforms, though antiandrogenic activity was not assessed in this study, despite environmental antiandrogens being more common (68).
Studies reporting an association.
Eight of the 10 studies reported elevated AR activity near oil and gas operations (Table 4) (14–16, 55, 57–62). Two studies reported an increase in androgenic activity in oil and gas impacted sample types (16, 55). Using a yeast cell line with human AR, Vrabie et al. (55) reported <11% androgenic activity by several crude oil samples. Our laboratory reported <5% androgenic activity in 12% of surface and groundwater samples from a dense-drilling region, though mainly in reference samples (16).
Antiandrogenic activity was frequently detected, with eight studies using diverse assays and reporting AR inhibition for various oil and gas matrices (14–16, 55, 58, 60–62). Vrabie et al. (55) used a reporter gene assay in human cells, detecting 60% to 90% AR inhibition for a range of crude and refined oil products that was rescued with increased concentrations of testosterone. Kizu et al. (62) used a prostate specific antigen gene expression assay to report 80% inhibition for several crude oil extracts and a similar degree of antiandrogenicity for several constituent PAHs. Thomas et al. (60) used yeast assays (human AR) to assess produced water extracts from North Sea oil production platforms, reporting ≤7,000 mg flutamide EQ/L, and identifying constituent carboxylic and naphthenic acids as antiandrogens. Tollefsen et al. (61) used a similar assay to report ≤8,170 flutamide EQ/L in 18 of 20 produced water samples (61). Oil sands process affected water exhibited <75% AR antagonism in a reporter gene assay, though the assay was performed in an atypical manner by varying positive control concentration (58).
Finally, three studies from our laboratory assessed AR disruption associated with oil and gas activities (14–16). Surface water and groundwater near drilling-dense UOG fluid spill sites exhibited AR inhibition in 46% of samples, with greater antagonism near spill sites relative to reference sites (16). Surface water on and downstream of an UOG wastewater injection disposal site exhibited up to 700 μg flutamide EQ/L water, whereas upstream and reference samples exhibited no antagonism (14). We have also shown that 21 of 24 UOG chemicals showed antiandrogenic activity, with six reaching an IC50 concentration; a mixture of these chemicals showed additive antagonism for AR (15).
Progesterone receptor disruption.
Disruption of the progesterone receptor has been infrequently assessed. Our search identified three studies that evaluated the impacts of oil and gas operations on progesterone receptor disruption in vitro (Table 4) (14, 15, 57).
Studies reporting no association.
One of the three studies, Tang et al. (57) reported no agonist or antagonist activity for the progesterone receptor (PR) in groundwater from a coal seam gas mining region (Table 4).
Studies reporting an association.
Two of the three studies, both performed by our laboratory, reported increased PR antagonism associated with nearby UOG operations (Table 4) (14, 15). Surface water on and downstream of an UOG wastewater injection disposal site exhibited up to 5.5 μg mifepristone EQ/L water whereas upstream and reference samples exhibited no antagonism (14). We have also shown that 12 of 24 UOG chemicals were antagonists for PR with four chemicals, reaching an IC50 concentration, and a mixture of these chemicals showed additive antagonism (15).
DISCUSSION OF INTEGRATION OF EVIDENCE FOR REPRODUCTIVE HEALTH EFFECTS
Evidence for each endpoint is integrated here and summarized in Figure 2.
FIGURE 2.
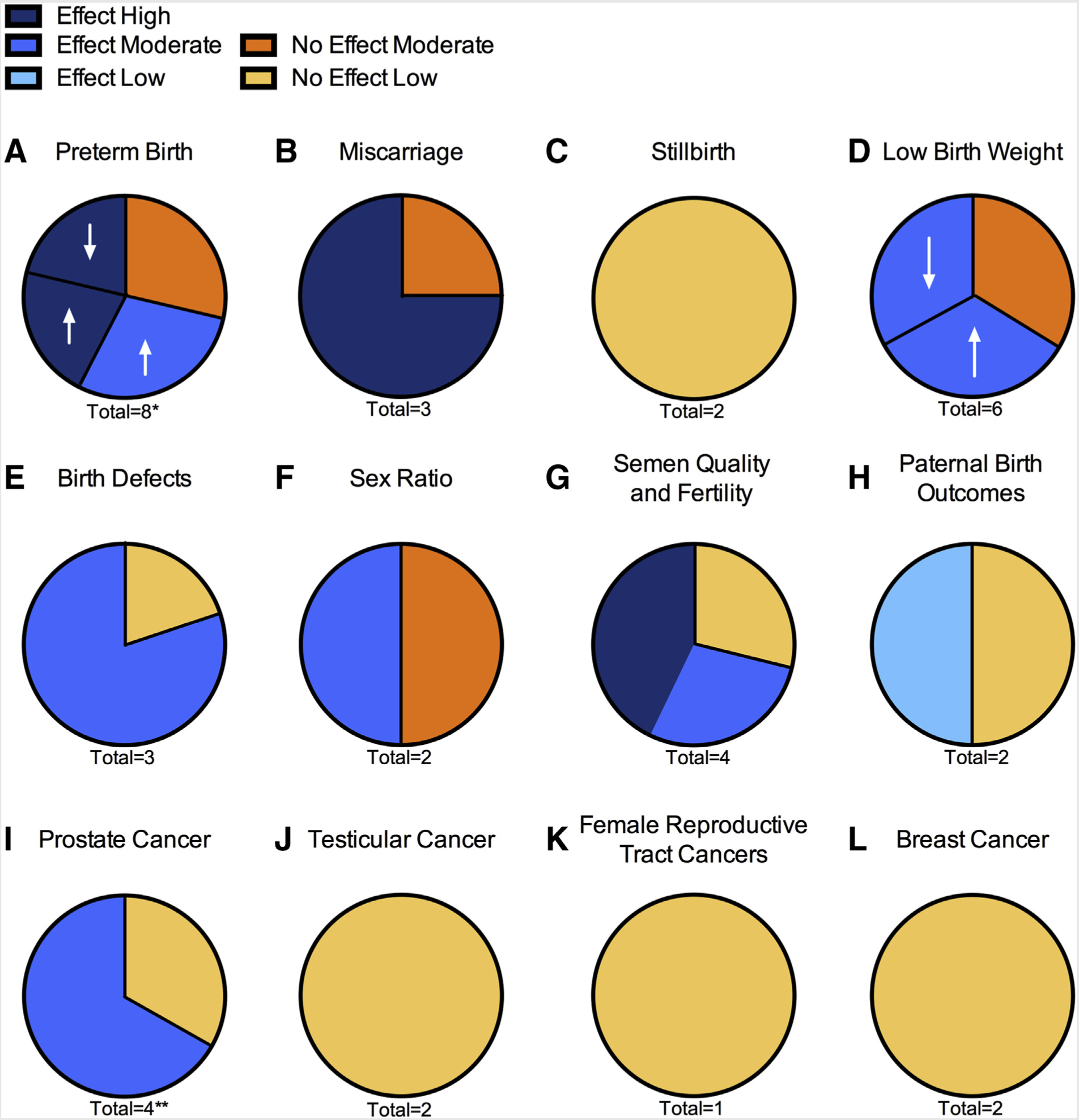
Integration of evidence of health effect. Visualization of integration of the evidence of a health effect based on each articles finding and the level of evidence and confidence ratings for endpoints (A) Preterm Birth, (B) Miscarriage, (C) Stillbirth, (D) Low Birth Weight, (E) Birth Defects, (F) Sex Ratio, (G) Semen Quality and Fertility, (H) Paternal Birth Outcomes, (I) Prostate Cancer, (J) Testicular Cancer, (K) Female Reproductive Tract Cancers, and (L) Breast Cancer. The total number of articles for each endpoint is indicated below each chart. Evidence was integrated for each endpoint based on each article’s level of evidence and level of confidence. High, moderate, and low level of confidence findings were weighted by multiplying the number of articles by 3 for high, 2 for moderate, and 1 for low for all studies except where noted. *Four studies were not independent of each other and integration of evidence was adjusted (Materials and Methods). ↑ Indicates increased risk. ↓ Indicates decreased risk. **Two studies analyzed the same cohort, only the most recent study was included (Materials and Methods).
Birth Outcomes Associated with Maternal Exposure Preterm birth
The integration of available evidence suggests that exposure to oil and gas industry activities increases the risk of preterm birth (Fig. 2A). Casey et al. (29), ranked at high level of evidence, found a risk of preterm birth (OR 1.4; 95% CI, 1.0–1.9) for the lowest versus highest exposure quartiles, which was exacerbated after controlling for year of birth (OR 1.9; 95% CI, 1.2–2.9) (29). Four studies conducted in Taiwan reported an increased risk of preterm birth (Table 3) (23–26). Another study, by McKenzie et al. (32), ranked high level of evidence found a decreased risk with increased exposure level; the magnitude of effect is small, with OR ranging between 0.96 and 0.91. It is important that the Casey study incorporated many more aspects relevant to natural gas activities—drilling and fracturing dates, fracturing volumes, etc.—into their model than the McKenzie study, as previously described (29, 32). Two studies did not provide adequate evidence to suggest any association (Fig. 2A) (35, 39).
Miscarriage.
The integration of available evidence suggests that an increased risk of miscarriage is associated with oil and gas industry activities (Fig. 2B). Two studies ranked high level of evidence for the large magnitude of effect (OR 2.47 and OR 2.7 based on employment history recorded exposure or 2.9 based on self-reported exposure, respectively) found an increased risk of miscarriage in women either residentially or occupationally exposed to oil and gas industry activities (34, 36). One study reported no health effect, but its level of evidence was inadequate (27).
Stillbirth.
The integration of evidence from two studies with inadequate levels of evidence is insufficient to evaluate the potential health effect of oil and gas industry activities on stillbirth (Fig. 2C) (33, 34). Stillbirth occurs rarely and as a result has been regarded as a weak indicator of developmental toxicity resulting from environmental pollutants (69).
Low birth weight.
The results from the six studies investigating low birth weight are inconsistent (27,31–33,35,39). The integration of evidence is inadequate to determine whether low birth weight is associated with oil and gas industry activities (Fig. 2D). Two studies ranked moderate level of evidence found an increased risk for either low-birth-weight (OR 1.77) or small-for-gestational-age children (OR 1.34) of women residentially exposed to oil and gas industry activities (31, 35). Two studies ranked moderate level of evidence found a decreased risk of low birth weight (27, 32). Two studies ranked inadequate level of evidence did not find any association between exposure and low birth weight (33, 39).
Birth defects.
The integration of evidence indicates that maternal exposure to oil and gas industry activities is likely to be associated with birth defects (Fig. 2E). Two studies with moderate level of evidence found a correlation between birth defects and maternal residential (n = 1) (32) or occupational (n = 1) (30) exposure to oil and gas industry activities. Among these two studies, one is a retrospective cohort study with a large sample size (n = 19,793 in the highest exposure group vs. n = 66,626 in the reference group) (32), and the other one is a case control study with a sample size of 17 cases versus 10 controls (30). A case control study (n = 159) ranked inadequate level of evidence reported no health effect of exposure (33). Another study done by Axelsson and Rylander (28) was rated as having very low confidence for the reasons stated earlier.
Sex ratio.
There is not enough evidence to evaluate the effects of exposure to oil and gas industry activities on sex ratio (Fig. 2F). Two studies performed by the same group that investigated the possible effect of maternal residential exposure to petrochemical or petroleum air pollution (37, 38). Both applied the Z-statistic using the national Taiwanese sex ratio. One reported no health effect (37), and the other reported a health effect (38). The discrepancy might be explained by the different number of municipalities studied (n=2 vs. n=16) (Supplemental Table 1) (37, 38).
Semen Quality, Fertility, and Birth Outcomes Associated with Adult Male Exposures
The integration of evidence suggests occupational and experimental exposure to oil and gas industry activities and related chemicals has a health impact on semen quality, including sperm concentration, motility, viability, and DNA integrity (Fig. 2G).
Semen quality and fertility.
The evidence suggests that sperm concentration and count are impacted by occupational exposure to oil and gas industry activities. One study of moderate evidence rating reported a decrease between 24% to 29% in sperm count after exposure to oil and gas industry activities (41). Another study with an inadequate evidence rating reported no health effect, but this study was underpowered (40). Despite being underpowered, the latter study reported a 17% decrease in sperm concentration (40). Although that result was not significant, it is consistent with other findings (40). The integration of the evidence also suggests occupational and experimental exposure to oil and gas industry activities and related chemicals has a health impact on sperm motility. Two articles of high to moderate evidence ratings indicated that there is a decrease in sperm motility upon exposure to oil and gas industry activities and related chemicals ranging from 16% to 97% (41, 43).
Evidence suggests occupational exposure to oil and gas industry activities and related chemicals may have a health effect on sperm viability. However, there were only two studies that reported on this endpoint, and they reported opposite findings (41, 43). One article with a high evidence rating reported that exposure to benzene metabolites can result in a 59% to 78% decrease in sperm viability (43). Another article with an inadequate evidence rating reported no health effect on sperm viability (41). Due to the inconsistency in results more research may be required to support a health effect on sperm viability.
Available evidence suggests that experimental exposure to chemicals related to oil and gas industry activities has a health effect on sperm DNA integrity. One study with a high evidence rating reported a 208% to 336% increase in denatured single-stranded DNA upon treatment with benzene metabolites (43).
Mandani et al. (43) speculated that benzene metabolites may impact semen quality by the production of reactive oxygen species, disruption of the plasma membrane, and inhibition of oxidative reactions, preventing the generation of the ATP necessary for cellular functions. They also hypothesized that exposure may disrupt important events during spermatogenesis; this may also explain many of the effects on semen quality (43).
The integration of evidence is inadequate to determine the health effects of occupational exposure to oil and gas industry activities on sperm morphology or male fertility. One article with an inadequate evidence rating reported no health effect on sperm morphology, and another article with an inadequate evidence rating reported no health effect on male fertility; both of these studies were underpowered to detect a health effect (Supplemental Table 1) (40, 44).
Birth outcomes arising from paternal exposure.
The integration of evidence is inadequate to determine the health effects of paternal occupational exposure to oil and gas industry activities on miscarriage (Fig. 2H). One article with an inadequate evidence rating reported no health effect on miscarriage in relation to paternal occupational exposure to oil and gas industry (44). However, this study was underpowered to detect an effect (44). Available evidence suggests that exposure to oil and gas industry activities may have a health effect on birth defects. However, only one article with a low evidence rating reported on this endpoint, and further research is needed to support this health effect (45). Birth defects resulting from paternal occupational exposure could be a result of DNA damage or epigenetic changes in the sperm that may lead to negative health outcomes in offspring (Fig. 2H) (63).
Reproductive Cancers
A total of eight articles were systematically reviewed and evaluated for four reproductive cancers (21, 22, 47–52). The only parameter with studies reporting evidence of a health effect was prostate cancer (21, 22, 49). The remaining evaluated endpoints found no evidence of a health effect or were rated with a very low level of confidence (47, 48, 50–52). The latter were not further evaluated for evidence of health effects and were not factored into the overall integration of the evidence (50–52). Six of the articles assessed were based on occupational oil and/or gas industry exposures, and two evaluated possible risks associated with residential exposure (21, 22, 47–52).
Prostate cancer.
The integration of the evidence suggests a moderate level of evidence for a health effect of prostate cancer related to oil and gas activities, with an overall effect size of approximately 20% (Fig. 2I) (21, 22, 47–52). Two studies by Gun et al. (21, 22) evaluated the possible risk of prostate cancer associated with oil and gas industry occupational exposure in the same cohort, so only the most recent data were considered for assessing the overall integration of evidence. Kaldor et al. (49) examined prostate cancer risks related to residential oil and gas activities exposure. These study endpoints were rated with moderate levels of evidence for a health effect (21, 22, 49). Two studies that reported no health effect yielded low confidence levels and inadequate levels of evidence for no health effect (47, 48).
Testicular cancer.
The level of evidence was inadequate to determine integration of evidence for health effect on testicular cancer in relation to oil and gas industry activities (Fig. 2J). Two articles that investigated whether exposure to oil and gas industry activities was associated with testicular cancer were rated at a low confidence level and with an inadequate level of evidence to deduce a health effect (21, 22, 49). Of the two studies by Gun et al. (21, 22), only the most recent data were considered in integration of the evidence.
Female reproductive tract cancer incidence.
The level of evidence was inadequate to determine integration of evidence for health effect on female reproductive tract cancer in relation to oil and gas industry activities (Fig. 2K). Only one of the four studies (49) that examined potential links between female reproductive tract cancer and exposure to oil and gas industry activities could be evaluated for level of evidence for a health effect. The endpoints of cervical and uterine cancer in this study were rated with low confidence levels and inadequate levels of evidence to support a lack of health effect (49).
Breast cancer incidence.
The level of evidence was inadequate to determine health effect for breast cancer risk associated with oil and gas industry exposure (Fig. 2L). Two studies that investigated possible associations between breast cancer and oil and gas industry activities were rated low confidence and inadequate levels of evidence to determine health effect (22, 49).
Disruption of Human Sex Steroid Hormone Receptors
Evidence for each endpoint is integrated below and summarized in Figure 3.
FIGURE 3.
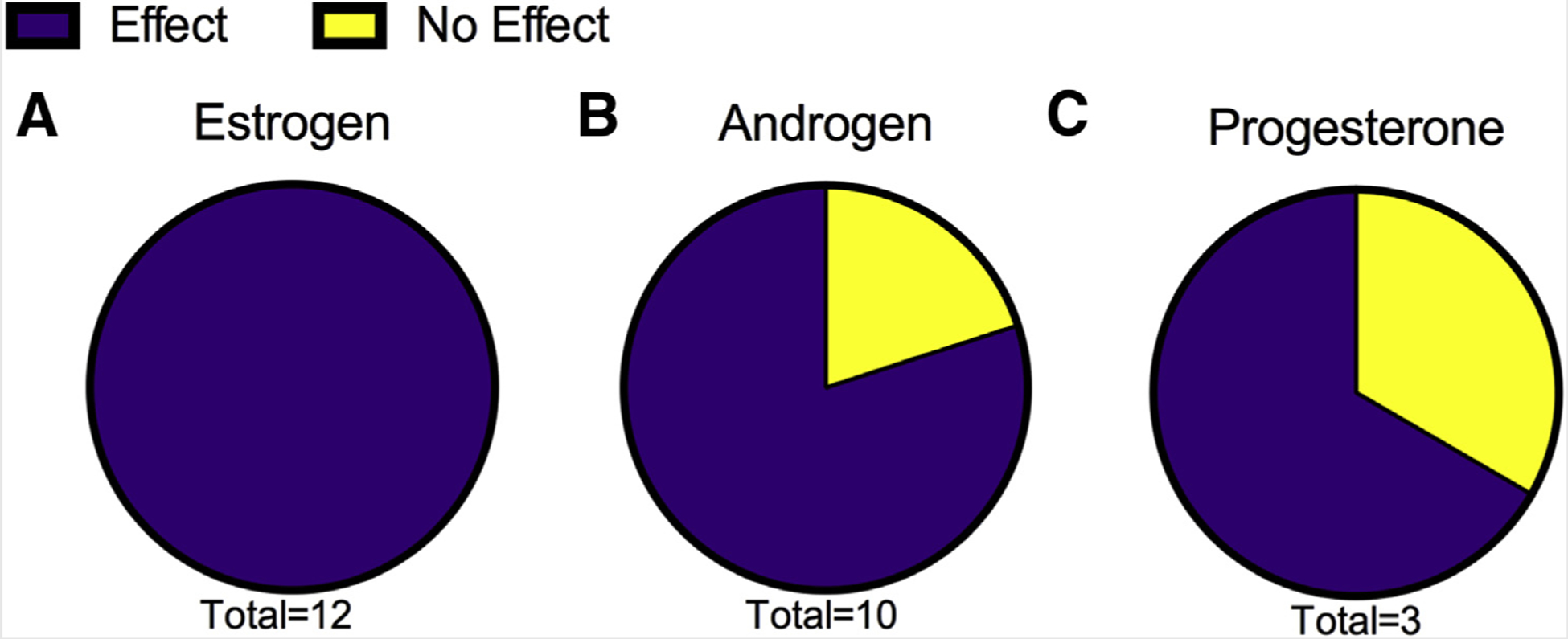
In vitro effect/no effect summary. Summary of the number of in vitro studies reporting effect or no effect on individual steroid hormone receptors including (A) estrogen, (B) androgen, and (C) progesterone. The total number of articles for each endpoint is indicated below each chart.
Estrogen receptor disruption.
The integration of the evidence suggests that many chemicals used in and produced by oil and gas operations can disrupt ER as all 12 studies reported ER disruption (Fig. 3B) (14–16, 53–61). The vast majority of studies used reporter gene assays in human cell lines, and supplemental relative binding affinity assays performed in whole human cells confirmed that many oil and gas chemicals could directly bind the ER (14–16, 53–61). Considering that all of the identified studies reported ER disruption through either agonist or antagonist interactions, the integration of the evidence strongly suggests that oil and gas operations contribute ER-active chemicals to the environment (Fig. 3B) (14–16, 53–61).
Androgen receptor disruption.
The integration of the evidence suggests that AR disruption is a likely impact from oil and gas operations (Fig. 3A). Eight out of 10 studies reported an effect of oil and gas products on AR disruption (14–16, 55, 57–62). Two studies using yeast assays with human receptors detected between 6,000 and 7,000 mg flutamide-EQ/L in produced water (60, 61), while another assessing UOG wastewater contamination into a surface stream detected approximately 10-fold lower equivalent concentrations, perhaps due to dilution of the raw effluents into the receiving stream (14). Further, reporter gene and gene expression assays both detected approximately 80% AR inhibition by crude oils (55, 62). The available evidence suggests that oil and gas operations likely contribute antiandrogenic chemicals to the environment (Fig. 3A).
Progesterone receptor disruption.
The integration of the evidence suggests that PR disruption is a possible impact from oil and gas operations (Fig. 3C). Far fewer studies have assessed PR, with only two reporting disruption and one reporting no effects (14, 15, 57). Notably, all three studies assessing PR activity tested unconventional oil and gas impacted samples (14, 15, 57). Further work should also address potential PR disruption by conventional oil and gas operations. Environmental contaminants that antagonize PR are uncommon, which bolsters the potential association between oil and gas activities and PR disruption. The available evidence suggests that oil and gas operations possibly contribute PR-disrupting chemicals to the environment (Fig. 3C).
Limitations
We conducted a systematic review to the best of our knowledge, but there are several limitations. The literature search strategies were designed to be as inclusive of oil and gas related articles as possible, but this was limited by the commonality of the term “oil” by itself because the return product presents an exceptionally high number of false positives. Although specific chemicals found in the oil and gas industry are known to cause reproductive toxicity, it was beyond the scope of the current review to systematically evaluate the research on each of the over 1,000 chemicals known to be used within unconventional oil and gas production (19). For each endpoint there were insufficient numbers of studies to individually evaluate the occupational versus residential exposures. Finally, none of these studies quantified actual exposure to any chemical or mixture of chemicals; some routes of exposures are suggested, but none are demonstrated.
CONCLUSIONS
The integration of the evidence suggests negative health impacts on reproduction from occupational and residential exposure to oil and gas extraction activities. Specifically, there is moderate evidence for increased risk of miscarriage, prostate cancer, birth defects, and decreased semen quality. However, no conclusions can be drawn for the following endpoints. The evidence is low and inadequate for testicular, breast, or female reproductive cancers, birth outcomes associated with paternal exposures, and stillbirth. The evidence for both sex ratio and low birth weight is inconsistent.
There is ample evidence for disruption of the estrogen, androgen, and progesterone receptors with individual chemicals and complex mixtures of chemicals and waste products related to oil and gas extraction. These data provide a strong mechanistic rationale for how oil and gas activities may increase the health risks outlined herein.
Importantly, few studies have been conducted to evaluate the impact of unconventional oil and gas operations on human health. The majority of the articles identified in our search and included in this review examined potential effects from exposure to conventional oil and gas activities. The rapid rise in unconventional extraction began around 2000, and research has only recently begun to be conducted and published. The results from this systematic review suggest negative impacts on human reproduction from exposure to oil and gas activities. The impacts from unconventional oil and gas activities will likely be greater, given that unconventional activities have many similarities to conventional ones and employ dozens of known endocrine-disrupting chemicals in the process of hydraulic fracturing.
Supplementary Material
Acknowledgments:
The authors thank Rebecca Graves for sharing her knowledge of systematic reviews and database searching; Drs. Kris Thayer, Kembra Howedeshell and Katherine Pelch from Office of Hazard Assessment and the National Toxicology Program, for sharing their expertise; and Sierra Baxter and Katelyn Cinnamon for retrieving articles.
Supported by the National Institutes of Health [Grants R21-ES026395 and R01-ES021394-04S1 (to S.C.N.)] and the University of Missouri.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/11535-systematic-review-of-the-association-between-oil-and-natural-gas-extraction-processes-and-human-reproduction
V.D.B. has nothing to disclose. C.-X.M. has nothing to disclose. J.C.-G. has nothing to disclose. C.D.K. has nothing to disclose. R.K. has nothing to disclose. S.C.N. has nothing to disclose.
REFERENCES
- 1.U.S. Energy Information Administration. Monthly crude oil and natural gas production. Washington, DC: U.S. Department of Energy; 2016. Available at: http://www.eia.gov/petroleum/production/. Accessed August 17, 2016. [Google Scholar]
- 2.U.S. Energy Information Administration. Annual energy outlook 2016 with projections to 2040. Washington, DC: U.S. Department of Energy; 2016. Available at: http://www.eia.gov/forecasts/aeo/index.cfm. Accessed August 17, 2016. [Google Scholar]
- 3.U.S. Energy Information Administration. Number of producing gas wells. Washington, DC: U.S. Department of Energy; 2016. Available at: https://www.eia.gov/dnav/ng/ng_prod_wells_s1_a.htm. Accessed August 17, 2016. [Google Scholar]
- 4.Colborn T, Schultz K, Herrick L, Kwiatkowski C. An exploratory study of air quality near natural gas operations. Hum Ecol Risk Assess 2014;20:86–105. [Google Scholar]
- 5.Kassotis CD, Tillitt DE, Lin CH, McElroy JA, Nagel SC. Endocrine-disrupting chemicals and oil and natural gas operations: potential environmental contamination and recommendations to assess complex environmental mixtures. Environ Health Perspect 2016;124:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vengosh A, Jackson RB, Warner N, Darrah TH, Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol 2014;48: 8334–48. [DOI] [PubMed] [Google Scholar]
- 7.Burton GA Jr, Basu N, Ellis BR, Kapo KE, Entrekin S, Nadelhoffer K. Hydraulic “fracking”: are surface water impacts an ecological concern? Environ Toxicol Chem 2014;33:1679–89. [DOI] [PubMed] [Google Scholar]
- 8.Ingraffea AR, Wells MT, Santoro RL, Shonkoff SB. Assessment and risk analysis of casing and cement impairment in oil and gas wells in Pennsylvania, 2000–2012. Proc Natl Acad Sci USA 2014;111:10955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauter MS, Alvarez PJ, Burton A, Cafaro DC, Chen W, Gregory KB, et al. Regional variation in water-related impacts of shale gas development and implications for emerging international plays. Environ Sci Technol 2014; 48:8298–306. [DOI] [PubMed] [Google Scholar]
- 10.Rozell DJ, Reaven SJ. Water pollution risk associated with natural gas extraction from the Marcellus Shale. Risk Anal 2012;32:1382–93. [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitz PM, Slizovskiy IB, Lamers V, Trufan SJ, Holford TR, Dziura JD, et al. Proximity to natural gas wells and reported health status: results of a household survey in Washington County, Pennsylvania. Environ Health Perspect 2015;123:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinzor N, Subra W, Sumi L. Investigating links between shale gas development and health impacts through a community survey project in Pennsylvania. New Solut 2013;23:55–83. [DOI] [PubMed] [Google Scholar]
- 13.Werner AK, Vink S, Watt K, Jagals P. Environmental health impacts of unconventional natural gas development: a review of the current strength of evidence. Sci Total Environ 2015;505:1127–41. [DOI] [PubMed] [Google Scholar]
- 14.Kassotis CD, Iwanowicz LR, Akob DM, Cozzarelli IM, Mumford AC, Orem WH, et al. Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci Total Environ 2016;557–558:901–10. [DOI] [PubMed] [Google Scholar]
- 15.Kassotis CD, Klemp KC, Vu DC, Lin CH, Meng CX, Besch-Williford CL, et al. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 2015;156:4458–73. [DOI] [PubMed] [Google Scholar]
- 16.Kassotis CD, Tillitt DE, Davis JW, Hormann AM, Nagel SC. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 2014;155: 897–907. [DOI] [PubMed] [Google Scholar]
- 17.Webb E, Bushkin-Bedient S, Cheng A, Kassotis CD, Balise V, Nagel SC. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev Environ Health 2014;29: 307–18. [DOI] [PubMed] [Google Scholar]
- 18.Elliott EG, Ettinger AS, Leaderer BP, Bracken MB, Deziel NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. J Expo Sci Environ Epidemiol 2016. Published online 2016. [DOI] [PubMed] [Google Scholar]
- 19.Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 2014;122:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Office of Health Assessment and Translation (OHAT). Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. Research Triangle Park, NC: National Toxicology Program, U.S. Department of Health and Human Services; 2015. Available at: http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf. Accessed August 17, 2016. [Google Scholar]
- 21.Gun RT, Pratt NL, Ryan P, Roder D. Update of mortality and cancer incidence in the Australian petroleum industry cohort. Occup Environ Med 2006;63: 476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gun RT, Pratt NL, Griffith EC, Adams G, Bisby J, Robinson K. Update of a prospective study of mortality and cancer incidence in the Australian petroleum industry. Occup Environ Med 2004;61:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai SS, Yu HS, Liu CC, Yang CY. Increased incidence of preterm delivery in mothers residing in an industrialized area in Taiwan. J Toxicol Environ Health A 2003;66:987–94. [DOI] [PubMed] [Google Scholar]
- 24.Yang CY, Chiu HF, Tsai SS, Chang CC, Chuang HY. Increased risk of preterm delivery in areas with cancer mortality problems from petrochemical complexes. Environ Res 2002;89:195–200. [DOI] [PubMed] [Google Scholar]
- 25.Lin MC, Chiu HF, Yu HS, Tsai SS, Cheng BH, Wu TN, et al. Increased risk of preterm delivery in areas with air pollution from a petroleum refinery plant in Taiwan. J Toxicol Environ Health A 2001;64:637–44. [DOI] [PubMed] [Google Scholar]
- 26.Yang CY, Chang CC, Chuang HY, Ho CK, Wu TN, Chang PY. Increased risk of preterm delivery among people living near the three oil refineries in Taiwan. Environ Int 2004;30:337–42. [DOI] [PubMed] [Google Scholar]
- 27.Axelsson G, Molin I. Outcome of pregnancy among women living near petrochemical industries in Sweden. Int J Epidemiol 1988;17:363–9. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson G, Rylander R. Outcome of pregnancy in women engaged in laboratory work at a petrochemical plant. Am J Ind Med 1989;16:539–45. [DOI] [PubMed] [Google Scholar]
- 29.Casey JA, Savitz DA, Rasmussen SG, Ogburn EL, Pollak J, Mercer DG, et al. Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology 2016;27:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevrier C, Dananche B, Bahuau M, Nelva A, Herman C, Francannet C, et al. Occupational exposure to organic solvent mixtures during pregnancy and the risk of nonsyndromic oral clefts. Occup Environ Med 2006;63:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin MC, Yu HS, Tsai SS, Cheng BH, Hsu TY, Wu TN, et al. Adverse pregnancy outcome in a petrochemical polluted area in Taiwan. J Toxicol Environ Health A 2001;63:565–74. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ Health Perspect 2014;122:412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira LM, Stein N, Sanseverino MT, Vargas VM, Fachel JM, Schuler L. Reproductive outcomes in an area adjacent to a petrochemical plant in southern Brazil. Rev Saude Publica 2002;36:81–7. [DOI] [PubMed] [Google Scholar]
- 34.Sebasti an MS, Armstrong B, Stephens C. Outcomes of pregnancy among women living in the proximity of oil fields in the Amazon basin of Ecuador. Int J Occup Environ Health 2002;8:312–9. [DOI] [PubMed] [Google Scholar]
- 35.Stacy SL, Brink LL, Larkin JC, Sadovsky Y, Goldstein BD, Pitt BR, et al. Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS One 2015;10:e0126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Cho SI, Sammel M, You L, Cui S, Huang Y, et al. Association of petrochemical exposure with spontaneous abortion. Occup Environ Med 1998; 55:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang CY, Cheng BH, Hsu TY, Tsai SS, Hung CF, Wu TN. Female lung cancer mortality and sex ratios at birth near a petroleum refinery plant. Environ Res 2000;83:33–40. [DOI] [PubMed] [Google Scholar]
- 38.Yang CY, Tsai SS, Cheng BH, Hsu TY, Wu TN. Sex ratio at birth associated with petrochemical air pollution in Taiwan. Bull Environ Contamin Toxicol 2000;65:126–31. [DOI] [PubMed] [Google Scholar]
- 39.Yang CY, Cheng BH, Hsu TY, et al. Association between petrochemical air pollution and adverse pregnancy outcomes in Taiwan. Arch Environ Health 2002;57:461–5. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg MJ, Wyrobek AJ, Ratcliffe J, Gordon LA, Watchmaker G, Fox SH, et al. Sperm as an indicator of reproductive risk among petroleum refinery workers. Br J Ind Med 1985;42:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SL, Wang XR, Chia SE, Shen HM, Song L, Xing HX, et al. A study on occupational exposure to petrochemicals and smoking on seminal quality. J Androl 2001;22:73–8. [PubMed] [Google Scholar]
- 42.Khalifa EA-DM, El-Hussein E-SA, Abdul-Maboud KH. Possible effect of petroleum oil on male reproduction. Adv Contracept Deliv Syst 1997;13:85–8. [Google Scholar]
- 43.Mandani P, Desai K, Highland H. Cytotoxic effects of benzene metabolites on human sperm function: an in vitro study. ISRN Toxicol 2013;2013: 397524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bull N, Riise T, Moen B. Influence of paternal exposure to oil and oil products on time to pregnancy and spontaneous abortions. Occup Med 1999;49: 371–6. [DOI] [PubMed] [Google Scholar]
- 45.Desrosiers TA, Herring AH, Shapira SK, Hooiveld M, Luben TJ, Herdt-Losavio ML, et al. Paternal occupation and birth defects: findings from the National Birth Defects Prevention Study. Occup Environ Med 2012;69: 534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CC, Annegers JF, Frankowski RF, Spitz MR, Buffler PA. Childhood nervous system tumors—an evaluation of the association with paternal occupational exposure to hydrocarbons. Am J Epidemiol 1987;126:605–13. [DOI] [PubMed] [Google Scholar]
- 47.Christie D, Robinson K, Gordon I, Bisby J. A prospective study in the Australian petroleum industry. II. Incidence of cancer. Br J Ind Med 1991;48:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rybicki BA, Neslund-Dudas C, Nock NL, Schultz LR, Eklund L, Rosbolt J, et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev 2006;30:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaldor J, Harris JA, Glazer E, Glaser S, Neutra R, Mayberry R, et al. Statistical association between cancer incidence and major-cause mortality, and estimated residential exposure to air emissions from petroleum and chemical plants. Environ Health Perspect 1984;54:319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Järvholm B, Mellblom B, Norrman R, Nilsson R, Nordlinder R. Cancer incidence of workers in the Swedish petroleum industry. Occup Environ Med 1997;54:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis RJ, Schnatter AR, Drummond I, Murray N, Thompson FS, Katz AM, et al. Mortality and cancer morbidity in a cohort of Canadian petroleum workers. Occup Environ Med 2003;60:918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schechter MT, Spitzer WO, Hutcheon ME, Dales RE, Eastridge LM, Steinmetz N, et al. Cancer downwind from sour gas refineries: the perception and the reality of an epidemic. Environ Health Perspect 1989;79:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arcaro KF, O’Keefe PW, Yang Y, Clayton W, Gierthy JF. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology 1999;133:115–27. [DOI] [PubMed] [Google Scholar]
- 54.Arcaro KF, Gierthy JF, Mackerer CR. Antiestrogenicity of clarified slurry oil and two crude oils in a human breast-cancer cell assay. J Toxicol Environ Health A 2001;62:505–21. [DOI] [PubMed] [Google Scholar]
- 55.Vrabie CM, Candido A, van Duursen MB, Jonker MT. Specific in vitro toxicity of crude and refined petroleum products: II. Estrogen (a and d) and androgen receptor-mediated responses in yeast assays. Environ Toxicol Chem 2010;29:1529–36. [DOI] [PubMed] [Google Scholar]
- 56.Vrabie CM, Candido A, van den Berg H, Murk AJ, van Duursen MB, Jonker MT. Specific in vitro toxicity of crude and refined petroleum products: 3. Estrogenic responses in mammalian assays. Environ Toxicol Chem 2011; 30:973–80. [DOI] [PubMed] [Google Scholar]
- 57.Tang J, Taulis M, Edebeli J, Leusch F, Jagals P, Jackson G, et al. Chemical and bioanalytical assessment of coal seam gas associated water. Environ Chem 2015;12:267–85. [Google Scholar]
- 58.He Y, Wiseman SB, Hecker M, Zhang X, Wang N, Perez LA, et al. Effect of ozonation on the estrogenicity and androgenicity of oil sands process-affected water. Environ Sci Technol 2011;45:6268–74. [DOI] [PubMed] [Google Scholar]
- 59.Thomas KV, Balaam J, Hurst MR, Thain JE. Identification of in vitro estrogen and androgen receptor agonists in North Sea offshore produced water discharges. Environ Toxicol Chem 2004;23:1156–63. [DOI] [PubMed] [Google Scholar]
- 60.Thomas KV, Langford K, Peterson K, Smith AJ, Tollefsen KE. Effect-directed identification of naphthenic acids as important in vitro xeno-estrogens and anti-androgens in North Sea offshore produced water discharges. Environ Sci Technol 2009;43:8066–71. [DOI] [PubMed] [Google Scholar]
- 61.Tollefsen KE, Harman C, Smith A, Thomas KV. Estrogen receptor (ER) agonists and androgen receptor (AR) antagonists in effluents from Norwegian North Sea oil production platforms. Mar Pollut Bull 2007;54:277–83. [DOI] [PubMed] [Google Scholar]
- 62.Kizu R, Ishii K, Kobayahi J, Hashimoto T, Koh E, Namiki M, et al. Antiandrogenic effect of crude extract of C-heavy oil. Mater Sci Eng C Mater Biol Appl 2000;12:97–102. [Google Scholar]
- 63.Olshan AF, Faustman EM. Male-mediated developmental toxicity. Annu Rev Public Health 1993;14:159–81. [DOI] [PubMed] [Google Scholar]
- 64.Colborn T,Kwiatkowski C,Schultz K,Bachran M.Naturalgas operationsfroma public health perspective. Int J Hum Ecol Risk Assess 2011;17:1039–56. [Google Scholar]
- 65.U.S. Environmental Protection Agency. Assessment of the potential impacts of hydraulic fracturing for oil and gas on drinking water resources External Review Draft EPA/600/R-15/047. Washington, DC: U.S. EPA; 2015. Available at: https://cfpub.epa.gov/ncea/hfstudy/recordisplay.cfm?deid=244651#. Accessed August 17, 2016. [Google Scholar]
- 66.Hughey CA, Rodgers RP, Marshall AG. Resolution of 11,000 compositionally distinct components in a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of crude oil. Anal Chem 2002;74:4145–9. [DOI] [PubMed] [Google Scholar]
- 67.Akob DM, Mumford AC, Orem WH, Engle MA, Klinges JG, Kent DB, et al. Wastewater disposal from unconventional oil and gas development degrades stream quality at a West Virginia injection facility. Environ Sci Technol 2016;50:5517–25. [DOI] [PubMed] [Google Scholar]
- 68.Sonnenschein CA, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 1998;65: 143–50. [DOI] [PubMed] [Google Scholar]
- 69.IPCS Task Group on Principles for Evaluating Health Risks to Progeny Associated with Exposure to Chemicals during Pregnancy. Principles for evaluating health risks to progeny associated with exposure to chemicals during pregnancy Environmental Health Criteria 30. Geneva: International Programme on Chemical Safety, World Health Organization, 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


