Abstract
Objectives:
Micromolar concentrations of the pro-angiogenic metabolite deoxyribose-1-phosphate (dRP) were detected in platelet supernatants by mass spectrometry. In this study, we assessed whether the release of dRP by platelets stimulates endothelial cell migration and angiogenesis.
Methods and Results:
Protein-free supernatants from thrombin-stimulated platelets increased human umbilical vein endothelial cell (HUVEC) migratory activity in transmigration and monolayer repair assays. This phenomenon was ablated by genetic silencing of dRP-generating uridine phosphorylase (UP) and thymidine phosphorylase (TP) or pharmacological inhibition of UP and restored by exogenous dRP. The stimulation of endothelial cell migration by platelet-derived dRP correlated with upregulation of integrin β3, which was induced in a reactive oxygen species (ROS)-dependent manner, and was mediated by the activity of the integrin heterodimer αvβ3. The physiological relevance of dRP release by platelets was confirmed in a chick chorioallantoic membrane (CAM) assay, where the presence of this metabolite in platelet supernatants strongly induced capillary formation.
Conclusions:
Platelet-derived dRP stimulates endothelial cell migration by upregulating integrin β3 in a ROS-dependent manner. As demonstrated by our in vivo experiments, this novel paracrine regulatory pathway is likely to play an important role in the stimulation of angiogenesis by platelets.
Keywords: angiogenesis, endothelial cell, platelet, deoxyribose-1-phosphate, integrin
INTRODUCTION
Besides their well-established physiological role in haemostasis, platelets are known to release paracrine factors that facilitate vascular repair and angiogenesis 1–3 and platelet-based preparations (e.g. autologous platelet preparation, platelet-rich plasma, and platelet-derived wound healing formula or PDWHF) have been investigated for their pro-angiogenic potential and utilised for tissue regenerative purposes in clinical practice 4, 5. Platelet-derived angiogenic factors, including basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) have been shown to stimulate angiogenesis in vitro by enhancing both endothelial cell motility 1 and proliferation 6. Similarly, heparanase, platelet-derived growth factor (PDGF) and stromal cell-derived factor 1 alpha (SDF-1α), all of which are released by activated platelets, can increase endothelial motility and angiogenesis 1, 7–9, and there is also evidence that platelet-derived SDF-1α directly promotes migration and differentiation of endothelial progenitor cells 10, 11 (EPCs). With the exception of platelet-derived sphingosine-1-phosphate, which stimulates endothelial cell migration and angiogenesis by upregulating the expression of adhesion molecules 12, the majority of research in this area has focused on platelet secretion of protein factors.
Here, we present evidence that platelets release low molecular weight molecules (<2 kDa) that are capable of stimulating endothelial cell migration, a key component of angiogenesis 13. Small molecules released into platelet supernatants were characterised by gas chromatography-mass spectrometry (GC-MS) and direct injection-mass spectrometry (DIMS), which revealed the presence of deoxyribose-1-phosphate (dRP), a known pro-angiogenic molecule which stimulates endothelial cell migration 14–16. DRP is the product of the phosphorolytic degradation of deoxynucleosides in the “nucleotide salvage pathway”, which is catalysed by three enzymes in mammalian cells: thymidine phosphorylase (TP, or Platelet-Derived Endothelial Cell Growth Factor or PD-ECGF, EC 2.4.2.4), uridine phosphorylase (UP, EC 2.4.2.3), and purine nucleoside phosphorylase (PNP, EC 2.4.2.1) 17. We have used pharmacological inhibitors of TP, UP or PNP in human platelets and the genetic ablation of TP and UP in mouse platelets to investigate whether platelet-derived dRP stimulates human endothelial cell motility and angiogenesis. Taken together, our results show for the first time that platelet-derived dRP induces the upregulation of endothelial integrin β3 in a ROS-dependent manner, leading to increase endothelial cell motility in vitro and angiogenesis in vivo. These observations highlight a novel function of platelets in the regulation of vascular homeostasis and may facilitate the development of novel therapeutic approaches for modulating angiogenesis and tissue repair.
METHODS
A concise description of the experimental procedures is presented in this section. For an expanded Materials and Methods section refer to the online supplement.
Platelet isolation and supernatant preparation – Blood was drawn from healthy volunteers under the approval of the local Ethics Committee or from wild type (WT) and TP−/−/UP−/− mice, generated as previously described 18. Washed platelets were prepared as described 19. Where indicated, platelets were stimulated with human thrombin (0.1unit/ml for human platelets; 1unit/ml for mouse platelets) for 5 minutes.
Gas chromatography-mass spectrometry (GC-MS) and direct injection-mass spectrometry (DIMS) – In preparation for the MS analysis, supernatants were obtained without permeabilization by precipitation of platelet suspensions (1000g), followed by separation from platelet pellets and filtration (2kDa cut-off). Detailed methods for GC-MS and DIMS are given in the supplementary section.
Endothelial cell transmigration and endothelial monolayer repair assays – For transmigration assays, 105 HUVEC/well (medium M-199 plus Tyrode’s HEPES buffer – ratio 1:1, final foetal calf serum (FCS) concentration = 5%) were plated onto 24 well-transmigration inserts (membrane pore size 8 μm) pre-coated with 1% gelatin. The lower chamber contained medium M-199 plus protein-free platelet supernatant or Tyrode’s HEPES buffer (ratio 1:1 - final FCS concentration = 5%). Transmigration was quantified 5 hours after assembly of the chemotaxis microchamber by hematoxylin staining and phase-contrast microscopy.
For endothelial monolayer repair assays, HUVECs were cultured to confluence in 12 well-plates. A uniform wound was created by scratching the cell monolayer with a microtip, and medium M-199 plus platelet supernatant or Tyrode’s HEPES buffer was added (ratio 2:1 - final FCS concentration = 6.6%). Wound surface area was monitored at time 0, 12, and 24 hours post-wounding by phase contrast microscopy.
ROS production assay – The generation of ROS in HUVECs was investigated using dihydroethidium (DHE) staining and real-time single-cell fluorescence analysis, as previously described 20.
CAM assay – On day 7 of development, sterile filters soaked with vehicle, VEGF or the appropriate protein-free platelet supernatant were applied to relatively avascular regions of the CAM. The newly capillarised area in the region of each filter was quantified after 48 hours.
Statistical analysis – Data are expressed throughout as mean ± SEM. The results were analysed by t-test (for comparisons of two groups), one-way ANOVA with Bonferroni post-test (for multiple comparisons), or two-way ANOVA with Bonferroni post-test (for multiple comparisons of time courses).
RESULTS
Initial characterisation by GC-MS of human platelet supernatants revealed the presence of dRP, a pro-angiogenic molecule 14–16. The presence of this metabolite in platelet supernatants was confirmed using a dRP standard (figure 1A) and platelet expression of dRP-generating enzymes (TP, UP, and PNP) was established by immunoblotting (supplementary figure I). These data showed that human platelets express all three nucleoside phosphorylases, whereas mouse platelets express TP and UP, but not PNP, and HUVECs express PNP at high levels and UP at low levels, but no TP. The pattern of dRP secretion was then investigated by DIMS, which allows the relative quantitative determination of the ions, thus permitting a relative estimation of the change in concentration of the originating molecules (supplementary figure II). Thrombin-stimulation significantly increased the release of dRP by platelets (figure 1B), while decreasing its levels within platelets (platelet extracts, figure 1C), suggesting a storage-release mechanism rather than de-novo synthesis upon stimulation. Exposure of human platelets to collagen (10µg/ml) also significantly increased the release of dRP, whereas the thromboxane A2 analogue U46619 (100nM) did not (supplementary figure IIIA). Based upon the sensitivity of the experimental procedure and equipment utilised, the concentration of dRP in platelet supernatants is estimated to be in the micromolar range (50–400µM). Interestingly, the level of dRP in human plasma is approximately ten times lower than in platelet supernatants (figure 1B), suggesting that activated platelets are likely to change local concentrations of this metabolite at sites of vascular injury.
Figure 1.

Detection of dRP in platelet supernatants and whole cell extracts by mass spectrometry. (A) Gas chromatography-mass spectrometry single ion chromatogram (m/z 204) showing platelet extracts and a commercially available dRP standard. The dRP content of human platelet supernatants (B) or whole cell extracts (C) was investigated further by DIMS (from 4ml of platelet suspension). Thrombin-stimulated platelets were compared to resting platelets or plasma. The statistical significance of the differences was tested by one-way ANOVA or t-test (*= p<0.05, n=5).
The functional relevance of dRP release by platelets was assessed in transmigration experiments using protein-free platelet supernatants (figure 2A). Filtration (2 kDa cut-off), which eliminated membrane bodies, proteins and nucleic acids from platelet supernatants, reduced but did not abolish the pro-migratory activity of thrombin-stimulated platelet supernatants (figure 2B), suggesting that platelet stimulation leads to the release of small molecules (<2kDa) able to promote endothelial cell transmigration. To assess the importance of dRP in the stimulation of endothelial cell motility by platelet supernatants, protein-free supernatants from thrombin-stimulated mouse platelets were utilised. As expected, platelet supernatants from TP−/−/UP−/− mice were characterised by depleted levels of dRP compared to WT platelets (Supplementary Figure IIIB). Thrombin-stimulation of WT platelets significantly increased HUVEC transmigration, whereas the effects of thrombin-stimulated TP−/−/UP−/− supernatants did not reach statistical significance compared to supernatants from resting platelets (figure 2C). The addition of 200µM dRP to supernatants from TP−/−/UP−/− platelets significantly increased HUVEC transmigration to levels similar to WT platelet supernatants, suggesting that the ablation of dRP is responsible for the lack of HUVEC transmigration observed with TP−/−/UP−/− supernatants. Human platelets were also utilised and treated with specific nucleoside phosphorylase inhibitors: 100µM 6-(2-aminoethyl)amino-5-chlorouracil (AEAC) was used as an inhibitor of TP 21, 50µM 5-phenylthiocyclouridine (PTAU) was used to inhibit UP 22, whereas the activity of PNP was ablated with 50µM 8-aminoguanosine (AG). Supernatants from resting and thrombin-stimulated platelets treated with inhibitor(s) were used in HUVEC transmigration experiments (figure 2D). PTAU or the simultaneous use of the three inhibitors (ALL) significantly reduced the increase in HUVEC transmigration by protein-free supernatants, while the addition of 200µM dRP restored the stimulation of transmigration.
Figure 2.
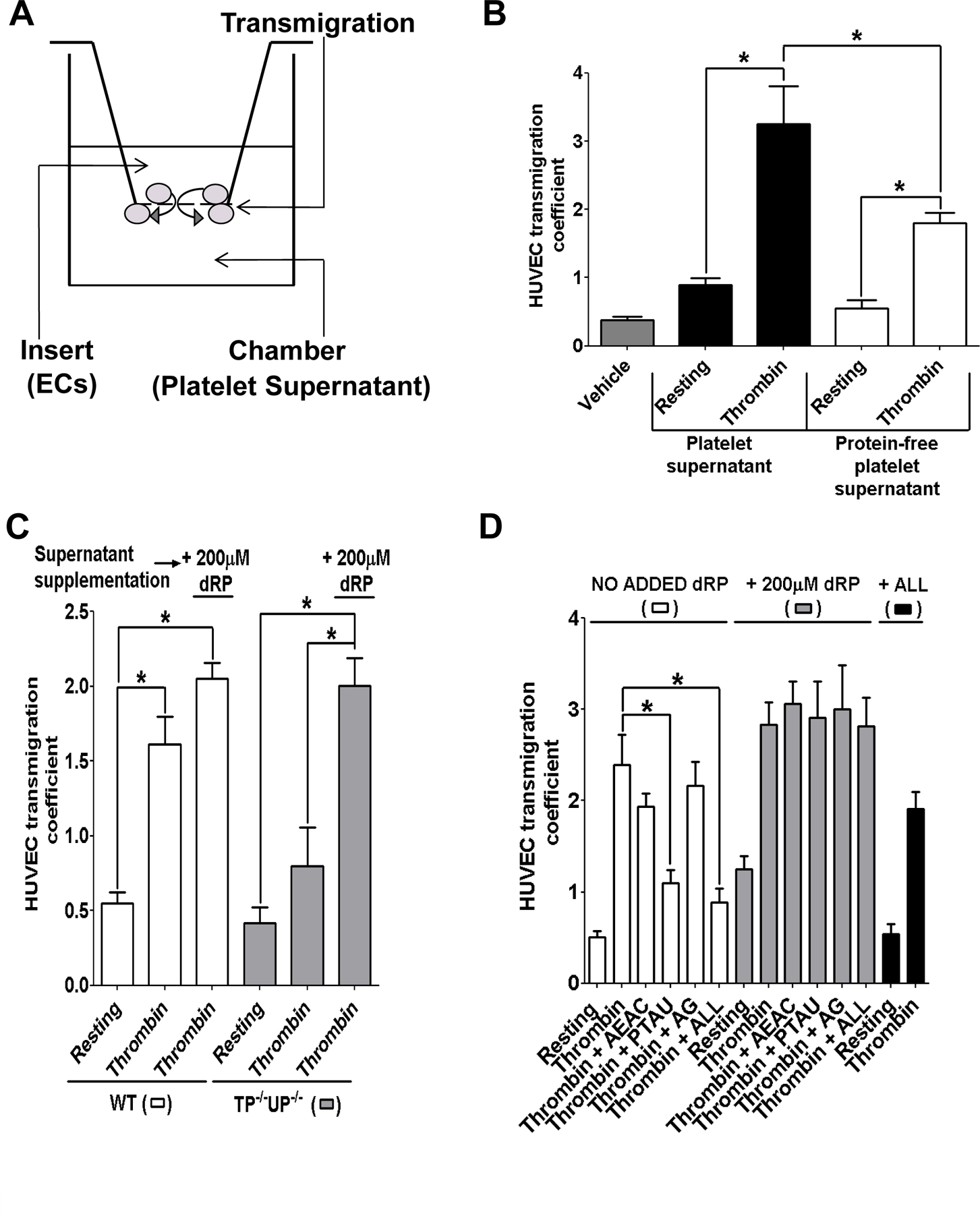
Release of dRP by mouse and human platelets stimulates HUVEC transmigration. (A) Schematic diagram of a transmigration chamber. (B) Complete (black bars) and protein-free supernatants (2 kDa cut-off; white bars) from resting and thrombin-stimulated platelets were added to the bottom of the transmigration chamber, and HUVEC transmigration was measured after 5 hrs. (C) Mouse platelets from WT (WT, white bars) and transgenic TP−/−/UP−/− platelets (grey bars) were treated with either vehicle solution (Tyrode’s buffer) or 1unit/ml thrombin for 5 minutes., whereas (D) human platelets were incubated with 100µM AEAC, 50µM PTAU, 50µM AG, or all three inhibitors (ALL) for 30 minutes, then treated with either vehicle solution or 0.1unit/ml thrombin for 5 minutes. Protein-free supernatants obtained as described were utilised for HUVEC transmigration assays (white bars). Where indicated 200µM dRP (grey bars) or 100µM AEAC, 50µM PTAU and 50µM AG (ALL, black bars) was added to protein-free supernatants immediately before use in the transmigration chambers. Transmigration was quantified after 5 hours and results were analysed by one-way ANOVA with Bonferroni post-test (*= p<0.05, n=4).
The migratory activity of HUVECs in response to protein-free platelet supernatants was also examined using a monolayer repair assays. HUVEC monolayers were physically wounded and wound closure in the presence of protein-free platelet supernatants was measured after 0, 12, and 24 hours (figure 3). The rate of closure was significantly faster in the presence of protein-free supernatants from WT mouse platelets compared to vehicle (Tyrode’s buffer). Protein-free supernatants from TP−/−/UP−/− platelets showed significantly reduced ability to accelerate wound-closure compared to WT platelet supernatants (even though rate and extent of wound closure were ameliorated by TP−/−/UP−/− platelet supernatants compared to vehicle). The addition of 200µM dRP to supernatants from TP−/−/UP−/− platelets restored HUVEC wound-closure rates to the same levels observed for WT supernatants.
Figure 3.

Release of dRP by mouse platelets stimulates HUVEC monolayer repair in vitro. Platelets from WT and transgenic TP−/−/UP−/− mice were stimulated with 1unit/ml thrombin for 5 minutes. Vehicle solution (Tyrode’s buffer) or protein-free platelet supernatants were added to HUVECs after manual creation of a wound in the monolayer. Where indicated, 200µM dRP was added to the protein-free supernatants. Wound size at 0, 12, and 24 hours post wounding is expressed as surface area (pixels). Results from 4 independent experiments were analysed by two-way ANOVA with Bonferroni post-test (*= p<0.05, compared to WT supernatants).
Upregulation of integrin β3 has been proposed as the molecular mechanism underlying the stimulation of endothelial cell migration by endothelial progenitor cell (EPC)-derived dRP 23. In accordance with this previous study, HUVECs cultured in the presence of dRP-depleted protein-free supernatants from thrombin-stimulated TP−/−/UP−/− platelets expressed significantly lower levels of integrin β3 compared to HUVECs cultured in the presence of WT supernatants (figure 4A and supplementary figure IVB for densitometric analysis). Supplementation of TP−/−/UP−/− supernatants with 200µM dRP restored integrin β3 to the level evident in HUVECs exposed to WT supernatants. Interestingly, inhibition of UP with 50µM PTAU in thrombin-stimulated human platelets also reduced the subsequent upregulation of integrin β3 expression in HUVECs, which was restored by addition of 200µM dRP to the supernatants, suggesting that UP is primarily responsible for generating dRP in human platelets (figure 4B and supplementary figure IVA for densitometric analysis). Expression of integrin β1, integrin αv, integrin α5 and focal adhesion kinase (FAK) by HUVECs was unaffected by incubation with platelet supernatants (supplementary figure IV A–B). Importantly, the dRP-dependent upregulation of integrin β3 was also observed in human microvascular endothelial cells (HMVECs), suggesting that platelet supernatant stimulates the migratory activity of different endothelial cell types (figure 4C–D).
Figure 4.

Platelet secretion of dRP induces integrin β3 upregulation in HUVECs in a ROS-dependent manner. HUVECs (A, B, F, and G) or HMVECs (C and D) were cultured for 6 hours in the presence of vehicle solution or protein-free supernatants from mouse platelets from WT or TP−/−/UP−/− animals (A, C, F, and G) or from human platelets treated with vehicle solution (resting) or stimulated with 1 unit/ml thrombin in the absence and presence of 50µM PTAU (B and D). Where indicated, 200µM dRP (A, B, D, and F) or 10mM N-acetyl-cysteine (G) were added to the supernatants immediately before incubation with HUVEC. After cell lysis, the expression of integrin β3 (A, B, C, D, F, and G) or HO-1 (F and G) were assessed by immunoblotting. All blots were also reprobed for actin. (E) The rate of intracellular ROS generation in HUVEC cells was measured using dihydroethidium (DHE, 10µM). Traces shown in panel i and ii indicate the rates of ROS production before and after addition of 100µM dRP or 1mM glucose, respectively. The rate of ROS generation in the absence or presence of dRP and glucose is measured as fluorescence unit/second and is presented as mean ± SEM (n=6) in panel iii. Statistical significance was tested by one-way ANOVA with Bonferroni post-test (*= p<0.05).
Incubation of HUVECs with dRP accelerated ROS production, as assessed using a dihydroethidium (DHE)-based assay (figure 4E). The generation of ROS by platelet-derived dRP was also confirmed by immunoblotting for heme oxygenase-1 (HO-1, or heat shock protein 32), a protein known to be upregulated in response to ROS generation in endothelial cells 24. As expected, supernatants from WT platelets increased HO-1 expression, whereas supernatants from TP−/−/UP−/− platelets did not (figure 4F). The addition of exogenous dRP restored the upregulation of HO-1 in HUVECs exposed to TP−/−/UP−/− platelets, confirming that the release of this metabolite by platelets is responsible for the enhancement of ROS generation. The ROS scavenger N-acetyl-cysteine (10mM) abolished the upregulation of HO-1 and integrin β3 (Figure 4G), suggesting that ROS generation is a critical step towards the upregulation of integrin β3 by platelet supernatants. Importantly, the integrin αvβ3 dependency of HUVEC migration promoted by protein-free platelet supernatants was confirmed using an inhibitory antibody for this integrin 25, which reduced the pro-migratory effects of WT and TP−/−/UP−/− platelet supernatants to similar levels (Figure 5).
Figure 5.
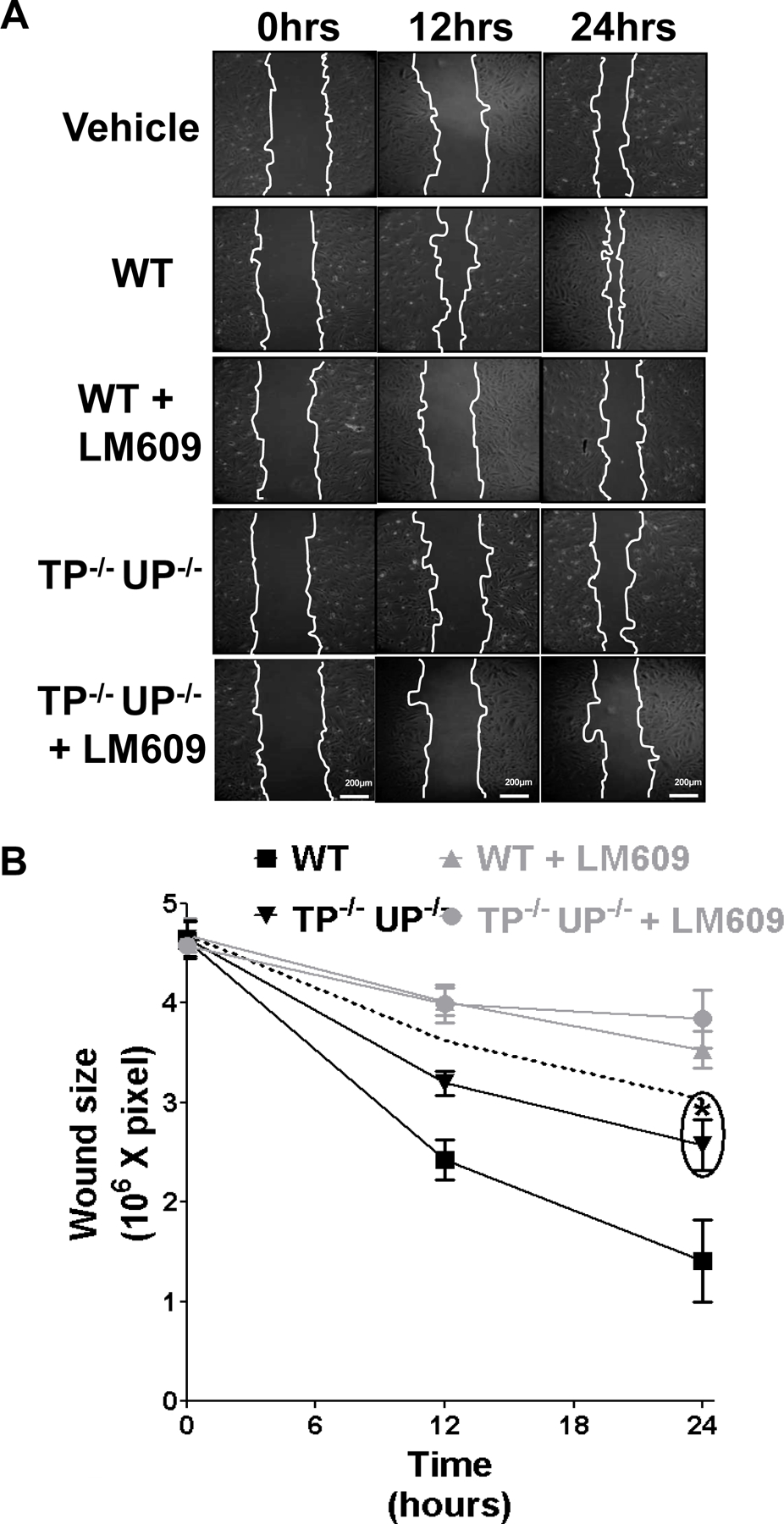
Inhibition of integrin αvβ3 abolishes the stimulation of HUVEC monolayer repair by protein-free WT platelet supernatants. Protein-free supernatants were obtained from WT and TP−/−/UP−/− mouse platelets stimulated with 1unit/ml thrombin for 5 minutes. The supernatants were added to HUVECs after manual creation of a wound in the monolayer with and without supplementation with 12.5µg/ml LM609. Wound size at 0, 12, and 24 hours after wounding is expressed as surface area (pixels). The closure rate in the presence of vehicle (Tyrode’s buffer) is also represented by the dashed line. Results from 4 independent experiments were analysed by two-way ANOVA with Bonferroni post-test (*= p<0.05, compared to WT supernatants).
Finally, the physiological relevance of dRP release by platelets was investigated in vivo using a chick chorioallantoic membrane (CAM) vascularisation assay 26. In these experiments, filtered supernatants from WT thrombin-stimulated platelets strongly stimulated local vascularisation of the CAMs, whereas supernatants from TP−/−/UP−/− mouse platelets did not (Figure 6). The addition of exogenous dRP (200µM) to TP−/−/UP−/− supernatants restored the level of vascularisation observed with WT platelet supernatants.
Figure 6.

Platelet-derived dRP stimulates angiogenesis in a chick chorioallantoic membrane (CAM) assay. Sterile filters soaked with vehicle, VEGF (100ng/filter) or the appropriate filtered platelet supernatant (2kDa cut off, 109 platelets/ml) were applied to relatively avascular regions of the CAM and incubated for 48 hours before analysis of capillary formation as surface area occupied by the vascular network (angiogenic index). Representative images (A) and mean ± SEM from 15 CAMs/treatment (B) are shown (ANOVA with Bonferroni post-test, *= p<0.05).
DISCUSSION
Our results demonstrate that platelets release small molecules capable of increasing endothelial cell migration in vitro and provide evidence for the importance of dRP in mediating this response. Thus, the genetic ablation of dRP-generating TP and UP in mouse platelets or the specific inhibition of dRP-generating UP by PTAU in human platelets resulted in significantly reduced ability of protein-free supernatants to induce endothelial transmigration. Similarly, the genetic ablation of dRP-generating TP and UP in mouse platelets reduced the ability of protein-free platelet supernatants to promote endothelial monolayer repair in vitro. Both observations supported the conclusions that dRP at the concentrations released by platelets stimulates endothelial cell migration and UP is the most important enzyme for platelet generation of this metabolite. In accordance with these conclusions, the addition of exogenous dRP to protein-free platelet supernatants reversed the inhibitory effects that pharmacological or genetic UP impairment produced on the stimulation of endothelial cell migration. Interestingly, the addition of dRP to the supernatants from resting platelets only partially restored the transmigration levels compared supernatants from stimulated platelets, which suggested that other small molecules released by stimulated platelets synergise with dRP and are also necessary for maximal stimulation of endothelial cell motility (e.g. sphingosine-1-phosphate 12).
The upregulation of integrin β3 in response to dRP has been reported to account for the increase in HUVEC motility induced by EPCs 23. Here, we observed that platelet dRP also promotes endothelial cell motility via upregulation of integrin β3, which is known to play a pivotal role in the stimulation of endothelial cell motility and angiogenesis 27. Our data obtained using the inhibitory antibody LM609 show that the integrin heterodimer αvβ3 mediates the promotion of endothelial cell motility by protein-free platelet supernatants, and suggest that the activity of this macromolecular complex is enhanced by dRP-dependent upregulation of integrin β3. Interestingly, LM609 reduced the HUVEC monolayer repair rate below levels observed in the presence of vehicle, suggesting a role for integrin αvβ3 in endothelial cell motility independently of platelet-dependent modulation of integrin β3 expression. In common with dRP, dR has also been shown to stimulate endothelial cell migration through integrins, in particular αvβ3 and α5β1 28. Since the functional interaction of integrin αvβ3 with vascular endothelial growth factor receptor-2 (VEGFR-2) has been proposed to regulate VEGF-induced endothelial cell migratory activity 29, it is not unreasonable to speculate that the upregulation of endothelial cell integrin β3 by platelet-derived dRP may enhance responsiveness to endogenous VEGF, resulting in increased endothelial cell motility and stimulation of angiogenesis. Interestingly, platelet preparations employed to enhance tissue repair in clinical practice have been shown to stimulate angiogenesis via upregulation of integrin αvβ3 30.
Previous studies in cancer cells have linked dRP production to protein glycation and generation of ROS 31. Here, we showed that dRP increases ROS production in HUVECs, which is accompanied by induction of the anti-oxidant defence enzyme HO-1. Our experiments with the ROS inhibitor N-acetyl-cysteine suggest that the generation of ROS is necessary for dRP-dependent upregulation of integrin β3 by platelet supernatants. Although the precise mechanism coupling increased ROS production with enhanced integrin expression requires further investigation, our results provide strong support for ROS-dependent regulation of integrin β3 as a key pathway mediating the pro-angiogenic effects of platelet-derived dRP on endothelial cells.
Our data on the stimulation of neoangiogenesis in vivo by platelet-derived dRP are in accordance with previous studies highlighting a link between dRP and angiogenesis. TP, for example, was initially cloned as platelet-derived endothelial cell growth factor (PDECGF) and characterised for its pro-angiogenic properties 32. The local injection of TP or TP-expressing cells has also been shown to induce tissue neovascularisation in vivo, and this depends on the enzymatic activity of TP and the generation of dRP and its dephosphorylation product dR 33–36. Taken together, the data presented in this study show, for the first time that platelets are capable of inducing endothelial cell migration and angiogenesis by releasing dRP, a pro-angiogenic metabolite that promotes the upregulation of integrin β3 in a ROS-dependent manner. These findings shed new light on the emerging importance of platelets as regulators of endothelial cell function and angiogenesis. In addition to stimulating angiogenesis during tissue repair, the effects of platelet-derived dRP on endothelial cell motility may also be relevant in the context of angiogenesis-dependent pathologies characterised by increased local and systemic platelet activation (e.g. cancer, atherosclerosis, and inflammation).
Supplementary Material
ACKNOWLEDGEMENTS
The platelet studies were funded by a Royal Society grant (RG090131) and an internal grant from the Royal Veterinary College (to GP). The endothelial cell studies were supported by a project grant (to CW-J) from the British Heart Foundation (PG/07/063/23289). WD is grateful for financial support from the BBSRC and EPSRC (BB/C008219/1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brill A, Elinav H, Varon D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc Res. 2004;63:226–235. [DOI] [PubMed] [Google Scholar]
- 2.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. [DOI] [PubMed] [Google Scholar]
- 3.Rhee JS, Black M, Schubert U, Fischer S, Morgenstern E, Hammes HP, Preissner KT. The functional role of blood platelet components in angiogenesis. Thromb Haemost. 2004;92:394–402. [DOI] [PubMed] [Google Scholar]
- 4.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. [DOI] [PubMed] [Google Scholar]
- 5.Langer HF, Gawaz M. Platelets in regenerative medicine. Basic Res Cardiol. 2008;103:299–307. [DOI] [PubMed] [Google Scholar]
- 6.Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). Thromb Haemost. 2002;88:834–842. [PubMed] [Google Scholar]
- 7.Thommen R, Humar R, Misevic G, Pepper MS, Hahn AW, John M, Battegay EJ. PDGF-BB increases endothelial migration on cord movements during angiogenesis in vitro. J Cell Biochem. 1997;64:403–413. [PubMed] [Google Scholar]
- 8.Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536–23541. [DOI] [PubMed] [Google Scholar]
- 9.Pi X, Wu Y, Ferguson JE 3rd, Portbury AL, Patterson C. SDF-1alpha stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc Natl Acad Sci U S A. 2009;106:5675–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–10. [DOI] [PubMed] [Google Scholar]
- 12.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. [DOI] [PubMed] [Google Scholar]
- 13.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. [DOI] [PubMed] [Google Scholar]
- 14.Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, Akiyama S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995;55:1687–1690. [PubMed] [Google Scholar]
- 15.Sengupta S, Sellers LA, Matheson HB, Fan TP. Thymidine phosphorylase induces angiogenesis in vivo and in vitro: an evaluation of possible mechanisms. Br J Pharmacol. 2003;139:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003;63:527–533. [PubMed] [Google Scholar]
- 17.Tozzi MG, Camici M, Mascia L, Sgarrella F, Ipata PL. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 2006;273:1089–1101. [DOI] [PubMed] [Google Scholar]
- 18.Lopez LC, Akman HO, Garcia-Cazorla A, Dorado B, Marti R, Nishino I, Tadesse S, Pizzorno G, Shungu D, Bonilla E, Tanji K, Hirano M. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet. 2009;18:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pula G, Crosby D, Baker J, Poole AW. Functional interaction of protein kinase Calpha with the tyrosine kinases Syk and Src in human platelets. J Biol Chem. 2005;280:7194–7205. [DOI] [PubMed] [Google Scholar]
- 20.Campanella M, Seraphim A, Abeti R, Casswell E, Echave P, Duchen MR. IF1, the endogenous regulator of the F(1)F(o)-ATPsynthase, defines mitochondrial volume fraction in HeLa cells by regulating autophagy. Biochim Biophys Acta. 2009;1787:393–401. [DOI] [PubMed] [Google Scholar]
- 21.Klein RS, Lenzi M, Lim TH, Hotchkiss KA, Wilson P, Schwartz EL. Novel 6-substituted uracil analogs as inhibitors of the angiogenic actions of thymidine phosphorylase. Biochem Pharmacol. 2001;62:1257–1263. [DOI] [PubMed] [Google Scholar]
- 22.el Kouni MH, Goudgaon NM, Rafeeq M, Al Safarjalani ON, Schinazi RF, Naguib FN. 5-phenylthioacyclouridine: a potent and specific inhibitor of uridine phosphorylase. Biochem Pharmacol. 2000;60:851–856. [DOI] [PubMed] [Google Scholar]
- 23.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, Astroulakis Z, Xiao Q, Hill J, Xu Q, Mayr M. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104:32–40. [DOI] [PubMed] [Google Scholar]
- 24.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther. 2009;329:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 26.Ribatti D Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol. 2008;270:181–224. [DOI] [PubMed] [Google Scholar]
- 27.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. J Biol Chem. 2003;278:19272–19279. [DOI] [PubMed] [Google Scholar]
- 29.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 30.Herouy Y, Mellios P, Bandemir E, Stetter C, Dichmann S, Idzko M, Hofmann C, Vanscheidt W, Schopf E, Norgauer J. Autologous platelet-derived wound healing factor promotes angiogenesis via alphavbeta3-integrin expression in chronic wounds. Int J Mol Med. 2000;6:515–519. [DOI] [PubMed] [Google Scholar]
- 31.Brown NS, Jones A, Fujiyama C, Harris AL, Bicknell R. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res. 2000;60:6298–6302. [PubMed] [Google Scholar]
- 32.Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W, Heldin CH. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557–562. [DOI] [PubMed] [Google Scholar]
- 33.Brown NS, Bicknell R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem J. 1998;334:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchimiya H, Furukawa T, Okamoto M, Nakajima Y, Matsushita S, Ikeda R, Gotanda T, Haraguchi M, Sumizawa T, Ono M, Kuwano M, Kanzaki T, Akiyama S. Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose. Cancer Res. 2002;62:2834–2839. [PubMed] [Google Scholar]
- 35.Liekens S, Hernandez AI, Ribatti D, De Clercq E, Camarasa MJ, Perez-Perez MJ, Balzarini J. The nucleoside derivative 5’-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. J Biol Chem. 2004;279:29598–29605. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita S, Nitanda T, Furukawa T, Sumizawa T, Tani A, Nishimoto K, Akiba S, Miyadera K, Fukushima M, Yamada Y, Yoshida H, Kanzaki T, Akiyama S. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999;59:1911–1916. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


