Abstract

A new secalonic acid derivative, F-7 (1), was isolated from the endophytic Aspergillus aculeatus MBT 102, associated with Rosa damascena. The planar structure of 1 was established on the basis of 1D and 2D NMR and ESI-TOF-MS spectra. The relative configuration of 1 was determined applying a combined quantum mechanical/NMR approach and, afterward, the comparison of calculated and experimental electronic circular dichroism spectra determined the assignment of its absolute configuration. The compound possesses strong cytotoxic activity against triple negative breast cancer (TNBC) cells. It was found to induce apoptosis, as evidenced by scanning electron microscopy and phase contrast microscopy. Furthermore, flow cytometry analyses demonstrated that 1 induced mitochondrial damage and reactive oxygen species mediated apoptosis, arresting the G1 phase of the cells in a dose-dependent manner. Also, the compound causes significant microtubule disruption in TNBC cells. Subsequently, 1 restricted the cell migration leading to the concomitant increase in expression of cleaved caspase and PARP.
Introduction
Microorganisms give rise to a vast wealth of specialized natural products, with a broad spectrum of structural diversity and biological activities.1 Natural products are captivating molecules for drug discovery as more than 70% antibiotics and 60% anticancer drugs in clinical use are of natural origin.2 Interestingly, more than 40% of new chemical entities have been derived from microorganisms. Thus, the investigation of microorganisms, ideally endophytes, has become imperative to expedite the development of novel pharmaceutical because of their unrevealing benefits and being the least explored bioresource for chemical diversity.3 Endophytes represent an important group of diverse plant symbionts that reside inside the plant tissues without causing any symptom of disease in the host under normal conditions.4 Fungal endophytes perform diverse ecological functions and support the host in numerous ways, such as imparting stress tolerance both biotic and abiotic, promoting growth and yield, disease resistance, survival, protection from other microbes, herbivores, and insects, and influencing distribution of host plants.4,5 In addition to their indispensable roles in nature, endophytes represent a massive and unexplored resource for the isolation of novel biomolecules and biocatalysts.1a,5,6 Secondary metabolites of endophytes are diverse and often bioactive because of the multitude of roles that they play in nature.7
As per the reports of the World Health Organization (WHO), one of the leading causes of mortality in the world is cancer.8 The most prevalent form of cancer among women resulting in about 450,000 deaths worldwide annually is breast cancer.9 Among all breast cancer types, triple negative breast cancer (TNBC) is the most threatening because of lack of estrogen receptor, progesterone receptor, and the human epidermal growth factor receptor 2 (HER2) expressions, which explains the unresponsiveness toward targeted therapy.10 Patients with TNBC are difficult to treat because of poor prognosis with limited treatment options and resistance to several anticancer drugs. Therefore, the identification of novel therapeutic agents to treat this complex malignant cancer is highly desirable.11 The genus Aspergillus is considered one of the dominant endophytes associated with plants, liverworts, mosses, hornworts, and ferns, from tropics to the arctic tundra with the capability to produce an array of secondary metabolites.12Aspergillus aculeatus, a member of Aspergillus section Nigri the black aspergilla, is an efficient producer of various bioactive compounds. So far, there are several reports documenting the bioactive potential of its compounds including secalonic acids A, D, and F which have shown incredible anticancer activities.13 The secalonic acid family (SAF), natural mycotoxins, including dimers of six different monoxanthones (secalonic acids A–F) with a dimeric tetrahydroxanthenone skeleton exhibits a wide range of anticancer activities including inhibition of DNA topoisomerase I and protein kinase C.14 Previous studies have highlighted that SAF inhibits the proliferation and promotes the apoptosis of HepG2 cells, repressing the progression of hepatocellular carcinoma via MARCH1 regulation of the PI3K/AKT/β-catenin signaling pathway and also the SAF inhibits VEGF-mediated angiogenesis through the Akt/mTOR pathway in breast cancer.14,15 In the present study, we report a novel derivative of secalonic acid from A. aculeatus MBT-102, an endophyte of Rosa damascena, and its cytotoxic potential against TNBC cells.
Results and Discussion
Majority of the drugs in clinical use are natural products or their derivatives.16,17 However, because of the emergence of new diseases and drug resistance, it is imperative to run sustainable drug discovery programs for the development of new clinical molecules.18,19 Because one of the most important sources of natural products, the plant kingdom, has been extensively explored, it becomes necessary to screen other diverse sources for discovery of potential bioactive molecules. As an alternative, the focus has been shifted to the enormous microbial biodiversity owing to the fact that it is poorly explored and underutilized.20 Metagenomic studies have revealed that only about 1% of bacteria and 5% of fungi have been characterized. Thus, a huge biological resource waits to be characterized and explored for human welfare.21 Endophytic fungi are regarded to possess a remarkable metabolic process because of the constant challenges they face inside the plant tissues and from microorganisms competing for endophytism. Thus, they have been found to produce numerous molecules with diverse bioactivities.22 The fungus, MBT102, was found to be associated with R. damascena in nature as an endophyte. The isolated strain showed rich growth on potato dextrose agar (PDA), chickpea dextrose agar, and malt extract agar (MEA). The aerial mycelia were numerous and blackish brown in color (Figure 1A,B) with acquisition of the ITS4-5.8S-ITS5 ribosomal gene sequence showing the highest homology of 99% with A. aculeatus. The phylogenetic position of the strain is depicted in Figure 2. The sequence was submitted to NCBI under the GenBank accession number MK886612. Compound 1 strongly inhibited breast cancer cell proliferation and is the most potent as compared to the MBT extract. It caused cancer cell apoptosis by reactive oxygen species (ROS) formation via increasing caspase-3 activity.23 Because it is well-reported that ROS plays an important role in the induction of cell death, we further examined the ROS levels in control and 1-treated MDA-MB-231 cancer cells and observed that 1-administered cells showed very high levels of ROS associated with the increased expression of MMP loss. Excessive ROS triggers the apoptosis pathway by altering the MMP and damaging the respiratory chain. Considering the connection between ROS and MMP, it is possible that 1 elicited ROS production, which permitted loss of MMP, thus initiating 1-induced apoptosis. ROS production is needed for proper cell migration and chemotaxis, which are tubulin-dependent processes. In vitro assay suggested that an oxidative environment created by 1 inhibited tubulin polymerization. Thus, polymerization assay was designed to recapitulate in vitro the exact oxidative environment response to inhibit actin polymerization.241 also caused G1 cell cycle arrest and led to a considerable increase in G1 cells dose-dependently. The cell cycle is considered as an important mechanism for inhibiting cell division. These findings are promising because it is well-established that breast cancer is one of the deadly cancers and 1 could suppress its growth through cell cycle arrest.25 Additionally, 1 also inhibited the cell migration of MDA-MB-231 cells as evidenced from the wound healing assay. Cell migration is the key feature of cancer progression and metastasis and suppression of cell migration may prove crucial in inhibition of metastasis in vivo. This may ensure comparatively longer survival of patients. Therefore, the potential of 1 to suppress migration of MDA-MB-231 cancer cells indicates that this compound may prove as an efficient molecule in inhibiting the metastasis of cancer cells in vitro.26 To clarify the role of 1 in cancer cell apoptosis and chemoresistance, we examined cell viability and apoptosis rate using 4′,6-diamidino-2-phenylindole (DAPI) staining and cell cytotoxicity assay [sulforhodamine B (SRB)].27 Our data demonstrated that 1 overexpression was able to inhibit cell viability, which was downregulated by 1 treatment. Next, we examined the change in cleaved caspase 3 and cleaved PARP. The outer membrane of mitochondria becomes permeable with the downregulation of MMP and this acts to trigger caspase and PARP cleavage/activation. Our results demonstrated that 1 enhanced the release of the expression of cleaved caspase 3/PARP, supporting the role of 1 in apoptosis and MMP loss with the release of ROS disrupting polymerization of tubulin integrity.23
Figure 1.

Morphology of A. aculeatus, MBT102 (A) 7 day-old culture on PDA and (B) microscopic structures of A. aculeatus, MBT102.
Figure 2.
Evolutionary history was inferred using the maximum likelihood method based on the Hasegawa–Kishino–Yano model. There were a total of 418 positions in the final dataset. Evolutionary analyses were conducted in MEGA6. Rhizopus microsporus was used as an out group.
Fermentation, Isolation, and Characterization of the Isolated Compound
The whole fermentation (20 L) broth of fungal strain MBTF-102 was
extracted with equal volumes of ethyl acetate, resulting in a yellow
color extract which was fractionated on a silica column, followed
by C18 HPLC (MeCN/H2O) to afford 1 [9 mg;
[α]25D +117 (c 0.1, CHCl3)] as yellow amorphous
powder. Compound 1 displayed an [M – H]− ion in the (−)-HRESIMS spectrum at m/z 653.1510 corresponding to the formula C32H30O15. This formula was consistent with 18°
of unsaturation. The proton NMR spectrum of 1 in CDCl3 (Table 1)
showed proton signals for two methyl doublets (δH 1.21 and 1.17) and (δH 1.21 and 1.17), two methyl
singlets (δH 3.69 and and 3.72), and two oxygen-bearing
methines (δH 3.93 and δH 4.38).
The aromatic region of the 1H NMR also indicated signals
for four one-proton doublets (at δH 7.50, 7.46, 6.61,
and 6.60) and two −OH protons (at δH 11.88
and 11.71). The 13C NMR/HSQC spectra of 1 (Table 1) displayed 32 signals,
which corresponded to 4 methyl groups, 2 methylene groups, 4 methine
groups (of which 2 were oxygenated), and 3 sp3 and 10 sp2 quaternary carbons. In addition, carbon signals pertaining
four olefinic (δC 107.6, 106.9, 140.4, and 140.3),
two ester (δC 168.5 and 170.3), and two ketone (δC 177.6 and 198.7) functionality appeared in the downfield
region of the spectrum. Inspection of the 1H and 13C NMR data of 1 (Table 1) in the literature suggested a close relationship
with secalonic acids (Figure S1). Detailed
analyses of 2D NMR data including the 1H–1H COSY, HMQC, and HMBC spectra measured in CDCl3 (Figures 3 and S3–S9) indicated that the gross structure
of 1 was similar to that of secalonic acid F (Figure S1),29 likely
only differing at the C-8a–C-8 carbons: C-8a (δC 72.5)–C-8 (δC 198.7);  in 1 and C-8a (δC 179.8)–C-8 (δC 99.9); and
in 1 and C-8a (δC 179.8)–C-8 (δC 99.9); and 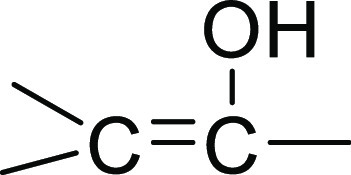 in secalonic acid F. The COSY
correlations for H-7 (δH 3.17)/H-6 (δH 2.04–1.99)/H-5 (δH 4.38) and HMBC correlation
from H-7 (δH 3.17) to C-8 (δC 198.7),
C-6 (δC 31.3), and C-11 (δC 18.01)
and from H-5 (δH 4.38) to C-8a (δC 72.5), C-7 (δC 39.7), and C-10 (δC 86.2) confirmed this hypothesis (Figure 3). All of the remaining HMBC correlations
(Figure 3) and NMR
data (Table 1) are
in full agreement with structure 1, as shown in Figure 3. Moreover, chemical
shifts of C-6 (C-6′), C-5 (C-5′), and C-10 (C-10′),
coupling constants of 3JH6–H5 (∼0 Hz) and 3JH6′–H5′ (11 Hz), and a positive cotton effect at 333 nm in the circular
dichroism (CD) spectra29a of 1 were almost the same as those for secalonic acid F (Tables 1 and S10). Therefore, compound 1 has the same relative configurations
at C-6 (C-6′), C-5 (C-5′), and C-10 (C-10′) as
those of secalonic acid F. The relative configuration at C-8a was
determined applying the quantum mechanical (QM)/NMR approach,30 specifically comparing the experimental 13C/1H NMR chemical shift data and the related predicted
values for the two possible solutions 1a (8aS*) and 1b (8aR*) (Figure 4). Prior to the prediction
of the 13C/1H NMR spectra, we also investigated
the possible hindered rotation along the dimeric tetrahydroxanthenone-related
ortho-disubstituted biaryl axis (linking C-2 and C-2′ atoms)
that could give rise to one preferential arrangement of the two phenyl
moieties and then leading to two possible atropisomers (featuring M* or P* relative configurations) to be
potentially accounted in this stereochemical investigation.30,31
in secalonic acid F. The COSY
correlations for H-7 (δH 3.17)/H-6 (δH 2.04–1.99)/H-5 (δH 4.38) and HMBC correlation
from H-7 (δH 3.17) to C-8 (δC 198.7),
C-6 (δC 31.3), and C-11 (δC 18.01)
and from H-5 (δH 4.38) to C-8a (δC 72.5), C-7 (δC 39.7), and C-10 (δC 86.2) confirmed this hypothesis (Figure 3). All of the remaining HMBC correlations
(Figure 3) and NMR
data (Table 1) are
in full agreement with structure 1, as shown in Figure 3. Moreover, chemical
shifts of C-6 (C-6′), C-5 (C-5′), and C-10 (C-10′),
coupling constants of 3JH6–H5 (∼0 Hz) and 3JH6′–H5′ (11 Hz), and a positive cotton effect at 333 nm in the circular
dichroism (CD) spectra29a of 1 were almost the same as those for secalonic acid F (Tables 1 and S10). Therefore, compound 1 has the same relative configurations
at C-6 (C-6′), C-5 (C-5′), and C-10 (C-10′) as
those of secalonic acid F. The relative configuration at C-8a was
determined applying the quantum mechanical (QM)/NMR approach,30 specifically comparing the experimental 13C/1H NMR chemical shift data and the related predicted
values for the two possible solutions 1a (8aS*) and 1b (8aR*) (Figure 4). Prior to the prediction
of the 13C/1H NMR spectra, we also investigated
the possible hindered rotation along the dimeric tetrahydroxanthenone-related
ortho-disubstituted biaryl axis (linking C-2 and C-2′ atoms)
that could give rise to one preferential arrangement of the two phenyl
moieties and then leading to two possible atropisomers (featuring M* or P* relative configurations) to be
potentially accounted in this stereochemical investigation.30,31
Table 1. 1H NMR (400 MHz) and 13C NMR (100 MHz) Spectroscopic Data for Compound F-7 (1) in CDCl3.
| c.·no. | δH | δC | HMBC correlations |
|---|---|---|---|
| 1 | 159.3 | ||
| 1′ | 160.2 | ||
| 9a | 106.9b | ||
| 9a′ | 106.3 | ||
| 9 | 190.3 | ||
| 9′ | 187.1 | ||
| 8a | 72.5 | ||
| 8a′ | 101.5 | ||
| 8 | 198.7 | ||
| 8′ | 177.6 | ||
| 7 | 3.17 dd (15.1, 13.6), 2.37–2.33 s | 39.7 | 198.7, 31.3, 18.01 |
| 7′ | 2.74 dd (18.8, 5.9) 2.33–2.29 | 36.3 | 177.6, 101.5, 77.2, 17.07 |
| 6 | 2.04–1.99 m | 31.3 | |
| 6′ | 2.47–2.37 m | 29.7 | 17.07 |
| 5 | 4.38 brs | 75.0 | 72.5, 39.7, 86.2 |
| 5′ | 3.93 d (11) | 77.2 | 29.7, 84.2, 17.07, 170.3 |
| 10 | 86.2 | ||
| 10′ | 84.2 | ||
| 4a | 158.4 | ||
| 4a′ | 156.7 | ||
| 4a | 6.61 d (8.6) | 107.6 | 106.9, 119.1 |
| 4′a | 6.60 d (8.4) | 106.9b | 106.3, 117.7 |
| 3 | 7.50 d (8.5) | 140.4 | 117.7, 159.3, 158.4 |
| 3′ | 7.46 d (8.5) | 140.3 | 119.1, 160.2, 156.7 |
| 2 | 119.1 | ||
| 2′ | 117.7 | ||
| 11 | 1.21 d (6.6) | 18.01 | 39.7, 31.3, 75.0 |
| 11′ | 1.17 d (6.3) | 17.07 | 36.3, 29.7, 77.2 |
| 12 | 168.5 | ||
| 12′ | 170.3 | ||
| 13 | 3.69 s | 53.2 | 168.5 |
| 13′ | 3.72 s | 53.8 | 170.3 |
| –OH-1 | 11.88 s | 160.2, 106.3, 117.7 | |
| –OH-2 | 11.71 s | 159.3, 106.9, 119.1 |
Overlapped signals in 1H NMR spectra.
Overlapped signals in 13C NMR spectra.
Figure 3.
Structure and key COSY and HMBC correlations of F-7 (1).
Figure 4.
Chemical structures of 5S*,6S*,8aS*,10S*,5′R*,6′S*,10′R*-1 (1a) and 5S*,6S*,8aR*,10S*,5′R*,6′S*,10′R*-1 (1b).
It is important to note that in the specific case of compound 1, the presence of only one hydroxyl substituent on the ortho position of each aromatic aryl function should not determine the hindered rotation along the biaryl axis. In order to corroborate this hypothesis, an extensive sampling of the conformations related to 1a and 1b was performed for the subsequent phases of stereochemical elucidation. In particular, the analysis of the conformational ensembles for compounds 1a and 1b disclosed sampled geometries related to both the putative atropisomeric species. In order to shed light about the possible hindered rotation along the biaryl axis, density functional theory (DFT) calculations were then performed for computing the rotational energy barrier related to the interconversion between the two species (see the Experimental Section), specifically on the M* and P* species of compound 1a as representative systems. The difference between the energy of the most energetically favored conformer of 1a and the energy of the related transition state indicated that ΔG⧧ = 17.4 kcal/mol, suggesting the absence of atropisomerism32 and, accordingly, conforming the hypothesized free rotation between the biaryl axis. Furthermore, the inspection of the sampled atropisomers pointed out that the M* arrangement pattern is preferred for this molecule on the final Boltzmann distribution. Starting from these data, the NMR chemical shift data were then computed for compounds 1a and 1b, in order to assign the relative configuration at the C-8a stereocenter. Specifically, 13C and 1H NMR chemical shifts were predicted at the DFT, accounting the contributions of each conformer for each stereoisomer on the basis of the related energies calculated at the DFT level on the final Boltzmann distribution. The comparison between the experimental and calculated NMR chemical shifts clearly indicated compound 1a as the most probable isomer of 1 because it featured the lowest mean absolute error (MAE) values (see the Computational Details) (13C MAE = 1.97 ppm vs 2.21 ppm and 1H MAE = 0.21 ppm vs 0.24 ppm) for 1a and 1b, respectively (Tables S1 and S2) and, accordingly, the best fit between the experimental and computed 13C/1H chemical shift data. These data thus allowed the assignment of the relative configuration of 1 as 5S*,6S*,8aS*,10S*,5′R*,6′S*,10′R*. Finally, we then predicted the absolute configuration of 1 by the comparison between the experimental and calculated electronic CD (ECD) spectra, the latter related the two possible enantiomeric species of 1a, namely, 5S,6S,8aS,10S,5′R,6′S,10′R-1 and 5R,6R,8aR,10R,5′S,6′R,10′S-1.33 Starting from the previously sampled and selected conformers of 1a, QM calculations at the TD-DFT MPW1PW91/6-31G(d,p) level were performed in methanol IEFPCM to reproduce the experimental solvent environment. As shown in Figure 5, the comparison of the experimental and calculated ECD curves for both the possible enantiomeric species related to 1a led to the assignment of the absolute configurations of 1 as 5S,6S,8aS,10S,5′R,6′S,10′R, in line with the stereochemical arrangement of secalonic acid F, as expected.
Figure 5.
Superposition between the experimental ECD spectrum of 1 and those calculated for 5S,6S,8aS,10S,5′R,6′S,10′R-1 (panel A) and 5R,6R,8aR,10R,5′S,6′R,10′S-1 (panel B).
Biological Activity
The SRB cytotoxicity assay was performed using the isolated compound 1 and the positive controls on a panel of human cancer cell lines, viz., MCF-7, MDA-MB-231, HT-29, SW-620, A549, PC-3, and PANC-1 of various origins. Among all cell lines, 1 displayed a range of cytotoxicity, as shown in Table 2. However, TNBC, MDA-MB-231 was relatively more sensitive with an IC50 of 6.86 ± 0.09 μM. Moreover, 1 was found to be nontoxic in normal human breast epithelial FR-2 cells with a 4–7 times higher IC50 value than in TNBC. Further experiments were carried out against MDA-MD-231 as the cytotoxic effect was more pronounced for this cell line.
Table 2. In Vitro Screening Data of F7 (1) and Its Extract MBT102 Have Been Executed on Various Cancer Cell Lines, Which Determined Its Potent and Selectivity Activity in the MDA-MB-231 Breast Cancer Cell Line and in Normal Breast Cell Linea.
| lung | prostate | breast | coloncolon | breast | pancreatic | normal breast epithelial | selectivity index | |||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | ||||||||||
| cell line | A549 | PC-3 | MCF-7 | HT-29 | SW620 | MDA-MB-231 | PANC-1 | FR-2 | ||
| F-7 (1) | μM/mL | 10.38 ± 0.14 | 12.97 ± 0.02 | 30.23 ± 0.54 | 22.8 ± 0.14 | 16.6 ± 0.28 | 6.86±0.09 | 10.14 ± 0.43 | 29.33 ± 1.47 | 4.27 ± 1.55 |
| MBT102 | μg/mL | 1.67 ± 0.08 | 3.41 ± 0.07 | 2.78 ± 0.72 | 1.06 ± 0.27 | 4.42 ± 0.1 | 2.31 ± 0.40 | 2.96 ± 0.60 | 1.48 ± 1.07 | 0.64 ± 2.18 |
| Paclitaxel | μM/mL | 0.01 ± 1.21 | 0.065 ± 2.36 | 0.01 ± 0.32 | 0.03 ± 0.68 | 0.140 ± 2.20 | 0.01 ± 1.41 | 0.037 ± 1.09 | 0.07 ± 2.38 | 7 ± 0.11 |
Paclitaxel was used as a positive control.
Compound 1 Induces Apoptosis in MDA-MB-231 Cells Investigated Using Microscopic Analysis
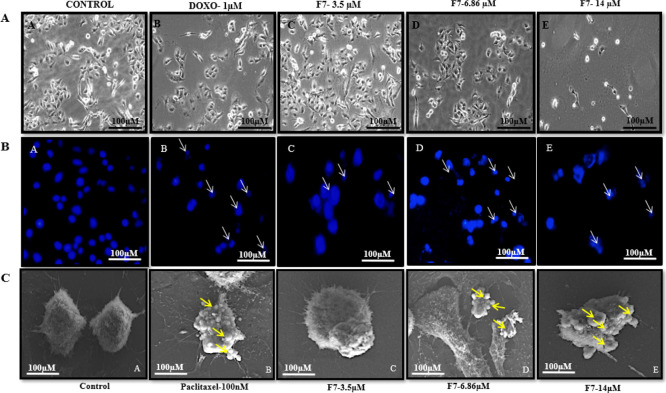
To gain an insight into cell death, compound 1 was evaluated for morphological changes induced by apoptosis. In the present study, phase contrast microscopy revealed characteristic morphological changes such as cell density decrease in treated cells in a concentration-dependent manner (Figure 6). Furthermore, a fluorescence microscope was used to evaluate its ability of inducing apoptosis. Treated MDA-MB-231 cells subsequently stained with DAPI caused nuclear condensation, fragmentation of nuclei, shrinkage of cell size, and formation of scattered apoptotic bodies in a concentration-dependent manner (Figure 6). This confirmed that 1 induced cell death through induction of apoptosis in MDA-MB-231 cells. The results were further corroborated by scanning electron microscopy (SEM). Treatment induced smoothening of the cell surface and condensation and blebbing of the plasma membrane in a concentration-dependent manner compared to the untreated cells (Figure 6) because of apoptosis.
Figure 6.
Cell morphology and DNA staining with DAPI after 48 h of treatment with F7 (1), observed the morphological changes in MDA-MB-231 cells under a phase contrast microscope (top panel-A) and fluorescence inverted microscope (below panel-B) at the magnification of 20×. Representative SEM images (C) showed concentration-dependent onset of apoptosis characterized by membrane blebbing and apoptotic body formation in MDA-MB-231 cells compared to control cells.
Compound 1 Inhibits Tubulin Polymerization in MDA-MB-231 Cells
Microtubules play an important role in the regulation of mitotic progression and as such have become an attractive target for chemotherapy. Disrupting the assembly of microtubules can trigger apoptosis and also can induce cell cycle arrest in the M-phase by either stabilizing or destabilizing tubulin polymerization.34 We investigated the effect of 1 on tubulin polymerization of MDA-MB-231 cells using confocal microscopy. As shown in Figure 7, 1 interferes with tubulin formation in a concentration-dependent manner. Treatment of MDA-MB-231 cells with a concentration of 6.8 and 14 μM of 1 showed a remarkable loss and disruption of microtubule formation. This result clearly demonstrates that 1 has potency of inhibiting tubulin polymerization which eventually results in apoptotic cell death.
Figure 7.
Confocal microscopic immunofluorescent images of the microtubule structure within MDA-MB-231 cells. (A) Alexa 488 florescent-labeled tubulin-α antibody fluoresce green allowing visualization of the tubulin network, while DAPI stained nuclear material blue. MDA-MB-231 cells exposed to Paclitaxel as a positive control for a microtubule depolymerizing agent, demonstrated compromised cell density with a disorganized tubulin structure. A total of 48 h F7 (1) exposure showed complete tubulin disintegration together with apoptotic body formation and decreased cell density. (B) Data represent means ± SD (n = 3). ***P < 0.001 vs control.
Compound 1 Inhibited Cell Proliferation during In Vitro Clonogenic Assay in TNBC Cells (MDA-MB-231)
In vitro colony formation assay was performed to gain further insight into the antiproliferative activity of 1 on MDA-MB-231 cells treated with different concentrations at 3.5, 6.8, and 14 μM for 14 days. It was observed that 1 significantly decreased the colony formation in MDA-MB-231 cells in a concentration-dependent manner (Figure 8). This assay revealed that MDA-MB-231 cells have been restricted for their colony formation capability as a decrease in the number of colonies was observed after the treatment. It concludes that 1 has an inhibitory effect on colony formation of MDA-MB-231 cells.
Figure 8.
Colony formation assay (A) effect of F7 (1) inhibition on cell proliferation in the MDA-MB-231 cell line was analyzed using colony formation. Colonies formed after 14 days of treatment were measured. F7 significantly reduced colony formation in a concentration-dependent manner compared with the untreated control (B). Data represent means ± SD (n = 3). ***P < 0.001, **P < 0.01 vs control.
Compound 1 Inhibited Cell Migration during In Vitro Wound Healing Assay in TNBC Cells
Cell migration is the key feature of cancer progression in which cancer cells invade extracellular matrix and basement membrane which further migrates into new tissue.35 To investigate the inhibitory effect of 1 on migration of MDA-MB-231, an in vitro wound healing assay was performed. MDA-MB-231 cell confluent monolayer was scratched and treated with 1 at different concentrations for 48 h. It significantly suppressed invasion of MDA-MB-231 cells to the denuded zone in a dose-dependent manner (Figure 9). The migration percentage was reduced from 100 to 40% at 14 μM concentration of 1. These results depict that 1 inhibited the motility of MDA-MB-231 cells significantly.
Figure 9.
In vitro wound healing assay (A) ML of MDA-MB-231 cells was scratched and treated with different concentrations of F7 for 48 h. The area of the wound was measured at the two time points (0 and 48 h), and % reduction in cell migration was assessed by recovery of the scratch as compared with 0 h post scratch. (B) Estimation of migration was assessed to the corresponding control. Data represent means ± SD (n = 3). ***P < 0.001, **P < 0.01 vs control.
Compound 1 Disrupts Mitochondrial Membrane Potential (ΔΨmt) in MDA-MB-231 Cells
Mitochondrial membrane potential (MMP) (ΔΨmt) dissipation is the central mechanism for investigating initiation of drug-induced apoptosis. It has been reported that the intrinsic or mitochondrial pathway through death signals to mitochondria results in the release of mitochondrial intermembrane proteins such as cytochrome c, which associate with apoptotic protease-activating factor-1-Apaf-1 to form the apoptosome in order to activate caspase-3.36 In accordance, the change in the permeability of the mitochondrial membrane was accessed by flow cytometry after labeling cells with RH-123, a fluorescent cationic dye. Loss of mitochondrial integrity is detected by decrease in fluorescence intensity. As shown in Figure 10, MDA-MB-231 treated with 1 revealed significant depolarization of mitochondria and loss of MMP in a concentration-dependent manner as indicated by a dramatic loss in the RH-123 signal intensity. These results indicate that 1 dose-dependently reduced the MMP (ΔΨm) in MDA-MB-231 cells and induced cancer cell apoptosis associated with mitochondrial dysfunction.
Figure 10.
F7 (1) induced changes in MMP. The loss of MMP was measured by rhodamine-123 (Rh-123) staining and analyzed by flow cytometry. Cells were treated with F7 (3.5, 6.8, and 14) for 48 h and then loaded with Rh-123 (5 mg/mL) for 30 min. (A) Inserted histogram demonstrated a left shift of the histogram peak representing the decrease in Rh-123 fluorescence intensity because of the loss of MMP. (B) Percentage of cells with reduced fluorescence intensity was calculated. ***P < 0.001, **P < 0.01 vs control.
Compound 1 Induces ROS Generation in MDA-MB-231 Cells
Several studies have demonstrated that the chemotherapeutic agents induce ROS generation which consequently leads to oxidative damage and further sensitizes apoptosis. Excessive ROS triggers the apoptosis pathway by altering the MMP and damaging the respiratory chain.24 Considering the connection between ROS and MMP, it is possible that 1 will elicit ROS production, which permitted loss of MMP. We investigated the effect of 1 on ROS generation, and treated MDA-MB-231 cells were analyzed by flow cytometry using 2,7-dichlorofluoresceindiacetate (DCFH-DA), an oxidation-sensitive fluorescent dye. The right shift in the histogram confirmed increased production of ROS generation in the treated cells (Figure 11). These results provide an evidence that 1 promotes apoptosis by oxidative stress because of production of ROS generation in a dose-dependent manner.
Figure 11.
Intracellular ROS level was measured by flow cytometry analysis using DCFH-DA. (A) MDA-MB-231 cells were treated with indicated concentration of F7 (1) and 0.05% H2O2 and incubated with 5 μM DCFH-DA. (B) Bar representation showing a remarkable increase in cell death with increased generation of intracellular ROS. ***P < 0.001, **P < 0.01 vs control.
Compound 1 Induces G1 Phase Arrest in MDA-MB-231 Cells during Cell Cycle Progression
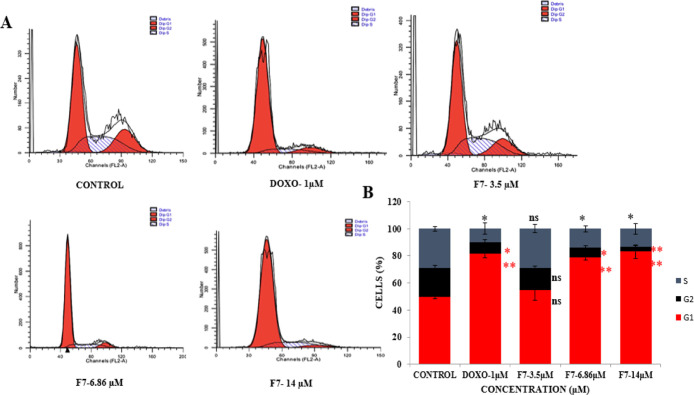
Cell cycle regulation is considered as a powerful strategy for inhibiting cell division in cancer cells, as cancer development is cognate with the cell cycle machinery dysregulation.25 Cell cycle arrest represents a survival mechanism that helps in activation of apoptotic cascade, leading to the cell death and also provides an opportunity for cancer cells to repair its damaged DNA. Therefore, cell cycle phase distribution was analyzed to determine whether the antiproliferative effects of 1 were mediated via cell cycle blockade. MDA-MB-231 cells exposed to 3.5, 6.8, and 14 μM concentration of 1 for 48 h were subjected to flow cytometric analysis. The result showed a distinct dose-dependent G1 arrest in MDA-MB-231 cells as compared to the control cells (Figure 12). The cell cycle evaluation revealed that 1 significantly halted the progression of cells by restricting the G1 cell cycle phase.
Figure 12.
Cell cycle analysis of MDA-MB-231 breast cancer cells. (A) F7 (1)-treated cells at 3.5, 6.8, and 14 μM showed an increased G1 arrest peak. (B) Percent of cells in different phases of the cell cycle are represented using the bar diagram. Data are from three independent experiments. ns-nonsignificant,***P < 0.001, **P < 0.01 vs control. PI fluorescence was measured using a flow cytometer with an FL-2 filter.
Compound 1 Induces the Intrinsic Pathway of Apoptosis in MDA-MB-231 Cells
In order to better understand the mechanism underlying the induction of apoptosis by 1, alteration in protein expression was analyzed by western blotting studies. MDA-MB-231 cells were treated with different concentrations of 1 (3, 7, 14, and 28 μM) for 48 h. Results showed an increased level of caspase-3 and cleaved caspase-3 and concomitant increase in the expression level of cleaved PARP (biomarker of apoptosis); the data were normalized with respect to β-actin (Figure 13). The observed increase in protein expression further confirms that 1 induces apoptosis in TNBC, MDA-MB-231 cells. Based on these findings, 1 induced programmed cell death in MDA-MB-231 cells through the mitochondria-dependent pathway by increased ROS leading to loss of MMP (ΔΨm) with increased expression of apoptosis-linked proteins. ROS production is needed for proper cell migration and chemotaxis, which are tubulin-dependent processes. In vitro assay suggested that an oxidative environment created by 1 inhibited tubulin polymerization. This study emphasized 1 as a persuasive resource of a natural anticancer agent that exhibited antimigratory properties and employed decrease in the number of subdiploid cells at the G1 phase. Therefore, the potential of 1 to suppress migration of MDA-MB-231 cancer cells indicates that this compound may prove as an efficient molecule in inhibiting the proliferation and metastasis of TNBC cancer cells.
Figure 13.

Western blot analysis of apoptosis-related proteins (Parp and caspase-3) after 48 h of treatment with F7 (1) on various concentrations on MDA-MB-231 breast cancer cells. Actin was taken as an internal control.
Experimental Section
General Experimental Procedures
High-resolution mass spectra were obtained on an Agilent 6540 (Q-TOF) mass spectrometer. Optical rotation was measured on a PerkinElmer 341 polarimeter in a 1 dm cell at 25 °C. Column chromatography was performed using silica gel (100–200 mesh). Semipreparative HPLC was performed on an Agilent HPLC with an Ascentis Si (Supelco, USA, 5 μm, 25 cm × 4.6 mm), a photodiode array detector, and autoinjector function (Agilent 1260 series). 1H NMR spectra were recorded (Brucker AVANCE) at 400 MHz and 13C NMR at 100 MHz in CD3OD chemical shifts values were reported in δ (ppm) units and coupling constants values in hertz. Tetramethylsilane (TMS) was used as the internal standard. However, other regents were purchased commercially and used without further purification, unless otherwise stated.
Biological Material Collection and Isolation of the Endophytic Fungus
The fungal endophyte, MBT102, was isolated from the leaves of an aromatic plant, R. damascena, collected from the Yarikha experimental farm (34°04′26.4″N74° 25′43.9″E) of CSIR-IIIM, located in the Kashmir Valley as described previously.37 Morphological characteristics and growth pattern, along with its microscopic structures, were examined. The culture was submitted to the MRCJ repository (WDCM 1117), CSIR-IIIM-Jammu, under the voucher no. MRCJ-800.
Genomic DNA Extraction and Phylogenetic Analysis by ITS1-5.8S-ITS2 Ribosomal Gene Sequencing
The DNA template was prepared from 7 day-old culture grown on PDB using a modification of previously described protocol.38 The ITS region of the fungus was amplified with the universal ITS primers, ITS4 (5′TCCTCCGCTTATTGATATGC3′) and ITS5 (5′GGAAGTAAAAGTCGTAACAA3′) (Sigma-Aldrich), using the polymerase chain reaction (PCR)4a with a slight modification in the PCR mixture by adding 2 μL of dimethyl sulfoxide in the 20 μL reaction. The amplicon was sequenced and aligned with the sequences in the GenBank database via the BLASTN program to find out sequence homology.39 Relevant sequences were downloaded and aligned using the maximum likelihood method based on the Tamura–Nei model40 and a phylogenetic tree was constructed using MEGA6.28
Fermentation, Extraction, and Isolation of Compounds
The endophyte, MBT102, was grown in PDB for 5 days with a constant shaking at 100 rpm. The 20 L volume of fermented whole broth was homogenized with methanol (2 L) to disrupt fungal cells, extracted with ethyl acetate (20 L × 3) and concentrated under reduced pressure. It yielded a total of 27 g of the extract which was further dissolved in H2O/ACN (0.1:1) to form a suspension. The aqueous suspension was successively partitioned with petroleum ether and ethyl acetate to obtain a petroleum ether soluble fraction (3.4 g) and an ethyl acetate soluble fraction (11 g), respectively. The ethyl acetate extract was fractionated over a silica (100–200 mesh) column (CHCl3/MeOH, 1:0/0:1) to yield 24 major fractions, F1–F24. Fraction F-2 (130 mg) was purified over several runs using preparative HPLC using an Ascentis Si (Supelco, USA), particle size 5 μm, dimensions 25 cm × 4.6 mm, mobile phase: acetonitrile (B) and water (A), a flow rate of 20 mL min–1 with a gradient from 20 to 30% B in 10 min, 30 to 45% B in 5 min, 45 to 80% B in 40 min, and 80 to 100% B in 5 min. Although total seven compounds were purified, only compound 1 (20.3 mg, tR = 27.8 min) was major in quantity that was characterized using HRESIMS, 1D, and 2D data analyses.
Compound 1
Yellow amorphous powder; [α]25D +117 (c 0.1, CHCl3); 1H NMR (CDCl3, 400 MHz) and 13C NMR data, see (Table 1); (−) HRESIMS m/z 653.1510 [M – H]− (calculated for C32H29O15–, 653.1512).
Computational Details
Maestro 11.1 was used for generating the starting 3D chemical structures of compounds 1a and 1b.41 Optimization of the 3D structures was performed with Macro model 11.5 using the OPLS force field and the Polak-Ribier conjugate gradient algorithm (PRCG, maximum derivative less than 0.001 kcal/mol).42
The full exploration of the conformational space was performed at the empirical molecular mechanics (MM) level with the Monte Carlo multiple minimum method (50,000 steps), low-mode conformational search method (50,000 steps), and molecular dynamics simulations, the latter was performed at 450, 600, 700, and 750 K, with a time step of 2.0 fs, an equilibration time of 0.1 ns, and a simulation time of 10 ns.
For both stereoisomers 1a and 1b, all the conformers obtained from the conformational searches were minimized (PRCG, maximum derivative less than 0.001 kcal/mol) and compared. The “redundant conformer elimination” module of Macro model 11.5 was used to select nonredundant conformers, excluding those differing more than 21.0 kJ/mol (5.02 kcal/mol) from the most energetically favored conformation and setting a 0.5 Å root-mean-square deviation minimum cutoff for saving structures. The possible hindered rotation along the dimeric tetrahydroxanthenone-related ortho-disubstituted biaryl axis (linking C-2 and C-2′ atoms) was assessed by means of QM calculations (see the Results and Discussion) that were performed using Gaussian 09 software.43
Specifically, the obtained conformers were optimized at the QM level using the MPW1PW91 functional and the 6-31G(d) basis set.44 After this step at the QM level, the optimized geometries were visually inspected in order to remove redundant conformers. First, the possible hindered rotation along the dimeric tetrahydroxanthenone-related ortho-disubstituted biaryl axis was investigated by computing the rotational energy barrier required for the putative interconversion between the M*and P* atropisomers of compound 1a as a representative system. The starting geometry model representing the transition state was built with the two phenyl moieties occupying the same plane that was subsequently optimized at the QM level using the Berny algorithm and the MPW1PW91 functional and the 6-31G(d) basis set, followed by vibrational frequency calculations (TS, CalcAll, Freq keywords for Gaussian calculations). Comparison of the energies between the lowest energy-associated conformer found for 1a and the transition state indicated the absence of the hindered rotation around the ortho-disubstituted biaryl axis (see the Results and Discussion). The computation of the 13C and 1H NMR chemical shifts was performed for compounds 1a and 1b using the MPW1PW91 functional and the 6-31G (d,p) basis set. Final 13C and 1H NMR spectra for each of the stereoisomers were built considering the influence of each conformer on the total Boltzmann distribution taking into account the relative energies. Calibrations of calculated 13C and 1H chemical shifts were performed following the multistandard approach (MSTD).45 Specifically, sp213C and 1H NMR chemical shifts were computed using benzene as a reference compound, sp213C ketone carbonyl NMR chemical shifts were computed using acetone, sp213C ester carbonyl NMR chemical shifts were computed using methyl acetate, while TMS was used for computing sp313C and 1H chemical shift data.
Then, experimental and calculated 13C and 1H NMR chemical shifts were compared computing the Δδ parameter (Tables S1 and S2): Δδ = |δexp – δcalc| where δexp (ppm) and δcalc (ppm) are the 13C/1H experimental and calculated chemical shifts, respectively.
The MAE values for stereoisomers 1a and 1b were computed using the following equation
defined as the summation (∑) of the n computed absolute error values (Δδ), normalized to the number of chemical shifts considered (n) (Tables S1 and S2).
Finally, the absolute configuration of 1 was established through the prediction of ECD spectra for both the enantiomers related to 1a. Specifically, all the conformers previously obtained from the DFT calculations for 1a were accounted for performing QM calculations at the TDDFT (Nstates = 40) MPW1PW91/6-31g (d,p) level in MeOH IEFPCM to reproduce the experimental solvent environment. The final ECD spectra for both the enantiomeric species related to 1a were computed and graphically plotted using SpecDis software,46 considering the influence of each conformer on the total Boltzmann distribution taking into account the relative energies. In order to simulate the experimental ECD curve, a Gaussian band shape function was applied with the exponential half-width (σ/γ) of 0.35 eV. All the QM calculations were performed using Gaussian 09 software.43
Cell Lines, Growth Medium, and Treatment Conditions
MCF-7 and MDA-MB-231 (breast), HT-29 and SW-620 (colon), A549 (lung), PC-3 (prostate), and PANC-1 (pancreatic) cancer cell lines were procured from U.S. National Cancer Institute (NCI, USA) and normal human breast epithelial-FR-2 was procured from EACC. Paclitaxel was used as the positive control and purchased from Sigma-Aldrich, India. All the cells were cultured in complete growth medium (RPMI-1640) supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 units/mL penicillin in a carbon dioxide incubator (New Brunswick, Galaxy 170R, Eppendorf) at 37 °C with 98% humidity and 5% CO2 gas environment. All the biochemicals and reagents used in this study were AR grade and purchased from Sigma-Aldrich.
Cell Inhibition Assay
The SRB assay was performed, in which a cell suspension of optimum cell density (7000–14,000 cells/100 μL) was seeded and exposed to a test material at various concentrations from 1 to 50 μg/mL for extract and 1–50 μM of compound 1 for 24 and 48 h along with the positive controls at 37 °C. Furthermore, fixation of cells was carried out by adding ice-cold trichloroacetic acid for 1 h at 4 °C. After an hour, the plates were washed thrice with distilled water and air-dried. This was followed by the addition of 100 μL of 0.4% SRB dye for half an hour at room temperature. To remove unbound SRB, plates were washed thrice with 1% v/v acetic acid and the remaining cell-bound dye was solubilized by adding 100 μL of 10 mM Tris buffer (pH 10.4) to each well. To solubilize the protein-bound dye completely, the plates were put on a shaker platform for 5 min. The absorbance of the samples was measured at 540 nm using a microplate reader (Thermo Scientific), and IC50 was determined using GraphPad Prism Software Version 5.0.34
In Vitro Clonogenic Cell Survival Assay
Clonogenic cell survival (CFU) assay was performed to determine the ability of MDA-MB-231 cancer cells to form colonies after treatment with F-7 compound using a standard protocol as described previously.14,47 Briefly, 8 × 104/mL cells per well were seeded in each well of a six-well plate. After 24 h, cells were treated with the compound 1 at different concentrations of 3.5, 6.8, and 14 μM and incubated for 14 days. The colonies were fixed in 4% formaldehyde and stained with 0.5% crystal violet (Hi Media #K001-1KT). Colonies of 50 or more cells were counted from three independent experiments and imaged. Clonogenic survival was expressed as the percentage of colony formation by treated cells in comparison with the untreated control cells.
In Vitro Scratch Assay
Wound healing migration was executed to study in vitro directional migration according to a previously described study.35 In brief, MDA-MB-231 cancer cells were plated in a six-well plate to achieve required growth of about ∼70 confluency. The cells were serum-starved for 24 h and confluent monolayer culture was scrape-wounded with a sterile microtip to create a denuded horizontal line with a constant width. After being washed with phosphate-buffered saline (PBS) to remove cellular debris, cells were treated with different concentrations (3.5, 6.8, and 14 μM) of compound 1 for 48 h. MDA-MB-231 cell migration to close the wounded region was monitored and photographed at 0 and 48 h using a photomicroscope at 20× magnification. The length of the wound was determined using ImageJ software (version 1.46), and percentage of wound healing was calculated using the following equation
Morphological Characteristics of the Cell Death Mechanism
MDA-MB-231 breast cancer cells were treated with 3.5, 6.8, and 14 μM concentration of 1 for 48 h. After treatment, cells were photographed using a phase contrast microscope.48 To assess the nuclear morphological studies, samples were stained with DAPI (1 μg/mL), incubated at room temperature for 20 min in the dark, and examined under an inverted fluorescence microscope (Olympus, 1 × 81) at 20× magnification.49
Immunofluorescence Assay
MDA-MB-231 breast cancer cells were cultured on the sterile coverslips in a six-well plate for the detection of the tubulin network for 24 h. Adhered cells were treated with 3.5, 6.8, and 14 μM concentration of compound 1 for 48 h, and paclitaxel at 50 nM was used as a positive control. Cells were fixed with 4% paraformaldehyde for 10 min at room temperature. Furthermore, for the permeabilization, the cells were treated with 0.5% Triton X in 1× PBS for 5 min. Subsequently, the cells were blocked with blocking buffer (10% normal goat serum, 1× PBS) for 20 min at room temperature.50 Coverslips were incubated with the primary antibody, α-tubulin (Sigma), in dilution buffer (1× PBS, 0.1% Triton X-100) for 1 h followed by the fluorochrome-conjugated secondary antibody, Alexa Fluor 488 (Invitrogen), at room temperature for 1 h.34 Cells were washed and counter-stained with DAPI (1 μg/mL in PBS) for 20 min in the dark.51 The coverslips were mounted over glass slides and analyzed under a laser scanning confocal microscope (Olympus Fluoview FV1000) under 20× magnification.49
Scanning Electron Microscopy
For SEM, MDA-MB-231 cells were grown on coverslips with different concentrations of 1 (3.5, 6.8, and 14 μM) for 48 h.52 The cells on the coverslip were fixed immediately in 2.5% glutaraldehyde in phosphate buffer for 1 h at 4 °C, washed with PBS thrice, and postfixed in 1% osmium tetroxide (OsO4) in the same buffer for another hour. After dehydration in a graded ethanol/PBS (35, 50, 75, 90, and 100%) for 5–10 min, the specimen was dried in a critical point drier using CO2 (Balzer’s Union) and coated with gold using a sputter coater (Polaron).53 After cell fixation, images were acquired with a JEOL-100CXII electron microscope with ASID at 40 kV.34
Detection of MMP (Δψm)
MDA-MB-231 cancer cells were exposed to 3.5, 6.8, and 14 μM concentration of 1 and doxorubicin (1 μM) for 48 h. Rhodamine-123 (200 nM), a fluorescent probe used in the estimation of MMP (Ψmt), was added 40 min before termination. Cells were collected immediately, washed with PBS, and studied on a flow cytometer.54
Intracellular ROS Accumulation
The generation of intracellular ROS was measured by flow cytometry using DCFH-DA, an oxidative sensitive probe which gets converted to highly fluorescent 2′,7′-dichlorofluorescin (DCF) in the presence of peroxides.51a MDA-MB-231 breast cancer cells were seeded at a cell density of 1 × 105/mL/well and incubated for 24 h. After incubation, cells were treated with 3.5, 6.8, and 14 μM of compound 1 for 48 h. Following the treatment, the cells were further incubated with 5 μM DCFH-DA for 30 min at 37 °C in the dark. H2O2 was added to the medium 2 h prior to the termination. Then, cells were washed with sterile 1× PBS and fluorescence intensity of DCF was analyzed in the FL-1 channel (excitation λ 488 nm; emission λ 535 nm) using a flow cytometer.55
Cell Cycle Phase Analysis
Cell cycle phase distribution was studied by propidium iodide (PI) staining.56 After cells reach 70–80% confluence, cells were treated with different doses of 1 (3.5, 6.8, and 14 μM) for 48 h. To examine the distribution of MDA-MD-231 in the sub-G1, G0/G1, S, and G2/M phases of the cell cycle, the cells were washed with PBS twice, fixed in 70% ethanol, and stored overnight at 4 °C. The fixed cells were incubated in PI (50 mg/mL) containing RNase A (10 mg/mL) at room temperature in the dark for 30 min.57 Fluorescence intensity was analyzed on a flow cytometer. The percentage of cells in G0, G1, S, and G2 phases was calculated. Results were the representative of three independent experiments with triplicate samples for each assay.
Immunoblotting Assay
The MDA-MB-231 cells were treated with 1 at 3, 7, 14, and 28 μM for 48 h. Equal amount of protein (30–70 mg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and transferred to a polyvinylidene difluoride membrane.15b Nonspecific binding was blocked by incubation with 3% BSA-TBST or 5% nonfat milk-TBST (Tris-buffered saline containing 0.1% Tween-20).55a The membranes were probed with the respective primary antibody for 4 h and washed with TBST thrice. After that, blots were incubated with apoptotic antibodies (PARP and caspase-3) for 1 h and again washed thrice with TBST. Analysis of protein expression was measured using ECL chemiluminescence and the values were normalized to those of β-actin.54
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi, India, through the Major Lab Project, MLP1008 of the institute. S.F. acknowledges the Department of Science and Technology, New Delhi, India, for INSPIRE Senior Research Fellowship. G.B. acknowledges the financial support of MIUR Italy PRIN 2017 project (2017A95NCJ) “Stolen molecules–Stealing natural products from the depot and reselling them as new drug candidates” and a 2014 to 2020 POR CAMPANIA FESR grant from the Regional Council of Campania Region, entitled “Campania OncoTerapie-Combattere la resisten zatumorale: piatta forma integrate multi disciplinare per un approccio tecnologico. We also thank D. Singh for running and processing NMR experiments. We also acknowledge the institutional publication number CSIR-IIIM/IPR/00201.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02505.
1H, 13C NMR, DEPT, HSQC, HMBC, COSY, NOESY, and HRESIMS spectroscopic data of the secalonic acid derivative F-7 (1) (PDF)
Author Present Address
∇ A.A.: CSIR-Traditional Knowledge Digital Library (TKDL), New Delhi 110067, India.
The authors declare no competing financial interest.
Supplementary Material
References
- a Keller N. P.; Turner G.; Bennett J. W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937. 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]; b Strobel G. Harnessing endophytes for industrial microbiology. Curr. Opin. Microbiol. 2006, 9, 240–244. 10.1016/j.mib.2006.04.001. [DOI] [PubMed] [Google Scholar]
- McAlpine J. B.; Bachmann B. O.; Piraee M.; Tremblay S.; Alarco A.-M.; Zazopoulos E.; Farnet C. M. Microbial genomics as a guide to drug discovery and structural elucidation: ECO-02301, a novel antifungal agent, as an example. J. Nat. Prod. 2005, 68, 493–496. 10.1021/np0401664. [DOI] [PubMed] [Google Scholar]
- a Qadri M.; Johri S.; Shah B. A.; Khajuria A.; Sidiq T.; Lattoo S. K.; Abdin M. Z.; Riyaz-Ul-Hassan S. Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. SpringerPlus 2013, 2, 8. 10.1186/2193-1801-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu H.-Y.; Yang F.-L.; Li L.-H.; Rao Y. K.; Ju T.-C.; Wong W.-T.; Hsieh C.-Y.; Pivkin M. V.; Hua K.-F.; Wu S.-H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018, 8, 17956. 10.1038/s41598-018-36411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Arora P.; Wani Z. A.; Nalli Y.; Ali A.; Riyaz-Ul-Hassan S. Antimicrobial Potential of Thiodiketopiperazine Derivatives Produced by Phoma sp., an Endophyte of Glycyrrhiza glabra Linn. Microb. Ecol. 2016, 72, 802–812. 10.1007/s00248-016-0805-x. [DOI] [PubMed] [Google Scholar]; b Potshangbam M.; Devi S. I.; Sahoo D.; Strobel G. A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. 10.3389/fmicb.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri M.; Rajput R.; Abdin M. Z.; Vishwakarma R. A.; Riyaz-Ul-Hassan S. Diversity, molecular phylogeny, and bioactive potential of fungal endophytes associated with the Himalayan blue pine (Pinus wallichiana). Microb. Ecol. 2014, 67, 877–887. 10.1007/s00248-014-0379-4. [DOI] [PubMed] [Google Scholar]
- El-Sayed E. R.; Ahmed A. S.; Abdelhakim H. K. A novel source of the cardiac glycoside digoxin from the endophytic fungus Epicoccum nigrum: isolation, characterization, production enhancement by gamma irradiation mutagenesis and anticancer activity evaluation. J. Appl. Microbiol. 2020, 128, 747–762. 10.1111/jam.14510. [DOI] [PubMed] [Google Scholar]
- Selim K.; Nagia M.; el Ghwas D. E.. Endophytic fungi are multifunctional biosynthesizers: ecological role and chemical diversity. Endophytic Fungi: Diversity, Characterization and Biocontrol; NOVA Science, 2017; p 39. [Google Scholar]
- Nordin N.; Yeap S. K.; Rahman H. S.; Zamberi N. R.; Abu N.; Mohamad N. E.; How C. W.; Masarudin M. J.; Abdullah R.; Alitheen N. B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. 10.1038/s41598-018-38214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61. 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Prado-Vázquez G.; Gámez-Pozo A.; Trilla-Fuertes L.; Arevalillo J. M.; Zapater-Moros A.; Ferrer-Gómez M.; Díaz-Almirón M.; López-Vacas R.; Navarro H.; Maín P. A novel approach to triple-negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci. Rep. 2019, 9, 1538. 10.1038/s41598-018-38364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rashmi K. C.; Harsha Raj M.; Paul M.; Girish K. S.; Salimath B. P.; Aparna H. S. A new pyrrole based small molecule from Tinospora cordifolia induces apoptosis in MDA-MB-231 breast cancer cells via ROS mediated mitochondrial damage and restoration of p53 activity. Chem.-Biol. Interact. 2019, 299, 120–130. 10.1016/j.cbi.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Razak N. A.; Abu N.; Ho W. Y.; Zamberi N. R.; Tan S. W.; Alitheen N. B.; Long K.; Yeap S. K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. 10.1038/s41598-018-37796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Arnold A. E. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. 10.1016/j.fbr.2007.05.003. [DOI] [Google Scholar]; b Tawfike A. F.; Tate R.; Abbott G.; Young L.; Viegelmann C.; Schumacher M.; Diederich M.; Edrada-Ebel R. Metabolomic tools to assess the chemistry and bioactivity of endophytic Aspergillus strain. Chem. Biodiversity 2017, 14, e1700040 10.1002/cbdv.201700040. [DOI] [PubMed] [Google Scholar]
- Yodsing N.; Lekphrom R.; Sangsopha W.; Aimi T.; Boonlue S. Secondary Metabolites and Their Biological Activity from Aspergillus aculeatus KKU-CT2. Curr. Microbiol. 2018, 75, 513–518. 10.1007/s00284-017-1411-y. [DOI] [PubMed] [Google Scholar]
- Xie L.; Li M.; Liu D.; Wang X.; Wang P.; Dai H.; Yang W.; Liu W.; Hu X.; Zhao M. Secalonic Acid-F, a Novel Mycotoxin, Represses the Progression of Hepatocellular Carcinoma via MARCH1 Regulation of the PI3K/AKT/β-catenin Signaling Pathway. Molecules 2019, 24, 393. 10.3390/molecules24030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang W.; Krohn K.; Zia-Ullah U.; Flörke U.; Pescitelli G.; Di Bari L.; Antus S.; Kurtán T.; Rheinheimer J.; Draeger S.; Schulz B. New Mono-and Dimeric Members of the Secalonic Acid Family: Blennolides A–G Isolated from the Fungus Blennoria sp. Chem.—Eur. J. 2008, 14, 4913–4923. 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]; b Guru S. K.; Pathania A. S.; Kumar S.; Ramesh D.; Kumar M.; Rana S.; Kumar A.; Malik F.; Sharma P. R.; Chandan B. K.; Jaglan S.; Sharma J. P.; Shah B. A.; Tasduq S. A.; Lattoo S. K.; Faruk A.; Saxena A. K.; Vishwakarma R. A.; Bhushan S. Secalonic acid-D represses HIF1α/VEGF-mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res 2015, 75, 2886–2896. 10.1158/0008-5472.can-14-2312. [DOI] [PubMed] [Google Scholar]; c Gao X.; Sun H. L.; Liu D. S.; Zhang J. R.; Zhang J.; Yan M. M.; Pan X. H. Secalonic acid-F inhibited cell growth more effectively than 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Neoplasma 2017, 64, 344–350. 10.4149/neo_2017_304. [DOI] [PubMed] [Google Scholar]
- Clardy J.; Walsh C. Lessons from natural molecules. Nature 2004, 432, 829–837. 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- Sieber S. A.; Marahiel M. A. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. 10.1021/cr0301191. [DOI] [PubMed] [Google Scholar]
- Gould K. Antibiotics: from prehistory to the present day. J. Antimicrob. Chemother. 2016, 71, 572–575. 10.1093/jac/dkv484. [DOI] [PubMed] [Google Scholar]
- Vasan N.; Baselga J.; Hyman D. M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Staley J. T.; Castenholz R. W.; Colwell R. R.; Holt J. G.; Kane M. D.; Pace N. R.; Salyers A. A.; Tiedje J. M.. The Microbial World: The Foundation of the Biosphere; American Academy of Microbiology, 1997.; b Sun Y.-Z.; Zhang D.-H.; Cai S.-B.; Ming Z.; Li J.-Q.; Chen X. MDAD: a special resource for microbe-drug associations. Front. Cell. Infect. Microbiol. 2018, 8, 424. 10.3389/fcimb.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Heywood V. H.; Watson R. T.. Global Biodiversity Assessment; Cambridge University Press: Cambridge, 1995; Vol. 1140. [Google Scholar]; b Demain A. L.; Sanchez S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Aly A. H.; Debbab A.; Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]; b Wani Z. A.; Ashraf N.; Mohiuddin T.; Riyaz-Ul-Hassan S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. 10.1007/s00253-015-6487-3. [DOI] [PubMed] [Google Scholar]; c Arora P.; Ahmad T.; Farooq S.; Riyaz-Ul-Hassan S.. Endophytes: A Hidden Treasure of Novel Antimicrobial Metabolites. Antibacterial Drug Discovery to Combat MDR; Springer, 2019; pp 165–192. [Google Scholar]
- Lyakhovich A.; Surrallés J. Constitutive activation of caspase-3 and Poly ADP ribose polymerase cleavage in fanconi anemia cells. Mol. Cancer Res. 2010, 8, 46–56. 10.1158/1541-7786.mcr-09-0373. [DOI] [PubMed] [Google Scholar]
- Kim S. J.; Jegal K. H.; Im J.-H.; Park G.; Kim S.; Jeong H. G.; Cho I. J.; Kang K. W. Involvement of ER stress and reactive oxygen species generation in anti-cancer effect of CKD-516 for lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 685. 10.1007/s00280-020-04043-x. [DOI] [PubMed] [Google Scholar]
- Murad H.; Hawat M.; Ekhtiar A.; AlJapawe A.; Abbas A.; Darwish H.; Sbenati O.; Ghannam A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. 10.1186/s12935-016-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Decker C. C.; Zechner L.; Krstin S.; Wink M. In vitro wound healing of tumor cells: inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. 10.1186/s40360-018-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H.; Qin Y.-x.; Zhou Y.-l.; Tong L.-j.; Lin L.-p.; Geng M.-y.; Duan W.-h.; Ding J. GA3, a new gambogic acid derivative, exhibits potent antitumor activities in vitro via apoptosis-involved mechanisms. Acta Pharmacol. Sin. 2009, 30, 346–354. 10.1038/aps.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K.; Stecher G.; Peterson D.; Filipski A.; Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Andersen R.; Buechi G.; Kobbe B.; Demain A. L. Secalonic acids D and F are toxic metabolites of Aspergillus aculeatus. J. Org. Chem. 1977, 42, 352–353. 10.1021/jo00422a042. [DOI] [PubMed] [Google Scholar]; b Tang X.; Yi Z.; Li N.. Deep-sea-sourced penicillium F11 capable of producing compound secalonic acid F with cytotoxic activity. CN 102408997 A, 2012.
- a Bifulco G.; Dambruoso P.; Gomez-Paloma L.; Riccio R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem. Rev. 2007, 107, 3744–3779. 10.1021/cr030733c. [DOI] [PubMed] [Google Scholar]; b Di Micco S.; Chini M. G.; Riccio R.; Bifulco G. Quantum mechanical calculation of NMR parameters in the stereostructural determination of natural products. Eur. J. Org. Chem. 2010, 2010, 1411–1434. 10.1002/ejoc.200901255. [DOI] [Google Scholar]; c Lauro G.; Bifulco G. Elucidating the Relative and Absolute Configuration of Organic Compounds by Quantum Mechanical Approaches. Eur. J. Org. Chem. 2020, 2020, 3929. 10.1002/ejoc.201901878. [DOI] [Google Scholar]
- a Bringmann G.; Price Mortimer A. J.; Keller P. A.; Gresser M. J.; Garner J.; Breuning M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem., Int. Ed. 2005, 44, 5384–5427. 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]; b Smyth J. E.; Butler N. M.; Keller P. A. A twist of nature - the significance of atropisomers in biological systems. Nat. Prod. Rep. 2015, 32, 1562–1583. 10.1039/c4np00121d. [DOI] [PubMed] [Google Scholar]
- a Bringmann G.; Price Mortimer A. J.; Keller P. A.; Gresser M. J.; Garner J.; Breuning M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem., Int. Ed. 2005, 44, 5384–5427. 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]; b LaPlante S. R.; Edwards P. J.; Fader L. D.; Jakalian A.; Hucke O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 2011, 6, 505–513. 10.1002/cmdc.201000485. [DOI] [PubMed] [Google Scholar]
- a Masullo M.; Bassarello C.; Bifulco G.; Piacente S. Polyisoprenylated benzophenone derivatives from the fruits of Garcinia cambogia and their absolute configuration by quantum chemical circular dichroism calculations. Tetrahedron 2010, 66, 139–145. 10.1016/j.tet.2009.11.034. [DOI] [Google Scholar]; b Liu H.-B.; Lauro G.; O’Connor R. D.; Lohith K.; Kelly M.; Colin P.; Bifulco G.; Bewley C. A. Tulongicin, an Antibacterial Tri-Indole Alkaloid from a Deep-Water Topsentia sp. Sponge. J. Nat. Prod. 2017, 80, 2556–2560. 10.1021/acs.jnatprod.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nadmid S.; Plaza A.; Lauro G.; Garcia R.; Bifulco G.; Müller R. Hyalachelins A–C, Unusual Siderophores Isolated from the Terrestrial Myxobacterium Hyalangium minutum. Org. Lett. 2014, 16, 4130–4133. 10.1021/ol501826a. [DOI] [PubMed] [Google Scholar]; d Cerulli A.; Lauro G.; Masullo M.; Cantone V.; Olas B.; Kontek B.; Nazzaro F.; Bifulco G.; Piacente S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017, 80, 1703–1713. 10.1021/acs.jnatprod.6b00703. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Qadri M.; Sharma P. R.; Kumar A.; Andotra S. S.; Kaur T.; Kapoor K.; Gupta V. K.; Kant R.; Hamid A.; Johri S.; Taneja S. C.; Vishwakarma R. A.; Riyaz-Ul-Hassan S.; Shah B. A. Tubulin inhibitors from an endophytic fungus isolated from Cedrus deodara. J. Nat. Prod. 2013, 76, 194–199. 10.1021/np3006666. [DOI] [PubMed] [Google Scholar]
- Lu M.-K.; Shih Y.-W.; Chang Chien T.-T.; Fang L.-H.; Huang H.-C.; Chen P.-S. α-Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. 10.1248/bpb.33.1685. [DOI] [PubMed] [Google Scholar]
- Koul M.; Meena S.; Kumar A.; Sharma P.; Singamaneni V.; Riyaz-Ul-Hassan S.; Hamid A.; Chaubey A.; Prabhakar A.; Gupta P.; Singh S. Secondary metabolites from endophytic fungus Penicillium pinophilum induce ROS-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Med. 2016, 82, 344–355. 10.1055/s-0035-1558308. [DOI] [PubMed] [Google Scholar]
- Wani Z. A.; Mirza D. N.; Arora P.; Riyaz-Ul-Hassan S. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: An insight into the microbiome of Crocus sativus Linn. Fungal Biol. 2016, 120, 1509–1524. 10.1016/j.funbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Wani Z. A.; Kumar A.; Sultan P.; Bindu K.; Riyaz-Ul-Hassan S.; Ashraf N. Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Sci. Rep. 2017, 7, 8598. 10.1038/s41598-017-08974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.; Madden T. L.; Schäffer A. A.; Zhang J.; Zhang Z.; Miller W.; Lipman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K.; Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Maestro 11.1; Schrödinger, LLC: NY, 2017.
- MacroModel; Schrödinger, LLC: NY, 2017.; b Jorgensen W. L.; Tirado-Rives J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. 10.1021/ja00214a001. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E. J.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford CT, 2009.
- Cimino P.; Gomez-Paloma L.; Duca D.; Riccio R.; Bifulco G. Comparison of different theory models and basis sets in the calculation of 13C NMR chemical shifts of natural products. Magn. Reson. Chem. 2004, 42, S26–S33. 10.1002/mrc.1410. [DOI] [PubMed] [Google Scholar]
- a Sarotti A. M.; Pellegrinet S. C. Application of the Multi-standard Methodology for Calculating 1H NMR Chemical Shifts. J. Org. Chem. 2012, 77, 6059–6065. 10.1021/jo3008447. [DOI] [PubMed] [Google Scholar]; b Sarotti A. M.; Pellegrinet S. C. A Multi-standard Approach for GIAO 13C NMR Calculations. J. Org. Chem. 2009, 74, 7254–7260. 10.1021/jo901234h. [DOI] [PubMed] [Google Scholar]
- Bruhn T.; Schaumlöffel A.; Hemberger Y.; Bringmann G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
- Gupta N.; Qayum A.; Raina A.; Shankar R.; Gairola S.; Singh S.; Sangwan P. L. Synthesis and biological evaluation of novel bavachinin analogs as anticancer agents. Eur. J. Med. Chem. 2018, 145, 511–523. 10.1016/j.ejmech.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Rello S.; Stockert J. C.; Moreno V.; Gämez A.; Pacheco M.; Juarranz A.; Cänete M.; Villanueva A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. 10.1007/s10495-005-6075-6. [DOI] [PubMed] [Google Scholar]
- Sharma N.; Kumar A.; Sharma P. R.; Qayum A.; Singh S. K.; Dutt P.; Paul S.; Gupta V.; Verma M. K.; Satti N. K.; Vishwakarma R. A new clerodane furano diterpene glycoside from Tinospora cordifolia triggers autophagy and apoptosis in HCT-116 colon cancer cells. J. Ethnopharmacol. 2018, 211, 295–310. 10.1016/j.jep.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Hong X.; Li S.; Li W.; Xie M.; Wei Z.; Guo H.; Wei W.; Zhang S. Disruption of protein neddylation with MLN4924 attenuates paclitaxel-induced apoptosis and microtubule polymerization in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2019, 508, 986–990. 10.1016/j.bbrc.2018.12.048. [DOI] [PubMed] [Google Scholar]
- a Ham J.; Lim W.; Bazer F. W.; Song G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. J. Cell. Physiol. 2018, 233, 1638–1649. 10.1002/jcp.26069. [DOI] [PubMed] [Google Scholar]; b Lo Y.-C.; Cormier O.; Liu T.; Nettles K. W.; Katzenellenbogen J. A.; Stearns T.; Altman R. B. Pocket similarity identifies selective estrogen receptor modulators as microtubule modulators at the taxane site. Nat. Commun. 2019, 10, 1033. 10.1038/s41467-019-08965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Singh B.; Sharma P. R.; Bharate S. B.; Saxena A. K.; Mondhe D. M. A novel microtubule depolymerizing colchicine analogue triggers apoptosis and autophagy in HCT-116 colon cancer cells. Cell Biochem. Funct. 2016, 34, 69–81. 10.1002/cbf.3166. [DOI] [PubMed] [Google Scholar]
- Jiang X.-J.; Cretoiu D.; Shen Z.-J.; Yang X.-J. An in vitro investigation of telocytes-educated macrophages: morphology, heterocellular junctions, apoptosis and invasion analysis. J. Trans. Med. 2018, 16, 85. 10.1186/s12967-018-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan S.; Kumar A.; Malik F.; Andotra S. S.; Sethi V. K.; Kaur I. P.; Taneja S. C.; Qazi G. N.; Singh J. A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis 2007, 12, 1911–1926. 10.1007/s10495-007-0105-5. [DOI] [PubMed] [Google Scholar]
- a Pathania A. S.; Guru S. K.; Ul Ashraf N.; Riyaz-Ul-Hassan S.; Ali A.; Abdullah Tasduq S.; Malik F.; Bhushan S. A novel stereo bioactive metabolite isolated from an endophytic fungus induces caspase dependent apoptosis and STAT-3 inhibition in human leukemia cells. Eur. J. Pharmacol. 2015, 765, 75–85. 10.1016/j.ejphar.2015.08.018. [DOI] [PubMed] [Google Scholar]; b Agarwal A.; Kasinathan A.; Ganesan R.; Balasubramanian A.; Bhaskaran J.; Suresh S.; Srinivasan R.; Aravind K. B.; Sivalingam N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species–independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nut. Res. 2018, 51, 67–81. 10.1016/j.nutres.2017.12.011. [DOI] [PubMed] [Google Scholar]
- a Focaccetti C.; Bruno A.; Magnani E.; Bartolini D.; Principi E.; Dallaglio K.; Bucci E. O.; Finzi G.; Sessa F.; Noonan D. M.; Albini A. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PloS One 2015, 10, e0115686 10.1371/journal.pone.0115686. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao J.; Liu Y.; Zhang W.; Zhou Z.; Wu J.; Cui P.; Zhang Y.; Huang G. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle 2015, 14, 3112–3123. 10.1080/15384101.2015.1078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Somasagara R. R.; Hegde M.; Nishana M.; Tadi S. K.; Srivastava M.; Choudhary B.; Raghavan S. C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.