Abstract

A ubiquitous example of DNA and proteins inspires the scientific community to design synthetic systems that can construct various self-assembled complex nano-objects for high-end physiological functions. To gain insight into judiciously designed artificial amphiphilic structures that through self-assembling form various morphological architectures within a single system, herein, we have studied self-aggregation of amide-functionalized surface-active ionic liquids (AFSAILs) with different head groups in the DMSO/water mixed system. The AFSAIL forms stimuli-responsive reversible micelle and vesicle configurations that coexist with three-dimensional (3D) network structures, the organogel in the DMSO/water mixed system. The self-assembly driving forces, self-organization patterns, network morphologies, and mechanical properties of these network structures have been investigated. With the proven biodegradability and biocompatibility, one can envisage these AFSAILs as the molecules with a new dimension of versatility.
Introduction
Self-assembled artificial network structures that can compete with the natural systems such as DNA and proteins in their complexity and functionality are yet to be constructed. Strategically designed amphiphiles that can form various structural architectures including three-dimensional (3D) network structures, that is, gels, make an exciting bottom-up approach for fabricating nano- and microstructured materials with advanced functional properties.1,2 These supramolecular structures have attracted significant attention in various fields including food, cosmetics, removal of toxic pollutants, vehicles for drug delivery, biomineralization, templates to synthesis nanomaterials, as chemo- and biosensors, in solar cells, as catalysts, as scaffolds for tissue engineering, in enzyme immobilization, as optical and electronic components for wearable devices, and oil spill recovery to cite few.3,4 These network structures are formed as a result of various noncovalent interactions including hydrogen bonding, π–π stacking, and van der Waals interactions.5,6 These noncovalent interactions and so the network structure could be rationally designed through judicious selection of various building blocks and through placing them under external stimuli such as pH, temperature, light, solvents, magnetic field, metal ions, and others.7−9
Among the spectrum of available surfactants, ionic surfactants have edge over others because of their ability to form well-organized discrete nano- and microaggregates such as vesicles, wormlike micelles, microtubes, lamellar sheets, and 3D fibrous gels within a single system through ease and their ability to sequester a range of molecules including biological and pharmaceutical ones that can mimic the natural systems.10−15 In an attempt to have novel surfactant structures that have ionic liquid (IL) character, the better known as surface-active ionic liquids (SAILs) are studied for their aggregation behavior in an aqueous medium.16−21 Traditional surfactants, although having similar molecular architectures to ILs, do not fit in this category because of their higher melting points (>100 °C). Several groups including our own group have reported the formation of various structural architectures, viz., micelles, wormlike micelles, lamellar, vesicles, coacervates, and gels through (i) self-assembling of these SAILs in aqueous and nonaqueous media, (ii) synergistic interactions of SAILs with various additives, and (iii) stimuli response.22−28 Among these, solvent-induced aggregation is interesting as it is controlled by a delicate balance of various noncovalent interactions as stated above.5,6 Self-aggregation in these system leads to the formation of various 1D arrays such as micelles and vesicles that through topological entanglement at higher concentrations leads to the formation of 3D soft semisolid-like material also known as organogels.5,6,28,29 The properties and the morphologies of these organogels could be tailored through changing the solvent conditions, that is, polarity, dielectric constant, concentration, and pH for the same SAIL or through the SAIL with a slightly modified structure for the same solvent. Because of their characteristic stimuli-responsive properties, they have found applications in diverse fields and are included in the group of smart materials.30−32
Pursuant to continued research in designing SAILs with tailor-made properties including functionalized SAILs, herein, we had studied aggregation behavior of three amide-functionalized SAILs (AFSAILs) with the same terminal long alkyl side chain (C16) and different head groups (imidazolium, morpholinium, and pyridinium) (Figure 1) in the DMSO/water binary mixture. The AFSAILs used here are 4-methyl-4-(2-(hexadecylamino)-2-oxoethyl)morpholin-4-ium bromide (C16AMorphBr), 3-(2-(hexadecylamino)-2-oxoethyl)-1-methyl-1H-imidazole-3-ium bromide (C16AMeImBr), and 1-(2-(hexadecylamino)-2-oxoethyl)-1-methylpyridin-1-iumbromide (C16APyrBr). These AFSAILs exhibited unprecedented reversible aggregation behavior through the transformation of spherically shaped micellar aggregates into vesicles and then organogels (Figure 1) beyond their respective critical gelation concentrations (CGC). Introducing an amide functionality such as in the case of carboxylic, hydroxyl, ester, and ether within the alkyl chain close to the hydrophilic head group increases biodegradability and biocompatibility, making them better choices against their nonfunctionalized and traditional analogous surfactants.33,34 Because of the intermolecular hydrogen bonding within the AFSAILs, the organization of molecules in the AFSAILs is affected, which substantially improves the thermal stabilities than their ester-functionalized counterparts.33,34 The amide group that is in the vicinity to the head group along with the long alkyl chain (C16) provides flexibility, decreases the counterion binding, and exposes the amide group to water that leads to higher hydration of the head group as compared to its lower alkyl chain counterparts, that is, C8 and C12.34 Self-assembling behavior leading to the formation of distinct micellar structures of C16AMorphBr has been reported. Transformation of the aggregated structures with concentration is yet to be investigated and is one of the prime objectives of this work. Among the range of self-organized structures within the single amphiphilic system, vesicles, because of their special bilayer structures, can mimic the biological membranes.35 Furthermore, vesicles prepared from the cationic surfactants are superior than the nonionic surfactant-based niosomes and lipid-based liposomes because of their simple formulation, chemical stability against hydrolytes, and oxidative degradation in aqueous media and their ability to sequester a range of functional molecules irrespective of their charge, size, and shape.36,37 However, 3D network structures, the gels, because of their unique physicochemical properties, exhibited application in diverse fields from biomedical to electronics. In this work, we have studied the stimuli-responsive morphological structures including vesicles and gels within a single system in the DMSO/water system. Nevertheless, despite the extensive literature on the self-assembly behavior of SAILs in aqueous solution, the research on binary mixtures and that too on the transformation of stimuli-responsive aggregated structures is unavailable.
Figure 1.
Chemical structures of ionic liquids (A) C16AMeImBr, (B) C16AMorphBr, and (C) C16APyBr and (D) stimuli-responsive structural transition.
Results and Discussion
Rationale for the Effect of DMSO on the Micellar Properties of AFSAILs
In the present work, micellization of three AFSAILs, C16AMorphBr, C16AMeImBr, and C16APyBr, has been studied in two different solvent systems, (1) in water and (2) in the binary solvent system of DMSO/water (1:3 v/v). Studied AFSAILs have limited solubility in water, that is, C16AMeImBr, C16AMorphBr, and C16APyBr are soluble up to 2.25, 1.72, and 2.26 mM, respectively, in water. It has been observed that all the three ILs are freely soluble in polar aprotic solvents such as DMSO and DMF, whereas they are soluble in hot alcohols (∼50–55 °C) at and beyond their CGCs. At the selected ratio of study (1:3, DMSO/water), all AFSAILs form clear isotropic solution at and beyond the CGC, that is, 21 mM (10 mg/mL), 27 mM (12 mg/mL), and 31 mM (14 mg/mL) for C16AMorphBr, C16AMeImBr, and C16APyBr at room temperature (RT), respectively. AFSAILs studied herein have identical tails and functionality, but they differ in their head groups, promoting different levels of interfacial and micellar properties in general.
We used surface tension measurement as the tool to investigate the self-assembly in an aqueous medium and found that the cmc obtained is in good agreement with the reported literature (Table 1).34 Herein, hydrophobicity of the head group plays a dominant role in deciding cmc; imidazolium and pyridinium heads having higher hydrophobicity exhibited lower cmc than the morpholinium-based SAIL. Furthermore, it is manifested from cmc data that the cmc of the amide-functionalized SAILs is threefold–fourfold lower than the nonfunctionalized SAILs with the same alkyl chain, whereas somewhat lower than the ester-functionalized SAILs with the same alkyl chain length.38−43 The micellar properties of the studied AFSAILs in DMSO/water systems have not been studied previously and hence not compared. DMSO is a polar aprotic solvent, completely miscible in water and has a dielectric constant less than that of water (εDMSO = 46.7; εwater = 80.4). It is having a partially negatively charged oxygen atom that forms a hydrogen bond with water and breaks the 3D structure of water to form the stoichiometric hydrates close to the ratio 1:3 (1DMSO·2H2O).44 With an increased mass fraction of DMSO in water, the dielectric constant decreases; this eventually increases the electrostatic repulsion between the head groups, decreases the hydrophobic interactions, and causes an increase in cmc (Figure S2; Table 1).44 Surface-active parameters for all the three AFSAILs derived from the surface tension measurement in water and the DMSO/water system are reported in Table S1 along with the nonfunctionalized, ester-functionalized, and traditional surfactants.
Table 1. cmc of the Studied AFSAILs in Water and the DMSO/Water Mixture.
| cmc (mM) | |||
|---|---|---|---|
| solvent | C16AMeImBr | C16AMorphBr | C16APyBr |
| water | 0.18 | 0.20 | 0.18 |
| DMSO/water | 0.35 | 0.29 | 0.24 |
Concentration- and Temperature-Responsive Reversible Structural Transition
Looking at the impact of concentration and temperature on the self-assembling behavior of surfactants in aqueous and nonaqueous media,45−49 we hereby preliminary investigated the concentration- and temperature-induced phase transformation of AFSAILs in the DMSO/water system (Figure S3A) using visual observation and turbidity measurements (at 650 nm). All the three AFSAILs show concentration-dependent phase transition from transparent solution with negligible absorbance to a bluish turbid solution (Tyndall effect) with higher absorbance to the 3D gel with the highest absorbance (Figures S3A and 2). Herein, the gel phase was analyzed by the “tube inversion test”.50−52 The wavelength of absorbance was 650 nm as neither the solvent nor the AFSAILs show any absorbance at this wavelength. Concentrations associated with the first, second, and third transitions through visual observation are reported in Figure S3.
Figure 2.
Turbidity of the AFSAILs as a function of (A) concentration and (B) temperature.
Gel formation was observed in the ester-functionalized SAIL, 3-methyl-1-(hexadecyloxycarbonylmethyl)imidazolium bromide (C16EMeImBr), in the equimolar composition of DMSO/water with a CGC of 8.80% (w/v).25 The lower Lewis basicity of the ester group relative to the amide group and the lower solubility of the later SAIL lead to the formation of organogels at lower concentration.25 The gelation behavior of the three studied AFSAILs in different solvents is reported in Table S2. It was found that the studied AFSAILs form a gel in DMF/water and alcohol/water systems apart from the DMSO/water system (1:3 v/v). It is inferred from the solvatochromic parameters that protic solvents, because of their ability to form a hydrogen bond with the AFSAILs, induce gelation through a delicate balance between the solvent-AFSAIL and AFSAIL–AFSAIL interactions.28,47 For the imidazolium-based amide-functionalized surfactant N-cetyl-N′-acetamidoimidazolium bromide, Cheng et al. reported gelation in polar protic and aprotic solvents with a range of CGCs.48 For the ester-functionalized SAILs, the temperature-responsive ionogel was recently reported with different ranges of CGCs.15
The organogel obtained through varying the concentration of the AFSAILs in the DMSO/water system exhibited unprecedented reversible phase behavior on increasing the temperature from RT (25 °C) to 80 °C, transforming the organogel into a free-flowing bluish turbid liquid, may be of vesicles at 40 °C, and then transparent solution, likely of smaller sized spherical micelles at 80 °C (Figures S3 and 2). The dramatic change in absorbance is associated with the change in aggregate sizes or shapes or both. The phase transition associated with the visual observation and turbidity may be due to the transformation of the spherically shaped micellar aggregates with 18–26 nm sizes to vesicles of sizes of >350 nm to organogels (lamellar shaped), as characterized by small-angle neutron scattering (SANS) measurement, vide infra. It is to be noted here that the CGCs were 10, 12, and 14 mg/mL for C16AMorphBr, C16AMeImBr, and C16APyBr, respectively (Figure S3B–D). Wang et al. reported a similar behavior for the nonfunctionalized SAILs with different alkyl chains, that is, from dodecyl to hexadecyl in an aqueous medium where the micellar aggregates are transformed into vesicles,45 whereas Du et al. observed micelles to vesicles to gel transformation in the binary solvent system.49 Temperature-induced transition data through visual observation and turbidity measurements are further confirmed through the differential scanning calorimetry (DSC) thermogram of the organogel (Figure S4). As shown in the thermogram, the gel to sol transition was observed at 39.30 °C for C16AMorphBr, 37.89 °C for C16APyrBr, and 38.26 °C for C16AMeImBr.
The concentration- and temperature-induced behaviors of the AFSAILs were examined through measuring the surface potential on the aggregated structures for the possible interactions within the amphiphiles. Stability of the colloidal system depends on the repulsive interaction between the charged micellar aggregates that inhibit their aggregation to form larger sized aggregates. This could be characterized through measuring the charge on the colloidal particles through zeta potential (ζ), that is, if ζ is large enough (>30), the system is more stable; conversely, if ζ is small (<30), the system is more pronounced to agglomeration. By decreasing the surface potential, repulsion among the head groups decreases, packing at the air/solution interface increases, and critical concentration for the curvature decreases. This could result in the increased size of the aggregates and probably the shape of the aggregates too. To strengthen our hypothesis from the turbidity and visual observation, we herewith employed ζ of the studied AFSAIL systems throughout the investigated concentration regime. We observed a decrease in ζ as the concentration of all the positively charged AFSAILs increases (Figures 3 and S5). A reverse trend was observed for the ζ values once the temperature was increased from 25 to 80 °C (Figures 3 and S5), confirming the clear correlation between long fibrous 3D structures and surface potential: larger sizes have small surface potentials (vice versa).53,54 It is interesting to mention here that the value of ζ is lower in C16AMorphBr as compared to other two AFSAILs because of the strong AFSAIL–AFSAIL and AFSAIL–solvent interaction in the former (Figures 3 and S5). The morpholinium head group is lacking the aromatic π–π interaction potential and provides the lowest lipophilic interaction potential compared to other two head groups and therefore exhibits the low inhibitory potential. Free electron pairs at the oxygen atom of the morpholinium head group are weak donors or acceptors for π–π interactions. It is more likely that they are involved in strong hydrogen bonding interactions to DMSO/water molecules, making the morpholinium head group even more hydrophilic.
Figure 3.

Zeta potential of the C16AMeImBr system as a function of concentration and temperature.
Compactness and polarity of the colloidal system depend on the pattern of self-assembled structures present in the system, for example, compactness of the system increases on transforming the aggregates from micelles to vesicles to gels. This could be characterized through measuring the ratio of the first and third vibronic bands I1/I3 of pyrene. The cybotactic region of pyrene experiences shift upon the formation of various morphological aggregates, the gel being the most compact with the least I1/I3 and micelles being the least compact, I1/I3 will be the highest among micelles, vesicles, and gels. With increasing concentration of the amphiphile, I1/I3 decreases, indicating that pyrene senses a more hydrophobic environment. This is attributed to the transformation of micellar aggregates from lower concentration to organogels at higher concentration with intermediate vesicular aggregates (Figure 4A).55,56 With increasing temperature, compactness of the system decreases and pyrene gets exposed to the dehydrated alkyl chains that lead to the conformational changes and a concomitant increase in the I1/I3 value, showcasing the structural transformation. It was inferred from the temperature-induced changes (Figure 4B) that pyrene senses a more polar environment, that is, I1/I3 increases with increasing the temperature of the organogel from 25 to 80 °C, suggesting the reversible phase transformation from the organogel to micellar suspension with temperature.54,57I1/I3 values at lower concentration and higher temperature indicate that the polarity of the cybotactic region of pyrene is between those of benzyl alcohol (I1/I3 = 1.22) and ethanol (I1/I3 = 1.18).55 At intermediate concentration and 40 °C, I1/I3 values are close to hydrocarbon solvents (i.e., benzene, I1/I3 = 0.80–1.00),55 whereas at high concentration and RT, that is, at 25 °C, I1/I3 values are close to aromatic hydrocarbon solvents. Among the three AFSAILs studied, I1/I3 decreases in the order C16AMorphBr > C16AMeImBr > C16APyrBr, indicating the higher polar nature of the morpholinium-based AFSAIL.
Figure 4.
I1/I3 values of the studied systems as a function of (A) concentration and (B) temperature.
To reaffirm our results from the abovementioned techniques, SANS measurements have been employed to verify the concentration- and temperature-responsive reversible structural aggregates from a single system, the AFSAILs in the DMSO/D2O solvent system. SANS, an experimental technique that uses elastic neutron scattering at a small scattering angle and measures the differential scattering cross section (dΣ/dΩ) per unit volume as a function of wave vector transfer (Q), gives insight into the organization of microstructures. All the samples have been prepared in DMSO/D2O at a ratio of 1:3 to achieve the needed contrast between the scatterer and solvent. The SANS curves for all the systems as a function of concentration and temperatures with experimental and fitted data are shown in Figures 5, S6, and S7, Tables S3 and S4. Three distinct structural aggregates have been observed based on the varying concentrations of C16AMeImBr at 25 °C (Figure 5A). At lower concentration (3 mg/mL), scattering is less and C16AMeImBr has been found to form spherical micelles (characterized by the core radius). With increasing concentration to 7 mg/mL, the scattering intensity in the low-Q region increases and shows a Q–2 functionality. Modeling shows that the system consists of vesicles, and the absence of any Bragg peak in the data confirms the formation of unilamellar vesicles at this concentration. The measurement of the radius of vesicles is limited by the Qmin of the SANS instrument, and the absence of lower cutoffs in the data indicates that the radius of the vesicle could be much larger than what can be determined from the present Qmin, that is, 2π/Qmin ∼ 350 Å. By increasing the concentration further to 12 mg/mL, the scattering pattern shows higher scattering and a distinct behavior with two correlation peaks. The ratio of peak positions (1:2) at higher q is attributed to the structural transition from vesicles to the lamellar structure (fibrous gel) and suggests that these lamellar structures are made up of tubes composed of polar and nonpolar layers.58,59 On the other hand, the role of temperature in C16AMeImBr, as depicted in Figure 5B, gives an interesting result, where the temperature-dependent SANS measurements have been carried out for a particular concentration system of C16AMeImBr (12 mg/mL). The trend of structural transition from micelle to vesicle to lamellar for increasing concentrations (3–7–12 mg/mL) is found to follow for decreasing temperatures (80 to 40 to 25 °C) too. At 25 °C, C16AMeImBr (12 mg/mL) forms lamellar, then forms vesicles at 40 °C, and transitions to spherical micelles at 80 °C. The SANS data for the concentration- and temperature-dependent behavior for C16AMorphBr and C16APyrBr are shown in Figures S6 and S7, respectively. The fitted parameters for concentration-dependent SANS data for all the AFSAILs are given in Table S3, whereas those from temperature-dependent SANS data are in Table S4. The onset of changes in SANS coincides with the onset of transitions in turbidity, phase behavior, zeta potential, and pyrene fluorescence results, vide supra. Furthermore, the micellar radius for all the three systems follow the order C16APyrBr < C16AMeImBr < C16AMorphBr which signifies the dominant role of head group hydrophobicity.60 Thus, SANS data support the reversible transformation from micelles to vesicles to-gels as a function of concentration and temperature. We have schematically explained the transformation in Scheme 1.
Figure 5.
(A) Concentration- (at 25 °C) and (B) temperature-dependent structural transition for the representative C16AMeImBr system through experimental (O) and fitted (lines) SANS curves.
Scheme 1. Representation of Concentration- and Temperature-Dependent Reversible Transformation.

Formation of the Organogel and Its Morphology and Mechanical Properties
Encouraged by the abovementioned results, we then extended our strategy to gelate the AFSAIL molecules at its CGC by initially dissolving them in DMSO and then gelating through the addition of the cosolvent, water. The strategy was to construct the surfactant-based organogel that can be useful for diverse applications. In a separate set of experiments, we prepared solutions of AFSAILs in respective solvents at their respective CGCs. To this, the cosolvent, water, was added so that the final composition of the mixture was 1:3. The representative gelation in the DMSO/water (1:3) system through employing the experimental condition as stated above is shown in Video I of the manuscript. The organogel thus formed exhibited identical structural characteristic that was observed through concentration-dependent organogel formation.
The organogel morphology was further examined through inverted microscopy, scanning electron microscopy (SEM), atomic force microscopy (AFM), and transmission electron microscopy (TEM). We observed that upon the addition of water into DMSO solution of ILs, the transparent solution was transformed into the fibrous network or 3D gel network (Figure 6A–I; Video I). This is a dynamic process, taking approximately 10 min under controlled conditions of temperature (25 °C) and atmospheric pressure. The SEM images of the gel formed in the representative DMSO/water system show physically cross-linked 3D fibrous networks (Figure 7J–L),61,62 whereas AFM images (Figure 7M–O) show the formation of short sponge-like fibers.63 As shown in AFM images of the organogels (Figure 7M–O), bundles of fibers are linked through physical interactions. A magnified view of these organogels through TEM images shows the presence of the 3D self-assembled fiber network (Figure 7P–R).64 SEM images of the organogels prepared in DMF, methanol, and ethanol are also presented in Figure S8.
Figure 6.

Inverted microscopy images taken during the gelation process (0, 5, and 10 min duration): for C16AMeImBr (A–C), for C16AMorphBr (D–F), and for C16APyBr (G–I).
Figure 7.
SEM (J–L), AFM (M–O), and TEM (P–R) images of C16AMeImBr (J,M,P), C16AMorphBr (K,N,Q), and C16APyBr (L,O,R) organogels.
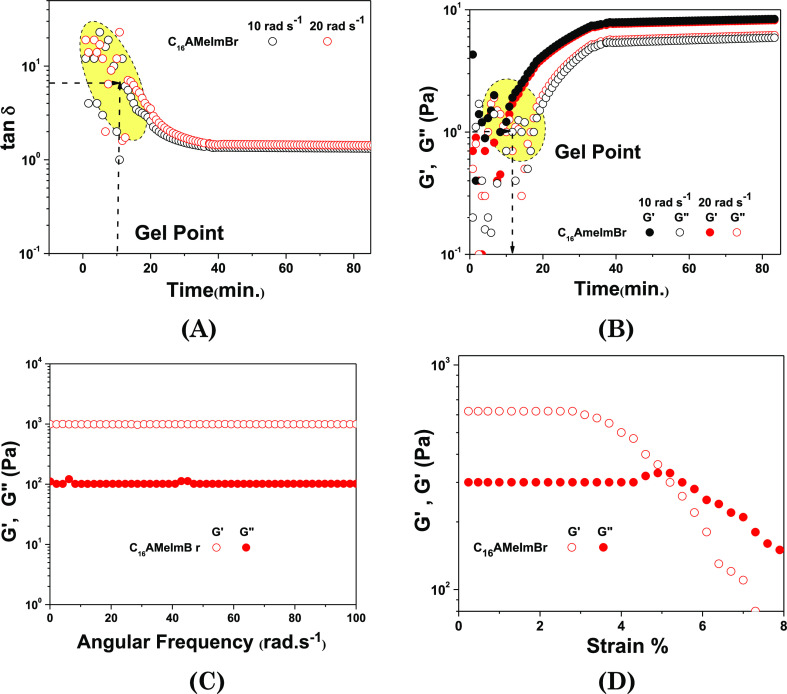
To further elucidate the time-dependent gelation and mechanical properties of the organogel, we resorted to shear rheology experiments. In the main text of the manuscript, we have given data of representative C16AMeImBr organogels. Rest two systems’ data are given in the electronic Supporting Information. Based on Winter–Chambon criteria, shear rheology at a constant strain of 0.1% was performed as the sol to gel transition took place.65 The tan δ for all organogels at both of the frequencies (10 and 20 rad s–1) crossover at ∼10 min (Figures 8A, S9A and S10A), representing a critical point, that is, tan δc, which is the corresponding gel point from where the ratio of G″ and G′ becomes independent of the applied frequency. The scattered data after tan δc, that is, after 10 min, indicate that tan δ decreases with time and then becomes constant, representing the completion of gelation.
Figure 8.
Rheological behavior of C16AMeImBr organogels (in DMSO/water, 1:3 v/v) at their respective CGCs. Evolution of rheological parameters (A) tan δ (B) G′, and G″ as a function of time and storage and loss moduli as a function of (C) frequency and (D) strain.
Furthermore, tan δ was used to estimate the (i) relaxation exponent, n, and (ii) fractal dimension df at the fibrous gel point from the time versus tan δ graph at two frequencies (10 and 20 rad s–1) (Figures 8A, S9A and S10A).65,66 The relaxation exponent is given by tan δ = tan nπ/2, where 0 < n < 1. Based on the n values, three different regions could be assigned of n; 0.5 < n < 1, where G′ < G″; n ∼ 0.5, where G′ ∼ G″; and n < 0.5, where G′ > G″. In our system, we obtain n ≈ 0.84, 0.82, and 0.71 for C16AMorphBr, C16AMeImBr, and C16APyBr, respectively, suggesting the highly elastic nature of the organogels at this point (Figures 8A, S9A and S10A). Fractal dimension (df) was further determined from the relaxation exponent to define the 3D network structure of the organogels studied. A percolation-based theory for the fully screened excluded volume has been proposed to relate n and df using the correlation n = d(d + 2 – 2df)/2(d + 2 – df), where d = 3 for 3D structures.68 Using the abovementioned relationship, df values of C16AMorphBr-, C16AMeImBr-, and C16APyBr-based organogels have been estimated and were found to be 6.49, 6.26, and 5.56, respectively, which attributed to the hyperbranched fibers present in the organogel.
For the peptide-based hydrogels, Yucel et al. observed n = 0.47, whereas for poly(N-isopropylacrylamide)-based microgels, where the gel was formed through physical cross-linking, n was found to be very less, that is, 0.06.67 For the fluorenylmethyloxycarbonyl chloride (Fmoc)-based organogel, n was found to be 0.2, suggesting the reasonable elastic nature of the gel.64 For the Fmoc-based organogel, Hashemnejad and Kundu get 2.32 df, suggesting that branched fibers are present in the gel.69
As shown in Figures 8B, S9B, and S10B, G′ and G″ crossover occurs close to the increasing time corresponding tan δc, where G′ surpasses G″ at ∼10 min and then almost levels off, indicating the formation of the soft-solid-like 3D fibrous gel. The results indicate that the gel point, as determined by the Winter–Chambon criteria, is slightly different from the one defined from the G′–G″ crossover. At this stage, G′ was about an order of magnitude higher than G″, which further confirms the formation of ionic gel or soft-solid-like materials.65 The ionic supramolecular gel also displays an almost frequency-independent nature.
Figures 8C, S9C, and S10C show shear modulus as a function of frequency. Results indicate that for all the organogels, G′ is higher than G″ for the studied frequency range, and they are independent of the frequency that confirms a soft-solid-like behavior. Figures 8D, S9D, and S10D display changes in G′ and G″ as a function of strain. Supramolecular ionic gels display a softening behavior at a low strain value, and they lose their structural integrity once they reach a large strain value or critical strain value (ϒc). Critical strain values for C16AMorphBr, C16AMeImBr, and C16APyBr are 4.8, 5.7, and 6.0%, respectively. The higher ϒc value for C16APyBr is in agreement with the fluorescence data that signify the higher hydrophobicity of the organogel as compared to the imidazolium- and morpholinium-based organogels.
As observed from the SANS measurements, the organogels are made up of the lamellar structures that are made up of tubes composed of polar and nonpolar layers with thicknesses of 1.5–1.9 nm. Furthermore, the thickness of the lamellar sheets is 1 order of magnitude smaller than its d spacing; thus, the fibers of these organogels can be considered relatively stiff. At higher concentration, that is, at CGC, population of the fibers is high, which eventually increases the thickness, but at the same time, an increase in d spacing, albeit small, also occurred. These phenomena lead to higher bending modulus, which in turn leads to the higher shear modulus as observed in several molecular gels where physical cross-linking is the main cause of gelation and the fiber diameter is much smaller than its length.69 In contrast to this, in the polymeric gels, fiber branching and local heterogeneity are not available, which leads to the concentration-dependent structural defects in the molecular gels.
To verify the cause of structural transitions and to understand the AFSAIL–AFSAIL and solvent–AFSAIL interactions during phase transitions, we recorded the FT-IR spectra of the AFSAILs in suspension and the gel form. When the FT-IR spectra of the suspension and organogel samples were prepared in solvent/water, they exhibited peaks at 1650 and 3400 cm–1 which are overlapped with the characteristic bands of the respective AFSAILs.70 To avoid this and get better insights into the cause of structural transition, we replace water with D2O and prepared the gel samples in D2O as the cosolvent. The FT-IR spectra were recorded for the solid AFSAILs and AFSAILs in the solution state (in DMSO) and in the gel state (D2O in IL-DMSO solution) (Figure S10). Peaks associated with the −N–H and −C=O stretching frequencies within the AFSAIL get shifted to the lower frequencies (Table 2) for the AFSAIL solutions in DMSO. The shifts are more prominent in C16AMorphBr as compared to the other two AFSAILs because of strong AFSAIL–solvent interactions. Once D2O was added, the organogel was formed, and the peaks are further shifted to lower frequencies (Table 2). This band shifting to lower frequency (red shift) in gels is attributed to the presence of intermolecular hydrogen bonding between the amide −NH– and carbonyl −C=O groups with the D2O (Figure S11; Table 2). Thus, FTIR results indicate that hydrogen bonding plays a dominant role in the phase transformation from sol to fibrous gel.71
Table 2. Frequency Values for Solid AFSAILs, AFSAILs in the Suspension Form, and Organogels.
| frequency (cm–1) |
||||
|---|---|---|---|---|
| functional group | ILs | solid AFSAILs | AFSAILs in suspension | organogel |
| –N–H | C16AMeImBr | 3442 | 3437 | 3375 |
| C16AMorphBr | 3439 | 3425 | 3273 | |
| C16APyBr | 3445 | 3419 | 3325 | |
| –C=O | C16AMeImBr | 1680 | 1653 | 1649 |
| C16AMorphBr | 1675 | 1651 | 1643 | |
| C16APyBr | 1662 | 1660 | 1641 | |
We further tested whether gelation is disturbed in the presence of a saturated salt solution such as NaCl and acidic or basic medium for its practical application potential.72,73 We observed that the gel remains intact up to 800 mM solution of NaCl, above which the gel was transformed into the viscous solution (Figure 9). Any structural transformation of the organogel in the highly saline medium is above the scope of this manuscript and will be communicated separately. Similarly, the organogel stability was tested in the buffer solutions. It was observed that the gel remains intact in buffer solution with 7.4, whereas at acidic (2–4) and alkaline (10–12) pH, it gets transformed into the viscous solution (Figure 9).
Figure 9.
Stability of organogels with respect to pH and NaCl addition.
Conclusions
In conclusion, AFSAILs exhibited stimuli-responsive reversible self-assembling behavior in the DMSO/water solvent system and formed a variety of nano-objects including 3D interpenetrating network structures. The organogels in the DMSO/water system have proven to be a very attractive method to create nanostructures that are unattainable by a single component previously. The self-assembled structures that change their shapes and sizes based on the stimuli, that is, concentration and temperature, are formed as a result of hydrogen bonding and competitive AFSAIL–AFSAIL and solvent–AFSAIL interactions. The nanostructures can mimic the complexity and functionality of natural systems such as DNA and proteins. The knowledge of structural transition within the single component was used to develop the organogels through the addition of water as the cosolvent in the isotropic solution of SAILs in the aprotic solvent, here DMSO. In principle, the self-assembly approach within the surfactant-based gelators can be extended to other molecular building blocks that can open an entirely new research field with a broad and unexplored range of nanostructures exclusively based on “weak” interactions. In future investigations, our emphasis will be on designing SAILs with tailor-made functionalization that forms nano-objects with unique characteristics. This will serve as a unique and powerful approach toward the formation of novel architectures on demand and can also act as a substitute for conventional ionic surfactants in various applications. A graphical summary of all the results is shown in Figure 10.
Figure 10.
A graphic summary of our results for the three AFSAILs.
Experimental Section
Materials
Hexadecyl amine and bromoacetyl bromide were obtained from TCI and used as received. DMSO, DMF, diethyl ether, acetone, methanol, ethanol, and ethyl acetate were obtained from Sigma-Aldrich. All solvents are of high purity (<∼99%).
Synthesis of C16AMeImBr, C16APyrBr, and C16AMorphBr
2-Bromo-N-hexadecylacetamides were synthesized according to the earlier procedure reported.34 1-Aminohexadecane (25 mmol, 6.02 g) in dichloromethane (30 mL) was added dropwise to a stirred solution of bromoacetyl bromide (27.5 mmol, 5.50 g) in dichloromethane (30 mL) cooled in an ice bath. After 30 min, the ice bath was removed, and the reaction mixture was stirred for another 4 h at RT followed by neutralization with NaOH solution. The dichloromethane layer was separated using a separating funnel and was removed from the crude reaction mixture under reduced pressure in a rotary flash evaporator at 40° to 50 °C. The crude reaction mixture was then washed with 100 mL of warm aqueous ethanol. The lower layer consisting of 2-bromo-N-hexadecylacetamides was allowed to separate in the separating funnel. It was then separated and dried using a vacuum rotary flash evaporator at 80 °C for 30 min.
2-Bromo-N-hexadeylacetamides (10 mmol, 3.61 g) were then reacted with N-methyl morpholine (11 mmol, 1.11 g) or N-methyl imidazole (11 mmol, 0.90 g) or pyridine (11 mmol, 0.86 g) at 80 °C for 1 h. The resulting crude mixture was cooled to 20 °C. The product was washed thrice with 50 mL of diethyl ether and then recrystallized with 30 mL of ethyl acetate to get pure amide group-appended imidazolium-, pyridinium-, and morpholinium-based ILs. The structures of all of these products were confirmed by 1H NMR spectroscopy, Figure S1A–C.
C16AMeImBr: 83% yield; light brown solid; 1H NMR (CDCl3): 0.8 (t, 3H), 1.19–1.27 (m, 26H), 1.6 (m, 2H), 3.4 (t, 2H), 4.4 (s, 3H), 5.6 (s, 2H), 8.1 (s, 1H), 8.6 (s, 1H), 9.1 (1H), 9.4 (s, 1H).
C16AMorphBr: 71% yield; light brown solid; 1H NMR (CDCl3): 0.8 (t, 3H), 1.24–1.66 (s, 26H), 2.2 (m, 2H), 3.6 (m, 2H), 3.7 (s, 3H), 4.1–4.3 (m, 8H), 5.2 (s, 2H), 8.8 (s, 1H).
C16APyrBr: 74% yield; light brown solid; 1H NMR (CDCl3): 0.8 (t, 3H), 1.18–1.28 (m, 26H), 1.6 (m, 2H), 4.2 (t, 2H), 6.0 (s, 2H), 8.1 (m, 2H), 8.6 (m, 1H), 9.2 (1H), 9.6 (d, 2H).
Surface Tension Measurements
Surface tension of the AFSAIL solution was determined using a K9 tensiometer (Krüss) with a platinum–iridium ring at 25.0 ± 0.1 °C. The instrument was calibrated using triple-distilled water.
DSC Measurements
The DSC measurements were performed using a METTLER TOLEDO DSC 1 STARe instrument. The DSC cell was calibrated using indium (mp 156.6 °C; ΔHfusion = 28.42 J/g) and zinc (mp 419.6 °C; ΔHfusion = 112.0 J/g). An amount of 30–40 mg of the organogel samples was introduced into the equipment aluminum “pan” that was heated in a temperature range of 25–55 °C at a heating rate of 1 °C min–1 under a N2 atmosphere.
Steady-State Fluorescence Measurements
A Cary Eclipse fluorescence spectrophotometer (Varian Ltd., U.S.) was used to acquire the steady-state fluorescence spectra. The emission spectra of pyrene, the polarity probe (1 × 10–3 mM), were recorded between 350 and 500 nm at an excitation wavelength of 334 nm. The excitation and emission slit widths were selected to be of 5 nm.
Zeta Potential Measurements
The zeta potential was determined using Horiba SZ-100 equipment with different concentrations and temperature (25, 40, and 80 °C) ranges at BARC, Mumbai, India. Each measurement was repeated at least five times.
SANS Analysis
SANS measurements were performed at BARC, Mumbai, at sample temperatures of 25, 40, and 80 °C. Data are presented as a function of the scattering vector, q
where λ is the incident neutron wavelength and q is the scattering angle. The effective q range obtained for a given experiment is therefore defined by the sample–detector distance and the detector size. Two sample–detector distances of 2 and 14 m were used with an incident wavelength of λ 5 Å (Δλ/λ = 10%), providing a q-range of 0.005–0.400 Å–1. The data were reduced from raw counts on the 2D detector to a radially averaged 1D scattering pattern with the assumption of radially isotropic scattering. The sensitivity of each detector pixel was calibrated by comparison of its response to a flat scatterer, and then, scattering from an empty SANS cell was subtracted. Scattering was then radially averaged (accounting for instrument configuration) to provide the intensity as a function of q. The absolute intensity scale was provided by normalizing each sample by its thickness (1 or 2 mm) and then compared to the scattering from an empty beam measurement. The data modeling program SASView was used to fit the SANS data. SASView software uses standard iterative least-square fitting in which selected parameters of the chosen model can be refined to optimize the fit. Parameters were refined from several starting points to ensure that a global (rather than a local) minimum was found. A prefactor, referred to as the “scale” factor, containing parameters such as the volume fraction and contrast, was left to float, and the value returned by the fit checked against its calculated value to confirm consistency of the fit.
Micelle
where scale is a volume fraction, V is the volume of the scatterer, r is the radius of the sphere, and background is the background level. sld and sld_solvent are the scattering length densities (SLDs) of the scatterer and the solvent, respectively, whose difference is Δρ.
Vesicle
where ϕ is the volume fraction of the shell material, Vshell is the volume of the shell, Vcor is the volume of the core, Vtot is the total volume, Rcore is the radius of the core, Rtot is the outer radius of the shell, ρsolvent is the scattering length density of the solvent (which is the same as for the core in this case), ρscale is the scattering length density of the shell, background is a flat background level (e.g., due to incoherent scattering in the case of neutrons), and j1 is the spherical Bessel function j1=(sin(x) – x cos(x))/x2.
Lamellar Gel
The scattering intensity I(q) is calculated as
The form factor of the bilayer is approximated as the cross section of an infinite, planar bilayer of thickness t (compare the equations for the lamellar model)
ZN(q) describes the interference effects for aggregates consisting of more than one bilayer.
where
and
for the layer spacing distribution  .
.
Noninteger numbers of stacks are calculated as a linear combination of the lower and higher values
Inverted Optical Microscopy
Time-induced gelation behavior was investigated using a Nikon Eclipse TS 100 inverted optical microscope with a high-intensity light-emitting diode eco-illumination system. After the addition of water into IL/DMSO solution, samples immediately were placed between two glass slides at RT, and the time interval gel texture was recorded with a Nikon camera.
Scanning Electron Microscopy
To investigate the surface morphology transformation by water, the 3D microstructures of all three amide-functionalized LMWGs were studied by SEM (Hitachi, S-3400N). Before SEM analysis, gel samples were mounted onto a copper disk using double-sided carbon tape and the surface was sprayed with gold coating.
Transmission Electron Microscopy
A transmission electron microscope operating at 200 KeV was used for internal gel morphology. Carbon-coated copper grids with a mesh size of 200 were placed on the freshly prepared gel for about a minute and were then removed. After carefully removing excess gel from the grid using a filter paper, samples were dried under vacuum for 1–2 h before collecting the images.
Atomic Force Microscopy
Morphological analysis was conducted using a Bruker atomic force microscope operating in the Peak Force mode with a probe. A thin layer (∼1 mm) of a gel was sliced using a razor blade and was then placed on a clean microscope cover glass. The sample was then allowed to dry in a desiccator at RT for at least 1–2 h. AFM scans were performed with a scan rate of 0.5 Hz, at 512 × 512 pixels resolution, and also first-order flattened.
Rheology
Rheological measurements were carried out using a Physica MCR 301 rheometer (Anton Paar). A plate–plate geometry of 49.973 mm diameter and a default gap of 0.4 mm were used. The sol sample was transferred to the rheometer after the addition of water, and a small strain amplitude and 10 and 20 rad s–1 frequencies were applied to study the sol to gel transformation as a function of time. After the formation of the 3D structure or gelation process, frequency sweep experiment (ϒ0 = 0.1%) and strain sweep (frequency = 1 rad s–1) experiments were conducted. All the experiments were performed at least two times, and respective results were displayed.
Fourier Transform Infrared Spectroscopy
A Thermo Scientific Nicolet 6700 FTIR instrument was used to collect spectra at 4 cm–1 resolution by averaging over 64 scans over the range of 4000–600 cm–1. A solution consisting of the AFSAIL in DMSO/D2O (immediately after the addition of D2O) was placed on the liquid sample holder, and time-lapse spectra were collected. To avoid solvent evaporation, the chamber was covered with parafilm. The recorded spectrum was background-subtracted. The FTIR spectra were collected at 25 °C.
Acknowledgments
M.K. acknowledges financial assistance of UGC-DAE for the Collaborative Research Scheme (UDCSR/MUM/AO/CRS-M-276/2017). Naved Malek acknowledges financial assistances through the Department of Science and Technology, New Delhi (SR/FT/CS-014/2010), Institute Research Grants to the Assistant Professors by SVNIT and Council of Scientific and Industrial Research (CSIR), New Delhi (grant no. 01 (2545)/11/EMR-II). Omar El Seoud acknowledges financial support from FAPESP through grant No. FAPESP-2014-22136-4.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02397.
The authors declare no competing financial interest.
Supplementary Material
References
- Li Y.; Wang T.; Liu M. Gelating-Induced Supramolecular Chirality of Achiral Porphyrins: Chiroptical Switch between Achiral Molecules and Chiral Assemblies. Soft Matter 2007, 3, 1312–1317. 10.1039/b710165a. [DOI] [PubMed] [Google Scholar]
- Ajayaghosh A.; Praveen V. K. π-Organogels of Self-Assembled p-Phenylenevinylenes: Soft Materials with Distinct Size, Shape, and Functions. Acc. Chem. Res. 2007, 40, 644–656. 10.1021/ar7000364. [DOI] [PubMed] [Google Scholar]
- Okesola B. O.; Smith D. K. Applying Low-Molecular Weight Supramolecular Gelators in an Environmental Setting-Self-Assembled Gels as Smart Materials for Pollutant Removal. Chem. Soc. Rev. 2016, 45, 4226–4251. 10.1039/c6cs00124f. [DOI] [PubMed] [Google Scholar]
- Chen S.; Tong X.; He H.; Ma M.; Shi Y.; Wang X. Body Temperature Controlled Optical and Thermal Information Storage Light Scattering Display with Fluorescence Effect and High Mechanical Strength. ACS Appl. Mater. Interfaces 2017, 9, 11924–11932. 10.1021/acsami.7b03092. [DOI] [PubMed] [Google Scholar]
- Duan P.; Cao H.; Zhang L.; Liu M. Gelation Induced Supramolecular Chirality: Chirality Transfer, Amplification and Application. Soft Matter 2014, 10, 5428–5448. 10.1039/c4sm00507d. [DOI] [PubMed] [Google Scholar]
- Chen S.; He H.; Tang G.; Wu B.; Ma M.; Shi Y.; Wang X. Topological Structure Influences on the Gel Formation Process and Mechanical Properties of L-Lysine Based Supramolecular Gels. RSC Adv. 2015, 5, 101437–101443. 10.1039/c5ra17991b. [DOI] [Google Scholar]
- Sutton S.; Campbell N. L.; Cooper A. I.; Kirkland M.; Frith W. J.; Adams D. J. Controlled Release from Modified Amino Acid Hydrogels Governed by Molecular Size or Network Dynamics. Langmuir 2009, 25, 10285–10291. 10.1021/la9011058. [DOI] [PubMed] [Google Scholar]
- Vaid Z. S.; Rajput S. M.; Shah A.; Kadam Y.; Kumar A.; El Seoud O. A.; Mata J. P.; Malek N. I. Salt-Induced Microstructural Transitions in Aqueous Dispersions of Ionic-Liquids Based Surfactants. ChemistrySelect 2018, 3, 4851–4858. 10.1002/slct.201800041. [DOI] [Google Scholar]
- Geng H.; Ye L.; Zhang A.-y.; Shao Z.; Feng Z.-g. Ultrasound-Induced Gelation of Fluorenyl-9-Methoxycarbonyl-L-Lysine(Fluorenyl-9-Methoxycarbonyl)-OH and Its Dipeptide Derivatives Showing Very Low Minimum Gelation Concentrations. J. Colloid Interface Sci. 2017, 490, 665–676. 10.1016/j.jcis.2016.11.106. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S.; Samanta S. K. Surfactants Possessing Multiple Polar Heads. A Perspective on their Unique Aggregation Behavior and Applications. J. Phys. Chem. Lett. 2011, 2, 914–920. 10.1021/jz2001634. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kalur G. C.; Ziserman L.; Danino D.; Raghavan S. R. Wormlike Micelles of a C22-Tailed Zwitterionic Betaine Surfactant: From Viscoelastic Solutions to Elastic Gels. Langmuir 2007, 23, 12849–12856. 10.1021/la7028559. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wang P.; Yan Y.; Wang R.; Zhang J.; Dai C.; Hu S. Tuning and Designing the Self-Assembly of Surfactants: The Magic of Carbon Nanotube Arrays. J. Phys. Chem. Lett. 2013, 4, 3962–3966. 10.1021/jz402111h. [DOI] [Google Scholar]
- Shah A.; Kuddushi M.; Ray D.; Aswal V. K.; Malek N. I. Sodium Salicylate Mediated Ionic Liquid Based CatanionicCoacervates as the Membrane Free Microreactors for the Selective Sequestration of Dyes and Curcumin. ChemSystemsChem 2020, 2, e1900029 10.1002/syst.201900029. [DOI] [Google Scholar]
- Smith A. M.; Lovelock K. R. J.; Gosvami N. N.; Licence P.; Dolan A.; Welton T.; Perkin S. Monolayer to Bilayer Structural Transition in Confined Pyrrolidinium-Based Ionic Liquids. J. Phys. Chem. Lett. 2013, 4, 378–382. 10.1021/jz301965d. [DOI] [PubMed] [Google Scholar]
- Kuddushi M.; Patel N. K.; Rajput S.; Mata J.; Kumar A.; El Seoud O. A.; Malek N. I. Temperature Responsive Ionic Liquid Based Gelator: An Approach to Fabricate Anti-Cancer Drug Loaded Hybrid Ionogel. ChemSystemsChem 2020, 10.1002/syst.201900053. [DOI] [Google Scholar]
- Qi L.; Gong Y.; Fang M.; Jia Z.; Cheng N.; Yu L. Surface-Active Ionic-Liquid-Encapsulated Polyoxometalate Nanospheres: Construction, Self-Assembly, Adsorption Behavior, and Application for Dye Removal. ACS Appl. Nano Mater. 2020, 3, 375–383. 10.1021/acsanm.9b02012. [DOI] [Google Scholar]
- Wu A.; Lu F.; Sun P.; Gao X.; Shi L.; Zheng L. Photoresponsive Self-Assembly of Surface Active Ionic Liquid. Langmuir 2016, 32, 8163–8170. 10.1021/acs.langmuir.6b01937. [DOI] [PubMed] [Google Scholar]
- Ao M.; Xu G.; Pang J.; Zhao T. Comparison of Aggregation Behaviors between Ionic Liquid-Type Imidazolium Gemini Surfactant [C12-4-C12im]Br2 and Its Monomer [C12mim]Br on Silicon Wafer. Langmuir 2009, 25, 9721–9727. 10.1021/la901005v. [DOI] [PubMed] [Google Scholar]
- Garcia M. T.; Ribosa I.; Perez L.; Manresa A.; Comelles F. Aggregation Behavior and Antimicrobial Activity of Ester-Functionalized Imidazolium- and Pyridinium-Based Ionic Liquids in Aqueous Solution. Langmuir 2013, 29, 2536–2545. 10.1021/la304752e. [DOI] [PubMed] [Google Scholar]
- Malek N. I.; Vaid Z. S.; More U. U.; El Seoud O. A. Ionic-Liquid-Based Surfactants with Unsaturated Head Group: Synthesis and Micellar Properties of 1-(n-Alkyl)-3-Vinylimidazolium Bromides. Colloid Polym. Sci. 2015, 293, 3213–3224. 10.1007/s00396-015-3746-x. [DOI] [Google Scholar]
- Ali M. K.; Moshikur R. M.; Wakabayashi R.; Moniruzzaman M.; Kamiya N.; Goto M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustainable Chem. Eng. 2020, 8, 6263–6272. 10.1021/acssuschemeng.9b07773. [DOI] [Google Scholar]
- Tourné-Péteilh C.; Coasne B.; In M.; Brevet D.; Devoisselle J.-M.; Vioux A.; Viau L. Surfactant Behavior of Ionic Liquids Involving a Drug : From Molecular Interactions to Self-Assembly. Langmuir 2014, 30, 1229–1238. 10.1021/la404166y. [DOI] [PubMed] [Google Scholar]
- Banerjee C.; Mandal S.; Ghosh S.; Kuchlyan J.; Kundu N.; Sarkar N. Unique Characteristics of Ionic Liquids Comprised of Long-Chain Cations and Anions: A New Physical Insight. J. Phys. Chem. B 2013, 117, 3927–3934. 10.1021/jp4015405. [DOI] [PubMed] [Google Scholar]
- Wang H.; Tan B.; Wang J.; Li Z.; Zhang S. Anion-Based PH Responsive Ionic Liquids: Design, Synthesis, and Reversible Self-Assembling Structural Changes in Aqueous Solution. Langmuir 2014, 30, 3971–3978. 10.1021/la500030k. [DOI] [PubMed] [Google Scholar]
- Shah A.; Kuddushi M.; Rajput S.; El Seoud O. A.; Malek N. I. Ionic Liquids Based Catanionic Coacervates: The Novel Microreactors for Membrane Free Sequestration of Dyes and Curcumin. ACS Omega 2018, 3, 17751–17761. 10.1021/acsomega.8b02455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput S. M.; More U. U.; Vaid Z. S.; Prajapati K. D.; Malek N. I. Impact of Organic Solvents on the Micellization and Interfacial Behavior of Ionic Liquid Based Surfactants. Colloids Surf., A 2016, 507, 182–189. 10.1016/j.colsurfa.2016.08.008. [DOI] [Google Scholar]
- Kuddushi M.; Rajput S.; Shah A.; Mata J.; Aswal V. K.; El Seoud O.; Kumar A.; Malek N. I. Stimuli Responsive, Self-Sustainable, and Self-Healable Functionalized Hydrogel with Dual Gelation, Load-Bearing, and Dye-Absorbing Properties. ACS Appl. Mater. Interfaces 2019, 11, 19572–19583. 10.1021/acsami.9b01129. [DOI] [PubMed] [Google Scholar]
- Kuddushi M.; Patel N. K.; Rajput S.; Shah A.; El Seoud O. A.; Malek N. I. Thermo-Switchable de Novo Ionic Liquid-Based Gelators with Dye-Absorbing and Drug-Encapsulating Characteristics. ACS Omega 2018, 3, 12068–12078. 10.1021/acsomega.8b01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.; Ulijn R. V. Design of Nanostructures Based on Aromatic Peptide Amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. 10.1039/c4cs00247d. [DOI] [PubMed] [Google Scholar]
- Babu S. S.; Praveen V. K.; Ajayaghosh A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. 10.1021/cr400195e. [DOI] [PubMed] [Google Scholar]
- Hirst A. R.; Escuder B.; Miravet J. F.; Smith D. K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem., Int. Ed. 2008, 47, 8002–8018. 10.1002/anie.200800022. [DOI] [PubMed] [Google Scholar]
- Ajayaghosh A.; Praveen V. K.; Vijayakumar C. Organogels as Scaffolds for Excitation Energy Transfer and Light Harvesting. Chem. Soc. Rev. 2008, 37, 109–122. 10.1039/b704456a. [DOI] [PubMed] [Google Scholar]
- Garcia M. T.; Ribosa I.; Perez L.; Manresa A.; Comelles F. Self-Assembly and Antimicrobial Activity of Long-Chain Amide-Functionalized Ionic Liquids in Aqueous Solution. Colloids Surf., B 2014, 123, 318–325. 10.1016/j.colsurfb.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Kamboj R.; Bharmoria P.; Chauhan V.; Singh S.; Kumar A.; Mithu V. S.; Kang T. S. Micellization Behavior of Morpholinium-Based Amide-Functionalized Ionic Liquids in Aqueous Media. Langmuir 2014, 30, 9920–9930. 10.1021/la501897e. [DOI] [PubMed] [Google Scholar]
- Šegota S.; Težak D. Spontaneous Formation of Vesicles. Adv. Colloid Interface Sci. 2006, 121, 51–75. 10.1016/j.cis.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wiesman Z.; Dom N.; Sharvit E.; Grinberg S.; Linder C.; Heldman E.; Zaccai M. Novel Cationic Vesicle Platform Derived from Vernonia Oil for Efficient Delivery of DNA through Plant Cuticle Membranes. J. Biotechnol. 2007, 130, 85–94. 10.1016/j.jbiotec.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Lv H.; Zhang S.; Wang B.; Cui S.; Yan J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Controlled Release 2006, 114, 100–109. 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- El Seoud O. A.; Pires P. A. R.; Abdel-Moghny T.; Bastos E. L. Synthesis and Micellar Properties of Surface-Active Ionic Liquids: 1-Alkyl-3-Methylimidazolium Chlorides. J. Colloid Interface Sci. 2007, 313, 296–304. 10.1016/j.jcis.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Łuczak J.; Hupka J.; Thöming J.; Jungnickel C. Self-Organization of Imidazolium Ionic Liquids in Aqueous Solution. Colloids Surf., A 2008, 329, 125–133. 10.1016/j.colsurfa.2008.07.012. [DOI] [Google Scholar]
- Cornellas A.; Perez L.; Comelles F.; Ribosa I.; Manresa A.; Garcia M. T. Self-Aggregation and Antimicrobial Activity of Imidazolium and Pyridinium Based Ionic Liquids in Aqueous Solution. J. Colloid Interface Sci. 2011, 355, 164–171. 10.1016/j.jcis.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Quagliotto P.; Barbero N.; Barolo C.; Artuso E.; Compari C.; Fisicaro E.; Viscardi G. Synthesis and Properties of Cationic Surfactants with Tuned Hydrophylicity. J. Colloid Interface Sci. 2009, 340, 269–275. 10.1016/j.jcis.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Yue X.; Wang X.; Huang D.; Chen X. Micelle Formation by N-Alkyl-N-Methylpiperidinium Bromide Ionic Liquids in Aqueous Solution. Colloids Surf., A 2012, 412, 90–95. 10.1016/j.colsurfa.2012.07.021. [DOI] [Google Scholar]
- Zhao M.; Zheng L. Micelle Formation by N-Alkyl-N-Methylpyrrolidinium Bromide in Aqueous Solution. Phys. Chem. Chem. Phys. 2011, 13, 1332–1337. 10.1039/c0cp00342e. [DOI] [PubMed] [Google Scholar]
- Das S.; Mondal S.; Ghosh S. Physicochemical Studies on the Micellization of Cationic, Anionic, and Nonionic Surfactants in Water-Polar Organic Solvent Mixtures. J. Chem. Eng. Data 2013, 58, 2586–2595. 10.1021/je4004788. [DOI] [Google Scholar]
- Wang H.; Zhang L.; Wang J.; Li Z.; Zhang S. The First Evidence for Unilamellar Vesicle Formation of Ionic Liquids in Aqueous Solutions. Chem. Commun. 2013, 49, 5222–5224. 10.1039/c3cc41908h. [DOI] [PubMed] [Google Scholar]
- Prieto Kullmer C. N.; Ta D.; Chen C. Y.; Cieker C. J.; Annunziata O.; Dzyuba S. V. Hexadecyl-Containing Organic Salts as Novel Organogelators for Ionic, Eutectic, and Molecular Liquids. ACS Omega 2019, 4, 9400–9406. 10.1021/acsomega.9b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Yan D. Supramolecular Self-Assembly of Giant Polymer Vesicles with Controlled Sizes. Angew. Chem., Int. Ed. 2004, 43, 4896–4899. 10.1002/anie.200460325. [DOI] [PubMed] [Google Scholar]
- Cheng N.; Kang Q.; Xiao J.; Du N.; Yu L. Supramolecular Gels: Using an Amide-Functionalized Imidazolium-Based Surfactant. J. Colloid Interface Sci. 2018, 511, 215–221. 10.1016/j.jcis.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Du P.; Kong J.; Wang G.; Zhao X.; Li G.; Jiang X.; Li Z. Hydrogen Bonded Supramolecular Polymers in Both Apolar and Aqueous Media: Self-Assembly and Reversible Conversion of Vesicles and Gels. Chin. J. Chem. 2011, 29, 2597–2605. 10.1002/cjoc.201100254. [DOI] [Google Scholar]
- Chen X.; Wang M.; Yang X.; Wang Y.; Yu L.; Sun J.; Ding J. Injectable Hydrogels for the Sustained Delivery of a HER2-Targeted Antibody for Preventing Local Relapse of HER2+ Breast Cancer after Breast-Conserving Surgery. Theranostics 2019, 9, 6080–6098. 10.7150/thno.36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Ci T.; Chen L.; Yu L.; Ding J. Salt-Induced Reentrant Hydrogel of Poly(Ethylene Glycol)-Poly(Lactide-Co- Glycolide) Block Copolymers. Polym. Chem. 2014, 5, 979–991. 10.1039/c3py01107k. [DOI] [Google Scholar]
- Xu W.-K.; Tang J.-Y.; Yuan Z.; Cai C.-Y.; Chen X.-B.; Cui S.-Q.; Liu P.; Yu L.; Cai K.-Y.; Ding J.-D. Accelerated Cutaneous Wound Healing Using an Injectable Teicoplanin-Loaded PLGA-PEG-PLGA Thermogel Dressing. Chin. J. Polym. Sci. 2019, 37, 548–559. 10.1007/s10118-019-2212-5. [DOI] [Google Scholar]
- Skoglund S.; Hedberg J.; Yunda E.; Godymchuk A.; Blomberg E.; Odnevall Wallinder I. Difficulties and Flaws in Performing Accurate Determinations of Zeta Potentials of Metal Nanoparticles in Complex Solutions - Four Case Studies. PLoS One 2017, 12, e0181735 10.1371/journal.pone.0181735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D. C.; Winnik M. A. The Py scale of solvent of Solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorscence and empirical correlations with ET and y values. Photochem. Photobiol. 1982, 35, 17–21. 10.1111/j.1751-1097.1982.tb03805.x. [DOI] [Google Scholar]
- Kalyanasundaram K.; Thomas J. K. Environmental Effects on Vibronic Band Intensities in Pyrene Monomer Fluorescence and Their Application in Studies of Micellar Systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. 10.1021/ja00449a004. [DOI] [Google Scholar]
- Vaid Z. S.; Rajput S. M.; Kuddushi M.; Kumar A.; El Seoud O. A.; Malek N. I. Synergistic Interaction between Cholesterol and Functionalized Ionic Liquid Based Surfactant Leading to the Morphological Transition. ChemistrySelect 2018, 3, 1300–1308. 10.1002/slct.201702561. [DOI] [Google Scholar]
- Parmar I. A.; Shedge A. S.; Badiger M. V.; Wadgaonkar P. P.; Lele A. K. Thermo-reversible sol-gel transition of aqueous solutions of patchy polymers. RSC Adv. 2017, 7, 5101–5110. 10.1039/c6ra27030a. [DOI] [Google Scholar]
- Kumari H.; Armitage S. E.; Kline S. R.; Damodaran K. K.; Kennedy S. R.; Atwood J. L.; Steed J. W. Fluorous “ponytails” Lead to Strong Gelators Showing Thermally Induced Structure Evolution. Soft Matter 2015, 11, 8471–8478. 10.1039/c5sm01865j. [DOI] [PubMed] [Google Scholar]
- Kumari H.; Kline S. R.; Kennedy S. R.; Garvey C.; Raston C. L.; Atwood J. L.; Steed J. W. Manipulating three-dimensional gel network entanglement by thin film shearing. Chem. Commun. 2016, 52, 4513–4516. 10.1039/c6cc00171h. [DOI] [PubMed] [Google Scholar]
- Kamboj R.; Bharmoria P.; Chauhan V.; Singh G.; Kumar A.; Singh S.; Kang T. S. Effect of Cationic Head Group on Micellization Behavior of New Amide-Functionalized Surface Active Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 26040–26050. 10.1039/c4cp04054f. [DOI] [PubMed] [Google Scholar]
- Singh Chandel A. K.; Kannan D.; Nutan B.; Singh S.; Jewrajka S. K. Dually crosslinked injectable hydrogels of poly(ethylene glycol) and poly[(2-dimethylamino)ethyl methacrylate]-b-poly(N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965. 10.1039/c7tb00848a. [DOI] [PubMed] [Google Scholar]
- Nguyen P. K.; Gao W.; Patel S. D.; Siddiqui Z.; Weiner S.; Shimizu E.; Sarkar B.; Kumar V. A. Self-Assembly of a Dentinogenic Peptide Hydrogel. ACS Omega 2018, 3, 5980–5987. 10.1021/acsomega.8b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari B.; Palui G.; Banerjee A. Self-Assembling Tripeptide Based Hydrogels and Their Use in Removal of Dyes from Waste-Water. Soft Matter 2009, 5, 3452–3460. 10.1039/b905985g. [DOI] [Google Scholar]
- Friggeri A.; Gronwald O.; van Bommel K. J. C.; Shinkai S.; Reinhoudt D. N. More than Just a Catalyst: A Novel Role for Benzylamine in the Sol–Gel Transcription of Organogels. Chem. Commun. 2001, 2434–2435. 10.1039/b108148a. [DOI] [PubMed] [Google Scholar]
- Winter H. H. Can the Gel Point of a Cross-Linking Polymer Be Detected by the G′ - G″ Crossover?. Polym. Eng. Sci. 1987, 27, 1698–1702. 10.1002/pen.760272209. [DOI] [Google Scholar]
- Zhao Y.; Cao Y.; Yang Y.; Wu C. Rheological Study of the Sol-Gel Transition of Hybrid Gels. Macromolecules 2003, 36, 855–859. 10.1021/ma020919y. [DOI] [Google Scholar]
- Yucel T.; Micklitsch C. M.; Schneider J. P.; Pochan D. J. Direct Observation of Early-Time Hydrogelation in α-Hairpin Peptide Self-Assembly. Macromolecules 2008, 41, 5763–5772. 10.1021/ma702840q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar M. Screening Effect on Viscoelasticity near the Gel Point. Macromolecules 1989, 22, 4656–4658. 10.1021/ma00202a050. [DOI] [Google Scholar]
- Hashemnejad S. M.; Kundu S. Probing Gelation and Rheological Behavior of a Self-Assembled Molecular Gel. Langmuir 2017, 33, 7769–7779. 10.1021/acs.langmuir.7b01531. [DOI] [PubMed] [Google Scholar]
- Venyaminov S.; Prendergast F. Water (H2O and D2O) Molar Absorptivity in the 1000–4000 cm-1 Range and Quantitative Infrared. Anal. Biochem. 1997, 248, 234–245. 10.1006/abio.1997.2136. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang T.; Liu M. Gelating-Induced Supramolecular Chirality of Achiral Porphyrins: Chiroptical Switch between Achiral Molecules and Chiral Assemblies. Soft Matter 2007, 3, 1312–1317. 10.1039/b710165a. [DOI] [PubMed] [Google Scholar]
- Kuddushi M.; Mata J.; Malek N. Self-Sustainable, Self-Healable, Load Bearable and Moldable Stimuli Responsive Ionogel for the Selective Removal of Anionic Dyes from Aqueous Medium. J. Mol. Liq. 2020, 298, 112048. 10.1016/j.molliq.2019.112048. [DOI] [Google Scholar]
- Kuddushi M.; Patel N. K.; Gawali S. L.; Mata J. P.; Montes-Campos H.; Varela L. M.; Hassan P. A.; Malek N. I. Thermo-switchable de Novo Ionogel as Metal Absorbing and Curcumin Loaded Smart Bandage Material. J. Mol. Liq. 2020, 306, 112922. 10.1016/j.molliq.2020.112922. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.