Abstract
Many precedents prove that fluorescent probes are promising candidates for detection of metal ions in the environment and biological systems. Herein, two novel photoinduced electron transfer (PET)-based fluorescent probes, CH3-R6G and CN-R6G, were rationally synthesized by incorporating a triazolyl benzaldehyde moiety into the rhodamine 6G fluorophore. The optical properties of these probes were studied using an ultraviolet–visible (UV–vis) absorption spectrophotometer and a fluorescence spectrophotometer. Through the analysis of the test results, it is concluded that the selectivity and sensitivity of these two probes to Hg2+ are better than to other metal ions (Ag+, Al3+, Ba2+, Cd2+, Co3+, Cu2+, Cr3+, Fe3+, Ga2+, K+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+). According to the standard curve diagram, the detection limits of CH3-R6G and CN-R6G were determined to be 1.34 × 10–8 and 1.56 × 10–8 M, respectively. Reaction of the probes with Hg2+ resulted in a color change of the solution from colorless to pink. The corresponding molecular geometric configuration, orbital electron distribution, and orbital energy of these two compounds were predicted by density functional theory (DFT). The two probes CH3-R6G and CN-R6G have been successfully used for imaging Hg2+ in live breast cancer cells, thereby indicating their great potential for the micro-detection of Hg2+ in vivo.
1. Introduction
The development of multiple heavy metal ion detection sensing technologies is a major undertaking because they are of paramount importance in multiple scientific branches of chemical, biological, electrical, and environmental sciences.1−5 Heavy metal ions are very harmful to the human body and the most harmful are As2+, Cd2+, Cr2+, Hg2+, Pb2+, etc.6−12 These metal compounds cannot be decomposed in water, nor can they complex with other toxins in the water to form more toxic compounds.13−18
Mercury ions are widely found in plastics, mine purification, stains, electrical appliances, and chemicals closely related to our lives. Mercury within the human body mainly affects the central nervous system, the digestive system, and the kidneys, and also has certain effects on the respiratory system, skin, blood, and eyes.19−24 Mercury ions can bind to many negatively charged groups, such as sulfhydryl in proteins or enzymes in the body, which affect many metabolic pathways in the cell, such as energy production and protein and nucleic acid synthesis, thus affecting cell function and growth.25−29 Due to the high toxicity of mercury, countries around the world have imposed strict limits on the amount of Hg2+ in drinking water. For example, the United States EPA stipulates that the limit of Hg2+ in drinking water is 2 μg/L and the limit of Hg2+ in drinking water in China is 1 μg/L. In addition, studies have shown that mercury poisoning can occur when urinary mercury level exceeds 0.05 mg/L. Therefore, development of fluorescent probes that meet the above detection limit criteria for systematic detection of Hg2+ would be highly important.30−37
In this aspect, fluorescent probe detection technology is widely used, as a micro-analysis technology with high sensitivity, selectivity recognition, and a wide measurement range. A fluorescence sensor can perform real-time and on-line detection of single (multiple) objects38,39 and thus overcome the difficulty of cumbersome steps with traditional environmental analysis and monitoring methods. In addition, with the emergence of new detection methods and technologies, it has now become an emerging hot topic of analysis-fluorescence chemical sensor science.40−42 Generally, the design of fluorescent chemical sensors involves a variety of signaling mechanisms, namely photoinduced electron transfer (PET), intramolecular charge transfer, fluorescence resonance energy transfer, and imine isomerization.43−52
Two new Schiff-based fluorescent probes with triazolyl and rhodamine hydrazide were synthesized (Scheme 1), which were CH3-R6G and CN-R6G. These two probes are mainly used for metal ion sensing research. Triazole derivatives have a wide range of biological activities and have good application prospects in the fields of pesticides and medicine. Due to its special multifunctional coordination structure, topological structure, and unique physiological activity, the triazole Schiff-based complex exhibits excellent activity in both antibacterial and antifungal properties. Rhodamine-based molecules are dyes derived from the oxonium moiety as a parent. In addition to excellent photochemical and photophysical properties, the unique “on–off” structure of the spiro lactam ring can occur when metal ions are recognized. Specific electron rearrangement forms a large and stable rigid plane, and fluorescence and color changes occur as a basis for the naked eye recognition during this process. Therefore, these two probes could recognize Hg2+ judiciously and the naked eye selected metal cations such as Ag+, Al3+, Ba2+, Cd2+, Co3+, Cu2+, Cr3+, Fe3+, Ga2+, Hg2+, K+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+ (metal ions used are all cyanogen compounds). The photophysical properties of CH3-R6G and CN-R6G after interaction with metal ions were investigated through both ultraviolet–visible (UV–vis) spectrophotometry and fluorescence spectrophotometry.
Scheme 1. Synthesis of Probes CH3-R6G and CN-R6G.

2. Results and Discussion
2.1. UV–Vis Absorption Spectra
The recognition ability of CH3-R6G and CN-R6G in different metal ion solutions was studied. The research was carried out in a dimethyl sulfoxide (DMSO)/H2O (7:3, v/v, 10 μM) solution of metal ions such as Ag+, Al3+, Ba2+, Cd2+, Co3+, Cu2+, Cr3+, Fe3+, Ga2+, Hg2+, K+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+. As shown in Figure 1a,c, the absorption peaks of CH3-R6G and CN-R6G in the solution of DMSO/H2O (7:3, v/v, 10 μM) exhibited a low energy centered at 536 and 537 nm, respectively. In Figure 1b,d, the piecemeal addition of Hg2+ (0–1.3 equiv) resulted in a distinct increase in the intensity of the absorption. And there was a significant color change of CH3-R6G and CN-R6G from a colorless clear liquid to pink; the addition of other metal ions will not cause significant color changes in the solution. These results proved that the probes CH3-R6G and CN-R6G show high selectivity toward Hg2+ on the UV–vis spectra, the lactam ring opening may be the cause of absorption at 536 and 537 nm, respectively (Figure 2).
Figure 1.
UV–visible absorption spectra of CH3-R6G (a) and CN-R6G (c) in the presence of different metal ions in a solution of DMSO/H2O (7:3, v/v) at 25 °C and (b, d) upon titration with Hg2+ (1 × 10–5 M, 0–1.3 equiv).
Figure 2.
Fluorescence spectra of CH3-R6G (a) (10 μM) and CN-R6G (b) (10 μM) in the presence and absence of different metal ions (10 μM) in DMSO/H2O (7:3, v/v), respectively.
2.2. Fluorescence Spectra
To research the optical properties of probes CH3-R6G and CN-R6G, as shown in the figure, the fluorescence spectra of various metal ions were explored in a mixed solution of DMSO/H2O (7:3, v/v, 10 μM). The excitation wavelength of probes CH3-R6G and CN-R6G was 480 nm (all experiments on fluorescence). Addition of 1.0 equiv of the Fe3+ ion inductive about 120-fold escalation in the fluorescence intensity of probe CH3-R6G at 582 nm, probe CN-R6G was enhanced 50-fold at 578 nm. However, addition of 1.0 equiv of Hg2+ ion inductive about 600-fold escalation in the fluorescence intensity of probe CH3-R6G at 582 nm, probe CN-R6G was enhanced 350-fold at 578 nm. This phenomenon was observed in a very short time. This obvious enhancement indicates that the two probes have a strong affinity with Hg2+. The ability of probes CH3-R6G and CN-R6G to detect Hg2+ is 5-fold that of Fe3+ (Figure 2). These results once again illustrate the high selectivity and specificity of the CH3-R6G and CN-R6G probes for Hg2+. Moreover, as shown in Figure 3a,b, the two probes were particularly stable and did not show a tendency to decrease in the fluorescence intensity within half an hour.
Figure 3.
Photostability experiments of probes CH3-R6G+Hg2+ (a) and CN-R6G+Hg2+ (b).
The sensitivities of probes CH3-R6G and CN-R6G toward Hg2+ were investigated through fluorescence titration experiments as shown in Figure 4. With the addition of the solution to increase the concentration of Hg2+, fluorescence spectra of probes CH3-R6G and CN-R6G showed significant enhancement (Figure 4a,c). This may be due to the coordination reaction between CH3-R6G and CN-R6G toward Hg2+, thereby reducing the PET process and C=N isomerization. Moreover, there is an excellent linear relationship (R1 = 0.99848, R2 = 0.99944) between the fluorescence intensity and Hg2+ concentration (Figure 4b,d). In addition, according to the formula DL = 3σ/k, the detection limit of CH3-R6G toward Hg2+ was calculated to be 1.34 × 10–8 M and that of CN-R6G toward Hg2+ was 1.56 × 10–8 M. These results demonstrate a high sensitivity and its potential application in the synthesis of probes in the detection of Hg2+.
Figure 4.
Fluorescence spectra of CH3-R6G (a) and CN-R6G (c) (10 μM) with the increasing concentration of Hg2+ ions (0–1.3 equiv) in DMSO/H2O (7:3, v/v); the linear relationship between CH3-R6G (b) and CN-R6G (d) with Hg2+ ions.
On addition of more than one equivalent of Hg2+, it was observed that the fluorescence intensity did not continue to increase. This result might testify that the binding stoichiometry of CH3-R6G and CN-R6G to Hg2+ was 1:1. Therefore, the combined stoichiometric ratio between the probe and metal ion is studied by drawing Job’s plot. As shown in Figure 5a,b, for measuring the fluorescence intensity of the solution with a concentration of about 10–5 M shows that CH3-R6G and CN-R6G form a complex with a molar ratio of 1:1 with Hg2+.
Figure 5.
Job’s plot for measuring the stoichiometry of CH3-R6G+Hg2+ (a) and CN-R6G+Hg2+ (b) in a DMSO/H2O (7:3, v/v) solution.
2.3. Competition Experiments
Excellent selectivity is one of the important factors affecting the performance of fluorescent probes. Therefore, to test the probes synthesized by ion competition experiments, a DMSO/H2O mixed solution was used to find the metal ion binding ability; the concentration of the metal used in this experiment is 10–5 M. As shown in Figure 6a,b, addition of Hg2+ to the CH3-R6G and CN-R6G solutions will cause the mixture to have a significant fluorescence enhancement, which will not be affected by the addition of other metal ions in the mixture, indicating that the probes CH3-R6G and CN-R6G have high selectivity.
Figure 6.
Black columns represent the fluorescence intensity of probe CH3-R6G (a) or CN-R6G (b) and various metal ions (1.0 equiv) in solution; the red columns represent the fluorescence intensity of probe CH3-R6G+Hg2+ (a) or CN-R6G+Hg2+ (b) and various metal ions (1.0 equiv) in solution.
2.4. pH Experiments
It has been well documented that pH fluorescent probe detection capability is essential. Thus, a study of the effect of pH on the fluorescence intensity of the probes to evaluate the potential applicability of CH3-R6G and CN-R6G is done, as shown in Figure 7. Clearly, their fluorescence intensity is significantly strong under acidic conditions, while under neutral to alkaline conditions, they were almost non-fluorescent. We speculate that the probe can detect H+ in addition to Hg2+. Therefore, under acidic conditions, probes CH3-R6G and CN-R6G showed strong fluorescence, while under neutral to alkaline conditions, they detected Hg2+ and were unaffected by OH–. Therefore, the probes CH3-R6G and CN-R6G could be applied to detect Hg2+ in neutral to alkaline conditions and could also detect H+. When both H+ and Hg2+ are present, the probe will preferentially detect H+. The probes show a strong fluorescence intensity after opening the loop. Also, when probes CH3-R6G and CN-R6G detect H+, the fluorescence intensity is twice that of the probe detecting Hg2+.
Figure 7.
Fluorescence response of probes CH3-R6G (a) and CN-R6G (b) (10 μM) as a function of pH (pH 1.0–13.0) in the absence and presence of Hg2+ (10 μM), receptively, at room temperature.
2.5. Colorimetric Experiments
The specific recognition of Hg2+ by the probes CH3-R6G and CN-R6G was studied by colorimetric experiments, respectively. As shown in Figure 8a,b, in the DMSO/H2O (7:3, v/v, 10 μM) solution, on adding CH3-R6G and CN-R6G probe solutions to the same concentration of Hg2+, the color of the solution changes instantly from colorless to pink. Therefore, the probes CH3-R6G and CN-R6G can be used to identify Hg2+ with the naked eye, with a concentration as low as 10 μM. However, addition of other metal ions (Ag+, Al3+, Ba2+, Cd2+, Co3+, Cu2+, Cr3+, Fe3+, Ga2+, Hg2+, K+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+) showed almost no color changes in solution, which demonstrated the high specificity of probes CH3-R6G and CN-R6G for detecting Hg2+. To research the practical application of probes CH3-R6G and CN-R6G, we immersed a filter paper in the CH3-R6G or CN-R6G solution and then dried in air. Subsequently, the Hg2+ solution (mM) was applied on the test paper, which caused a significant change in color from colorless to pink. The results are shown in Figure 9a,b. Therefore, the test paper can more conveniently detect Hg2+ solution.
Figure 8.
Color contrast of probes CN-R6G+Hg2+ (a) (10 μM) and CH3-R6G+Hg2+ (b) (10 μM) upon addition of different metal ions (1.0 equiv) in the DMSO/H2O (7:3, v/v) solution.
Figure 9.

Photographs showing the color changes of sensors CH3-R6G (a) and CN-R6G (b) before and after addition of Hg2+ under sunlight.
2.6. Binding Sites
The Fourier transform infrared (FTIR) spectrum of probe CH3-R6G and complex CH3-R6G+Hg2+ are shown in Figure 10a, the peaks at 3180 and 2971 cm–1 suggesting the presence of N–H stretching and C–H stretching and the peak at 1623 cm–1 suggesting the presence of C=N stretching. When Hg2+ is added, the peak of C=N shifted to 1607 cm–1. Similarly, the infrared (IR) spectra of probes CN-R6G and CN-R6G+Hg2+ are shown in Figure 10b, the peaks at 3436 and 3120 cm–1 suggesting the presence of O–H stretching and C–H stretching and the peak at 1621 cm–1 suggesting the presence of C=N stretching. When Hg2+ is added, the peak of C=N shifted to 1609 cm–1.
Figure 10.
FTIR spectra of probes CH3-R6G and CH3-R6G+Hg2+ (a) and CN-R6G and CN-R6G+Hg2+ (b).
To further investigate the probes CH3-R6G and CN-R6G to Hg2+ binding interactions, the 1H NMR spectra of CH3-R6G+Hg2+ and CN-R6G+Hg2+ have been presented in DMSO-d6, as shown in Figure 11a,b, respectively. As seen in Figure 11a, in the spectra of free probe CH3-R6G, the two H signals of amino N–H located at δ 5.08 ppm present a triple peak state. But, after the addition of Hg2+ (1.0 equiv) to the solution of sensor CH3-R6G, the two H proton signals of N–H transform into one H proton signal and it presents a unimodal state. Identical, under the same conditions, upon addition of Hg2+ to the solution of probe CN-R6G, as shown in Figure 11b, the two H proton signals of amino N–H that present a triple peak state transform into one H proton signal and it presents a unimodal state. These data prove that when the probe and metal ions form a complex, the rhodamine ring opens and proton H is hydrolyzed, so the rhodamine monomer shows strong fluorescence.
Figure 11.
1H NMR spectra of probe CH3-R6G only and complex probe CH3-R6G+Hg2+ in DMSO-d6 (a) and probe CN-R6G only and complex probe CN-R6G+Hg2+ in DMSO-d6 (b).
We predicted the possible binding mechanism between the probe and metal ions based on Job’s plot and FTIR, as shown in Scheme 2. For free probe CH3-R6G, the photoelectron transfer (PET) process leads to the fluorescence quenching of rhodamine. Hg2+ was added resulting in a ring-opened lactam block and PET process. To the best of our knowledge, CH3-R6G is likely to chelate with Hg2+ via carbonyl O and amido N atoms resulting in the ring-opening reaction of the spirocyclic moiety.53−59 Similarly, the complexation of Hg2+ with CN-R6G blocks the PET process, thereby causing a strong fluorescence enhancement.
Scheme 2. Proposed Sensing Mechanism of Probe CH3-R6G and Hg2+.
2.7. Theoretical Calculation
Using Gaussian 09 program and density functional theory (DFT), the optimal molecular structure, molecular orbital distribution, and excitation energy of the synthesized probe were calculated at the B3LYP/6-311++g(d,p) level. The optimized structures of probe CH3-R6G and CN-R6G are shown in Figure 12. The energy level, orbital distribution, and orbital energy are listed in Figure 13. The position of the highest occupied molecular orbital (HOMO) electron cloud of the probe CH3-R6G is mainly on the benzene ring, as shown in Figure 13a, the HOMO – 1 and lowest unoccupied molecular orbital (LUMO) electron clouds are scattered on the rhodamine 6G matrix, while the LUMO + 1 electron cloud is almost scattered on the entire structure. They show that the overlap between the electron transitions can be easily achieved. The orbital energy of probe CH3-R6G increased in the order of HOMO – 1 (−0.19389), HOMO (−0.15963), LUMO (−0.04338), and LUMO + 1 (−0.03741) were sequentially increased, also the energies were negative. It is expected that probe CH3-R6G can be easily combined with metal ions. Probe CN-R6G has an electronic conversion similar to probe CH3-R6G. In Figure 13b, the electron clouds of HOMO – 1 and HOMO were delocalized on the fluorescein matrix, LUMO, and LUMO + 1 primary electron cloud located throughout the structure. The orbital energy of probe CN-R6G increased in the order of HOMO – 1(−0.20641), HOMO (−0.17498), LUMO (−0.07746), and LUMO + 1(−0.05481) were in turn increase and negative. It is predicted that the probe may be easily combined to form the CN-R6G metal cation. In addition, we calculated that the difference in the orbital energy between the probes CH3-R6G and CN-R6G is very small. The results are shown in Table 1, which means that electrons can transition easily.
Figure 12.

Optimized molecular structures of CH3-R6G (a) and CN-R6G (b).
Figure 13.

Corresponding orbital electron distribution of probes CH3-R6G (a) and CN-R6G (b).
Table 1. Orbital Energy Differential of CH3-R6G and CN-R6G.
| compound | ΔEH→L (au) | ΔEH→L+1 (au) | ΔEH-1→L (au) |
|---|---|---|---|
| CH3-R6G | 0.11625 | 0.12222 | 0.15051 |
| CN-R6G | 0.09752 | 0.12017 | 0.12895 |
2.8. Cell Culture and Live Cell Imaging with CH3-R6G and CN-R6G
The live cell imaging of probes CH3-R6G and CN-R6G was carried out on MCF-7 cell lines. The cells were grown in 75 cm2 culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified chamber at 5% CO2 and 37 °C. The in vitro cytotoxicity of the probes CH3-R6G and CN-R6G was determined by the MTT assay (Figures 14 and 15). Briefly, MCF-7 cells were seeded in 96-well cell culture plates and allowed to adhere overnight. The probes CH3-R6G and CN-R6G were dissolved in DMSO and the required concentration for the study was made with RPMI-1640; the final concentration of DMSO did not exceed 1%. Probes CH3-R6G and CN-R6G were exposed to cells in the medium for 24 h. The cells treated with DMSO without probes CH3-R6G and CN-R6G were considered as the control. The MTT assay was performed as per the previous method and the absorbance of formazan crystals was determined at 670 nm using a plate reader and the attained results were stated with respect to the control (100%). The live cell imaging of probes CH3-R6G and CN-R6G that were grown in 6-well plates in the medium was performed. The probes CH3-R6G and CN-R6G were treated with cells in the medium for 30 min and washed with a phosphate buffer saline of pH 7.4 thrice to remove the excess of probes CH3-R6G and CN-R6G (Figures 14 and 15). Following, the cells were further incubated with Hg2+ (10 μM) for 30 min. The images were captured using phase-contrast and green fluorescent protein (GFP) filters using an inverted fluorescence microscope (EVOS FLC, Thermo Fisher Scientific).
Figure 14.
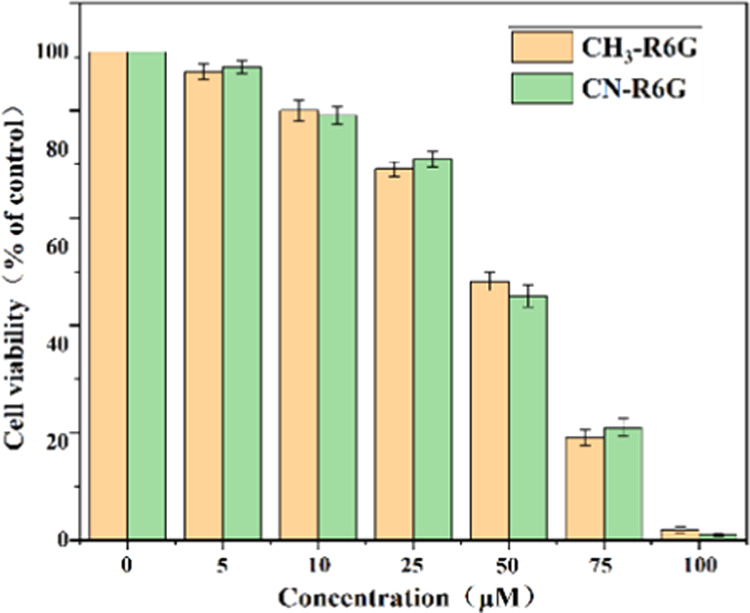
Cell viability graph of CH3-R6G and CN-R6G using Michigan Cancer Foundation-7 (MCF-7) cells by the MTT assay.
Figure 15.
Fluorescence images of MCF-7 cells treated with CH3-R6G (b) and CN-R6G (f). Bright-field images (a, e). Fluorescence images of MCF-7 cells treated with CH3-R6G+Hg2+ (d) and CN-R6G+Hg2+ (h). Bright-field images (c, g).
3. Conclusions
In summary, two kinds of novel fluorescent probes were synthesized and proved to have high applicability, low detection limit and easy-to-handle. The properties of the probes were predicted by theoretical calculation, their UV–vis and fluorescence properties were also investigated and the results demonstrated that the probes have specificity for Hg2+ over other detected metal ions based on the PET mechanism, respectively. In addition, these two probes are extremely resistant to interference from other metal ions, can visually detect metal ions from white to pink in solution or test paper. The probes were successfully employed in fluorescence imaging of Hg2+ in MCF-7 cells showing applications of the probe for biological-Hg2+ detection.
4. Experimental Section
4.1. Materials and Equipment
The solvents and chemicals used in the experiment can be obtained from commercial sources and are of analytical grade. (3-Methyl-1H-1,2,4-triazole, 3-cyano-1,2,4-triazole, and rhodamine 6G were purchased from Macklin.) 1H NMR (400 MHz) and 13C NMR (400 MHz) spectra were measured on a Bruker AV-400 spectrometer with DMSO-d6 used as the solvent and tetramethylsilane (TMS) used as the internal standard. The KBr precipitation technique was used to perform infrared measurements on a Bruker α FTIR spectrometer in the 4000–400 cm–1 region. The Agilent 6510 precision mass Q-TOF LC/MS system was used to perform high-resolution mass spectrometry (HRMS). Fluorescence spectra were recorded on a Hitachi F-4600 fluorescence spectrophotometer at a scan rate of 2400 nm/min. Ultraviolet–visible absorption was measured on a SHIMADZU UV-2600. All experiments were performed at room temperature.
4.2. Synthesis
4.2.1. Synthesis of Compound 1
3-Methyl-1H-1,2,4-triazole (1.70 g, 20 mmol), p-fluorobenzaldehyde (2.49 g, 20 mmol), and anhydrous potassium carbonate (3.44 g, 25 mmol) were dissolved in 100 mL of DMF. The mixture was stirred and reacted at 100 °C for 24 h. After the reaction was cooled to room temperature, 300 mL of ice water was added. The mixture was stirred for another 4 h to yield a yellow precipitate, which was subjected to filtration, and the filtrate was recrystallized with ethyl acetate and dried in vacuo to give compound 1 as a white solid (3.07 g, yield 82%); IR (KBr): 3104, 1695, 1608, 1315, 1211, 833 cm–1; 1H NMR (DMSO-d6, 400 MHz) δ 10.03 (s, 1H, −CHO), 9.33 (s, 1H, triazolyl-H), 8.07 (s, 4H, ArH), 2.39 (s, 3H, −CH3); 13C NMR(DMSO-d6, 100 MHz): δ 191.9, 161.7, 143.3, 140.8, 134.7, 131.2, 124.4, 119.0, 13.6, 13.2; ESI-TOF HRMS (m/z): calcd for C10H9N3O, [M + H]+, 188.0746; found, 187.0980.
4.2.2. Synthesis of Compound 2
3-Cyano-1,2,4-triazole (1.88 g, 20 mmol), p-fluorobenzaldehyde (2.49 g, 20 mmol), and anhydrous potassium carbonate (3.44 g, 25 mmol) were dissolved in 100 mL of DMF. The mixture was stirred and reacted at 100 °C for 24 h. After the reaction was cooled to room temperature, 300 mL of ice water was added. The mixture was stirred for another 4 h to yield a yellow precipitate, which was subjected to filtration, and the filtrate was recrystallized with ethyl acetate and dried in vacuo to give compound 2 as a white solid (3.49 g, yield 88%); IR (KBr): 3110, 2256, 1702, 1604, 1334, 1207,835 cm–1; 1H NMR (DMSO-d6, 400 MHz): δ 10.08 (s, 1H, −CHO), 9.79 (s, 1H, triazolyl-H), 8.15 (s, 4H, ArH); 13C NMR(DMSO-d6, 100 MHz): δ 163.8, 151.0, 147.8, 128.0, 120.5, 118.3, 104.9, 37.4, 16.9, 14.1; ESI-TOF HRMS (m/z): calcd for C10H6N4O, [M + H]+,199.0542; found, 198.0586.
4.2.3. Synthesis of Compound 3
Rhodamine 6G (11.98 g, 25 mmol) and hydrazine (2.55 g, 50 mmol) were placed in a flask and anhydrous ethanol was added as the solvent. The reaction mixture was stirred at 78 °C until the fluorescence disappeared and a solid precipitate was formed. When no new precipitates are produced, the mixture was filtered and the filtrate was washed with ethanol and dried under vacuum to give compound 3 as a colorless solid (9.42 g, yield 88%); IR (KBr): 3417, 3101, 2966, 1714, 1622, 1519, 1467, 1276 cm–1; 1H NMR (DMSO-d6, 400 MHz): δ 7.77–7.73 (m, 1 H, ArH), 7.49–7.42 (m, 2H, −NH2), 6.95–6.90 (m, 1H, ArH), 6.26 (s, 2H, ArH), 6.10 (s, 2H, ArH), 4.99 (t, J = 5.3 Hz, 2H, ArH), 4.21 (s, 2H, ArH), 3.13 (p, J = 6.8 Hz, 4H, −CH2−), 1.88 (d, J = 9.9 Hz, 6H, −CH3), 1.21 (t, J = 7.0 Hz, 6H, −CH3); 13C NMR (DMSO-d6, 100 MHz): δ 165.2, 152.1, 151.31, 147.3, 132.2, 129.5, 128.0, 127.0, 123.4, 122.1, 117.8, 105.0, 95.9, 65.0, 37.4, 17.0, 14.1; ESI-TOFHRMS (m/z): calcd for C26H28N4O2, [M + H]+, 429.5261; found, 428.5263.
4.2.4. Synthesis of CH3-R6G
Compound 1 (1.87 g, 10 mmol), compound 3 (4.28 g, 10 mmol), and glacial acetic acid were placed in a flask and DMF was added as the solvent. The reaction was stirred at 100 °C for 5 h. Then, a large amount of ice water was added to the solution, which resulted in the precipitation of some pink solids. The solid was resuspended with acetone, crystallized, and dried under high vacuum to give CH3-R6G as a light pink solid (4.05 g, yield 68%); IR (KBr): 2967, 2859, 1716, 1693, 1619, 1307, 1216, 842 cm–1; 1H NMR (DMSO-d6, 400 MHz): δ 9.15 (s, 1H, −CH=N−), 8.71 (s, 1H, ArH), 7.92 (d, J = 7.2 Hz, 1H, ArH), 7.80 (d, J = 8.4 Hz, 2H, imidazole-H), 7.63–7.51 (m, 4H, imidazole-H), 7.05 (d, J = 7.3 Hz, 1H, ArH), 6.34 (s, 2H, ArH), 6.18 (s, 2H, −NH2), 5.07 (t, J = 5.0 Hz, 2H, −NH−), 3.17–3.10 (m, 4H, −CH2−), 2.34 (s, 3H, −CH3), 1.84 (s, 6H, −CH3), 1.20 (t, J = 7.1 Hz, 6H, −CH3). 13C NMR (DMSO-d6, 100 MHz): δ 163.9, 151.4, 151.2, 147.8, 142.6, 140.1, 133.5, 128.8, 128.3, 126.8, 123.8, 123.0, 119.1, 118.2, 105.0, 95.8, 65.6, 37.5, 18.5, 14.1; ESI-TOFHRMS (m/z): calcd for C36H35N7O2, [M + H]+, 598.2852; found, 597.3959.
4.2.5. Synthesis of CN-R6G
Compound 2 (1.98 g, 10 mmol), compound 3 (4.28 g, 10 mmol), and glacial acetic acid were placed in a flask and hot ethanol was added as the solvent. The reaction was stirred at 100 °C for 5 h. Then, a large amount of ice water was added to the solution, which resulted in the precipitation of some buff solids. The solid was resuspended with acetone, crystallized, and dried under high vacuum to give CN-R6G as a buff solid (4.32 g, yield 71%); IR (KBr): 3438, 2971, 1691, 1637, 1623, 1371, 1201, 836 cm–1; 1H NMR (DMSO-d6, 400 MHz): δ 9.62 (s, 1H, −CH=N−), 8.77 (s, 1H, triazolyl-H), 7.95–7.85 (m, 3H, ArH), 7.65–7.54 (m, 4H, ArH), 7.05 (d, J = 7.3 Hz, 1H, ArH), 6.33 (s, 2H, ArH), 6.17 (s, 2H, −NH2), 5.06 (t, J = 5.1 Hz, 2H, −NH−), 3.16–3.09 (m, 4H, −CH2−), 1.83 (s, 6H, −CH3), 1.19 (t, J = 7.0 Hz, 6H, −CH3). 13C NMR (DMSO-d6, 100 MHz): δ 164.4, 151.8, 151.5, 148.3, 145.4, 139.1, 134.5, 129.3, 128.5, 127.3, 124.4, 123.6, 121.0, 118.8, 105.4, 96.2, 66.2, 38.0, 17.5, 14.6; ESI-TOFHRMS (m/z): calcd for C36H32N8O2, [M + H]+, 609.2648; found, 608.2765.
4.3. Preparation of Metal Ion Solutions for UV–Vis and Fluorescence Studies
Stock solutions of probes CH3-R6G and CN-R6G (1 mM) were also prepared in DMSO. Stock solutions (1 mM) of the cyanide salts of various metal ions, such as Ag+, Al3+, Ba2+, Cd2+, Co3+, Cu2+, Cr3+, Fe3+, Ga2+, Hg2+, K+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+ ions, in distilled water were prepared. Prepare a test solution by putting 1 mL of the probe stock solution into a test tube, adding an aliquot of each metal stock solution, and diluting the solution to 100 mL with DMSO and distilled water.
4.4. Theoretical Calculation
Gaussian 09 program and density functional theory (DFT) were used to calculate the optimal molecular structure of the synthesized probe on the B31YP/6-311++g(d,p) level and the distribution of the excitation energy molecular orbital.
4.5. Synthesis of Cell culture
Michigan Cancer Foundation-7 (MCF-7) cells were purchased from the Korean Cell Line Bank (Seoul, Korea). MCF-7 cells were cultured in a Dulbecco’s modified Eagle’s medium (DMEM, high glucose) supplemented with 10% fetal bovine serum. All mediums contain 100 U/mL penicillin and 100 U/mL streptomycin and the cells were kept under 5% CO2 at 37 °C.
Acknowledgments
The work was supported by the Shandong Provincial Natural Science Foundation of China (ZR2017LC005, ZR2018PB007) and the Major Science and Technology Innovation Project of Shandong Province (2018 CXGC1107).
The authors declare no competing financial interest.
References
- Zhang X.; Wang B.; Xiao Y.; Wang C.; He L. Targetable, Two-photon Fluorescent Probes for Local Nitric Oxide Capture in the Plasma Membranes of Live Cells and Brain Tissues. Analyst 2018, 143, 4180–4188. 10.1039/C8AN00905H. [DOI] [PubMed] [Google Scholar]
- Chen M.; Zhang F.; Wang Y.; Zhang X.; Li X.; Zhang C.; Zhao H. Synthesis and Application of Ratio Fluorescence Probe for Chloride. Anal. Bioanal. Chem. 2018, 410, 6507–6516. 10.1007/s00216-018-1250-0. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Wang X.; Xu L. Autofluorescent Hyperbranched Poly(amide amine) as Effective Fluorescent Probe for Label-free Detection of Copper(II) Ions. Anal. Sci. 2017, 33, 1345–1350. 10.2116/analsci.33.1345. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang F.; Ji Y.; Wei T.; Qiang J.; Ren X.; Shin I.; Yoon J. Recent Progress in the Development of Fluorescent, Luminescent and Colorimetric Probes for Detection of Reactive Oxygen and Nitrogen Species. Chem. Soc. Rev. 2016, 45, 2976–3016. 10.1039/C6CS00192K. [DOI] [PubMed] [Google Scholar]
- Song F.; Yang C.; Shao X.; Du L.; Zhu J.; Kan C. A Reversible “Turn-off-on” Fluorescent Probe for Real-time Visualization of Mercury(II) in Environmental Samples and its Biological Applications. Dyes Pigm. 2019, 165, 444–450. 10.1016/j.dyepig.2019.02.054. [DOI] [Google Scholar]
- Moriuchi-Kawakami T.; Mizuno Y.; Inoue T.; Matsubara S.; Moriuchi T. A C3-substituted Cyclotriveratrylene Derivative with 8-Quinolinyl groups as a Fluorescence-enhanced Probe for the Sensing of Cu2+ ions. Analyst 2019, 144, 1140–1146. 10.1039/C8AN01615A. [DOI] [PubMed] [Google Scholar]
- Safir Filho M.; Dao P.; Gesson M.; Martin A. R.; Benhida R. Development of Highly Sensitive Fluorescent Probes for the Detection of β-Galactosidase Activity-application to the Real-time Monitoring of Senescence in Live Cells. Analyst 2018, 143, 2680–2688. 10.1039/C8AN00516H. [DOI] [PubMed] [Google Scholar]
- Yang L.; Niu J. Y.; Sun R.; Xu Y. J.; Ge J. F. Rosamine with Pyronine-Pyridinium Skeleton: Unique Mitochondrial Targetable Structure for Fluorescent Probes. Analyst 2018, 143, 1813–1819. 10.1039/C7AN02041D. [DOI] [PubMed] [Google Scholar]
- Ogasawara A.; Kamiya M.; Sakamoto K.; Kuriki Y.; Fujita K.; Komatsu T.; Ueno T.; Hanaoka K.; Onoyama H.; Abe H.; Tsuji Y.; Fujishiro M.; Koike K.; Fukayama M.; Seto Y.; Urano Y. A Red Fluorescence Probe Targeted to Dipeptidylpeptidase-IV for Highly Sensitive Detection of Esophageal Cancer. Bioconjugate Chem. 2019, 30, 1055–1060. 10.1021/acs.bioconjchem.9b00198. [DOI] [PubMed] [Google Scholar]
- Baig M. Z. K.; Pawar S.; Tulichala R. N. P.; Nag A.; Chakravarty M. A Single Fluorescent Probe as Systematic Sensor for Multiple Metal Ions: Focus on Detection and Bio-imaging of Pd2+. Sens. Actuators, B 2017, 243, 226–233. 10.1016/j.snb.2016.11.116. [DOI] [Google Scholar]
- Momeni S.; Ahmadi R.; Safavi A.; Nabipour I. Blue-emitting Copper Nanoparticles as a Fluorescent Probe for Detection of Cyanide Ions. Talanta 2017, 175, 514. 10.1016/j.talanta.2017.07.056. [DOI] [PubMed] [Google Scholar]
- Xu H.; Gu B.; Li Y.; Huang Z.; Yao S.; et al. A Highly Selective, Colorimetric and Ratiometric Fluorescent Probe for NH2 NH2 and its Bioimaging. Talanta 2018, 180, 199–205. 10.1016/j.talanta.2017.12.039. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Wei L.; Kaibo Z.; Sasa Z. FRET-based Small-molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. 10.1021/ar300273v. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Liu Y.; Lee J.; Lee J. H.; Kim H. Y.; Park M. Rational Designed Strategy to Dispel Mutual Molestation of Mercuric and Ferric Ions towards Robust, pH-stable Fluorescent Carbon Nanodots. Analyst 2017, 142, 1149–1156. 10.1039/C6AN02601J. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Luo Y.; Wang H.; Wei H.; Guo T.; Tan H.; Yuan L.; Zhang X.-B. A Novel Ratiometric and Reversible Fluorescence Probe with a Large Stokes Shift for Cu2+ based on a New Clamp-on unit. Anal. Chim. Acta 2019, 1065, 134–141. 10.1016/j.aca.2019.03.029. [DOI] [PubMed] [Google Scholar]
- Hatai J.; Rahaman S. A.; Dasgupta D.; Bandyopadhyay S. Instant Detection of Cyanide in Seafood with a Tryptophan based Fluorescence Probe. Anal. Methods 2019, 11, 3563–3569. 10.1039/C9AY00936A. [DOI] [Google Scholar]
- Shirbhate M. E.; Jeong Y.; Ko G.; Baek G.; Kim G.; Kwon Y. U.; Kim K. M.; Yoon J. Selective Fluorescent Recognition of Zn2+ by Using Chiral Binaphthol-pyrene Probes. Dyes Pigm. 2019, 167, 29–35. 10.1016/j.dyepig.2019.03.063. [DOI] [Google Scholar]
- Song H.; Zhang Z. A Quinoline-based Ratiometric Fluorescent Probe for Discriminative Detection of Zn2+ and Cd2+ with Different Binding Modes, and its Zn2+ Complex for Relay Sensing of Pyrophosphate and Adenosine Triphosphate. Dyes Pigm. 2019, 165, 172–181. 10.1016/j.dyepig.2019.02.011. [DOI] [Google Scholar]
- Yang L.; Yuanan S.; Yani G.; Fengpei Q.; Xiaojie R.; Fan Z.; Xiangzhi S. An Instantaneous Near-infrared Trimethyl Lock Based Fluorescent Probe for Biothiols with a Large Stokes Shift. Anal. Chim. Acta 2018, 1034, 168–175. 10.1016/j.aca.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Wang J. B.; Wu Q. Q.; Min Y. Z.; Liu Y. Z.; Song Q. H. A Novel Fluorescent Probe for Au(III)/Au(I) Ions Based on an Intramolecular Hydroamination of a Bodipy Derivative and its Application to Bioimaging. Chem. Commun. 2012, 48, 744–746. 10.1039/C1CC16128H. [DOI] [PubMed] [Google Scholar]
- Wei T. B.; Zhang P.; Shi B.; Chen P.; Lin Q.; Liu J.; Zhang Y. A Highly Selective Chemosensor for Colorimetric Detection of Fe3+ and Fluorescence Turn-on Response of Zn2+. Dyes Pigm. 2013, 97, 297–302. 10.1016/j.dyepig.2012.12.025. [DOI] [Google Scholar]
- Jiao X.; Liu C.; He S.; Zhao L.; Zeng X. Highly Selective and Sensitive Ratiometric Near-infrared Fluorescent Probe for Real-time Detection of Hg2+ and its Bioapplications in Live Cells. Dyes Pigm. 2019, 160, 86–92. 10.1016/j.dyepig.2018.07.040. [DOI] [Google Scholar]
- Jiao X.; Xiao Z.; Hui P.; Liu C.; Wang Q.; Qiu X.; He S.; Zeng X.; Zhao L. A Highly Selective and pH-tolerance Fluorescent Probe for Cu2+ based on a Novel Carbazole-Rhodamine Hybid Dye. Dyes Pigm. 2019, 160, 633–640. 10.1016/j.dyepig.2018.08.060. [DOI] [Google Scholar]
- Hu L.; Yan X. W.; Li Q.; Zhang X.; Shan D. Br-PADAP Embedded in Cellulose Acetate Electrospun Nanofibers: Colorimetric Sensor Strips for Visual Uranyl Recognition. J. Hazard. Mater. 2017, 329, 205–210. 10.1016/j.jhazmat.2017.01.038. [DOI] [PubMed] [Google Scholar]
- Yousaf A.; Xu N.; Arif A. M.; Zhou J.; Sun C.; Wang X.; Su Z. A Triazine-based Metal-organic Framework with Solvatochromic Behaviour and Selectively Sensitive Photoluminescent Detection of Nitrobenzene and Cu2+ Ions. Dyes Pigm. 2019, 163, 159–167. 10.1016/j.dyepig.2018.11.039. [DOI] [Google Scholar]
- Cheng R.; Liu S.; Shi H.; Zhao G. A Highly Sensitive and Selective Aptamer-based Colorimetric Sensor for the Rapid Detection of PCB 77. J. Hazard. Mater. 2018, 341, 373. 10.1016/j.jhazmat.2017.07.057. [DOI] [PubMed] [Google Scholar]
- Liu S.; Yang M.; Liu Y.; Chen H.; Li H. A Novel “turn-on” Fluorescent Probe Based on Triphenylimidazole-hemicyanine Dyad for Colorimetric Detection of CN– in 100% Aqueous Solution. J. Hazard. Mater. 2018, 344, 875–882. 10.1016/j.jhazmat.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Niamnont N.; Khumsri A.; Promchat A.; Tumcharern G.; Sukwattanasinitt M. Novel Salicylaldehyde Derivatives as Fluorescence Turn-on Sensors for Cyanide Ion. J. Hazard. Mater. 2014, 280, 458–463. 10.1016/j.jhazmat.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhang J.; An X.; Duan H. Recent Progress on the Organic and Metal Complex-based Fluorescent Probes for Monitoring Nitric Oxide in Living Biological Systems. Org. Biomol. Chem. 2020, 18, 1522. 10.1039/C9OB02561H. [DOI] [PubMed] [Google Scholar]
- Babaee E.; Barati A.; Gholivand M. B.; Taherpour A.; Zolfaghar N.; Shamsipur M. Determination of Hg2+ and Cu2+ Ions by Dual-emissive Ag/Au Nanocluster/carbon Dots Nanohybrids: Switching the Selectivity by pH Adjustment. J. Hazard. Mater. 2019, 367, 437–446. 10.1016/j.jhazmat.2018.12.104. [DOI] [PubMed] [Google Scholar]
- Bartwal G.; Komal A.; Khurana J. M. A Highly Selective pH Switchable Colorimetric Fluorescent Rhodamine Functionalized Azo-phenol Derivative for Thorium Recognition up to Nano Molar Level in Semi-aqueous Media: Implication Towards Multiple Logic Gates. J. Hazard. Mater. 2018, 360, 51–61. 10.1016/j.jhazmat.2018.07.091. [DOI] [PubMed] [Google Scholar]
- Msj K.; Wang Y. W.; Senge M. O.; Peng Y. Sensitive Fluorescence on-off Probes for the Fast Detection of a Chemical Warfare Agent Mimic. J. Hazard. Mater. 2017, 342, 10–19. 10.1016/j.jhazmat.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Sukato R.; Sangpetch N.; Palaga T.; Jantra S.; Vchirawongkwin V.; Jongwohan C.; Sukwattanasinitt M.; Wacharasindhu S. New Turn-On Fluorescent and Colorimetric Probe for Cyanide Detection Based on BODIPY-Salicylaldehyde and its Application in Cell Imaging. J. Hazard. Mater. 2016, 314, 277–285. 10.1016/j.jhazmat.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Liu R. Q.; Ding G. H.; Li J. L.; Feng H. J.; He W. Y.; Wu L. Y. A Triazole-based Fluorescence Probe for Detecting Hg2+ Ions and its Biological Application. Luminescence 2019, 35, 3705–3711. 10.1002/bio.3705. [DOI] [PubMed] [Google Scholar]
- Pawar S.; Kaja S.; Nag A. Red-Emitting Carbon Dots as a Dual Sensor for In(3+) and Pd(2+) in Water. ACS Omega 2020, 5, 8362–8372. 10.1021/acsomega.0c00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.; Zhao W.; Yang H.; Huang Z.; Liu X.; Han A. Detection of Hg2+ by a FRET Ratiometric Fluorescent Probe based on a Novel BODIPY-RhB System. Tetrahedron Lett. 2016, 57, 2655–2659. 10.1016/j.tetlet.2016.05.015. [DOI] [Google Scholar]
- Khan B.; Hameed A.; Minhaz A.; Shah M. R. Synthesis and Characterisation of Calix 4 Arene based Bis(triazole)-bis (hexahydroquinoline): Probing Highly Selective Fluorescence Quenching Towards Mercury (Hg2+) Analyte. J. Hazard. Mater. 2018, 347, 349–358. 10.1016/j.jhazmat.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Pooja D.; Saini S.; Thakur A.; Kumar B.; Tyagi S.; Nayak M. K. A “Turn-On” Thiol Functionalized Fluorescent Carbon Quantum Dot Based Chemosensory System for Arsenite Detection. J. Hazard. Mater. 2017, 328, 117–126. 10.1016/j.jhazmat.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Promchat A.; Rashatasakhon P.; Sukwattanasinitt M. A Novel Indolium Salt as a Highly Sensitive and Selective Fluorescent Sensor for Cyanide Detection in Water. J. Hazard. Mater. 2017, 329, 255–261. 10.1016/j.jhazmat.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Xing Y.; Zhao D.; Gu T.; Liu H. L.; Yu W. D. Highly Efficient Fluorescence Probe for Copper (II) Ions Based on Gold Nanoclusters Supported on Wool Keratin. J. Mater. Sci. 2018, 53, 4056–4066. 10.1007/s10853-017-1830-y. [DOI] [Google Scholar]
- Arjunan S.; Gopalan S.; Selvaraj S.; Thangaraj S.; Krishna K.; Nanjan B.; Raju N.; Subramaniam M. P. A Selective Fluorescence Chemosensor: Pyrene Motif Schiff Base Derivative for Detection of Cu2+ Ions in Living Cells. J. Photochem. Photobiol., A 2018, 364, 424–432. 10.1016/j.jphotochem.2018.06.021. [DOI] [Google Scholar]
- Pawar S.; Teja B. R.; Nagarjuna R.; Ganesan R.; Nag A. Probing the Surface Composition Effect of Silver–Gold Alloy in SERS Efficiency. Colloids Surf., A 2019, 578, 123638–123642. 10.1016/j.colsurfa.2019.123638. [DOI] [Google Scholar]
- Fu C.; Hsieh C.; Juang R.; Yang J.; Gu S.; Gandomi Y. A. Highly Efficient Carbon Quantum Dot Suspensions and Membranes for Sensitive/Selective Detection and Adsorption/Recovery of Mercury Ions from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2019, 100, 127–136. 10.1016/j.jtice.2019.04.012. [DOI] [Google Scholar]
- Du C.; Fu S.; Ren X.; Wang X.; Zhong W.; Zhou J.; Wang H. Y. A Diketopyrrolopyrrole-based Fluorescent Probe for Investigating Mitochondrial Zinc Ions. New J. Chem. 2018, 42, 3493–3502. 10.1039/C7NJ04940D. [DOI] [Google Scholar]
- Chi F.; Wei X.; Qin Y.; Li F.; Yin M.; et al. Investigation on the Site Occupation of Rare-earth Ions in CaIn 2 O 4 with the Fluorescence Probe of Eu3+. Phys. Chem. Chem. Phys. 2017, 19, 12473–12479. 10.1039/C7CP01938F. [DOI] [PubMed] [Google Scholar]
- Farrokhnia M.; Karimi S.; Momeni S.; Khalililaghab S. Colorimetric Sensor Assay for Detection of Hydrogen Peroxide Using Green Synthesis of Silver Chloride Nanoparticles: Experimental and Theoretical Evidence. Sens. Actuators, B 2017, 246, 979–987. 10.1016/j.snb.2017.02.066. [DOI] [PubMed] [Google Scholar]
- Mahmoud W. E. Synthesis and Characterization of 2A-3SHPA Decorated ZnS@CdS Core–Shell Heterostructure Nanowires as a Fluorescence Probe for Antimony Ions Detection. Sens. Actuators, B 2017, 238, 1001–1007. 10.1016/j.snb.2016.07.150. [DOI] [Google Scholar]
- Shorie M.; Kaur H.; Chadha G.; Singh K.; Sabherwal P. Graphitic Carbon Nitride QDs Impregnated Biocompatible Agarose Cartridge for Removal of Heavy Metals from Contaminated Water Samples. J. Hazard. Mater. 2019, 367, 629–638. 10.1016/j.jhazmat.2018.12.115. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Liu L.; Xing Y.; Zhou X. Duplex Functional G-quadruplex/NMM Fluorescent Probe for Label-free Detection of Lead(II) and Mercury(II) ions. J. Hazard. Mater. 2018, 355, 50–55. 10.1016/j.jhazmat.2018.04.082. [DOI] [PubMed] [Google Scholar]
- Bhatt K. D.; Shah H. D.; Panchal M. A Switch-off Fluorescence Probe Towards Pb(II) and Cu(II) Ions based on a Calix[4]pyrrole Bearing Amino-quinoline Group. Luminescence 2017, 32, 1398–1404. 10.1002/bio.3336. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Verma N. C.; Khan S.; Tiwari S.; Chaudhary A.; Nandi C. K. Paper Strip Based and Live Cell Ultrasensitive Lead Sensor Using Carbon Dots Synthesized from Biological Media. Sens. Actuators, B 2016, 232, 107–114. 10.1016/j.snb.2016.03.110. [DOI] [Google Scholar]
- Mukherjee A.; Chakravarty M. Regioisomeric Monopyridine-functionalized Triarylethene: Small AIEgens with Isomeric Effect and an Efficient Platform for the Selective and Sensitive Detection of Pd2+ and Fe3+. New J. Chem. 2020, 44, 6173–6181. 10.1039/D0NJ00306A. [DOI] [Google Scholar]
- Adak A. K.; Dutta B.; Manna S. K.; Sinha C. Rhodamine-Appended Benzophenone Probe for Trace Quantity Detection of Pd(2+) in Living Cells. ACS Omega 2019, 4, 18987–18995. 10.1021/acsomega.9b01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Liu C.; Tian B.; Cai X.; Zhu H.; Jia P.; Li Z.; Zhang X.; Sheng W.; Zhu B. A Novel Highly Selective Ratiometric Fluorescent Probe with Large Emission Shift for Detecting Mercury Ions in Living Cells and Zebrafish. Dyes Pigm. 2020, 177, 108290 10.1016/j.dyepig.2020.108290. [DOI] [Google Scholar]
- Wu N.; Zhao L.-X.; Jiang C.-Y.; Li P.; Liu Y.; Fu Y.; Ye F. A Naked-eye Visible Colorimetric and Fluorescent Chemosensor for Rapid Detection of Fluoride Anions: Implication for Toxic Fluorine-containing Pesticides Detection. J. Mol. Liq. 2020, 302, 112549 10.1016/j.molliq.2020.112549. [DOI] [Google Scholar]
- Wechakorn K.; Prabpai S.; Suksen K.; Kanjanasirirat P.; Pewkliang Y.; Borwornpinyo S.; Kongsaeree P. A Rhodamine-triazole Fluorescent Chemodosimeter for Cu(2+) Detection and its Application in Bioimaging. Luminescence 2018, 33, 64–70. 10.1002/bio.3373. [DOI] [PubMed] [Google Scholar]
- Yan T.; Cheng G.; Yang H. 1,3,4-Oxadiazole based Thermostable Energetic Materials: Synthesis and Structure–property Relationship. New J. Chem. 2020, 44, 6643–6651. 10.1039/D0NJ00518E. [DOI] [Google Scholar]
- Fu Y.; Pang X. X.; Wang Z. Q.; Chai Q.; Ye F. A Highly Sensitive and Selective Fluorescent Probe for Determination of Cu (II) and Application in Live Cell Imaging. Spectrochim. Acta, Part A 2019, 208, 198–205. 10.1016/j.saa.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Ye F.; Liang X. M.; Xu K. X.; Pang X. X.; Chai Q.; Fu Y. A Novel Dithiourea-appended Naphthalimide “on-off” Fluorescent Probe for Detecting Hg(2+) and Ag(+) and its Application in Cell Imaging. Talanta 2019, 200, 494–502. 10.1016/j.talanta.2019.03.076. [DOI] [PubMed] [Google Scholar]















