Abstract
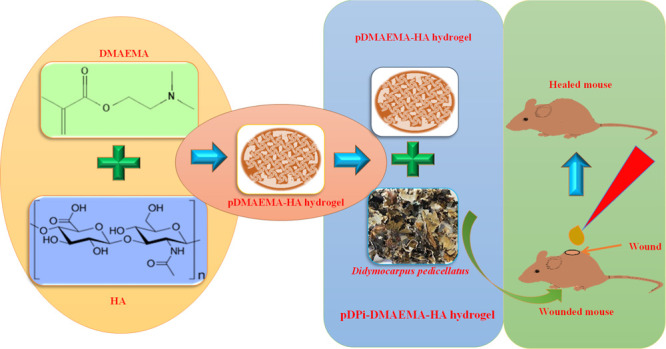
Wound is the major health problem associated with skin damages and arises because of various types of topical injuries. Furthermore, wounds in patients with diabetes take a relatively long time to heal. Currently, herbal medicines have been extensively used for wound care and management. Here, we engineered polymeric hybrid hydrogel of dimethylaminoethyl acrylate and hyaluronic acid (pDMAEMA–HA), which was impregnated with a herbal extract of Didymocarpus pedicellatus. The developed polymeric hybrid hydrogel system can be used for effective therapy of incurable wounds. Therefore, the development of D. pedicellatus-impregnated pDMAEMA–HA (pDPi-DMAEMA–HA) hybrid hydrogel was accomplished by the synthesis of pDMAEMA–HA hydrogel via the optimization of various reaction parameters followed by impregnation of herbal drugs D. pedicellatus. The developed hydrogel composite was well characterized via various techniques, and swelling kinetics was performed to analyze the water uptake property. The swelling ratio was found to be 1600% in both types of hydrogels. To evaluate the wound healing of these polymeric hydrogels, the Wistar rats full-thickness excision wound model was utilized. The healing strength of hydrogels was determined using measurement of wound contraction and histopathological study. The results of wound healing by these polymeric hydrogels revealed that animals treated with the pDPi-DMAEMA–HA hybrid hydrogel group were found to have a higher level of wound closure as compared to marketed formulation as well as polymeric hybrid hydrogel. The histopathologic examinations implied that pDPi-DMAEMA–HA hybrid hydrogel and polymeric hybrid hydrogel-treated groups exhibited enhanced cutaneous wound repair as well as high level of cellular repair and maintenance compared to the standard group because of hyaluronic acid roles in various stages of wound repair.
1. Introduction
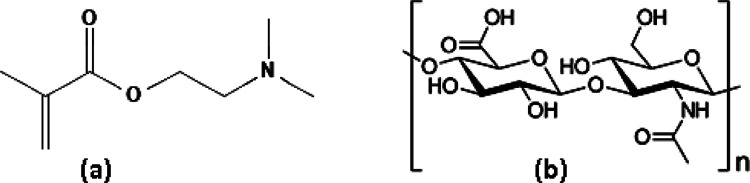
Biomaterials deliver distinctive unique tool including site specific drug delivery, early detection, therapy, imaging, and diagnosis of various diseases.1 The diversity of these biomaterials provides unique biological and physiochemical characteristics for the variety of potential biomedical applications.2 Hydrogel is a very commonly used material in the area of wound healing because of the ability to absorb a great amount of water and biological fluids, while remaining insoluble.2,3 The hydrogels have gained much attention in the last few decades because of their potential application in tissue engineering and regeneration of tissues.4 It is widely used as debriding agents in the management of a variety of wounds and wound dressing to accelerate the healing rate.5 The hydrogel helps to maintain the moist environment of the wound to recognize as being beneficial in wound healing, capable of healing of moist wound and reducing pain.6 Among hydrogels, dimethylaminoethyl methacrylate (pDMAEMA; Figure 1a) hydrogel is one of the most widely used due to several advantageous features such as nontoxicity, biocompatibility, and stability.7 Wound healing is a complex biological process comprised a series of sequential events aimed at repairing injured tissue.8 Furthermore, extracellular matrix components play an important role in the regulation of all phases of tissue repair, including cellular migration, inflammation, angiogenesis, remodeling, and scar formation.9 In addition, hyaluronic acid (HA; Figure 1b) is a natural biocompatible, biodegradable, and lacks an immunogenic polymer. HA works as a biologically active molecule, which regulates the tissue repair process on multiple levels and is considered as a safe and effective option to be used in skin repair.10,11 Treatment with HA was highly efficacious in reducing the various infections and ameliorated the inflammatory host response associated.12−16 HA is considered as one of the key players in tissue regeneration because of involvement in various steps of wound healing, that is, inflammation, granulation, re-epithelialization, and so forth.17−19 The proposed scientific rationale for the development of biomaterials based on HA relies on the characteristics of the material, which is a structural component of the ECM architecture and has an important role in water homeostasis that could favor the tissue hydration system, which is conducive to wound healing.20,21
Figure 1.
Chemical structure of (a) dimethylaminoethyl acrylate (DMAEMA) and (b) hylauronic Acid (HA).
In addition, herbal medicines have been extensively used on the basis of potential of curing multiple diseases and phytochemicals present in herbal medicines are being actively explored for their directly use as therapeutic agents.22,23D. pedicellatus is one of the beneficial and uncommon medicinal plant, whose use is reported in Ayurveda (Traditional system of medicine of India) as a common medication. From ancient times, D. pedicellatus is utilized in the remedy of renal disorder and kidney stones and therapeutic phytochemicals are flavonoids, steroids, terpenoids, saponins, tannins, proteins, phenols, alkaloids, glycosides, and carbohydrates.24 The main active phytochemicals in the extracts are pashanone, didymocarpol, didymacarpenol, didmyocarpin, isodidmyocarpin, pedicin, isopedicin, pediflavone, pedicellic acid, didymocarpene, and pedicellin. Previously, thermosensitive HA-based injectable hydrogels were developed using a corn silk extract (CSE) and nanosilver and their wound care potential was investigated.25 Keeping an importance of herbal medicine and phytochemicals of D. Pedicelliatus, herbal medicine-impregnated hydrogel needs are anticipated for wound healing applications. The approach would explore a possible interaction of hydrogel with phytoconstituents, which increases the stability of hydrogel and understanding of biomolecular interaction with living tissues. Furthermore, the impregnated process of the herbal extract with hydrogel will provide the better biocompatibility, adhesiveness, and efficacy because of a synergistic effect. Impregnation could also promote the higher product stability to achieve necessary efficacy toward controlled and site specific delivery with enhanced bioavailability. In the current study, D. pedicellatus extract-impregnated pDMAEMA–HA (pDPi-DMAEMA–HA) hybrid hydrogel was developed using an aqueous ethanolic extract of D. pedicellatus and hybrid hydrogel of DMAEMA and HA. Various reaction parameters and physicochemical parameters of synthesis were optimized of pDMAEMA–HA hydrogel and further impregnated with the D. pedicellatus extract. The developed composite were characterized using scanning electron microscopy (SEM) and Fourier-transform infrared (FTIR) spectroscopy. Swelling analysis of hydrogels was performed to understand and target the objective toward the tissue hydration system at the wound site. Additionally, the streptozotocin-induced incurable diabetic wounds healing were achieved by pDPi-DMAEMA–HA hydrogel. Various parameters of wound healings were evaluated using wound area contraction and histological assays.
2. Results and Discussion
2.1. Development of pDMAEMA–HA Hydrogel and Impregnation with the Extract of D. pedicellatus
The hydrogel development involved the conversion of the DMAEMA monomer and HA polymer to pDMAEMA–HA hydrogel. The optimized value of DMAEMA and HA to pDMAEMA–HA synthesis were found in a ratio of 1000:1. The synthesized hydrogels were found to be highly stable at pH 11. The synthesized hydrogel after the completion of reactions was a semisolid form and after drying was found to be a circular solid sheet form. Impregnation of D. pedicellatus occurred in the swelled pDMAEMA–HA hydrogel. Previously, poly-acrylic acid-diethylaminoethyl methacrylate (pAcD) and poly-methacrylic acid-diethylaminoethyl methacrylate (pMcD) hydrogels were developed by our groups via a free radical aqueous polymerization technique using starting materials of acrylic acid (AAc), methacrylic acid (MAAc), and diethylaminoethyl methacrylate (DEAEMA) to understand the mechanism of hydrogel formation and morphology and delineate the relevant mechanical properties.26,27
2.2. Characterization of pDMAEMA–HA and pDpi-DMAEMA–HA Hydrogels
2.2.1. Scanning Electron Microscopy
SEM images enlightening the morphological structure of pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels were taken after freeze drying are shown in Figure 2. Hydrogel of pDMAEMA–HA was seen with a large number of open porous structures (∼1 mm), while pDPi-DMAEMA–HA hydrogel was also seen with less numbers of open porous structures (∼1000 nm) and smaller size of particles (400–500 nm). These porous structures facilitate aqueous solution flow into the gel and plays an important role to achieve the pDMAEMA–HA hydrogel to absorb more aqueous solvent.26,28,29 The pore size of hydrogel might be increased because of the impregnation of phytochemicals in to the pDPi-DMAEMA–HA. In our previously reported study, pAcD and pMcD hydrogels were found to have a structural framework comprising inter-connected nanogels and continuous outer skin with macroporous interiors as visualized by SEM.26
Figure 2.
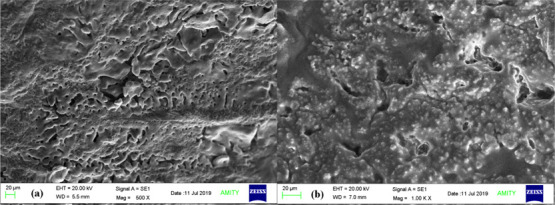
SEM micrographs of (a) Cross section of pDMAEMA–HA hydrogels and (b) cross section of pDPi-DMAEMA–HA hydrogels.
2.2.2. Fourier-Transform Infrared Spectroscopy
The FTIR spectrum of the D. pedicellatus extract, pDMAEMA–HA hybrid hydrogel and pDPi-DMAEMA–HA hydrogel are shown in Figure 3. The wide band at 3400 cm–1 originates from the −OH structural polymeric association and from the stretch in the N–H groups of the D. pedicellatus extract.28,30 Asymmetrical and symmetrical stretching of methyl groups in the pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels were observed as weak vibrations at a range of 2800–3000 cm–1.28,31 However, respective peaks of phenolics and flavonoids were found in the range of 1000–1600 cm–1 in the D. pedicellatus extract and present in the pDPi-DMAEMA–HA hydrogel.28 The characteristic peak in a sharp structure of the C=O group belonging to the carboxylic acid group in the pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels occurred at 1700 cm–1.28,30,31 In the pDMAEMA–HA hydrogel, DMAEMA interacts with >C=O with various −OH groups of HA using hydrogen bonds. The functional group analysis using this spectroscopy confirms the encapsulation of the D. pedicellatus extract in pDMAEMA–HA hydrogel.
Figure 3.
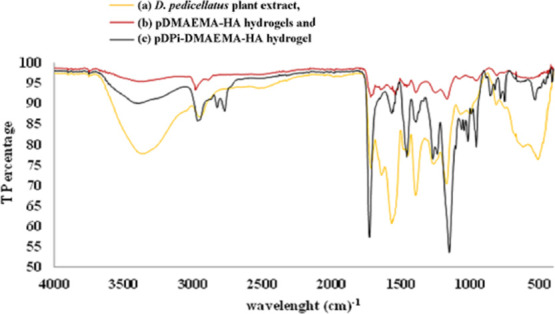
FTIR spectroscopy micrographs overlay of the (a) D. pedicellatus plant extract, (b) pDMAEMA–HA hydrogels, and (c) pDPi-DMAEMA–HA hydrogels.
2.2.3. Swelling Kinetics of pDMAEMA–HA and pDPi-DMAEMA–HA Hydrogels
The hydrogels exhibited high capacity to water absorption and swelling ratio was found to be strongly dependent on the composition of the composite hydrogels. Graph of the swelling study of the pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels are shown in Figure 4. Synthesized hybrid hydrogel demonstrated quite a high swelling ratio in compared to previously reported pDMAEMA hydrogels.26−29,32 The water binding affinity of pDPi-DMAEMA–HA hydrogel was found to be high compared to pDMAEMA–HA hydrogel alone, due to higher affinity of water toward phytochemicals. Uniformly distributed open pores in the hydrogels as confirmed by SEM play an active role in the diffusion of water molecules into the gel matrix. A swelling ratio reached its maximal rate upto 1200% in a short time of such as 8 h and 1600% in 24 h. Other previously reported study suggests that the swollen gels showed fairly strong viscoelastic behavior and underwent deformation from ∼70 to 85% before failure, indicating the formation of robust 3D structures.28
Figure 4.
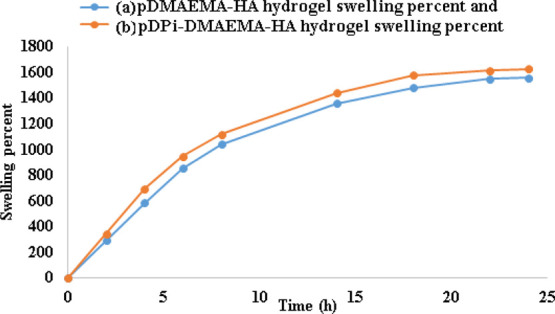
Swelling percentage with time (h) in buffer solution of (a) pDMAEMA–HA hydrogels and (b) pDPi-DMAEMA–HA hydrogels.
2.3. Effect of pDMAEMA–HA and pDPi-DMAEMA–HA Hydrogels on Diabetic Wound Healing
2.3.1. Wound Closure and Contraction
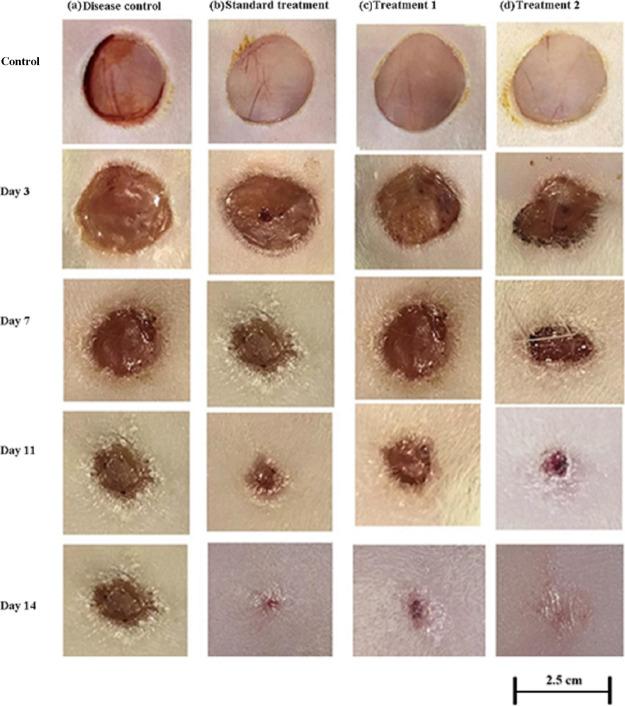
In order to determine the wound closure efficacy of the bioinspired hydrogels in vivo, full-thickness skin incisions were created on a rat of 2.5 cm. The prepared hydrogel samples were applied at the wound sites as our previously reported study.33Figure 5 shows the images of the rat skin wounds taken at different time intervals after treating with a marketed standard, pDMAEMA–HA hydrogel, and pDPi-DMAEMA–HA hydrogel. The control group (without treatment) was also included with no sign of inflammation and infection occurring. It was clearly observed the formation of new epidermis and extension of epidermis toward the wound centers in all the treated wound lesions, resulting in the reduced area of the wounds. The accelerated wound closure rate was found in pDMAEMA–HA hydrogel and pDPi-DMAEMA–HA hydrogel treated groups, when compared with the disease control and standard treatment group (Figure 5). Animal wounds treated with pDMAEMA–HA hydrogel, standard treatment and pDPi-DMAEMA–HA hydrogel demonstrated maximum rate of wound healing in comparison to the control group (lowest) on the 14th day, respectively. The results of wound closure after treatment with hydrogel(s) were as follows
Figure 5.
Visual inspection of wound healing in rats (a) disease group, (b) treated with standard marketed formulation, (c) treated with pDMAEMA–HA hydrogels (treatment 1), and (d) treated with pDPi-DMAEMA–HA hydrogels (treatment 2).
In order to determine the wound closure rate, wound areas were calculated in all groups. After three days of treatment, the wound closure rate in all groups was found to be negligible (Figure 6); however, from the 7th day of treatment, the wound closure rate was accelerated and reached 72 ± 2.6 and 56 ± 2.5% in pDPi-DMAEMA–HA and pDMAEMA–HA hydrogels, while 44 ± 2.0 and 26 ± 1.9% only in standard treatment and disease-controlled groups (Figure 6). pDMAEMA–HA hydrogel without D. pedicellatus still had a valuable wound closure effect, when compared with the disease and standard control group till the 7th day. On the 14th day of treatment, wound closure rate reached 98 ± 1.9, 93 ± 1.8, and 96 ± 1.5% in pDPi-DMAEMA–HA, pDMAEMA–HA, and standard treatment, which showed a high degree of difference in comparisons to the controlled group (55 ± 2.8%), respectively (Figure 6). Decisively, it is proposed that pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels could enhance the wound healing in vivo. These results were observed because of hydrogels could actually maintain a physiologically moist microenvironment and supply an adhesion effect at the wound site, enhancing the wound contraction and closure rate at the early stage of the wound healing process.33,34 Moreover, it was also reported that HA could promote fibroblast proliferation, angiogenesis and matrix deposition could contribute to generating the contractile force at the wound area, and this process mostly occurs at the initial stage of a wound healing process, which is in agreement with this study.35,36 Our previously reported study demonstrated that hydrogel nanotubes exhibited unique biocompatibility and helped in the rapid establishment of wound barrier properties with concomitant cell proliferation guided by the provisional matrix mimicking an extracellular matrix niche, which support permissive milieu for vascular sufficiency with a well-proliferated fibroblast at the wound bed.33 In addition, cell migration and proliferation efficacy in vitro and contractile force generated by HA could be the reason of the excellent healing performance of these hydrogels.33
Figure 6.
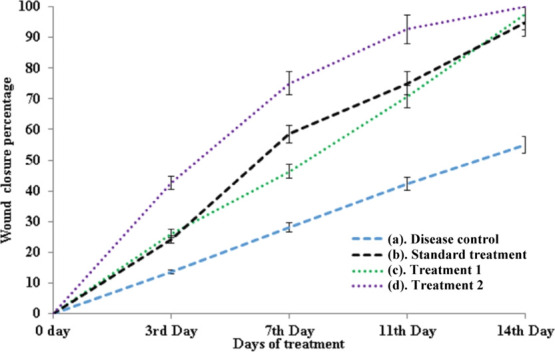
Percent of wound closure after days (a) disease group, (b) treated with standard marketed formulation, (c) treated with pDMAEMA–HA hydrogels (treatment 1), and (d) treated with pDPi-DMAEMA–HA hydrogels (treatment 2).
2.3.2. Histological Assessment
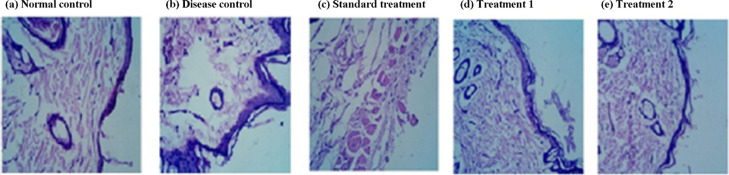
The histopathological analysis of animal wounds treated with various hydrogels displayed a significant level of alteration in the skin tissue architecture in to normal and disease control. Photomicrograph of hematoxylin- and eosin-stained sections showing accelerative effect on re-epithelization and granulation tissue formation in the skin wound, when treated with pDMAEMA–HA hydrogel and pDPi-DMAEMA–HA hydrogel (Figure 7). While, disease control and standard treated groups showed partial re-epithelization of the epidermis and luminized types of blood vessels (Figure 7). The pDPi-DMAEMA–HA hydrogel group demonstrated complete re-epithelization of the wound and which is very similar to the normal control. Differentiation of keratinocytes was confirmed through the presence of the keratinized layer (cells devoid of nuclei) above the nucleated epithelial cells after treatment, in which the results are in agreement with the previously reported study.36 The skin of the disease control animal group was comparatively unable to re-epithelization of the epidermis, pronounced leukocytic infiltration and disorganized granulation tissue filling the incisional space.11,36
Figure 7.
Histopathology of the rat skin (a) normal control, (b) disease control, (c) standard treatment, (d) treated with pDMAEMA–HA hydrogels (treatment 1), and (e) treated with pDPi-DMAEMA–HA hydrogels (treatment 2).
3. Conclusions
In the present study, herbal medicine-based polymeric hybrid polymeric hydrogels were developed to examine biocompatibility, accelerate tissue regeneration, and improve the efficiency in the diabetic animal tissue system. Polymeric hybrid hydrogel as well as pDPi-DMAEMA–HA hybrid hydrogel was found to have enhanced swelling and wound healing effectiveness properties as compared to the other available marketed formulations. As a consequence, with regard to its applicable impregnation ability, flexibility, and higher water absorption ability, it is suggested that this ratio of pDPi-DMAEMA–HA hybrid hydrogel can be utilized as a novel polymeric material for the management of wounds. It was also revealed that pDPi-DMAEMA–HA hybrid hydrogel played a significant role to improve the healing of the incurable diabetes wounds. An investigation on the biomarkers for tissue repair, cellular migration, inflammation, angiogenesis, remodeling, and mediators for wound healing needs to be explored. These hybrid formulations optimistically would be able to treat various types of wounds including burn, skin irritation, fracture, accidental wound, cut, skin rupture, diabetics’ wounds, and foot ulcers.
4. Experimental Section
4.1. Materials
The 2-(dimethylamino) ethyl methacrylate (DMAEMA) and HA sodium salt (Streptococcus equi) (Figure 1) were purchased from Sigma-Aldrich, India. Ammonium persulphate (APS) and 2-butanone were procured from Qualigen fine chemicals, India, and N,N,N′,N′-tetramethylethylenediamine (TEMED) was purchased from SRL Pvt. Ltd., India. All other reagents and chemicals wee used for the study were analytical grade with the highest level of purity. The deionized water was used throughout the experiments for the development of hydrogels, preparation of buffer solutions, and swelling kinetic studies. The plant material (stem part of D. pedicellatus) was procured from the local market (Khari baoli, near chandni chowk) of Delhi, India, and authenticated by the National Institute of Science Communication and Information Resources (NISCAIR), CSIR, New Delhi, India.
4.2. Preparation of HA Aqueous Solution
Aqueous basic solution (pH 11) was used to prepare HA solution and freshly prepared solution of HA was utilized for each reaction of hydrogel synthesis.
4.3. Synthesis of pDMAEMA–HA Polymers
pDMAEMA–HA hydrogels were synthesized with slight modification of our previously reported procedure of hydrogel synthesis.26 The free radical polymerization reaction was utilized to develop hybrid pDMAEMA–HA composite hydrogels with the different volume ratios of DMAEMA and HA. In a typical reaction, DMAEMA and HA after distillation were mixed at 0 °C (ice bath) along with deionized water. The prepared reaction mixture was taken in a 100 mL three-neck round bottom flask and kept in a heated water bath at 41 ± 1 °C placed over a magnetic stirrer. A thermometer and nitrogen line was attached to the necks of the flask and the nitrogen was purged into the mixture for few minutes. Aqueous solution of APS (100 mg/mL, 50 μL) and TEMED (5 μL) were added to the monomer mixtures and mixed along with a magnetic stirrer for about 30 min. After that, the reaction mixture was sonicated for 5 min to expel the entrapped air. The reaction mixture was then directly poured in a Petri-plate and incubated for 24 h at 41 ± 1 °C. The synthesized pDMAEMA–HA hydrogel was removed from the Petri-plate after the reaction. The obtained hydrogels were washed with basic water (pH 11) for five consecutive days, whereas water was replaced after every hour on the first day and twice a day for the remaining period, to remove any unreacted water-soluble constituents. The washed gel was dried in an oven at 50 °C. The dried gels were grounded to a powder form using liquid N2 and stored in a vacuum desiccator until further used. Various reaction conditions were optimized to obtain stable pDMAEMA–HA hydrogel.
4.4. Preparation of the Plant Extract
The stem parts of the D. pedicellatus plant was thoroughly washed with deionized water and completely dried under the shade. Dried materials were then grounded into small pieces and powdered using a grinder. The resulting powder (100 g) was extracted with 1:1 water and ethanol for 72 h using a Soxhlet apparatus (1000 mL). The extract solution was filtered, ethanol was evaporated using rotavapor and water was removed using freeze dryer. The final plant extract product was kept in desiccators until further use.
4.5. Impregnation of the D. pedicellatus Extract with pDMAEMA–HA Hybrid Hydrogel
Dried powder of pDMAEMA–HA hybrid hydrogel and D. pedicellatus dried extract was mixed in a ratio of 500 and 1000 mg, vortexed, and swelled with 10 mL of PBS buffer for 24 h. Initially, the solution was sonicated for 5 min to disperse the D. pedicellatus dried extract completely to buffer. Excess buffer was removed using a lyophilizer. The resultant pDPi-DMAEMA–HA hybrid hydrogel was kept in desiccators until further use.
4.6. Characterization of pDMAEMA–HA and pDPi-DMAEMA–HA Hydrogel Composites
The synthesized pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels were characterized by SEM and FTIR.
4.6.1. Scanning Electron Microscopy
To understand the morphology and absorbent structure of the developed hydrogels were studied using a SEM (ZEISS EVO 50). Hydrogel samples were first swollen in buffer solutions (PBS) of pH 7.4 and lyophilized in a freeze drier (FD Series, LabFreez instruments, China) for 24 h.27 The resultant hydrogel samples were fixed on aluminium stubs using double sided carbon adhesive tape and gold coated in a sputter coater (PolaronE 5100 Gold Sputter Coating unit) under an argon atmosphere. The electron microscopy images were obtained at 500× and 1000× magnification of pDMAEMA–HA and pDPi-DMAEMA–HA hydrogels, respectively.
4.6.2. Fourier-Transform Infrared Spectroscopy
FTIR spectroscopic analysis was performed to identify the functional groups present in the D. pedicellatus extract, pDMAEMA–HA, and pDPi-DMAEMA–HA hydrogels. The D. pedicellatus extract, pDMAEMA–HA hydrogel, and pDPi-DMAEMA–HA hydrogel were analyzed through a Bruker Alpha-PATR-FTIR, equipped with a temperature controller ATR–IR spectrophotometer (USA) in the range of 450–4000 cm–1.28
4.6.3. Swelling Kinetics of Hydrogels
Swelling analysis was performed by immersing hydrogel in a buffer solution at room temperature as previously reported in a study.29,32,37 An initial dry weight of 100 mg of the hydrogel powder and PBS buffer solution (pH 7.4) were utilized. The swollen samples were periodically weighted after wiping out excess solution. The swelling percentage was calculated through the following formula
where, as Ws and Wd are the weights of the hydrogel at swelling state and dry state, respectively.
4.7. Wound Healing Potential of pDMAEMA–HA and pDPi-DMAEMA–HA Hydrogels in Streptozotocin-Induced Diabetic Rats
4.7.1. Animals
Female Albino Wistar rats weigh ranging 150 to 200 g were procured for this experiment from the animal house of Amity Institute of Pharmacy (AIP); Amity University Noida, U.P. India. The study took place with the approval of protocol from the Institute Animal Ethical Committee (IAEC), Amity University, Noida, India. Rats were housed in an air conditioned environment under standard laboratory conditions (25 °C, RH 30–70%) with 12 h light/dark cycle. Two rats per cage were housed in standard suspended cages each having grill facilities and top of stainless steel with a facility for holding pelleted food and drinking water in polycarbonate bottles. Paddy husk was used as bedding materials and was changed at least twice a week. The rats were fed ad libitum pelleted food and filtered water.
4.7.2. Induction of Diabetes Mellitus
Albino wistar rats were fasted for 12 h and their body weight and blood glucose levels were measured. Diabetes was induced by injection of single dose of 45 mg/kg i.p streptozotocin (STZ) in 0.1 M citrate buffer.31 Subsequently, after STZ administration, the animal was allowed free access to feed and water for 48 h, and after that the animal was given 10% of sucrose solution to develop high blood glucose. Blood glucose was measured using a glucometer (Dr. Morpean, USA) at various intervals. After 21 days of STZ administration, animals with blood glucose levels above 250 mg/dL were defined as diabetic and used for the study.38
4.7.3. Experimental Design
Animals were randomly allocated into 5 groups. (n = 6 rats per group).
Group-1: no treatment and no diabetes (control).
Group-2: diabetes without treatment (disease control).
Group-3: diabetes with treatment (standard topical formulation).
Group-4: diabetes with treatment with pDMAEMA–HA hydrogel (treatment 1).
Group-5: diabetes with treatment with pDPi-DMAEMA–HA hydrogel (treatment 2).
4.7.4. Induction of Full Thickness Excision Wound
Animals were anesthetized by the intraperitoneal administration of xylazine hydrochloride (10 mg/kg body) and ketamine hydrochloride (25 mg/kg). The animal’s dorsal surface hair was trimmed with an electric clipper. The dorsum of all rats was rinsed with a 10% povidone-iodine solution followed by 70% isopropyl alcohol. A biopsy punch device (diameter, 6 mm) was used to induce the full-thickness round wound of 2.5 cm in the inter-scapular region of the upper back of each rat. The skin flap was cut out with the help of iris scissors and removed using angular forceps. The wound area of each animal was measured using a Vernier caliper. After recovering from anesthesia, rats were placed in their respective cages for further study.
4.7.5. Treatment with Hydrogels
Swollen hydrogel of pDMAEMA–HA hydrogel (1 g each) and pDPi-DMAEMA–HA hydrogel (1 g each) were applied topically in the wound area on alternate days (1, 3 and 5 days) for 3 days on Group-4 and Group-5, respectively, whereas for Group-3 standard control group, marketed formulation (Silverex Ionic gel 1 g) was applied topically in the wound area same as the treatment group. In Group-2 (disease controlled) animals, wound left open without any treatment.
4.7.6. Measurement of Wound Contraction
The wound area were measured on day 3, 7, 11, and 14 after treatment using a Vernier caliper and represented as wound contraction.33,39 Percentage of wound contraction was determined taking the initial size of the wound as 100% using the following formula
4.7.7. Histological Assessment
Animals from every group were sacrificed after 14 days of treatment. The wound area of the animal skin was collected, washed in cold normal saline and soaked, and dried on filter paper. For fixation of the skin, 10% formal saline buffer was used. The tissue was fixed in 10% buffered formal saline and processed for histological sections. This includes dehydration of the tissue in ascending concentration of alcohol followed by clearing in xylene and embedded in paraffin wax. Tissue sections were cut in rotatory microtome at 5 μm thickness and stained with hematoxylin and eosin.40 The histological tissue sections of organs were mounted on the slide using DPX fluid (Distyrene, Plasticizer and Xylene) and photographs were taken on a photomicrograph computerized microscope, Coslab, India, at different magnifications. The study was performed with the alliance of Singh Histology Processing Center, New Delhi, India.
4.7.8. Statistical Analysis
Results were expressed as mean ± SD for six rats in each experimental group. The statistical analysis was performed using SigmaPlot Version 13 software. All the data were analyzed using one-way analysis of variance (ANOVA) and P-values < 0.05 were considered as significant.41
Acknowledgments
The author A.K.M. and S.K.R. acknowledges to the Department of Biotechnology, New Delhi, Govt. of India for providing the Research Associate assistantship and Research grant (BT/PR21866/NNT/28/1145/2016).
Glossary
Abbreviations
- APS
ammonium persulphate
- D. Pedicelliata
Didymocarpus Pedicelliata
- pDPi-DMAEMA–HA
Didymocarpus pedicellatus impregnated pDMAEMA–HA
- pDMAEMA–HA
dimethylaminoethyl acrylate–hyaluronic acid
- DPX
distyrene plasticizer xylene
- FTIR
Fourier-transform infrared spectroscopy
- HA
hyaluronic acid
- pAcD
poly(AAc-co-DEAEMA)
- PBS
phosphate buffer saline
- pMcD
poly(MAAc-co-DEAEMA)
- SEM
scanning electron microscopy
- STZ
streptozotocin
- TEMED
N,N,N′,N′-tetramethylethylenediamine
The authors declare no competing financial interest.
References
- Liu Y.; Liu J.; Zhang J.; Li X.; Lin F.; Zhou N.; Yang B.; Lu L. Noninvasive Brain Tumor Imaging Using Red Emissive Carbonized Polymer Dots across the Blood–Brain Barrier. ACS Omega 2018, 3, 7888–7896. 10.1021/acsomega.8b01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. M.; Tandon S.; Kandasubramanian B. Advancements in Hydrogel-Functionalized Immunosensing Platforms. ACS Omega 2020, 5, 2060–2068. 10.1021/acsomega.9b03816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish C. S.; Satish K. P.; Shivakumar H. G. Hydrogels as controlled drug delivery systems: Synthesis, crosslinking, water and drug transport mechanism. Indian J. Pharm. Sci. 2006, 68, 133–140. 10.4103/0250-474x.25706. [DOI] [Google Scholar]
- Van Vlierberghe S.; Dubruel P.; Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 2011, 12, 1387–1408. 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- Madaghiele M.; Sannino A.; Ambrosio L.; Demitri C. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh B.; Ioannovich J.; Al-Amm C.; El-Musa K. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr. Pharm. Biotechnol. 2002, 3, 179–195. 10.2174/1389201023378283. [DOI] [PubMed] [Google Scholar]
- Diaz I. L.; Parra C.; Linarez M.; Perez L. D. Design of micelle nanocontainers based on PDMAEMA-b-PCL-b-PDMAEMA triblock copolymers for the encapsulation of amphotericin B. AAPS PharmSciTech 2015, 16, 1069–1078. 10.1208/s12249-015-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner G. C.; Werner S.; Barrandon Y.; Longaker M. T. Wound repair and regeneration. Nature 2008, 453, 314–321. 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Enoch S.; Leaper D. J. Basic science of wound healing. Surgery 2005, 23, 37–42. 10.1383/surg.23.2.37.60352. [DOI] [Google Scholar]
- Demidova-Rice T. N.; Hamblin M. R.; Herman I. M. Acute and Impaired Wound Healing. Adv. Skin Wound Care 2012, 25, 304. 10.1097/01.asw.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babensee J. E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008, 20, 101–108. 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Zaleski K. J.; Kolodka T.; Cywes-Bentley C.; McLoughlin R. M.; Delaney M. L.; Charlton B. T.; Johnson W.; Tzianabos A. O. Hyaluronic acid binding peptides prevent experimental staphylococcal wound infection. Antimicrob. Agents Chemother. 2006, 50, 3856–3860. 10.1128/aac.00082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong Y.-J.; Lin G.; Kim B. J.; Kim H.-E.; Kim S.; Jeong S.-H. Hyaluronic acid-based hybrid hydrogel microspheres with enhanced structural stability and high injectability. ACS Omega 2019, 4, 13834–13844. 10.1021/acsomega.9b01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi N.; Arad A.; Margalit R. Molecular and cellular studies of hyaluronic acid-modified liposomes as bioadhesive carriers for topical drug delivery in wound healing. Arch. Biochem. Biophys. 1994, 313, 267–273. 10.1006/abbi.1994.1387. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Kirker K. R.; Prestwich G. D. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- Khunmanee S.; Jeong Y.; Park H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 204173141772646. 10.1177/2041731417726464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin V. Growth factors in skin wound healing. Eur. J. Cell Biol. 1995, 68, 1–7. [PubMed] [Google Scholar]
- Mori M.; Yamaguchi M.; Sumitomo S.; Takai Y. Hyaluronan-based biomaterials in tissue engineering. Acta Histochem. Cytoc. 2004, 37, 1–5. 10.1267/ahc.37.1. [DOI] [Google Scholar]
- Gao Y.; Sun Y.; Yang H.; Qiu P.; Cong Z.; Zou Y.; Song L.; Guo J.; Anastassiades T. P. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int. J. Mol. Sci. 2019, 20, 3722. 10.3390/ijms20153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. D.; Myers S.; Leigh I. M.; Navsaria H. A. The role of hyaluronic acid in wound healing. Am. J. Clin. Dermatol. 2005, 6, 393–402. 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- Longinotti C. The use of hyaluronic acid based dressings to treat burns: A review. Burns Trauma 2014, 2, 162–168. 10.4103/2321-3868.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayemi O. E.; Ekennia A. C.; Katata-Seru L.; Ebokaiwe A. P.; Ijomone O. M.; Onwudiwe D. C.; Ebenso E. E. Antimicrobial and wound healing properties of polyacrylonitrile-moringa extract nanofibers. ACS Omega 2018, 3, 4791–4797. 10.1021/acsomega.7b01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. J.Health Promoting Herbs as Useful Adjuncts to Prevent Chronic Diseases. In Nutritional Health; Humana Press: Totowa, NJ, 2001; pp 237–252. [Google Scholar]
- Erickson S. B.; Vrtiska T. J.; Lieske J. C. Effect of Cystone on urinary composition and stone formation over a one year period. Phytomed 2011, 18, 863–867. 10.1016/j.phymed.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi P.; Ali G. W.; Della Sala F.; Abdel-Fattah W. I.; Borzacchiello A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. 10.1016/j.carbpol.2019.115023. [DOI] [PubMed] [Google Scholar]
- Shakeel A.; Singh A.; Das S.; Suhag D.; Sharma A. K.; Rajput S. K.; Mukherjee M. Synthesis and morphological insight of new biocompatible smart hydrogels. J. Polym. Res. 2017, 24, 113. 10.1007/s10965-017-1267-7. [DOI] [Google Scholar]
- Suhag D.; Bhatia R.; Das S.; Shakeel A.; Ghosh A.; Singh A.; Sinha O. P.; Chakrabarti S.; Mukherjee M. Physically cross-linked pH-responsive hydrogels with tunable formulations for controlled drug delivery. RSC Adv. 2015, 5, 53963–53972. 10.1039/c5ra07424j. [DOI] [Google Scholar]
- Taktak F.; Öğen Y. Preparation and characterization of novel silk fibroin/2-(N, N-dimethylamino) ethyl methacrylate based composite hydrogels with enhanced mechanical properties for controlled release of cefixime. J. Macromol. Sci. 2017, 54, 458–464. 10.1080/10601325.2017.1320750. [DOI] [Google Scholar]
- Yacob N.; Hashim K. Morphological effect on swelling behaviour of hydrogel. AIP Conf. Proc. 2014, 1584, 153–159. [Google Scholar]
- Chan E.-S.; Yim Z.-H.; Phan S.-H.; Mansa R. F.; Ravindra P. Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food Bioprod. Process. 2010, 88, 195–201. 10.1016/j.fbp.2009.09.005. [DOI] [Google Scholar]
- Gulsonbi M.; Parthasarathy S.; Bharat Raj K.; Jaisankar V. Green synthesis, characterization and drug delivery applications of a novel silver/carboxymethylcellulose–poly (acrylamide) hydrogel nanocomposite. Ecotoxicol. Environ. Saf. 2016, 134, 421–426. 10.1016/j.ecoenv.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Yanfeng C.; Min Y. Swelling kinetics and stimuli-responsiveness of poly (DMAEMA) hydrogels prepared by UV-irradiation. Radiat. Phys. Chem. 2001, 61, 65–68. 10.1016/s0969-806x(00)00374-1. [DOI] [Google Scholar]
- Singh A.; Bhattacharya R.; Shakeel A.; Sharma A. K.; Jeevanandham S.; Kumar A.; Chattopadhyay S.; Bohidar H. B.; Ghosh S.; Chakrabarti S.; Rajput S. K.; Mukherjee M. Hydrogel nanotubes with ice helices as exotic nanostructures for diabetic wound healing. Mater. Horiz. 2019, 6, 274–284. 10.1039/c8mh01298a. [DOI] [Google Scholar]
- Jiang H.; Zheng M.; Liu X.; Zhang S.; Wang X.; Chen Y.; Hou M.; Zhu J. Feasibility Study of Tissue Transglutaminase for Self-Catalytic Cross-Linking of Self-Assembled Collagen Fibril Hydrogel and Its Promising Application in Wound Healing Promotion. ACS Omega 2019, 4, 12606–12615. 10.1021/acsomega.9b01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming S. A.; Martin P.; Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M.; Jackson C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayo L. L.; Venault A.; Constantino V. G. R.; Caparanga A. R.; Chinnathambi A.; Ali Alharbi S.; Zheng J.; Chang Y. Design of hemocompatible poly (DMAEMA-co-PEGMA) hydrogels for controlled release of insulin. J. Appl. Polym. Sci. 2015, 132, 42365. 10.1002/app.42365. [DOI] [Google Scholar]
- Robbins M. J.; Sharp R. A.; Slonim A. E.; Burr I. M. Protection against streptozotocin-induced diabetes by superoxide dismutase. Diabetologia 1980, 18, 55–58. 10.1007/bf01228303. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Kang X.; Ji L.; Bai J.; Liu W.; Wang Z. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897. 10.2147/ijn.s168998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsarra I. A. Chitosan topical gel formulation in the management of burn wounds. Int. J. Biol. Macromol. 2009, 45, 16–21. 10.1016/j.ijbiomac.2009.03.010. [DOI] [PubMed] [Google Scholar]
- El-Kased R. F.; Amer R. I.; Attia D.; Elmazar M. M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 1–11. 10.1038/s41598-017-08771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]