Specifications Table
| Subject | Aquatic Science |
|---|---|
| Type of data | Image Table Figures Raw and analyzed DNA sequences Raw and analyzed HPLC data Raw and analyzed mass spectrometry data |
| How data were acquired | Metaphyton samples were provided from field collections in amber containers. Whole biomass and water samples were extracted, enriched, and analyzed for the presence of toxins. Strain identification was determined by 16S rRNA barcoding. Toxins were detected by HPLC-MS (6530 QTOF MS, Agilent, Santa Clara, CA). |
| Data source location | City/Town/Region: Austin, Texas Country: USA Name of Body of Water: Lady Bird Lake, a river-like reservoir on the (Texas) Colorado River Latitude and longitude (and GPS coordinates) for collected samples/data: Red Bud Isle Tip: 30.287850; -97.785967 Red Bud Isle W: 30.291115; -97.786422 Auditorium Shores: 30.264266; -97.754195 Metaphyton mats were collected in <1 m water accessed by boat or from shore. Collection sites were characterized as having silty sediments with some cobble and boulders. Hydrologic discharge data were provided by the Lower Colorado River Authority. Water quality parameters were collected with a Hydrolab MS5 Datasonde (OTT Hydromet, Loveland, CO, USA). Table 1 lists conditions documented on the day of collection. |
| Data format | Raw Analyzed |
| Identification | Individual cells/filaments were isolated for genetic and biochemical characterization. Genetic identification was performed using 16S rRNA PCR barcoding and Sanger sequencing. Results of the BLAST sequence analysis are shown in Table 2. |
| Strain Characteristics | Not Applicable – mixed community |
| Data accessibility | Repository name: Mendeley Data http://dx.doi.org/10.17632/7rv77w2yj3.1 |
1. Introduction
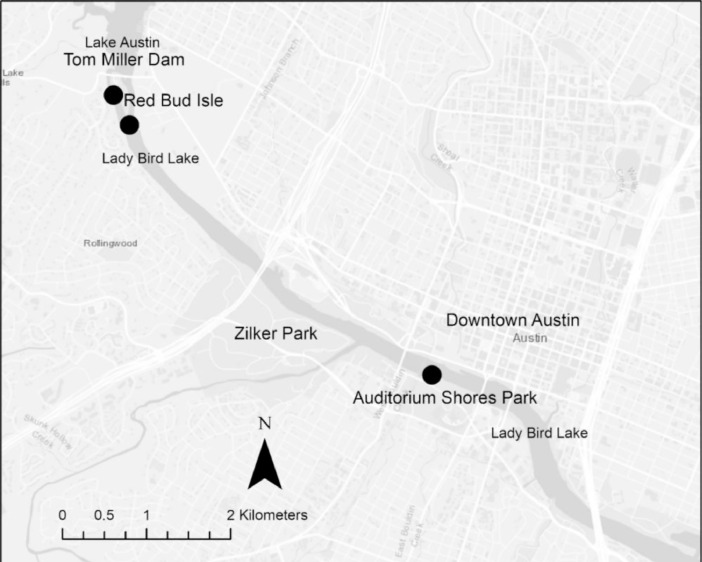
Mass canine deaths were first reported to City staff on August 3, 2019 after swimming and presumably ingesting toxin-producing cyanobacteria documented in Lady Bird Lake, Austin, Texas. Floating metaphyton mats were abundant and appeared to originate from benthic spires that concentrated near Red Bud Isle and Auditorium Shores, popular recreational destinations for the community (Fig. 1). There was up to 75% surface water coverage on the west side of Red Bud Isle, likely due to low hydrologic flow (Fig. 2). At Auditorium Shores, mats clustered along shorelines, increasing the likelihood for canine interactions. The mats persisted until November 12, 2019, at which point cooler temperatures and increased flows resulted in senescence.
Fig. 1.
Map of Lady Bird Lake, Austin, Texas and sampling sites at Red Bud Isle and Auditorium Shores.
Fig. 2.
Metaphyton in Lady Bird Lake, Austin, Texas, USA. The 2019 event resulted in dense surface water coverage near Red Bud Isle and Auditorium Shores.
Metaphyton mats were a complex assemblage of microalgae, bacteria, protozoa, and sediments, wherein filamentous cyanobacteria were dominant (Fig. 3). Microscopic inspection and DNA analyses support Geitlerinema, Limnothrix, Pseudanabaena, and Phormidium were common, and analysis of the mat materials by HPLC-MS detected high levels of dihydroanatoxin-a (dhATX) [1]. These metabolites were abundant in the algal biomass (Fig. 4), and only trace levels were detected in the water column. Dog mortalities were reported between 30 min to 2 h following exposure to algae-laden waters, suggesting that poisoning was due to accidental ingestion of algal cells containing anatoxins [2]. While there are numerous reports of dog neurotoxicosis from anatoxin-a (ATX) and homoanatoxin-a (HTX) exposure [3], [4], [5], [6], this may be among the first reports of dog fatalities associated with dhATX.
Fig. 3.
Micrographs of metaphyton mat materials at 100x (A) and 400x (B) magnification. Mats were a complex assemblage of several different species of filamentous cyanobacteria as well as unicellular microalgae, bacteria, protists, and sediments.
Fig. 4.
Total ion chromatogram of a crude biomass extract and the extracted ion chromatogram for dihydroanatoxin-a at 168.138 m/z (A). The EIC had two peaks eluting between 0.6 and 1.4 min, which correspond to the naturally-occurring isomers as shown by Puddick et al. [2]. The mass spectrum (B) shows dhATX eluting at 0.77 min, which had a parent ion at 168.1386 m/z using electrospray ionization in positive mode. The observed mass of analytes was within 0.5 ppm of the calculated mass (168.1383 u) for [dhATX + H]+.
Originally called Town Lake when impounded in 1960 (and then renamed in 2007), Lady Bird Lake is an 8.6 km constant-level, flow-through reservoir in the heart of downtown Austin and the last in-line impoundment of the Texas Colorado River. Initially the source of drinking water, power plant cooling, and industrial water, those functions have since been re-located. Lady Bird Lake now serves as a popular recreational destination welcoming over 2.5 million visitors annually for on-water activities and use of the 16 km hike-and-bike trail that runs along the shoreline.
Austin has conditions that are favorable for the growth of cyanobacteria, e.g., hot temperatures, low hydrologic flows, eutrophic conditions. Thus, it is likely these events may increase in frequency and duration. Ongoing research is characterizing individual strains from the mat materials to generate a genetic and biochemical database for watershed managers to rapidly detect harmful cyanobacteria and their toxins in local reservoirs. Ultimately, genetic and biochemical data will be compiled with hydrologic discharge and nutrient data from local agencies to outline the overall progression of the 2019 event.
2. Compositional Profile of the Strain's Biomass
| Lipid Profile | Not Available |
|---|---|
| CHNO Analysis (if available) | Not Available |
| Protein, Carbohydrate, Lipid, Ash Content (if available) | Not Available |
| Protein and Amino Acid Profile (if available) | Not Available |
| Carbohydrate Profile (if available) | Not Available |
| Toxin Concentrations (ug/L) (if available) | Not Available |
| References to Methods Used for Profiling | Not Available |
3. Environmental Impact
Dog fatalities occurred within 30 min to 2 h following exposure to cyanobacteria-dominated mat materials.
There were concerns of human contact and poisoning.
There were concerns of localized anoxia and release of intracellular toxins associated with senescence due to the dense surface coverage.
4. Toxicity Information
HPLC-MS analyses supported dhATX was the predominant cyanotoxin in metaphyton samples. While reports of ATX poisoning are more common with dog mortalities [3], [4], [5], others have demonstrated dhATX can be more toxic than ATX when delivered through oral routes [2,7]. Among the strains identified thus far, Phormidium was abundant in samples, which is a well-known producer of anatoxins [8], [9].
5. Economic Impact
-
•
The event closed recreational parks, reducing visits by locals and tourists.
-
•
Uncertainty and fears about toxins resulted in loss of revenue for companies that rent equipment, e.g., canoes and stand-up paddle boards, for recreation on the reservoir.
-
•
For the duration of the event, the City of Austin incurred increased expenditures for monitoring and testing, signage, and emergency meetings.
6. Experimental Design, Materials, and Methods
6.1. Sample collection
Sites were accessed from shore during the afternoon (14:30 h–17:45 h) on 5 August 2019. At each site, benthic mats were collected by scrapping cohesive material off the surface sediments, and surface mats, i.e., metaphyton, were scooped off the water surface and placed into 250 mL amber plastic bottles. Surface water samples were collected at 0.1 m depth with 250 mL amber plastic bottles, ensuring little to no mat material was simultaneously collected. At both sites, mat biomass was collected nearest shore, in 1 m or less of water, which would represent the material most likely to be encountered by a swimming canine or human. Samples were kept on ice, with split samples transported to the University of Texas at Austin the day of collection for species isolation, toxin extraction, and analyses.
6.2. HPLC-MS analysis
Biochemical analyses were performed to determine the presence/absence of targeted cyanotoxins (e.g., anatoxins, microcystins, cylindrospermopsin, saxitoxins) in water and biomass samples. All extraction and enrichment steps were performed in replicate in dimmed lighting, and sample tubes were wrapped in foil to minimize photolysis. Prior to processing, water samples were centrifuged for 5 min at 2100 g to sediment debris; metaphyton samples were sonicated for 30 min and the biomass was pelleted by centrifugation. The resulting supernatants were used for toxin enrichment and analysis. Clarified lake water and algal extracts (50 mL) were passed over a Waters Sep Pak C18 solid-phase extraction (SPE) cartridge to enrich targeted compounds. SPE cartridges were activated with 5 mL of 100% MeOH and conditioned using 5 mL of double-distilled water. Then 50 mL of water or algal sample supernatant was slowly pushed through the column to adsorb metabolites. The eluate was retained as the sample load, and 5 mL of 100% MeOH was pushed through the cartridge to collect metabolites adsorbed on the column. Methanolic fractions were dried under nitrogen at 40°C in pre-weighed glass vials. Once dried, extract residues were resuspended in 1 mL of 5% MeOH (aqueous), representing a 50 × enrichment from the original samples. For each sample, 100 µL of extract was transferred to a glass insert in a 2 mL amber glass vial in preparation for high-performance liquid chromatography-mass spectrometry (HPLC-MS).
Biomass and water enrichments were analyzed on an Agilent 6530 QTOF instrument. Samples (10 µL) were injected and separated on a Zorbax Eclipse Plus C18 column (50 mm length, 2.1 mm ID, 5 µm-particle size, packed) using a water:methanol gradient with 0.1% formic acid addition. The gradient was ramped from 1.0–95.0% MeOH over 12 min and held at 100% MeOH for 6 min. Mass spectrometry data were collected following electrospray ionization (ESI) operated in positive mode to produce the total ion chromatogram (TIC) and related extracted ion chromatograms (EIC). Data were analyzed using Agilent's Mass Hunter software and compared to the NIST database. All raw and processed mass spectrometry data are archived with Mendeley Data [1].
6.3. 16S rRNA barcode analysis
Individual cells and filaments were isolated from mat samples using a drawn-out glass Pasteur pipet, and isolates were maintained in 15 mL conical tubes, containing BG-11 medium (utex.org). Subsamples of isolates were transferred into pre-labeled 2 mL screw top centrifuge vials. Lysis buffer (200 µL; 1 M NaCl, 70 mM Tris, 30 mM Na2EDTA, pH = 8.6) was added to the samples, which were then vortexed and centrifuged for 1 min at 16,500 g. Following centrifugation, the supernatants were removed, and another 200 µL of lysis buffer was added along with acid-washed glass beads (300–500 µm, Fisher), 200 µL 24:1 chloroform:isoamyl alcohol, and 25 µL 10% dodecyltrimethylammonium bromide (w/v in ddH2O). Samples were then placed in a bead beater for 40 s. Aqueous and organic phases were partitioned by centrifugation for 2 min at 2100 g. Following, 100 µL of the top (aqueous) layer was removed and placed in a new 1.5 mL snap-cap centrifuge tube. The samples were then processed using a Geneclean Turbo (MP Biomedicals) DNA extraction kit per the manufacturer's instructions. Extracted DNA was quantified using 1 µL of purified DNA on a QubitTM 4 fluorometer using the QubitTM dsDNA high-sensitivity (HS) assay (ThermoFisher Scientific, USA).
The 16S region was amplified by PCR according to Janse et al. [10]. Each 25 µL reaction contained 20 ng DNA, 1 µL of 10 × buffer (ThermoFisher Scientific, USA), 1.5 µL of 25 mM MgCl2 (ThermoFisher Scientific, USA), 0.5 µL of 10 mM dNTP mix, 0.25 µL of 50 mM forward and reverse primer, 0.1 µL Taq (ThermoFisher Scientific, USA), and the remaining volume was nuclease-free ddH2O. Thermal cycler conditions were as follows: 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min, followed by 72 °C for 5 min, with a final step of 4 °C until the samples were removed from the thermal cycler. The presence of amplicons was confirmed by running 10 µL of PCR product with 2 µL of 6 × dye on 1.5% (w/v) agarose in 1 × Tris-acetate-EDTA gels. PCR samples containing a single band were purified using a GeneJET PCR kit per the manufacturer's instructions (Thermo Scientific, USA). Purified samples were submitted for Sanger sequencing to the University of Texas DNA Sequencing Facility. Raw sequences were trimmed prior to analysis using NCBI's nucleotide BLAST database (Table 2). Raw and trimmed sequence data are archived with Mendeley Data [1].
Table 1.
Site conditions and average discharge rates in cubic feet per second (CFS) from the Tom Miller Dam on 5 August 2019.
| Site | Dissolved Oxygen (mg/L) | Temperature (°C) | pH | Specific Conductance (μS/cm) |
|---|---|---|---|---|
| Red Bud Isle (tip) | 7.69 | 30.55 | 7.78 | 445.00 |
| Red Bud Isle (west) | 8.93 | 32.04 | 7.79 | 447.00 |
| Auditorium Shores | 8.30 | 31.47 | 7.73 | 521.00 |
| Average Discharge Rate (CFS) | 3-Day Average (CFS) | 5-Day Average (CFS) | 7-Day Average (CFS) | |
|---|---|---|---|---|
| Tom Miller Dam | 70.17 | 70.70 | 70.89 | 81.23 |
Table 2.
Results from NCBI-BLAST alignments and best assignments for both 16S forward (F) and reverse (R) sequencing reactions.
| Genus | species | Reaction | E-value | % ID |
|---|---|---|---|---|
| Limnothrix | sp. | F | 0 | 98.44 |
| R | 0 | 99.73 | ||
| Pseudanabaena | lonchoides | F | 0 | 100 |
| R | 0 | 99.75 | ||
| Phormidium | cf nigrum | F | 0 | 96.59 |
| R | 3.00E-158 | 99.68 | ||
| Geitlerinema | splendidum | F | 1.00E-175 | 95.8 |
| R | 0 | 97.38 |
Funding
This research was supported by a grant from the City of Austin (Phytoplankton Assessment Project ID 6660.063).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
The authors would like to thank Dr. Ian Riddington, Director of the UT Mass Spectrometry Core Facility, for his assistance.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.106344.
Appendix. Supplementary materials
References
- 1.Manning S.R., Perri K.A., Bellinger B. Mendeley Data; 2020. Metaphyton community and biochemical analysis of neurotoxic cyanobacterial mats in Lady Bird Lake, Austin, Texas (August 5, 2019) v(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.J. Puddick, S. Finch, P. Truman, R. van Ginkel, S. Murray, C. Page, A. Selwood, S. Wood, R. Munday (2018) The acute toxicity and biochemical assessment of anatoxin-a and dihydroanatoxin-a. Prepared for the New Zealand Ministry for the Environment. Cawthron Report No. 3094. 44 p.
- 3.Gugger M., Lenoir S., Berger C., Ledreux A., Druart J.C., Humbert J.F., Guette C., Bernard C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon. 2005;45:919–928. doi: 10.1016/j.toxicon.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Puschner B., Pratt C., Tor E.R. Treatment and diagnosis of a dog with fulminant neurological deterioration due to anatoxin-a intoxication. J. Vet. Emerg. Crit. Care. 2010;20:518–522. doi: 10.1111/j.1476-4431.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- 5.Fastner J., Beulker C., Geiser B., Hoffmann A., Kröger R., Teske K., Hoppe J., Mundhenk L., Neurath H., Sagebiel D., Chorus I. Fatal neurotoxicosis in dogs associated with tychoplanktic, anatoxin-a producing Tychonema sp. in mesotrophic lake Tegel, Berlin. Toxins. 2018;10:60. doi: 10.3390/toxins10020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood S.A, Selwood A.I., Rueckert A., Holland P.T., Milne J.R., Smith K.F., Smits B., Watts L.F., Cary C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon. 2007;50:292–301. doi: 10.1016/j.toxicon.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 7.J. Puddick, Acute toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a, accepted in Chemosphere. Please contact Jonathan.Puddick@cawthron.org.nz for a copy. [DOI] [PubMed]
- 8.McAllister T.G., Wood S.A., Hawes I. The rise of toxic benthic Phormidium proliferations: a review of their taxonomy, distribution, toxin content and factors regulating prevalence and increased severity. Harmful Algae. 2016;55:282–294. doi: 10.1016/j.hal.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Wood S.A., Smith F.M., Heath M.W., Palfroy T., Gaw S., Young R.G., Ryan K.G. Within-mat variability in anatoxin-a and homoanatoxin-a production among benthic Phormidium (cyanobacteria) strains. Toxins. 2012;4:900–912. doi: 10.3390/toxins4100900. https://doi:10.3390/toxins4100900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janse I., Meima M., Kardinaal W.E., Zwart G. High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl. Environ. Microbiology. 2003;69:6634–6643. doi: 10.1128/AEM.69.11.6634-6643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.