Abstract
An 85-year-old woman with antibiotics-resistant pneumonia after surgery for metastatic brain tumor from lung cancer was consulted to our department. Chest CT showed diffuse GGO bilaterally. BALF showed elevated ratios of lymphocytes and CD4/CD8. Tests for bacteria, mycobacteria, and fungi were negative. She improved following levetiracetam discontinuance and systemic corticosteroid administration, and we diagnosed levetiracetam-induced lung injury. Although levetiracetam is widely used, few reports of levetiracetam-induced pneumonia exist. Changes in chest images may occur after levetiracetam administration if patients have multiple risk factors for development of drug-induced interstitial lung disease. Bronchoscopy is useful for differential diagnosis if new lung lesions appear after starting levetiracetam.
Keywords: Drug-induced interstitial lung disease, Levetiracetam, Lung cancer
Abbreviations: AED, antiepileptic drug; BALF, bronchoalveolar lavage fluid; EGFR, epidermal growth factor receptor; GGO, ground-glass opacity; ILD, interstitial lung disease; LEV, levetiracetam
1. Introduction
Levetiracetam (LEV) is one of a newer type of antiepileptic drug (AED) that expresses efficacy by binding to synaptic vesicle protein 2A, which suppresses epileptic seizures [1]. The NICE (National Institute for Health and Care Excellence) guideline and expert opinion recommended its use for various types of epilepsy as a first-line treatment alternative to older AEDs [2,3]. AEDs including LEV is also widely used for the prevention of convulsions in patients with a primary or metastatic brain tumor and during the perioperative period for brain tumor surgery [[4], [5], [6], [7], [8]]. Typical adverse events of LEV are asthenia, dizziness, flu syndrome, headache, infection, rhinitis, and somnolence [9], but there are very few reports of lung injury. We report the case of LEV-induced interstitial lung disease (ILD) for which the patient was investigated by bronchoscopy.
2. Case report
A never-smoking woman aged 85 years was transferred to our hospital for further examination of a brain tumor. She had a history of right lower lobectomy with tuberculosis at age 27 years, a left lower lobe reduction surgery for non-small cell carcinoma at age 76, and bronchial asthma at age 80, with no recurrence of lung cancer and good control of bronchial asthma. She became aware of a headache in X-3 month and visited a nearby hospital with dysarthria in X-2 month, at which time a cerebral infarction was diagnosed by CT and an antiplatelet drug was started. However, right hemiplegia newly occurred, and the dysarthria worsened in X-1 month. She was transferred to our neurosurgery department after an additional brain MRI scan revealed a brain tumor in the left parietal lobe.
On admission, she had motor aphasia and right paresis without sensory disturbance. Brain MRI showed a single tumor with extensive edema around 19 × 25 × 21 mm in size in the left parietal lobe, and chest CT showed a 38-mm tumor adjacent to the sutures of the previous lobectomy in the lower lobe of the left lung (Fig. 1, Fig. 2A). We suspected intrapulmonary recurrence and metastatic brain tumor based on imaging findings started glycerol at 600 mg/day and performed CyberKnife therapy on day 12. After adding dexamethasone 6.6 mg/day, her dysarthria and right paresis improved, and glycerol and corticosteroid administration was terminated on day 23. However, hemiplegia and aphasia gradually worsened a few days later, and repeat brain MRI showed further exacerbation of edema (Fig. 1-B). On day 27, glycerol and corticosteroid administration were resumed preoperatively, and LEV administration was also begun. Left parietal tumor resection was performed on day 31. Postoperative pathology revealed that the tumor was positive for cytokeratin AE1/AE3, negative for thyroid transcription factor 1, and positive for p40, suggesting brain metastasis of lung squamous cell carcinoma (Fig. 2-D–G). We diagnosed the recurrence of non-small cell lung carcinoma that we determined to be stage IV A (cT2aN0M1b) and performance status 3. The brain tumor specimen removed was positive for epidermal growth factor receptor (EGFR) mutations (L858R). The tumor proportion score of anti-programmed cell death ligand 1 was 70%. Postoperatively, rehabilitation was resumed, corticosteroid therapy ended on day 54 due to improvement of the edema on brain MRI (Fig. 1-C), and only LEV was continued to prevent seizures.
Fig. 1.
Brain MRI three-dimensional fast spoiled gradient-echo images. (A) Single tumor with extensive edema around 19 × 25 × 21 mm in size was found in the left parietal lobe on admission. (B) Brain edema worsened after CyberKnife treatment. (C) Brain edema markedly improved after removal of the brain tumor. (D) Macroscopic specimen of the resected tumor showed a yellowish-white tumor of 20 × 15 × 10 mm in size and with a necrotic tendency. (E) A proliferation of atypical spindle cells, multinucleated cells, and multinucleated giant cells with eosinophilic cytoplasm was observed on hematoxylin and eosin staining. (F) Immunostaining with cytokeratin AE1/AE3 was extensively positive, suggesting an epithelial tumor. (G) Anti-p40 antibody was positive, suggesting lung squamous cell carcinoma.
Fig. 2.
Chest CT images. (A) A 38-mm tumor was found in the left lower lobe of the lung, and no specific lesion was found in the background lung. (B) Multiple GGOs were found in both lung fields with small bilateral pleural effusion. (C) GGOs disappeared after LEV discontinuation and corticosteroid treatment.
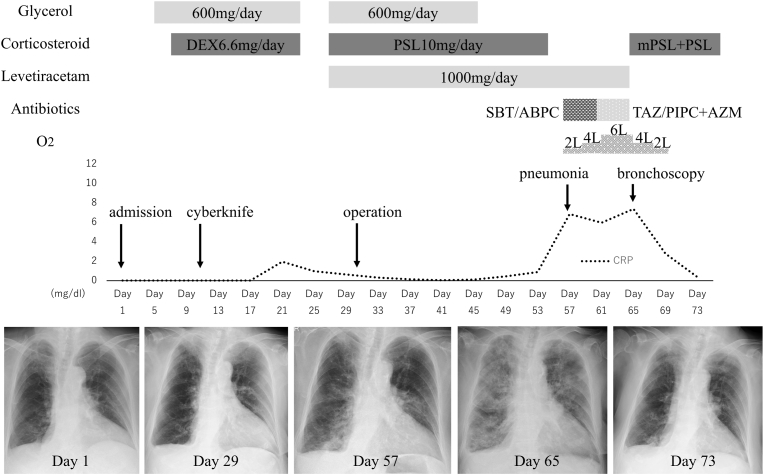
On day 57, fever, dyspnea, and cough appeared, and a chest X-ray showed ground-glass opacities (GGO) in the left middle, left lower, and right lower lung fields (Fig. 3). The patient was diagnosed as having pneumonia and was administered sulbactam/ampicillin, followed by tazobactam/piperacillin and azithromycin, but her X-ray findings continued to deteriorate. On day 64, a neurosurgeon consulted with the Department of Respiratory Medicine due to her worsening respiratory failure. Physical examination revealed no obvious abnormal auscultation findings and no rash or edema. Laboratory examinations revealed a significantly high serum level of C-reactive protein at 7.2 mg/dL and mildly high serum level of KL-6 at 583 U/ml. Her serum BNP (50.4 pg/mL), AST (22 U/L), ALT (14 U/L), creatinine (0.51 mg/dL) and peripheral blood eosinophil count (88/μL) were almost within normal range. Chest CT showed GGO in both lung fields and a small amount of pleural effusion (Fig. 2-B). Bronchoalveolar lavage fluid (BALF) was transparent with a 64% recovery rate. Its cell count is 2.5 × 10∧5 and contained 30.2% macrophages, 64.2% lymphocytes, 4.2% neutrophils, and 1.4% eosinophils, and the CD4/CD8 ratio was 3.5. Microbiological tests including for bacteria, mycobacteria, fungi, and polymerase chain reaction testing for Pneumocystis jirovecii in BALF were negative, as were cytomegalovirus antigenemia in serum and a rapid test for influenza virus. No autoantibody tests significantly associated with connective tissue diseases or vasculitis were positive. Based on the above test results, she was diagnosed as having LEV-induced ILD. Withdrawal of LEV and administration of methylprednisolone pulse therapy and prednisolone for 10 days improved her respiratory failure and chest CT findings (Fig. 2-C). She was discharged on day 79 after rehabilitation for right paresis and aphasia.
Fig. 3.
Changes over time in the C-reactive protein (CRP) level and the pulmonary lesion on chest X-ray. The patient underwent CyberKnife treatment on day 12 with glycerol and dexamethasone. However, improvement of neurological symptoms was not obtained, and tumor removal was performed on day 31 with the addition of LEV administration. She developed pneumonia on day 57 after she began to receive LEV only from day 55, and it worsened without responding to antibiotics. Drug-induced pneumonia was diagnosed with bronchoscopy, and systemic corticosteroid administration rapidly improved pneumonia. DEX, dexamethasone; PSL, prednisolone; mPSL, methylprednisolone; SBT/ABPC, sulbactam/ampicillin; TAZ/PIPC, tazobactam/piperacillin; AZM, azithromycin.
3. Discussion
We report a case of adult-onset LEV-induced ILD. Only one reported case of lung injury caused by LEV in an adult was found via a PubMed search [10]. This previous case was diagnosed as eosinophilic pneumonia based on non-segmental infiltrates detected by CT and findings of increased peripheral eosinophils. In our patient, lymphocytes without eosinophilia were dominant in the BALF, and CT showed findings reminiscent of a non-specific interstitial pneumonia pattern. Although the number of cases is small, phenytoin, carbamazepine, zonisamide, and lamotrigine have been reported as AEDs that can cause drug-induced ILD [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. CT findings in those cases have been reported to be mostly consolidation suggesting eosinophilic pneumonia or organizing pneumonia [[11], [12], [13], [14], [15], [16], [17], [18], [19]] and GGO or reticular shadows in a small number of cases [20,21]. From the CT findings which are mainly based on GGO, Pneumocystis jirovecii pneumonia, viral pneumonia, pulmonary edema including neurogenic pulmonary edema, Mendelson syndrome, drug reaction with eosinophilia and systemic symptom (DRESS) syndrome were considered as differential diseases in addition to drug-induced pneumonia. Pulmonary edema and Mendelson syndrome were ruled out because of the long period (25 days) following the last neurological surgery, lack of elevated BNP levels, and no apparent episodes of vomiting or aspiration. DRESS syndrome is a fatal hypersensitivity reaction and most commonly associated with AEDs, sulfonamides, and allopurinol [22]. Although this syndrome is known to be associated with pulmonary manifestations, we have ruled out the possibility of this disease due to the absence of a skin rash and peripheral blood eosinophilia. Antimicrobial agents such as sulbactam/ampicillin, tazobactam/piperacillin, and azithromycin were used during treatment for pneumonia. However, since all of these drugs were started after the appearance of GGO, the likelihood of drug-induced lung injury due to these drugs was considered low. The results of bronchoscopy were also particularly useful in differentiating pulmonary infectious diseases. Interstitial pneumonia developed 30 days after the start of LEV, but corticosteroid were concurrently being used for brain edema for 27 days from the start, and the manifestation of lung injury may have been masked by the corticosteroid (Fig. 3). We speculated that multiple factors for drug-induced ILD [23], such as old age, decreased lung function, pre-existing respiratory disease, advanced-stage malignancy, and poor performance status were involved in the development of ILD in this patient.
In general, it is very important to prevent convulsions in patients with brain tumors. Convulsions occur in approximately 20–45% of patients with brain tumor resulting in a poor quality of life [24]. The frequency of seizures is also reported to be 15% in patients with metastatic brain tumors and 15–20% in patients who have undergone supratentorial craniotomy [4,25]. The American Academy of Neurology, the Association for Neurological Surgeons, and the European Association of Neuro-Oncology recommended against seizure prophylaxis with AEDs for patients with primary or metastatic brain tumors without a history of seizures [[4], [5], [6]]. When limited to the perioperative period, there is still no comprehensive study that includes not only the old generation of AEDs but also the new generation of AEDs such as LEV, and the usefulness of the prophylactic administration of AEDs remains controversial [7,8,26,27].
LEV is one of a new generation of AEDs that is thought to express its effect by binding to synaptic vesicle protein 2A, which can inhibit epileptic seizure activity [1]. The most distinctive feature of LEV is that it is not metabolized by the hepatic cytochrome system but in the kidney, unlike the previous generation of enzyme-inducing AEDs such as carbamazepine, phenytoin, valproate, and barbiturates [28]. Therefore, LEV has very little drug interaction and can be easily used in combination with other AEDs. In recent years, LEV has been highly recommended for epilepsy treatment because of its efficacy [2,3]. In cancer cases, the interaction between anticancer drugs and AEDs is also clinically important. Our patient had EGFR mutation-positive lung cancer, and there is a possibility of introducing treatment with EGFR tyrosine kinase inhibitors in the future. Unlike CYP3A4-inducing AEDs such as carbamazepine or phenytoin, LEV does not promote the metabolism of tyrosine-kinase inhibitors and thus has the advantage that convulsion prevention treatment with LEV is easy to use together with cancer treatment [29]. Because similar problems occur with many anticancer agents, such as cyclophosphamide, camptothecin derivatives, taxanes, and topoisomerase inhibitors [30,31], the choice of LEV would be of great benefit in cases where chemotherapy with these agents may be administered for cancer treatment. There are no reports, to our knowledge, on the combination of immune checkpoint inhibitors and AEDs, and thus, it will be necessary to accumulate data in the future.
In conclusion, LEV is widely used as a new-generation AED to treat epilepsy and prevent seizures with fewer drug interactions including anticancer drugs. LEVs are generally considered to be associated with a low frequency of ILD, attention should be paid to the development of drug-induced lung injury when there are multiple risks of drug-induced ILD. Bronchoscopy may be useful for differential diagnosis when GGO newly appear during LEV administration.
Declaration of competing for interest
The authors declare no Conflicts of Interest (COI) in association with this article.
References
- 1.Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatric Dis. Treat. 2008;4:507–523. doi: 10.2147/ndt.s2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care excellence epilepsies: diagnosis and management. Clinical Guideline. 2012 https://www.nice.org.uk/guidance/cg137 [Cited 2020 August 27th]. Available from: [Google Scholar]
- 3.Shih J.J., Whitlock J.B., Chimato N., Vargas E., Karceski S.C., Frank R.D. Epilepsy treatment in adults and adolescents: expert opinion. Epilepsy Behav. 2016;69:186–222. doi: 10.1016/j.yebeh.2016.11.018. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Glantz M.J., Cole B.F., Forsyth P.A. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 5.Mikkelsen T., Paleologos N.A., Robinson P.D. The role of prophylactic anticonvulsants in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neuro Oncol. 2010;96:97–102. doi: 10.1007/s11060-009-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soffietti R., Abacioglu U., Baumert B. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO) Neuro Oncol. 2017;19:162–174. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeap M.C., Chen C.C., Liu Z.H. Postcranioplasty seizures following decompressive craniectomy and seizure prophylaxis: a retrospective analysis at a single institution. J. Neurosurg. 2018;131:936–940. doi: 10.3171/2018.4.JNS172519. [DOI] [PubMed] [Google Scholar]
- 8.Goldlust S.A., Hsu M., Lassman A.B., Panageas K.S., Avila E.K. Seizure prophylaxis and melanoma brain metastases. J. Neuro Oncol. 2012;108:109–114. doi: 10.1007/s11060-012-0802-y. [DOI] [PubMed] [Google Scholar]
- 9.Cereghino J.J., Biton V., Abou-Khalil B., Dreifuss F., Gauer L.J., Leppik I. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–242. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- 10.Fagan A., Fuld J., Soon E. Levetiracetam-induced eosinophilic pneumonia. BMJ Case Rep. 2017 doi: 10.1136/bcr-2016-219121. pii: bcr2016219121, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angle P., Thomas P., Chiu B., Freedman J. Bronchiolitis obliterans with organizing pneumonia and cold agglutinin disease associated with phenytoin hypersensitivity syndrome. Chest. 1997;112:1697–1699. doi: 10.1378/chest.112.6.1697. [DOI] [PubMed] [Google Scholar]
- 12.Tamada T., Nara M., Tomaki M., Ashino Y., Hattori T. Secondary bronchiolitis obliterans organizing pneumonia in a patient with carbamazepine-induced hypogammaglobulinemia. BMJ Case Rep. 2009 doi: 10.1136/bcr.2006.063842. bcr2006063842. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez Olivencia G., Martín Borge V., Sancho Bueso T. Bronchiolitis obliterans organizing pneumonia induced by carbamazepine. Med. Clin. 2007;128:198–199. doi: 10.1016/s0025-7753(07)72534-4. (in Spanish) [DOI] [PubMed] [Google Scholar]
- 14.Annoh Gordon R., Silhan L. A case report of phenytoin-induced eosinophilic pneumonia. Respir Med Case Rep. 2019;28:100922. doi: 10.1016/j.rmcr.2019.100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banka R., Ward M.J. Bronchiolitis obliterans and organizing pneumonia caused by carbamazepine and mimicking community acquired pneumonia. Postgrad. Med. 2002;78:621–622. doi: 10.1136/pmj.78.924.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orliaguet O., Langin T., Gavazzi G., Brambilla C. Acute eosinophilic pneumonia due to hypersensitivity to carbamazepine. Rev. Mal. Respir. 1998;15:797–799. (in French) [PubMed] [Google Scholar]
- 17.Milesi-Lecat A.M., Schmidt J., Aumaitre O., Kemeny J.L., Moinard J., Piette J.C. Lupus and pulmonary nodules consistent with bronchiolitis obliterans organizing pneumonia induced by carbamazepine. Mayo Clin. Proc. 1997;72:1145–1147. doi: 10.4065/72.12.1145. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya R., Tanizaki H., Nakajima S. DIHS/DRESS with remarkable eosinophilic pneumonia caused by zonisamide. Acta Derm. Venereol. 2015;95:229–230. doi: 10.2340/00015555-1863. [DOI] [PubMed] [Google Scholar]
- 19.Ghandourah H., Bhandal S., Brundler M.A., Noseworthy M. Bronchiolitis obliterans organizing pneumonia associated with anticonvulsant hypersensitivity syndrome induced by lamotrigine. BMJ Case Rep. 2016 doi: 10.1136/bcr-2014-207182. pii: bcr2014207182, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uji M., Sugama Y., Matsushita H. Carbamazepine-induced pneumonitis definitively diagnosed by accidental readministration. Nihon Kokyuki Gakkai Zasshi. 2005;43:150–154. (in Japanese) [PubMed] [Google Scholar]
- 21.Okamoto H., Ishimaru T., Ohgami N., Furugaki K., Emoto T., Ekimura A case of drug-induced interstitial pneumonia due to phenytoin. Nihon Kokyuki Gakkai Zasshi. 2009;47:758–762. (in Japanese) [PubMed] [Google Scholar]
- 22.Taweesedt P.T., Nordstrom C.W., Stoeckel J. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. BioMed Res. Int. 2019 doi: 10.1155/2019/7863815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skeoch S., Weatherley N., Swift A.J. Drug-induced interstitial lung disease: a systematic review. Clin. Med. 2018;7 doi: 10.3390/jcm7100356. pii: E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maschio M. Brain tumor-related epilepsy. Curr. Neuropharmacol. 2012;10:124–133. doi: 10.2174/157015912800604470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulman J., Greenhalgh J., Marson A.G. Antiepileptic drugs as prophylaxis for post-craniotomy seizures. Cochrane Database Syst. Rev. 2013;28:CD007286. doi: 10.1002/14651858.CD007286.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Kamenova M., Stein M., Ram Z. Prophylactic antiepileptic treatment with levetiracetam for patients undergoing supratentorial brain tumor surgery: a two-center matched cohort study. Neurosurg. Rev. 2019 doi: 10.1007/s10143-019-01111-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen C.C., Rennert R.C., Olson J.J. Congress of neurological Surgeons systematic review and evidence-based guidelines on the role of prophylactic anticonvulsants in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84:E195–E197. doi: 10.1093/neuros/nyy545. [DOI] [PubMed] [Google Scholar]
- 28.Patsalos P.N. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol. Ther. 2000;85:77–85. doi: 10.1016/s0163-7258(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 29.Thiessen B., Stewart C., Tsao M. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Canc. Chemother. Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 30.Vecht C.J., Kerkhof M., Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncol. 2014;19:751–759. doi: 10.1634/theoncologist.2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bénit C.P., Vecht C.J. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neurooncol Pract. 2016;3:245–260. doi: 10.1093/nop/npv038. [DOI] [PMC free article] [PubMed] [Google Scholar]