Abstract
The neuronal ceroid lipofuscinoses (NCL) are a collection of lysosomal storage diseases characterised by the accumulation of characteristic inclusions containing lipofuscin in various tissues of the body and are one of the causes of progressive myoclonic epilepsy. Mutations in at least thirteen genes have been identified as causes of NCL, which can present as infantile, late-infantile, juvenile or adult forms. CLN6 codes for an endoplasmic reticulum transmembrane protein of unknown function. Homozygous and compound heterozygous mutations of the gene are associated with both late-infantile (LINCL) and adult onset (ANCL) forms of NCL, including Kufs disease, comprising ANCL without associated visual loss. Moyamoya, a rare vasculopathy of the circle of Willis, has been reported in conjunction with a number of inflammatory and other diseases, as well as a handful of lysosomal storage diseases. To our knowledge, this is the first reported case of Moyamoya in the context of the neuronal ceroid lipofuscinoses or a CLN6-related disease.
Abbreviations: NCL, neuronal ceroid lipofuscinosis; LINCL, late-infantile neuronal ceroid lipofuscinosis; ANCL, adult neuronal ceroid lipofuscinosis; SEP, somatosensory evoked potentials; mtDNA, mitochondrial DNA; MERRF, mitochondrial epilepsy with ragged red fibres; WAIS-IV, Wechsler Adult Intelligence Scale (4th edition); BMIPB, the Brain Injury Rehabilitation Trust Memory and Information Processing Battery; Wiegl, Weigl Color Form Sorting Test; PPT1, palmitoyl-protein thioesterase 1; TPP1, tripeptidyl peptidase 1
Keywords: Neuronal ceroid lipofuscinosis, NCL, ANCL, Kufs disease, CLN6, Moyamoya
Highlights
-
•
There are many causes of progressive myoclonic epilepsy, including the neuronal ceroid lipofuscinoses
-
•
CLN6 homozygous and bi-allelic mutations are known to cause neuronal ceroid lipofuscinosis and Kufs disease
-
•
This appears to be the first reported case of Kufs disease due to CLN6 bi-allelic mutations in association with Moyamoya
1. Case report
A 16-year-old left-handed male patient of Caucasian British ancestry presented with a mild, non-progressive upper limb postural tremor but no other symptoms for several years until, without clear precipitant, he experienced two unprovoked generalised tonic–clonic seizures, both occurring in sleep. His antecedent history was unremarkable except for a viral respiratory infection at birth requiring 2 days of mechanical ventilation on the neonatal intensive care unit. He attained his cognitive and motor milestones appropriately, attended mainstream school and completed 14 GCSEs. He was otherwise well, except for ichthyosis. There is no history of parental consanguinity. There is a family history of neurofibromatosis type 1 in a nephew and Perthes disease in a brother, and a history of diabetes and hypertension in his father and mother, respectively. His father also had ichthyosis.
Following the nocturnal seizures, he underwent a number of investigations. His initial MRI and EEG were normal. Anticonvulsant medication, specifically sodium valproate, was commenced following a further convulsive seizure fourteen months later.
He continued to experience convulsive seizures every 3 to 6 months; these were initially nocturnal, but later arose from wakefulness. Some of the seizures were preceded by a visual aura, consisting of superimposition of peripheral vision into the central field (polyopia). There was also gradual worsening of his upper limb tremor, manifesting as an irregular jerky tremor at rest which worsened on action, especially in the right hand. It improved significantly on distraction. He also noticed lower limb tremor with associated unsteadiness, especially when descending stairs or slopes, with his left leg being more affected than the right. He described his legs as feeling ‘like jelly’ with occasional falls. He also noticed general cognitive slowing, fatigue and effortfulness when engaging in conversations with people less well known to him. The motor symptoms were initially attributed to valproate therapy and he was switched to levetiracetam with no clear improvement.
He underwent further investigations at 20 years of age. An MRI at this time showed some prominence of the cerebellar folia suggestive of mild generalised cerebellar parenchymal volume loss. White cell enzyme analysis was normal. Cerebrospinal fluid analysis revealed normal cell counts and protein values, with no evidence of unmatched oligoclonal bands. No mutations were identified in SLC2A1 or DYT-1a. Total cholesterol was elevated at 6.5 and he tested positive for pANCA but negative for serine proteinase 3 (PR3) antibodies. He was diagnosed with functional movement disorder and referred for specialist physiotherapy with some improvement in his symptoms.
However, his symptoms progressed and by the age of 22 years, he was wheelchair-bound. Fine motor skills had deteriorated such that he was unable to cut up food and his handwriting was illegible. He had prominent and widespread spontaneous and action myoclonus, to the extent that even dreaming about movement would precipitate myoclonus. His voice had developed a stuttering stammer. He had also developed ‘twitching’ and ‘rippling’ of muscles in his shoulders, upper limbs and torso. There were no sensory changes or sphincter disturbance. His convulsive seizures had also become more frequent, occurring on average once per week.
Clinical examination at this time revealed occasional facial myoclonus (resembling myokymia) and hypermetric saccades with nystagmus on horizontal gaze. Ophthalmic review revealed normal appearances of the discs and retina. Limb tone and power were normal. Deep tendon reflexes were present and symmetrical in the upper limbs, brisk in the lower limbs with flexor plantar responses. There was a postural tremor markedly exacerbated by action with some action myoclonus. Marked orthostatic tremor was noted on standing. There was subtle dysmetria and dysdiadokokinesia on the right. There was no evidence of telangiectasia or oculomotor apraxia. Systemic examination revealed marked ichthyoses but no organomegaly. Neuropsychometry, including screens of intellect (WAIS-IV), memory (BMIPB), language (graded naming test) and executive function (Wiegl and verbal fluency tests), showed him to be functioning within the normal range without any specific cognitive deficits.
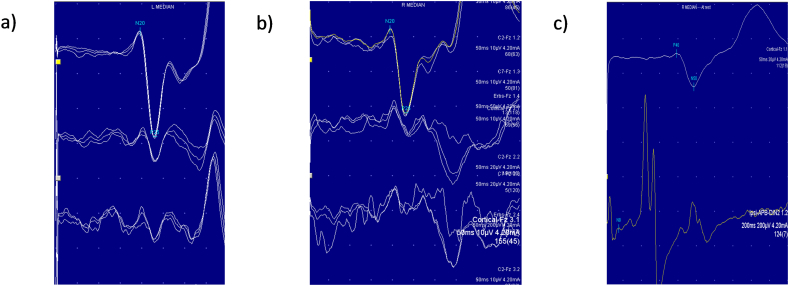
He was admitted for further investigations including prolonged EEG monitoring with video telemetry. There were independent runs of fast activity seen equally in both centroparietal regions. One seizure was captured with head turning to right and posturing of the left arm without a clear focus of onset (Fig. 1). There was no photosensitivity. EMG showed frequent interruptions of muscle tone intermixed with brief muscle jerks whilst holding his limbs against gravity. Short duration (20–30 ms) jerks, a rostrocaudal pattern of muscle activation, negative myoclonus and giant cortical somatosensory evoked potentials (SEPs) suggested a cortical origin of jerks, and a syndrome of progressive myoclonic epilepsy was suspected (Fig. 2).
Fig. 1.
a) Awake EEG showing left central spike discharges. b) Seizure onset with head version to the right and posturing of the left arm.
Fig. 2.
Nerve conduction studies. a) Presence of the C- reflex after stimulation of the left and (b) right median nerves c) Giant SEPs.
Genetic analysis was undertaken. There were no pathogenic mutations or large-scale rearrangements of mtDNA detected and no CSTB expansion suggestive of Unverricht–Lundborg disease. His muscle biopsy was unremarkable and a skin biopsy could not be processed due to technical issues.
Whole genome sequencing was performed as part of the 100,000 Genomes Project. This showed one pathogenic variant in CLN6 and two other variants of unknown significance in CLN6. Subsequent targeted sequence analysis of CLN6 showed compound heterozygosity for CLN6 c.13C > T p.(Arg5Trp) & c.150C > G p.(Tyr50*). His mother was heterozygous for the c.13C > T sequence variant and his father heterozygous for the c.150C > G sequence variant, providing evidence that these variants were on opposite alleles and therefore together likely to be pathogenic. The results fully explained the clinical phenotype and confirmed the diagnosis of Kufs disease/cerebral neuronal lipofuscinosis.
A repeat MRI brain scan showed no change in the degree of cerebellar volume loss. However, there were new T2 FLAIR hyperintensities in the right cerebral hemispheric white matter with minor neuroparenchymal volume loss in the right cerebral hemisphere, consistent with either a vascular or infective/inflammatory process. Imaging of the cord was normal (Fig. 3).
Fig. 3.
a) and b) FLAIR sequences showing T2 hyperintense lesions in the right cerebral hemisphere. c), d) and e) Magnetic resonance angiogram sequences showing stenoses of distal internal carotid arteries (white arrow) and proximal middle cerebral arteries with collateral vessel formation (red arrow).
Vascular imaging revealed stenoses of the terminal and para-ophthalmic internal carotid arteries (ICAs) and proximal middle cerebral arteries (MCAs) (right worse than left), with collateral vessels. Extracranial vasculature was unremarkable. The features were in keeping with intradural arterial occlusive vasculopathy/Moyamoya disease. He was commenced on a statin and amlodipine for blood pressure management, and bilateral superficial temporal artery to middle cerebral artery (extracranial-intracranial) vascular anastomoses were undertaken to reduce the risk of further ischaemic injury, without post-operative neurological sequelae. Additional genetic sequencing has identified no pathogenic variants in the RNF213 gene on the long arm of chromosome 17 (17q25), the most common genetic mutation associated with Moyamoya disease, and no significant variants in Cerebrovascular disorders (version 1.34) or Cerebral vascular malformations (version 1.36) 100,000 Genomes Project gene panels.
2. Discussion
Progressive myoclonic epilepsy represents a clinical syndrome of myoclonus and progressive neurological impairment. The most common diagnosis is Unverricht–Lundborg (EPM1) disease, caused by mutations in the cystatin B (CSTB) gene, which typically leads to symptoms arising in the second decade. Other causes include lysosomal storage diseases such as Lafora body disease, Sialidosis and Gaucher disease, and mitochondrial disorders such as MERRF [1].
The cerebral neuronal lipofuscinoses represent a family of lysosomal storage disorders that lead to the accumulation of autofluorescent lipopigments (lipofuscin) within various tissues in the body, including the brain, myocardium and kidneys. These granules have distinctive ultrastructural patterns on electron microscopy, described as curvilinear, rectilinear, fingerprint or granular osmiophilic deposit profiles. Classification of NCL on the basis of age at onset is conventional, with infantile, late-infantile, juvenile and adult subclassifications [2]. Diagnostic support can be provided by electrophysiological studies (including EEG, SEP and electroretinogram) and brain imaging (which may show cerebellar atrophy or reduced signal within bilateral thalami). Enzyme activity assays of PPT1 and TPP1 are helpful in the diagnosis of CLN1 and CLN2-related NCL, whilst skin or rectal biopsy may help provide a histological diagnosis [3]. At least 13 different genes have been implicated in the development of NCL, with next generation or whole-exome/whole-genome sequencing increasingly used for diagnosis, especially in adult-onset forms. Inheritance most commonly follows an autosomal recessive (either homozygous or compound heterozygous) pattern, although autosomal dominant inheritance can be seen, and incredible phenotypic heterogeneity exists even amongst patients with identical gene mutations [4].
CLN6 recessive mutations can lead to adult-onset NCL (ANCL), although more commonly these mutations cause a late-infantile form [4]. Unlike infantile and juvenile forms of NCL, ANCL is typically milder and not associated with visual loss. Kufs disease, often synonymous with adult-onset NCL, refers to ANCL without retinal involvement. There are two main subtypes: type A (most commonly caused by CLN6 recessive mutations) leads to progressive myoclonic epilepsy and type B (usually due to CTSF recessive mutations) causes additional dementia and motor signs [5].
Kufs disease secondary to CLN6 mutation typically manifests with myoclonic and, to a lesser extent, tonic–clonic seizures in the third decade, with bimodal peaks in teenage and early adult life. Motor function and cognition progressively deteriorate, with patients becoming wheelchair-bound approximately 12 years after symptom onset [6]. Cortical myoclonus is a universal feature, manifesting early in the course of disease and progressing to become the prominent disabling feature and causing severe motor impairment. Given their stimulus-sensitive nature, myoclonic and tonic–clonic seizures can be provoked by voluntary movements or somatosensory and visual stimuli, and EEG often shows significant photosensitivity [7]. “Giant” SEPs are confirmatory features seen on electrophysiological examination.
It is interesting to note that neuropsychometry was within normal limits 6 years after our patient's initial presentation, despite significant progression in his myoclonus. Dementia is widely considered to be a universal feature of Kufs disease, however, exceptions do exist in the literature. One case series of 20 patients with CLN6-related Kufs disease included 2 patients without evidence of cognitive impairment 7 and 20 years after disease onset [6]. These were Turkish siblings of consanguineous heritage, who developed generalised seizures at 18 and 26 years of age, respectively, and carried homozygous mutations of CLN6 (c.509A > G, p.Tyr170Cys) [8]. Despite normal neuropsychological testing, the siblings displayed obsessive–compulsive traits. Other behavioural abnormalities that have been noted in the context of CLN6-related Kufs disease include depression, aggression, and psychosis [6].
To our knowledge, this is the first reported case of CLN6-related disease in association with Moyamoya, a constrictive arteriopathy with a predilection for the distal internal carotid arteries and proximal cerebral arteries, a rare disease which most commonly affects patients from the far East. Moyamoya, although familial in about 10% of cases secondary to mutations in the RNF213 gene, usually occurs in conjunction with a range of conditions. These include genetic diseases such as Down's syndrome, sickle cell anaemia, fibromuscular dysplasia and neurofibromatosis type 1, and inflammatory pathologies such as systemic lupus erythematosus, and Grave's disease [9]. There are isolated case reports of Moyamoya in conjunction with certain lysosomal storage diseases, including glycogen storage disease 1a [10] and Mucolipidosis II [11]. Cerebral arteriopathy is also a common feature of Fabry disease, an X-linked disease caused by a deficiency of the lysosomal enzyme alpha galactosidase A.
CLN6 codes for a membrane protein that localises to the endoplasmic reticulum. Its function is unknown, although it may play a role in extracellular matrix remodelling, cell signalling and immuno/inflammatory responses [12]. It is unclear whether the development of Moyamoya in this case can be attributed to the observed pathological mutations in CLN6, and a further mutation cannot be excluded in explaining the clinical phenotype. It has been demonstrated that several lysosomal diseases, including NCL, are associated with prominent microglial and astrocyte activation [13], leading to cytokine release, immune cell recruitment and inflammation. Given Moyamoya's association with a wide range of inflammatory and other disorders, it is however not unreasonable to suspect that the observed mutations in CLN6 may have played a role in the pathogenesis.
Treatment strategies for progressive myoclonic epilepsy include pharmacological management of seizures and multidisciplinary team input to address the progressive disability. Sodium valproate is generally considered to be first-line pharmacological therapy, although should be avoided in patients with MERFF. Drugs such as carbamazepine, phenytoin, and vigabatrin are known to aggravate myoclonus and seizures, whilst lamotrigine can have an unpredictable effect [14]. Levetiracetam, clonazepam, zonisamide, brivaracetam and perampanel can be useful adjuncts. High dose piracetam was shown to result in significant amelioration in symptoms in one case series of two siblings with CLN6-related ANCL [8]. Revascularisation surgery, specifically extracranial-intracranial vascular anastomosis, can be considered in patients with Moyamoya to reduce the risk of ischaemic or haemorrhagic stroke. Revascularisation can be achieved directly, most commonly using the superficial temporal artery as the donor artery, or indirectly via pial synangiosis, using connective tissue as the supplier of neovascularisation [15]. The intent of the procedure is to minimise the risk of recurrent stroke, and as a result, this intervention had no immediate benefit on our patient's clinical state.
3. Conclusion
We describe a case of Moyamoya in conjunction with progressive myoclonic epilepsy caused by compound heterozygous mutations in CLN6. His case has various atypical features, including the absence of cognitive impairment, generally considered to be universal in ANCL, and Caucasian ethnicity, which is uncommonly observed in Moyamoya. Additional genetic testing failed to find any further mutation that could explain the development of Moyamoya. If the association of these two rare conditions reflects a causal relationship, the case raises some important questions about the role and function of CLN6, a gene about which little is known.
Despite the co-administration of sodium valproate, zonisamide, brivaracetam, clonazepam and piracetam, our patient continued to suffer with severe myoclonus with associated motor impairment and side effects of excessive fatigue. The stimulus- and action-sensitive nature of the myoclonus in particular confers significant disability, with symptoms provoked by any of kind of movement and also during sleep, presumably due to the widespread activation of motor and visual cortex.
Ethical statement
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. Informed consent for this case report was obtained from the patient and a written consent form was signed by his mother (next of kin) in the presence of the patient (this was in view of his physical disability and inability to sign written consent for himself).
Declaration of competing interest
None.
Acknowledgements
This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support.
Contributor Information
Jamie Talbot, Email: jamie.talbot1@nhs.net.
Priyanka Singh, Email: priyankasingh@nhs.net.
Clinda Puvirajasinghe, Email: clinda.puvirajasinghe@nhs.net.
Sanjay M. Sisodiya, Email: sanjay.sisodiya@nhs.net.
Fergus Rugg-Gunn, Email: f.rugg-gunn1@nhs.net.
References
- 1.Shahwan A., Farrell M., Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol. 2005;4(4):239–248. doi: 10.1016/S1474-4422(05)70043-0. [DOI] [PubMed] [Google Scholar]
- 2.Haltia M. The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol. 2003;62(1):1–13. doi: 10.1093/jnen/62.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Williams R.E., Aberg L., Autti T., Goebel H.H., Kohlschütter A., Lönnqvist T. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2006;1762(10):865–872. doi: 10.1016/j.bbadis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Cotman S.L., Karaa A., Staropoli J.F., Sims K.B. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurol Neurosci Rep. 2013;13(8):366. doi: 10.1007/s11910-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkovic S.F., Staropoli J.F., Carpenter S., Oliver K.L., Kmoch S., Anderson G.W. Diagnosis and misdiagnosis of adult neuronal ceroid lipofuscinosis (Kufs disease) Neurology. 2016;87(6):579–584. doi: 10.1212/WNL.0000000000002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkovic S.F., Oliver K.L., Canafoglia L., Krieger P., Damiano J.A., Hildebrand M.S. Kufs disease due to mutation of CLN6: clinical, pathological and molecular genetic features. Brain. 2019;142(1):59–69. doi: 10.1093/brain/awy297. [DOI] [PubMed] [Google Scholar]
- 7.Canafoglia L., Gilioli I., Invernizzi F., Sofia V., Fugnanesi V., Morbin M. Electroclinical spectrum of the neuronal ceroid lipofuscinoses associated with CLN6 mutations. Neurology. 2015;85(4):316–324. doi: 10.1212/WNL.0000000000001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Özkara Ç., Gündüz A., Coşkun T. Long-term follow-up of two siblings with adult-onset neuronal ceroid lipofuscinosis, Kufs type A. Epileptic Disord. 2017;19(2):147–151. doi: 10.1684/epd.2017.0911. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y.C., Liu C.H., Chang T.Y., Chin S.C., Chang C.H., Huang K.L. Coexisting diseases of moyamoya vasculopathy. J Stroke Cerebrovasc Dis. 2014;23(6):1344–1350. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Goutières F., Bourgeois M., Trioche P., Demelier J.F., Odievre M., Labrune P. Moyamoya disease in a child with glycogen storage disease type Ia. Neuropediatrics. 1997;28(2):133–134. doi: 10.1055/s-2007-973688. [DOI] [PubMed] [Google Scholar]
- 11.de Guzman P., Hamilton M., Khan A., Eesa M., Kirton A. Moyamoya syndrome associated with mucolipidosis-II. Can J Neurol Sci. 2014;41(4):522–524. doi: 10.1017/S0317167100018618. [DOI] [PubMed] [Google Scholar]
- 12.Kollmann K., Uusi-Rauva K., Scifo E., Tyynelä J., Jalanko A., Braulke T. Cell biology and function of neuronal ceroid lipofuscinosis-related proteins. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2013;183(11):1866–1881. doi: 10.1016/j.bbadis.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Bosch M.E., Kielian T. Neuroinflammatory paradigms in lysosomal storage diseases. Front Neurosci. 2015;9:417. doi: 10.3389/fnins.2015.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferlazzo E., Trenite D.K., Haan G.J., Felix Nitschke F., Ahonen S., Gasparini S. Update on pharmacological treatment of progressive myoclonus epilepsies. Curr Pharm Des. 2017;23(37):5662–5666. doi: 10.2174/1381612823666170809114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T., Oh C.W., Bang J.S., Kim J.E., Cho W.S. Moyamoya disease: treatment and outcomes. J Stroke. 2016;18(1):21–30. doi: 10.5853/jos.2015.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]