Abstract
We review radiation therapy (RT) options available for prostate cancer, including external beam (EBRT; with conventional fractionation, hypofractionation, stereotactic body RT [SBRT]) and brachytherapy (BT), with an emphasis on the outcomes, toxicities, and contraindications for therapies. PICOS/PRISMA methods were used to identify published English-language comparative studies on PubMed (from 1980 to 2015) that included men treated on prospective studies with a primary endpoint of patient outcomes, with ≥ 70 patients, and ≥ 5 year median follow up. Twenty six studies met inclusion criteria; of these, 16 used EBRT, and 10 used BT. Long-term freedom from biochemical failure (FFBF) rates were roughly equivalent between conventional and hypofractionated RT with intensity modulation (evidence level 1B), with 10-year FFBF rates of 45–90%, 40–60%, and 20–50% (for low-, intermediate-, and high-risk groups, respectively). SBRT had promising rates of BF, with shorter follow-up (5-year FFBF of >90% for low-risk patients). Similarly, BT (5-year FFBF for low-, intermediate-, and high- risk patients have generally been >85%, 69–97%, 63–80%, respectively) and BT + EBRT were appropriate in select patients (evidence level 1B). Differences in overall survival, distant metastasis, and cancer specific mortality (5-year rates: 82–97%, 1–14%, 0–8%, respectively) have not been detected in randomized trials of dose escalation or in studies comparing RT modalities. Studies did not use patient-reported outcomes, through grade 3–4 toxicities were rare (<5%) among all modalities. There was limited evidence available to compare proton therapy to other modalities. The treatment decision for a man is usually based on his risk group, ability to tolerate the procedure, convenience for the patient, and the anticipated impact on quality of life. To further personalize therapy, future trials should report (1) race; (2) medical comorbidities; (3) psychiatric comorbidities; (4) insurance status; (5) education status; (6) marital status; (7) income; (8) sexual orientation; and (9) facility-related characteristics.
Keywords: comparative effectiveness research, prostate cancer, technology, personalized medicine, radiation therapy, quality of life
Graphical Abstract
INTRODUCTION
Prostate cancer is the most prevalent cancer diagnosed in men in the United States, aside from skin cancer.1 Treatment options for non-metastatic prostate cancer typically include active surveillance (AS), radical prostatectomy (RP) and radiation therapy (RT).2 Within RT, treatment options include (1) external beam radiation therapy (RT), which may be conventionally fractionated (CFRT) with intensity modulated radiation therapy (IMRT) or protons, hypofractionated RT (HFRT) with IMRT or protons, or delivered as stereotactic body RT (SBRT); and (2) brachytherapy (BT), either high dose rate (HDR-BT) or low dose rate (LDR-BT). For reference, we define the various forms of RT in the Glossary. Although there are many standard treatment options for prostate cancer, randomized clinical trials (RCTs) to define the optimal therapy for patients with localized or locally advanced disease are limited.3
In modern medicine, it is crucial for primary care physicians and specialists (including oncologists) to work together to provide consistent, accurate information to patients regarding treatment options for prostate cancer. The goal of this systematic review article is to provide an understanding of the evolving definitive RT options available for prostate cancer by (1) comparing RT fractionation regimens (including external beam RT and brachytherapy) and applicability to risk groups; (2) comparing and contrasting outcomes, toxicities, and contraindications of the approaches; and (3) discussing future implications of these approaches and how they integrate into active surveillance. For the purposes of this review article, we do not include outcomes data on other treatments for localized prostate cancer, including RP, post-RP RT (e.g. in the adjuvant or salvage setting), or high intensity focused ultrasound. Since the choice of a patient for RT instead of RP is sometimes due to presence of comorbidities or age, we briefly juxtapose the appropriateness, contraindications, and toxicities of adjuvant/salvage RT.
METHODS
Key Questions:
We focused on three key questions:
What is the effectiveness of various forms of RT (e.g. conventionally fractionated RT ± IMRT, hypofractionated RT ± IMRT, SBRT, LDR-BT, HDR-BT), in terms of prostate cancer control outcomes, for clinically localized prostate cancer?
What is the effectiveness of various forms of RT (e.g. conventionally fractionated RT ± IMRT, hypofractionated RT ± IMRT, SBRT, LDR-BT, HDR-BT), in terms of toxicities, for clinically localized prostate cancer?
Based on the outcomes and toxicities, what should practitioners consider when discussing a particular type of RT with prostate cancer patients?
Data Sources and Searches
Three researchers searched the published English medical literature from 1980 through 2015 in MEDLINE and PubMed for full-text manuscripts (excluding abstracts) using the terms “prostate cancer,” and “radiation therapy,” along with any of the following: “external beam radiation therapy,” “hypofractionated radiation therapy,” “proton beam,” “stereotactic body radiation therapy,” “high dose rate brachytherapy,” and “low dose rate brachytherapy.” Terms were in titles or MeSH headings. The initial search resulted in 1,558 articles.
Study Selection
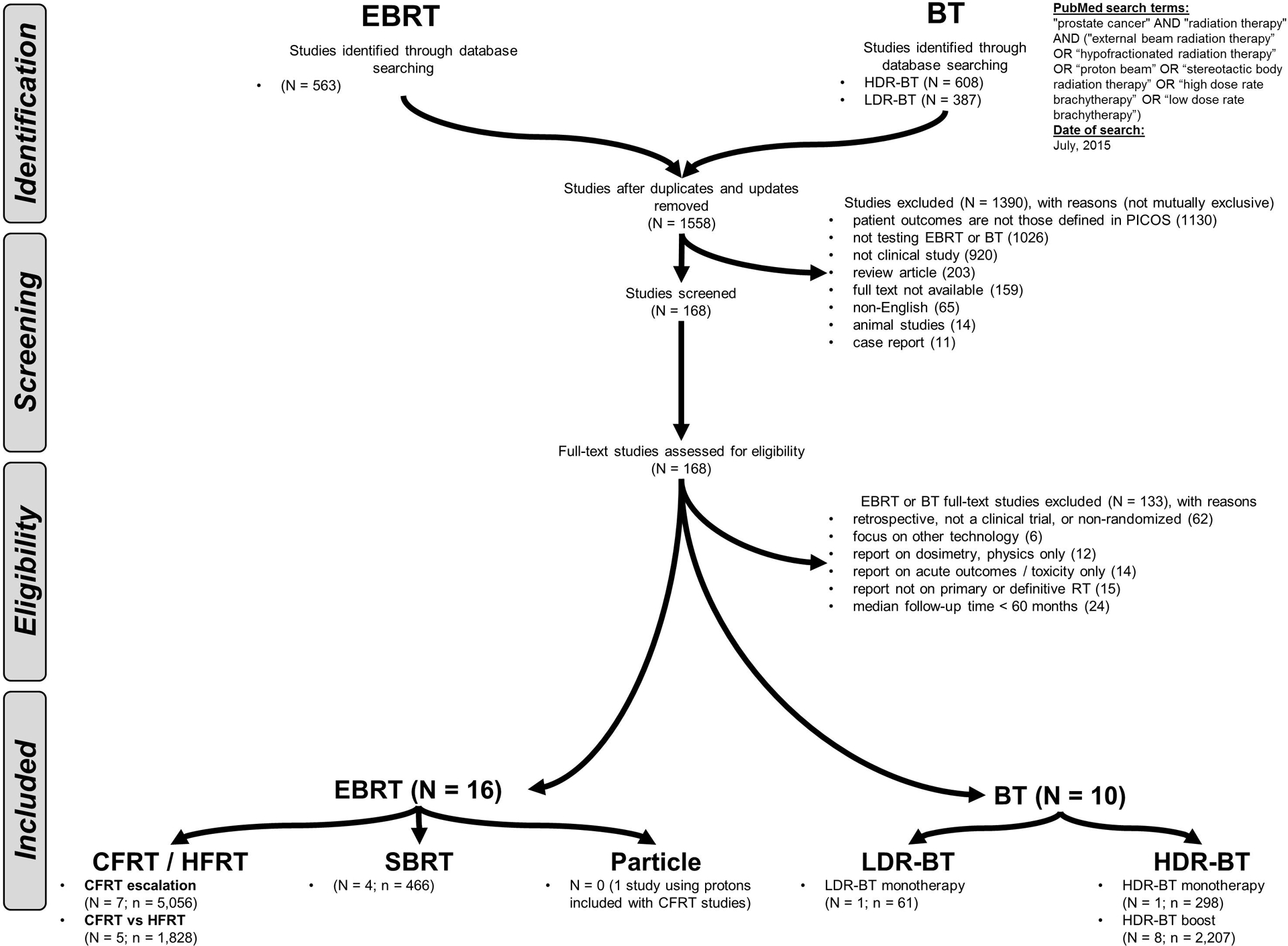
We defined inclusion criteria for the literature search using the Population, Intervention, Control, Outcome, Study (PICOS) design approach (Table 1). We conducted a systematic search using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) literature selection process (Figure 1).4 Three authors searched reference lists of identified papers to supplement the literature search.
Table 1.
PICOS: Participants, interventions, comparisons, outcomes, and study design.
| Population | Men with localized (T1-T2, N0-Nx, M0) and locally advanced (T3-T4, N0-Nx, M0) prostate cancer | ||||
| Intervention | Definitive brachytherapy: either high-dose rate (HDR) or low-dose rate (LDR) into the prostate Definitive external beam radiation therapy (EBRT), using conventional fractionation, hypofractionation, stereotactic body radiation therapy, or proton therapy |
||||
| Control | Multi-arm study that contains the intervention vs. another form of RT (e.g. EBRT vs. brachytherapy); or single-arm study of either | ||||
| Outcomes | |||||
| Efficacy | Clinical (surrogate outcomes) for all studies:
|
||||
| Safety | Late RTOG genitourinary and gastrointestinal toxicities | ||||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Genitourinary | None | Slight epithelial atrophy Minor telangiectasia (microscopic hematuria) |
Moderate frequency Generalized telangiectasia Intermittent macroscopic hematuria |
Severe frequency and dysuria Severe generalized telangiectasia (often with petechiae) Frequent hematuria Reduction in bladder capacity (<150 cc) |
Necrosis/ Contracted bladder (capacity <100 cc) Severe hemorrhagic cystitis |
| Gastrointestinal | None | Mild diarrhea Mild cramping Bowel movement 5 times daily Slight rectal discharge or bleeding |
Moderate diarrhea and colic Bowel movement >5 times daily Excessive rectal mucus or intermittent bleeding |
Obstruction or bleeding requiring surgery | Necrosis/Perforation Fistula |
| Study design | |||||
| Efficacy and/or safety | Prospective studies only; ≥ 70 patients; One or more arms; ≥ 5 y median follow-up; ≥ 5 y actuarial follow-up | ||||
Abbreviations: ASTRO: American Society of Therapeutic Radiology and Oncology; PSA: prostate specific antigen; RT: radiation therapy
Figure 1. PRISMA.
Patient populations of interest
We included studies of men with organ-confined (T1-T2, N0-Nx, M0) and locally advanced (T3-T4, N0-Nx, M0) prostate cancer, regardless of age, histologic grade, or PSA level. T-stage alone is a poor prognosticator, and >90% of patients are T1c and T2; thus, risk groups were defined by NCCN classification, the preferred prognostication system.5
We omitted studies comparing RT to RP and studies evaluating adjuvant and salvage RT post-RP for several reasons. First, our goal was to compare RT fractionation regimens, source types (i.e. external beam vs. brachytherapy), techniques (i.e. conformal vs. intensity modulation), and particle (i.e. photon vs. proton). Second, there is limited data comparing contemporary forms of RP (e.g. robotic, laparoscopic approaches) to contemporary forms of RT (i.e. RP vs. IMRT, RP vs. SBRT, RP vs. HDR-BT), particularly with controlling for the use of androgen deprivation therapy (ADT). Third, for patients with obstructive symptoms (either from tumor bulk or urinary comorbidities), initial therapy with RP (with or without adjuvant/salvage RT) may be most appropriate, and this should be considered for individual cases. Fourth, recommendations regarding adjuvant and salvage RT after RP have been published.6
Intervention and Control
The intervention was BT or external beam RT as definitive therapy. An included study may have multiple arms that contains the intervention vs. another form of RT (e.g. external beam RT vs. BT); or it may be a single-arm study of external beam RT or BT focusing on dose escalation. We organize studies for our discussion based on the type of RT employed, including (1) various forms of external beam RT: conventionally fractionated RT either with 3D-conformal radiation therapy (3D-CRT) or intensity modulated radiation therapy (IMRT),7–14 hypofractionated RT,15–19 SBRT,20–23 particle therapy (specifically, protons); and (2) BT: LDR-BT or HDR-BT, either as monotherapy or in combination with external beam RT24–34 (i.e. “BT boost”). Ongoing clinical trials by the Radiation Therapy Oncology Group (RTOG / NRG) that specify contraindications to therapy and address unanswered questions were identified.35–37
Outcomes of interest
The primary outcome measure was freedom from biochemical failure (FFBF) using the Phoenix definition (i.e. nadir + 2 ng/mL) because all major RCTs of RT for prostate cancer have historically used this endpoint rather than survival. If this was unavailable, then the ASTRO definition (i.e. 3 consecutive PSA rises) could be used, provided that the study had at least 5 years of follow-up. Secondary outcomes including overall survival (OS), distant metastasis (DM), prostate cancer specific mortality (PCSM) were analyzed when available. To assess toxicity, we used RTOG late genitourinary (GU) and RTOG late gastrointestinal (GI) scores (defined in Table 1). Socioeconomic characteristics and medical comorbidities are rarely reported among trials and thus could not be uniformly reviewed.
ADT was used for certain intermediate and high-risk patients. ADT may affect FFBF, DM, and OS; although the use of ADT was controlled for among RCTs included in this review, we could not control for it in the current systematic review because we are not analyzing individual patient data. Recommendations regarding ADT (in the context of RCTs that are included in this review) are provided.36–39
Study design
The search criteria were prospective design (e.g. phase II/III), minimum 60 month median and actuarial follow-up, and a sample size of > 70 patients. Retrospective studies were excluded so that we may compare the highest level evidence for each modality.
Data Extraction and Quality Assessment
We abstracted data on the number of patients, trial start/stop dates, study type (e.g. phase II/III), arms, number of patients, total RT dose, RT dose per fraction (of external beam RT or BT), median follow-up, actuarial follow-up, FFBF, OS, DM, PCSM, and late RTOG toxicities. Only one study used survival as the primary endpoint (RTOG 0126); all others used FFBF as the primary outcome measure. Other clinical endpoints (metastasis, cause-specific mortality) were not included since they are seldom reported. When comparing the endpoints across various RT techniques and fractionation schemes, the Centre of Evidence-Based Medicine levels were used (Supplementary Table 1). The strength of the overall body of evidence was rated by the entire group of reviewers, and disagreements were resolved by consensus.
Data Synthesis and Analysis
Subtypes of RT were compared. The level of evidence for each study was evaluated using the Center for Evidence-Based Medicine and recommendations from the NCCN (Supplementary Tables 1–3). Contraindications for RT were obtained from individual protocol eligibility criteria (when available), ongoing studies by the RTOG,35–37 and consensus statements.40–43
RESULTS
Studies were organized into two tables. Supplementary Table 2 lists those of external beam RT dose escalation with conventionally fractionated RT,7–14 those of hypofractionated RT vs. conventionally fractionated RT,15–19 and those of SBRT.20–23 Supplementary Table 4 lists studies of BT.24–34 Comparisons, based on levels of evidence (from Supplementary Table 1), were used to compare outcomes and toxicities for the various forms of RT (Table 2). Additionally, the following factors were summarized: applicability of each treatment modality for among the risk groups (Table 3); contraindications of each RT modality, based on guidelines and clinical trials (Table 4); and early and late toxicities of RT modalities (Table 5).
Table 2.
Levels of evidence for therapeutic studies of RT for prostate cancer, with respect to patient outcomes and toxicities.
| Key Question 1: What are the benefits for patient outcomes of different forms of EBRT for clinically localized prostate cancer? (#1 vs. #2) | |||
|---|---|---|---|
| Outcome measures | Option #1 | Option #2 | In favor of #1 or #2 |
| FFBF, OS*, DM*, or CSM* | EBRT with IMRT | EBRT with 3D-CRT | All outcomes: Untested in RCT, appear similar, level 2 |
| Dose escalated CFRT | non-dose escalated CFRT | FFBF: In favor of #1: level 1B7–14 OS, DM, CSM: Similar, level 1B7–14 |
|
| Dose escalated CFRT | HFRT | All outcomes: similar, level 1B15–19 | |
| CFRT | SBRT | All outcomes: current RCT35 | |
| HFRT | SBRT | All outcomes: current RCT35 | |
| Proton EBRT | Non-proton EBRT | All outcomes: Untested in RCT, appear similar, level 2 | |
| EBRT | LDR-BT | All outcomes: Untested in RCT, appear similar, level 2 | |
| EBRT | HDR-BT | All outcomes: Untested in RCT, appear similar, level 2 | |
| EBRT | BT + EBRT | FFBF: In favor of #2: level 1B (for intermediate, high risk)25 OS, DM, CSM: similar, level 1B25 |
|
| LDR-BT | HDR-BT | All outcomes: Untested in RCT, appear similar, level 2 | |
|
Key Question 2: From RCTs evaluating efficacy of RT, what are the benefits for late patient toxicities of different forms of RT for clinically localized prostate cancer? (#1 vs. #2) | |||
| RTOG toxicity | Option #1 | Option #2 | In favor of #1 or #2 |
| GU or GI late | EBRT with IMRT | EBRT with 3D-CRT | GU: similar, level 27–14,44, 45 GI: in favor of #1, level 1B7–14,44, 45 |
| Dose escalated CFRT | non-dose escalated CFRT | GU and GI: similar, level 1B7–14 | |
| Dose escalated CFRT | HFRT | GU and GI: similar, level 1B15–19 | |
| CFRT | SBRT | GU and GI: Current RCT35 | |
| HFRT | SBRT | GU and GI: Current RCT35 | |
| EBRT | LDR-BT | GU and GI: Untested in RCT | |
| Proton EBRT | Non-proton EBRT | GU and GI: untested in RCT; level II in favor of #2 | |
| EBRT | HDR-BT | GU and GI: untested in RCT | |
| EBRT | BT + EBRT | GU and GI: similar, level 1B25 | |
| LDR-BT | HDR-BT | GU and GI: untested in RCT | |
Abbreviations: BT: brachytherapy; CFRT: conventionally fractionated radiation therapy; CSM: cancer specific mortality; DM: distant metastasis; EBRT: external beam radiation therapy; GI: gastrointestinal; GU: genitourinary; HDR: high dose rate; HFRT: hypofractionated radiation therapy; LDR: low dose rate; OS: overall survival SBRT: stereotactic body radiation therapy.
Note:
= Not the primary outcome measure of RCTs.
Table 3.
RT treatment options for men with NCCN risk group-stratified prostate cancer, in relation to other treatment options.2
| Options and subtypes | NCCN risk group | ||||
|---|---|---|---|---|---|
| Low Gleason score ≤ 6, and PSA < 10 ng/ml, and clinical tumor classification T1, T2a |
Intermediate Gleason score 7, or PSA ≥ 10 ng/ml ≤ 20 ng/ml or clinical tumor classification of T2b, T2c |
High Gleason score 8 – 10, or PSA > 20 ng/ml, or clinical tumor classification of T3a |
Post-RP RT6 Adjuvant indications: pT3; positive SMs Salvage indications: suspected LR (e.g. rising PSA, imaging, biopsy-proven) |
||
| Active surveillance / watchful waiting / observation | Yes | Typically no | Typically no | N/A | |
| Radical prostatectomy (RP) | open, laparoscopic, robotic approaches78 | Monotherapy | Monotherapy | Monotherapy | N/A |
| Brachytherapy | low dose rate41 | Monotherapy or boost | Boost or monotherapy | Boost > monotherapy | No |
| high dose rate62, 75 | Monotherapy | Boost or monotherapy | Boost > monotherapy | No | |
| External beam radiation therapy (EBRT) | conventional fractionation49, 50 | Monotherapy | Monotherapy or boost | Monotherapy or boost | Yes |
| hypofractionation60 | Monotherapy | Monotherapy or boost | Monotherapy or boost | No | |
| stereotactic body radiation therapy (SBRT)61, 64 | Monotherapy* (sometimes) | Monotherapy* (sometimes) | Monotherapy (infrequently) * | No | |
| ± Androgen deprivation therapy | No | Sometimes*37 | Almost always, for 24–36 m38, 39 | Sometimes*36 | |
Abbreviations: PSA: prostate specific antigen; SBRT: stereotactic body radiation therapy; SMs: surgical margins
Note:
Green color refers to treatments that are accepted, NCCN Category 2A.2 Additionally, NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Yellow color refers to controversial topics, or those being explored on clinical trials.2
Denotes treatment options that are largely investigational or controversial (clinical trial reference provided, if available).
Red color refers to treatments that are not recommended.
“Boost” refers to the use of BT + EBRT, as opposed to either treatment alone.
Table 4.
Contraindications to prostate RT modalities
| As first-line therapy | Post-RP | ||||
|---|---|---|---|---|---|
| RT subtypes | |||||
| Possible contraindications | Brachytherapy40, 41 | External beam radiation therapy42 | External beam radiation therapy | ||
| Low dose rate or high dose rate | Conventional fractionation | Hypofractionation | Stereotactic body radiation therapy (SBRT)35 | Conventional fractionation | |
| Limited life expectancy (e.g. < 10 years; patient will not realize benefit of RT in lifetime) | |||||
| Unacceptable operative risks, or medically unsuited for anesthesia | ** | ** | *, ** | ** | |
| Distant metastases | *** | *** | *,*** | * | |
| Absence of rectum such that TRUS-guidance is precluded | ** | ** | ** | ** | |
| Large TURP defects which preclude seed placement and acceptable radiation dosimetry | ** | ** | * | N/A | |
| Ataxia telangiectasia | |||||
| Preexisting rectal fistula | |||||
| Risk of bleeding (e.g. from anticoagulants) | ** | ** | ** | ** | |
| Moderate-severe urinary symptoms (e.g. high IPSS score, typically defined as > 20) | Consider conventional fractionation | Consider conventional fractionation | Consider conventional fractionation | ||
| History of prior pelvic radiotherapy | * | * | |||
| Large prostate (e.g. > 60 cm3) | N/A | ||||
| Large median lobes | N/A | ||||
| Inflammatory bowel disease | * | * | |||
| Pubic arch interference (e.g. prior pelvic fracture, irregular pelvic anatomy, or a penile prosthesis) | |||||
| Patient peak flow rate < 10 cc/s and post void residual volume prior to brachytherapy > 100 cc | N/A | N/A | N/A | N/A | |
| Concurrent androgen deprivation therapy use | * | ||||
Abbreviations: N/A: not applicable; IPSS: International Prostate Symptom Score; TRUS: transrectal ultrasound; TURP: transurethral resection of prostate; RT: radiation therapy
Note:
Placement of fiducials for IGRT may be difficult
Depends on intra- vs. extra-prostatic disease burden
Green color refers to items that are not contraindications for RT.
Yellow color refers to items that may be contraindications for RT, or logistics.
Red color refers to items that are contraindications for RT.
Table 5.
Toxicities of prostate RT modalities
| As first-line therapy | Post-RP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT subtypes | |||||||||||||||
| Brachytherapy | Boost | External beam radiation therapy (with intensity modulation) | External beam radiation therapy | ||||||||||||
| Low dose rate | High dose rate | Brachytherapy + conventional fractionation | Conventional fractionation | Hypofractionation | Stereotactic body radiation therapy (SBRT) | Conventional fractionation (Toxicity below includes that of RP and RT) | |||||||||
| Quality of life domain | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | |
| Sexual | Limited data | Limited data | |||||||||||||
| Urinary incontinence | Limited data | ||||||||||||||
| Urinary irritative / obstructive | Limited data | Limited data | |||||||||||||
| Bowel / rectal | Limited data | Limited data | |||||||||||||
Abbreviations: RP: radical prostatectomy; RT: radiation therapy
white boxes: informative valid data with adequate sample size for conclusive analyses at the indicated time point is not available for the corresponding domain treatment
green boxes: domain not typically impacted, < 1% late Grade 3–4 toxicity
yellow boxes: domain impacted occasionally, 1 – 5% late Grade 3–4 toxicity
red boxes: domain impacted commonly at the indicated time point after the corresponding treatment, > 5% late Grade 3–4 toxicity
Key questions 1 and 2: outcomes and toxicities
EBRT
Almost all external beam RT RCTs have used FBFF as the primary outcome measure, with OS, DM, and CSM as secondary endpoints. In general, these external beam RT studies have shown an improvement in FFBF with higher biologically equivalent doses, evidence level 1B, with 5-year FFBF rates of >90%, 60–85%, and 50–70% (for low-, intermediate-, and high-risk groups, respectively) among modern trials. However, they have not shown inter-arm improvements in 5-year OS (82–97%), DM (1–14%), or CSM (0–8%), evidence level 1B. RTOG late grade 3–4 toxicities have been <5% on the studies, and the incidence of toxicity has depended largely on the use of IMRT (instead of 3D-CRT), evidence level 1B.44–46
Based on evidence level 1B, dose-escalated conventionally fractionated RT with IMRT appears to have similar outcomes at toxicities to hypofractionated RT with IMRT, with 5-year FFBF rates of >90%, 60–85%, and 50–70% (for low-, intermediate-, and high-risk groups, respectively); and 10-year FFBF rates of 45–90%, 40–60%, and 20–50% (for low-, intermediate-, and high-risk groups, respectively). SBRT had promising rates of BF, with shorter follow-up (5-year FFBF of >85% for low-risk patients). To date, no prospective studies have evaluated external beam RT vs. LDR-BT, or external beam RT vs. HDR-BT. No prospective studies of proton therapy for prostate cancer have been published, though one of the conventionally fractionated RT escalation studies did allow use of a proton boost.9 A randomized control trial of protons vs. conventionally fractionated RT with IMRT is currently ongoing;43 and RTOG/NRG 0938 allowed for treatment with protons (Supplementary Table 3).35
LDR-BT
There have been no prospective RCTs comparing LDR-BT to external beam RT or LDR-BT to HDR-BT (Supplementary Table 4). Several single-arm prospective studies have been published. RTOG/NRG 980531 was a multi-institutional phase II trial of LDR-BT. A total of 27 institutions accrued a total of 101 patients to this protocol. With a median follow-up of 5.3 years 5 patients had local failure, 1 had evidence of distant failure, and 6 had biochemical failures. These rates are comparable to other studies on BT and external beam RT. CALGB 99809, which evaluated LDR-BT boost with ADT, reported a 5-year FFBF of 85%.30 No study of LDR-BT has reported inter-arm differences in OS, DM, or CSM. The outcomes appear similar to those of patients treated with external beam RT, which had 5-year OS 82–97%, DM 1–14%, and CSM 0–8%.
HDR-BT
Results of efficacy and toxicity from two studies using HDR-BT as monotherapy are listed in Supplementary Table 4.24–29, 32–34 Five-year rates of FFBF for low-, intermediate-, and high- risk patients have generally been >85%, 69–97%, 63–80%, respectively. Five year rates of CSM, OS, local recurrence, and DM have been 99–100%, 85–100%, 0–8%, and 2–12%, respectively. A single study comparing HDR-BT boost vs. external beam RT reported improved FFBF with HDR-BT boost (10-year FFBF rates: 60% vs. 100% for low risk patients; 62% vs. 89% for intermediate-risk patients; 70% vs. 80% for high-risk patients),25 evidence level IB. Late grade 3–4 RTOG GU toxicities have been reported at 0–8%; GI toxicities, 0–3%. No study of HDR-BT has reported differences in OS, DM, or CSM.
Key Question 3
The NCCN2 provides RT options for low-, intermediate-, and high-risk groups. The various RT options from Key questions 1 and 2, as applicable to NCCN risk groups, are listed in Table 3, with the supporting categories of evidence from the NCCN (Supplementary Table 2). Notably, active surveillance is an acceptable treatment option for low-risk patients. Evidence suggests that conventionally fractionated RT with IMRT or hypofractionated RT with IMRT may be used for all risk groups, and rates of FFBF, OS, DM, and PCSM are roughly similar between the regimens. SBRT use is recommended on protocol. Similarly, BT monotherapy is an acceptable treatment option for select low- and intermediate-risk patients. FFBF rates among SBRT studies appear promising. For high risk patients, BT boost may have improved FFBF rates vs. conventionally fractionated RT alone (evidence level 1B). Additionally, ADT may be used with external beam RT or BT, in certain intermediate- and high-risk patients (also listed in Table 3).2, 38, 39
The contraindications to RT from the American College of Radiology and American Brachytherapy Society are listed in Table 4.40–42 There are no significant differences in late toxicities among the prospective studies that compare RT modalities for efficacy (e.g. conventionally fractionated RT vs. hypofractionated RT; evidence level 1b to 2); nonetheless, each RT modality has unique indications for the NCCN subgroups, contraindications, and unique toxicity profiles (Table 5).
DISCUSSION
In this analysis, we compared contemporary outcomes of various forms of RT for clinically localized prostate cancer. In terms of outcomes (Key Question/Answer 1; Table 2), long-term FFBF rates are roughly equivalent between conventionally fractionated RT and hypofractionated RT with IMRT (evidence level 1B) among all NCCN risk groups. Similarly, BT monotherapy is an acceptable treatment option for select low- and intermediate-risk patients. FFBF rates among SBRT studies appear promising. For certain intermediate- and high-risk patients, BT boost may have improved FFBF rates vs. conventionally fractionated RT with IMRT alone (evidence level 1B). No study testing definitive RT has reported differences in OS, DM, or PCSM (evidence level 1B), which is not attributable to ADT. In terms of toxicities (Key Question/Answer 2; Table 2), IMRT (as used with conventionally fractionated RT and hypofractionated RT) is associated with decreased toxicity compared to 3D-CRT (evidence level 1B to 2). Late GU and GI toxicities are otherwise similar between conventionally fractionated RT and hypofractionated RT (evidence level 1B). Many other modalities have not been tested in prospective RCTs to compare long-term toxicities, though grade 3–4 toxicities were rare (<5%). Thus, the treatment decision for a man is usually based on his risk group (Table 3), ability to tolerate the procedure (Table 4), anticipated impact on quality of life (Table 5), and convenience for the patient (Key Question/Answer 3).
The differences among modalities are small; thus, the treatments may not be adequately different to influence patient decisions. However, the amount of available evidence differs, and patients/providers may feel more comfortable about higher weight/quality of evidence. Finally, there might be some suggestion based on recent trials that elderly patients or those with baseline high urinary symptoms might fare worse with moderate hypofractionation than conventional fractionation. Sometimes, the relative merit of IMPRT lies simply in its existence as a choice rather than LDR-BT (due to relative contraindications) or RP (due to comorbidities/age).
Similarly, certain modalities have absolute contraindications (detailed in Table 5).35, 40, 41 For example, the presence of ataxia telangiectasia or preexisting rectal fistula are absolute contraindications to any type of RT. Additionally, BT typically has more contraindications (vs. conventional fractionation or hypofractionation), including absence of a rectum such that transrectal ultrasound guidance could not be performed, large gland size (a relative contraindication if > 60 cm3),47 pubic arch interference, large transurethral removal of prostate defect, and a low peak flow rate (< 10 cc/s and post void residual volume prior to BT > 100 cc). Additionally, the patient must be able to tolerate anesthesia, since multiple fractions (typically 2–6) are necessary; further, for LDR-BT, another dosimetric analysis should be performed 3 weeks after therapy,48 an inconvenience when considering 5 fractions of SBRT. However, SBRT also has more contraindications than conventional RT, and patients with certain contraindication (e.g. inflammatory bowel disease, large transurethral removal of prostate defect) are excluded on clinical trials.36
There are three important caveats to consider about the interpretation of RT modalities: PSA kinetics (applicable to Key Question 1); development of technology in prostate cancer RT (applicable to Key Question 2); and unknown factors about patients from the prospective data (applicable to Key Question 3). Clinicians should be aware of these when discussing outcomes, toxicities, and patient eligibility.
Key Question 1: PSA kinetics
With respect to Key question 1, there are no RT studies that use OS, DM, or PCSM as a primary endpoint in the localized setting; rather FFBF is used as a surrogate endpoint because there is a relatively long natural history of prostate cancer, with a long delay between BF, DM, and CSM.49, 50 The 5-year FFBF rate among a few of the trials has been above 90%7, 19 (Supplementary Tables 3,4). Assuming that from the date of BF to the date of DM is over 5 years, and from the date of DM to PCSM is an additional 5 years,51 there may not be difference seen in DM or CSM until 15 – 20 years of actuarial follow-up time.46 Moreover, with > 5–10 years after external beam RT, the subsequent rise in PSA may be secondary to benign prostate diseases and not to cancer recurrence.
Patient selection has changed from the 1980s - 2010s with the introduction of PSA screening (introduced in the mid-1990s). Before PSA screening, most patients who were treated had more advanced diseases than those detected with PSA. For example, Surveillance, Epidemiology, and End Results (SEER) data on prostate cancer incidence from 1988 through 1998 were consistent with overdiagnosis rates of approximately 29% for whites and 44% for blacks among men with prostate cancers detected by PSA screening.52 This overdiagnosis of indolent cancers may explain some differences between trials.
Additionally, there are typically very few events in overall patient outcomes (i.e. DM, CSM). For example, the 10-year CSM rates among some of the conventionally fractionated RT dose escalation trials have frequently been less than 10%.7, 8, 11, 12, 14 For the men with prostate cancer in these external beam RT RCTs, the median age has been in the late 60s; thus, waiting for an additional 10–20 years of follow-up may result in patient death due to non-cancer causes, precluding analysis of the intervention. Prostate cancer is typically diagnosed in the elderly and has an indolent course; accrual of a relatively large number of patients (i.e. thousands) is necessary to realize a change in FFBF of a few percent.
There are issues with using FFBF as a surrogate marker. Illustrative PSA curves after BT, external beam RT, and RP are displayed in Supplementary Figure 2. Regarding how FFBF is defined, the Phoenix definition following treatment is preferred for external beam RT.53 That is, after RT, the PSA is followed; the PSA nadir will be the lowest PSA value reached; if a value > 2 ng/mL above the nadir is detected, this is a BF. It is important to understand that this definition is for populations and not for individuals. External beam RT typically induces a slow and inconsistent decrease in PSA to levels that are typically still detectable. Moreover, 10–30% of patients exhibit a PSA bounce (i.e. a temporary elevation in PSA without disease recurrence) within 3 years after RT; bounces will normalize within about one year.54, 55 Bounces also occur with BT.56, 57 PSA bounces do not correlate with recurrence; they are associated with patient, cancer, and dosimetric factors.
Most imaging techniques in the recurrent setting are not yet sensitive or specific enough to establish a diagnosis of recurrence.58 Thus, most cancer recurrences should be documented by biopsy (rather than imaging alone) before salvage therapy is initiated.59 A biopsy would help rule out a PSA bounce and prevent overtreatment.
Key Question 2: Evolution of technology in prostate cancer RT
With respect to Key question 2, it is difficult to state which modalities have fewer long-term side effects; the RT options presented in this analysis have had unique patterns of implementation, certain modalities (e.g. SBRT) have been used for a relatively brief period of time.49 The timeline for the development of external beam RT and BT for prostate cancer is illustrated in Supplementary Figure 2. The shift from 3D conformal radiation therapy to IMRT during the 1990s, theoretical radiobiological models from 2001 (which support the use of high doses per fraction, such as those in hypofractionated RT,60 SBRT,61 and HDR-BT62), development of HDR-BT (to use in place of LDR-BT) in the 1980s, and advances in image guided radiation therapy (IGRT) for external beam RT since the 1980s have influenced the evolution of RT modalities, including hypofractionated RT, SBRT, HDR-BT, and particle beam therapy (e.g. protons).
EBRT is a cornerstone of curative management of localized prostate cancer. External beam RT is used in about 25% of patients younger than 65 years, and almost half of patients older than 65 years.63 Since the 1990s, there have been two central themes in localized prostate cancer external beam RT RCTs,49, 50 summarized in Supplementary Table 3. The first theme has been escalation of the total dose with conventionally fractionated RT,7–14 from 66 Gy to 70 Gray (Gy), up to a total of 76 to 80 Gy in 1.8 – 2 Gy fractions. The second theme has been the use of hypofractionated RT,15–19 up to a total dose of 50 to 66 Gy in 2.1 to 3.5 Gy fractions, compared to conventionally fractionated RT.60 More recent RCTs of prostate cancer external beam RT have been a continuation of the theme of dose escalation and hypofractionation: for example RTOG/NRG 0938 is examining the role of extreme hypofractionated RT with stereotactic body radiation therapy, using biologically equivalent doses higher than any conventionally fractionated RT RCTs.35, 61 Although biologically equivalent dose escalation has resulted in improved FFBF, other outcomes (OS, DM, and CSM) have not changed.46
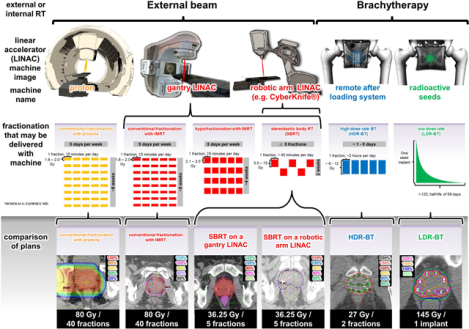
When the robotic arm linear accelerator (i.e. CyberKnife ®) was introduced (around 2001), it was marketed as being able to deliver “extremely” hypofractionated external beam RT, and it was shown to recapitulate HDR-BT plans to form a “virtual HDR-BT” (plans juxtaposed in Figure 2).64 Notably, however, SBRT may be delivered with any type of linear accelerator (robotic arm or gantry).61, 64 Although studies using SBRT have been published,20–23 they are limited by their exclusion of intermediate- and high-risk patients, and relatively small number of patients treated.61 Additionally, although SBRT is associated with lower treatment costs, there appears to be a greater rate of urinary toxicity for patients undergoing SBRT compared with conventionally fractionated RT with IMRT, and prospective studies are necessary.65 Treatment of patients with protons is more expensive, and possibly more toxic66, 67 than treatment with any of the other RT modalities. Each of the treatment modalities used to treat localized prostate cancer is associated with specific adverse effects and variable impact on quality of life68–72 (detailed in Table 5).
Figure 2. An illustration of the RT modalities available to treat prostate cancer.
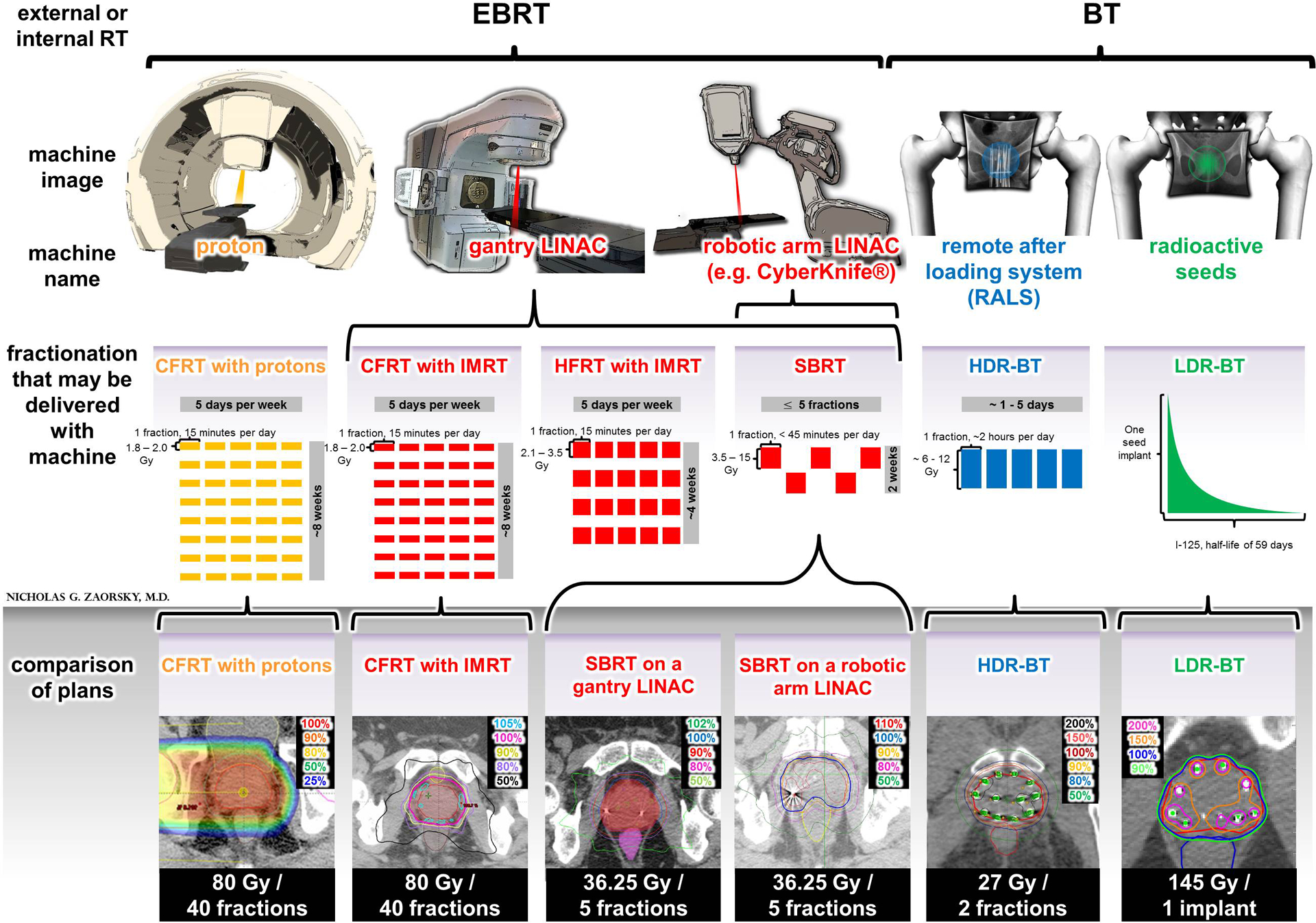
(TOP PANEL)
The two principal types of RT are external beam and brachythreapy. External beam RT is a form of radiation therapy where the patient sits or lies on a couch and an external source of radiation is pointed at the cancer. It includes conventional fractionation, hypofractionation, and stereotactic body radiation therapy (SBRT). External beam radiation therapy may be delivered with protons (yellow) or photons (red). Most men are treated with photon conventional fractionon with intensity modulated RT (IMRT) on a linear accelerator. SBRT may be delivered on a gantry or a robotic arm. Brachyhterapy is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment. For prostate cancer, brachytherapy is typically given as either high dose rate brachytherapy (delivered using a remote afterloading system) or low dose rate brachytherapy (delivered using permanently implanted radioactive seeds). High dose rate brachytherapy monotherapy is typically delivered in 1–5 fractions. Low dose rate brachytherapy monotherapy consists of one implant session.
(BOTTOM PANEL)
Plans from various fractionations and modalities are displayed. Proton plans are able to decrease incident dose (i.e. dose closer to the skin and femoral heads). Photon plans (red) pass through normal tissue and deliver some dose to normal structures; however, this dose is minimized using IMRT, or by adjusting incident beams of SBRT. brachytherapy plans generally deliver less dose to the rectum and have higher dose heterogeneity, with more areas of high doses in the prostate (vs. external beam radiation therapy).
Key Question 3: Unknown factors about prospective data
With respect to Key Question 3, the treatment decision for a man is usually based on his risk group (Table 3), ability to tolerate the procedure (Table 4), anticipated impact on quality of life (Table 5), and convenience for the patient. There are many other factors that influence outcomes and toxicities of prostate cancer patients treated with RT73 that were not reported among the studies included in this review, including: (1) race; (2) medical comorbidities; (3) psychiatric comorbidities; (4) insurance status; (5) education status; (6) marital status; (7) income; (8) sexual orientation; and (9) facility-related characteristics (e.g. number of centers involved, yearly hospital volumes).
Moreover, the scales used in the studies in this review are generally not detailed or tailored to patients; specifically, the RTOG score does not include the evaluation of anorectal symptoms, including fecal incontinence and urgency of defecation.74 Studies with limited follow up time (e.g. SBRT,61 HDR-BT monotherapy75) would not be able to accurately characterize late toxicities. Quality of life scales, including the SF-36 (introduced in 1994), Expanded Prostate Cancer Index Composite (EPIC, introduced in 2000), and the International Index of Erectile Function-15 (IIEF-15, introduced in 1999), are not routinely used among clinics or in clinical trials.46, 76, 77 These scales have been used to compare early and toxicities of external beam RT and BT in separate analyses.68, 70–72 The toxicities using these reports are summarized for all modalities included in this review in Table 5.
Limitations
This analysis has further limitations. First, we do not compare RT to RP (as per our methods). Recommendations of RP are covered in separate analyses;78 in general, RP is an option for most patients (Table 3). We anticipate that the results of this analysis may be used to compare RT options for various types of RP in future analyses. Next, we do not discuss recommendations regarding ADT; per Table 3, ADT is not recommended for low-risk patients, and it is almost always recommended for high-risk patients.38, 39 Novel risk-group stratifications (e.g. favorable and unfavorable intermediate risk)79 were not incorporated in this analysis; patients who fall into the unfavorable intermediate risk group may also benefit from more aggressive RT (e.g. BT boost) or the use of ADT. Similarly, genomic profiling of prostate cancers was not integrated in these studies; this may be beneficial in recommending therapy, similar to the post-operative setting.80
CONCLUSION
Studies of RT for prostate cancer have reported improved rates of FFBF with higher biologically equivalent RT doses. However, differences in OS, DM, and CSM have not been detected in RCTs of dose escalation or in studies comparing RT modalities. FFBF rates were roughly equivalent between conventional and hypofractionated RT with intensity modulation (evidence level 1B); SBRT had promising rates of BF, with shorter follow-up. Similarly, BT and BT + external beam RT were appropriate in select patients (evidence level 1B). There was limited evidence available to compare proton therapy to other modalities.
CONSENSUS / KEY ANSWERS
Key Answer 1:
Long-term FFBF rates are roughly equivalent between conventionally fractionated RT and hypofractionated RT with IMRT (evidence level 1B) among all NCCN risk groups. Similarly, BT monotherapy is an acceptable treatment option for select low- and intermediate-risk patients. FFBF rates among SBRT studies appear promising. For certain intermediate- and high-risk patients, BT boost may have improved FFBF rates vs. conventionally fractionated RT with IMRT alone (evidence level 1B). No study testing definitive RT has reported differences in OS, DM, or PCSM (evidence level 1B), which is not attributable to ADT.
Key Answer 2:
IMRT (as used with conventionally fractionated RT and hypofractionated RT) is associated with decreased toxicity compared to 3D-CRT (evidence level 1B to 2). Late GU and GI toxicities are otherwise similar between conventionally fractionated RT and hypofractionated RT (evidence level 1B). Many other modalities have not been tested in prospective RCTs to compare long-term toxicities, though grade 3–4 toxicities are rare (<5%).
Key Answer 3:
When making a decision for treatment, the patient and physician should consider the patient’s risk group, ability to tolerate the procedure, anticipated impact on quality of life, and convenience for the patient.
Supplementary Material
HIGHLIGHTS.
For prostate cancer, radiation therapy options include external beam and brachytherapy.
We compare the following radiation therapy modalities for prostate cancer using evidence grading: conventional fractionation, hypofractionation, stereotactic body radiation therapy, proton therapy, low dose rate brachytherapy, high dose rate brachytherapy, and brachytherapy boost.
Outcomes and toxicity profiles of the modalities are juxtaposed.
Contraindications of options are provided, based on current randomized trials.
Decision for a modality depends on risk group, tolerability of procedure, convenience, anticipated outcomes, and anticipated toxicities (i.e. quality of life).
GLOSSARY
| Term | Abbrev. | Definition |
|---|---|---|
| active surveillance, observation, watchful waiting | AS / WW | Choosing no immediate treatment as an alternative to immediate treatment at the time of diagnosis. These patients are closely monitored with and provided intervention, if necessary, at a time when cure is still possible. For the purposes of this review, we consider active surveillance, observation, and watchful waiting synonymous. For most patients undergoing active surveillance, a PSA is typically drawn every 6 months, digital rectal exam is performed yearly, and repeat biopsy is repeated at the discretion of the physician. |
| biologically effective dose | BED | A more conceptually useful measure of biological damage to cells than physical dose. It takes into account radiation sensitivity of cells, number of radiation fractions, and fraction size. |
| brachytherapy | BT | A form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment. For prostate cancer, it is typically given as either high dose rate (HDR; delivered using a RALS) or low dose rate (LDR; delivered using permanently implanted radioactive seeds). SBRT has been shown to recapitulate HDR-BT plans to form a “virtual HDR-BT.” |
| conventionally fractionated radiation therapy | CFRT | A type of EBRT typically defined as a single 1.8–2.0 Gy fraction lasting 15 minutes per day, five days per week, for about eight weeks to a total dose of 76–80 Gy. |
| external beam radiation therapy | EBRT | The most common form of radiation therapy. With EBRT, the patient sits or lies on a couch and an external source of radiation is pointed at the cancer. It includes CFRT, HFRT, and SBRT. EBRT may be delivered with photons or protons. |
| freedom from biochemical failure | FFBF | The time from which therapy for prostate cancer occurs until a rise in PSA hits a predefined threshold. For most randomized trials, the nadir + 2 ng/mL definition is used. FFBF helps to determine the efficacy of treatment. |
| Gray | Gy | The international unit of radiation dose for x-ray therapy (e.g. CFRT, HFRT, SBRT, LDR-BT, HDR-BT), 1 Joule/kilogram of matter. |
| hypofractionated radiation therapy | HFRT | A type of EBRT that is delivered as a single 2.1–3.5 Gy fraction lasting 15 minutes per day, five days per week, for about four weeks |
| image guided radiation therapy | IGRT | An integral component of RT systems that obtains imaging coordinates of a target and/or healthy tissues before or during treatment, detects and correct for random and systematic errors that occur in patient setup and organ motion, and increases accuracy and precision. Multiple types of IGRT systems exist. 2D/3D systems detect movements interfractionally (i.e. between two RT sessions); newer 4D systems detect movements intrafractionally (i.e. during one RT session). |
| intensity modulated radiation therapy | IMRT | An form of radiation that conforms the treatment volume to the shape of the tumor, using MLCs. The dose distribution created by IMRT is characterized by a concavity or invagination of the edge of the higher doses away from the rectum, rather than a straight edge through the rectum (as seen with 3D-CRT). IMRT may be envisioned as a flashlight, where the aperture may be adjusted to any shape; and beamlets of the flashlight may be made brighter or darker. |
| proton therapy | A type of EBRT that has a low incident energy and displays a spike at the tail end of its dose distribution. It theoretically spares the uninvolved tissues distal to the target and generally deposits a lesser dose than photons to tissues proximal to the target. | |
| prostate specific antigen | PSA | A soluble protein detected in the peripheral blood that is a surrogate biomarker used in both the initial detection and subsequent post treatment monitoring for prostate cancer. A rising PSA after therapy usually signifies local or metastatic recurrence, and it is used in calculating the FFBF. |
| remote afterloading system | RALS | Integral to HDR-BT, a RALS automatically deploys and retracts a single small radioactive source along the implant needle at specific positions delivering ≥ 12 Gy/hr. The RALS allows a physician to control the position where the HDR source stops for a predetermined time periods (i.e. the “dwell position” and “dwell time,” respectively). |
| radical prostatectomy | RP | The surgical removal of all or part of the prostate gland. |
| radiation therapy oncology group | RTOG | A national cooperative group set up for conduction of RT research and clinical investigations, it has helped to direct reporting of common outcome measures (e.g. FFBF) and toxicities (e.g. GI, GU; and their grades) among trials. |
| stereotactic body radiation therapy | SBRT | A type of EBRT delivered as a single 3.5–15.0 Gy fraction lasting up to 45 minutes per day, for up to 5 treatments, for about two weeks. |
Footnotes
Approval/disclosures: All authors have read and approved the manuscript. We have no financial disclosures. We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this paper. No text, text boxes, figures, or tables in this article have been previously published or owned by another party.
Conflicts of Interest Notification: We have no conflicts of interests.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw 2014;12(5):686–718. [DOI] [PubMed] [Google Scholar]
- 3.Shen X, Zaorsky NG, Mishra MV, et al. Comparative effectiveness research for prostate cancer radiation therapy: current status and future directions. Future Oncol 2012;8(1):37–54. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 5.Zaorsky NG, Li T, Devarajan K, Horwitz EM, Buyyounouski MK. Assessment of the American Joint Committee on Cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the National Comprehensive Cancer Network risk-stratification method. Cancer 2012;118(22):5535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 2013;190(2):441–9. [DOI] [PubMed] [Google Scholar]
- 7.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70(1):67–74. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;72(4):980–8. [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therpay in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J Clin Oncol 2010;28(7):1106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8(6):475–87. [DOI] [PubMed] [Google Scholar]
- 11.Michalski J, Winter K, Roach M, et al. Clinical outcome of patients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat Oncol Biol Phys 2012;83(3):e363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys 2010;76(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 2011;80(4):1056–63. [DOI] [PubMed] [Google Scholar]
- 14.Michalski JM, Moughan J, Purdy JA, et al. Initial Results of a Phase 3 Randomized Study of High Dose 3DCRT/IMRT versus Standard Dose 3D-CRT/IMRT in Patients Treated for Localized Prostate Cancer (RTOG 0126). Int J Radiat Oncol Biol Phys 2014;90(5):1263. [Google Scholar]
- 15.Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 2005;23(25):6132–8. [DOI] [PubMed] [Google Scholar]
- 16.Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 2011;81(5):1271–8. [DOI] [PubMed] [Google Scholar]
- 17.Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;84(5):1172–8. [DOI] [PubMed] [Google Scholar]
- 18.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013;31(31):3860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuban DA, Nogueras-Gonzalez GM, Hamblin L, et al. Preliminary Report of a Randomized Dose Escalation Trial for Prostate Cancer using Hypofractionation. Int J Radiat Oncol Biol Phys 2010;78(3):S58–S9. [Google Scholar]
- 20.Mantz C A Phase II Trial of Stereotactic Ablative Body Radiotherapy for Low-Risk Prostate Cancer Using a Non-Robotic Linear Accelerator and Real-Time Target Tracking: Report of Toxicity, Quality of Life, and Disease Control Outcomes with 5-Year Minimum Follow-Up. Front Oncol 2014;4(279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz AJ, Santoro M, DiBlasio F, Ashley R. Stereotactic Body Radiation Therapy for Low, Intermediate, and High-risk Prostate Cancer: Disease Control and Quality of Life. Int J Radiat Oncol 2011;81(2):S100–S. [Google Scholar]
- 22.Katz AJ, Santoro M, Ashley R, Diblasio F, Witten M. Stereotactic body radiotherapy as boost for organ-confined prostate cancer. Technol Cancer Res Treat 2010;9(6):575–82. [DOI] [PubMed] [Google Scholar]
- 23.Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations. Frontiers in oncology 2014;4(321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys 2005;61(5):1306–16. [DOI] [PubMed] [Google Scholar]
- 25.Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012;103(2):217–22. [DOI] [PubMed] [Google Scholar]
- 26.Duchesne GM, Williams SG, Das R, Tai KH. Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer: mature prospective phase I/II study results. Radiother Oncol 2007;84(2):128–34. [DOI] [PubMed] [Google Scholar]
- 27.Demanes DJ, Brandt D, Schour L, Hill DR. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am J Clin Oncol 2009;32(4):342–7. [DOI] [PubMed] [Google Scholar]
- 28.Galalae RM, Martinez A, Nuernberg N, et al. Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol 2006;182(3):135–41. [DOI] [PubMed] [Google Scholar]
- 29.Kalkner KM, Wahlgren T, Ryberg M, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol 2007;46(7):909–17. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz MD, Halabi S, Archer L, et al. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer 2011;117(24):5579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawton CA, DeSilvio M, Lee WR, et al. Results of a phase II trial of transrectal ultrasound-guided permanent radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (radiation therapy oncology group 98–05). Int J Radiat Oncol Biol Phys 2007;67(1):39–47. [DOI] [PubMed] [Google Scholar]
- 32.Tang JI, Williams SG, Tai KH, Dean J, Duchesne GM. A prospective dose escalation trial of high-dose-rate brachytherapy boost for prostate cancer: Evidence of hypofractionation efficacy? Brachytherapy 2006;5(4):256–61. [DOI] [PubMed] [Google Scholar]
- 33.Galalae RM, Loch T, Riemer B, et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther Onkol 2004;180(9):582–9. [DOI] [PubMed] [Google Scholar]
- 34.Demanes DJ, Martinez AA, Ghilezan M, et al. High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2011;81(5):1286–92. [DOI] [PubMed] [Google Scholar]
- 35.Lukka H RTOG 0938: A Randomized Phase II Trial Of Hypofractionated Radiotherapy For Favorable Risk Prostate Cancer. 2011.
- 36.Pollack A RTOG 0534: A Phase III Trial of Short Term Androgen Deprivation With Pelvic Lymph Node or Prostate Bed Only Radiotherapy (SPPORT) in Prostate Cancer Patients With a Rising PSA After Radical Prostatectomy. 2014.
- 37.Martinez AA. RTOG 0815: A Phase III Prospective Randomized Trial of Dose-Escalated Radiotherapy with or without Short-Term Androgen Deprivation Therapy for Patients with Intermediate-Risk Prostate Cancer. 2012.
- 38.Zaorsky NG, Trabulsi EJ, Lin J, Den RB. Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin Oncol 2013;40(3):308–21. [DOI] [PubMed] [Google Scholar]
- 39.Nomiya T, Tsuji H, Toyama S, et al. Management of high-risk prostate cancer: Radiation therapy and hormonal therapy. Cancer Treat Rev 2013;39(8):872–8. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 2012;11(1):20–32. [DOI] [PubMed] [Google Scholar]
- 41.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 2012;11(1):6–19. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen PL, Aizer A, Assimos DG, et al. ACR Appropriateness Criteria(R) Definitive External-Beam Irradiation in stage T1 and T2 prostate cancer. Am J Clin Oncol 2014;37(3):278–88. [DOI] [PubMed] [Google Scholar]
- 43.NCT01617161: Proton Therapy vs. IMRT for Low or Low-Intermediate Risk Prostate Cancer. 2013.
- 44.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2013;85(3):686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70(4):1124–9. [DOI] [PubMed] [Google Scholar]
- 46.Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamoah K, Eldredge-Hindy HB, Zaorsky NG, et al. Large prostate gland size is not a contraindication to low-dose-rate brachytherapy for prostate adenocarcinoma. Brachytherapy 2014;13(5):456–64. [DOI] [PubMed] [Google Scholar]
- 48.Shaikh T, Zaorsky NG, Ruth K, et al. Is it necessary to perform week three dosimetric analysis in low-dose-rate brachytherapy for prostate cancer when day 0 dosimetry is done? A quality assurance assessment. Brachytherapy 2015;14(3):316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaorsky NG, Harrison AS, Trabulsi EJ, et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol 2013;10(10):565–79. [DOI] [PubMed] [Google Scholar]
- 50.Wolff RF, Ryder S, Bossi A, et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer 2015. [DOI] [PubMed] [Google Scholar]
- 51.Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol 2015;67(6):1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94(13):981–90. [DOI] [PubMed] [Google Scholar]
- 53.Roach M 3rd, Hanks G, Thames H Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65(4):965–74. [DOI] [PubMed] [Google Scholar]
- 54.Zietman AL, Christodouleas JP, Shipley WU. PSA bounces after neoadjuvant androgen deprivation and external beam radiation: impact on definitions of failure. Int J Radiat Oncol Biol Phys 2005;62(3):714–8. [DOI] [PubMed] [Google Scholar]
- 55.Rosser CJ, Kuban DA, Levy LB, et al. Prostate specific antigen bounce phenomenon after external beam radiation for clinically localized prostate cancer. J Urol 2002;168(5):2001–5. [DOI] [PubMed] [Google Scholar]
- 56.Reis LO, Sanches BC, Zani EL, Castilho LN, Monti CR. PSA-nadir at 1 year as a sound contemporary prognostic factor for low-dose-rate iodine-125 seeds brachytherapy. World J Urol 2014;32(3):753–9. [DOI] [PubMed] [Google Scholar]
- 57.Hackett C, Ghosh S, Sloboda R, et al. Distinguishing prostate-specific antigen bounces from biochemical failure after low-dose-rate prostate brachytherapy. Journal of contemporary brachytherapy 2014;6(3):247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaorsky NG, Yamoah K, Thakur ML, et al. A paradigm shift from anatomic to functional and molecular imaging in the detection of recurrent prostate cancer. Future Oncol 2014;10(3):457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casalino DD, Remer EM, Arellano RS, et al. ACR Appropriateness Criteria(R) posttreatment follow-up of prostate cancer. J Am Coll Radiol 2011;8(12):863–71. [DOI] [PubMed] [Google Scholar]
- 60.Zaorsky NG, Ohri N, Showalter TN, Dicker AP, Den RB. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treat Rev 2013;39(7):728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaorsky NG, Studenski MT, Dicker AP, Gomella L, Den RB. Stereotactic body radiation therapy for prostate cancer: is the technology ready to be the standard of care? Cancer Treat Rev 2013;39(3):212–8. [DOI] [PubMed] [Google Scholar]
- 62.Zaorsky NG, Doyle LA, Yamoah K, et al. High dose rate brachytherapy boost for prostate cancer: A systematic review. Cancer Treat Rev 2014;40(3):414–25. [DOI] [PubMed] [Google Scholar]
- 63.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62(4):220–41. [DOI] [PubMed] [Google Scholar]
- 64.Zaorsky NG, Hurwitz MD, Dicker AP, Showalter TN, Den RB. Is robotic arm stereotactic body radiation therapy “virtual high dose ratebrachytherapy” for prostate cancer? An analysis of comparative effectiveness using published data [corrected]. Expert Rev Med Devices 2015;12(3):317–27. [DOI] [PubMed] [Google Scholar]
- 65.Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 2014;32(12):1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012;307(15):1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol 2011;60(5):908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358(12):1250–61. [DOI] [PubMed] [Google Scholar]
- 69.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Annals of internal medicine 2008;148(6):435–48. [DOI] [PubMed] [Google Scholar]
- 70.Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-stage prostate cancer. Semin Radiat Oncol 2008;18(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen RC, Zhang Y, Chen MH, et al. Patient-reported quality of life during radiation treatment for localized prostate cancer: results from a prospective phase II trial. BJU Int 2012;110(11):1690–5. [DOI] [PubMed] [Google Scholar]
- 72.Jarosek SL, Virnig BA, Chu H, Elliott SP. Propensity-weighted long-term risk of urinary adverse events after prostate cancer surgery, radiation, or both. Eur Urol 2015;67(2):273–80. [DOI] [PubMed] [Google Scholar]
- 73.Zaorsky NG, Egleston B, Horwitz EM, et al. The missing pieces in reporting of randomized controlled trials of external beam radiation therapy dose escalation for prostate cancer. Am J Clin Oncol 2016. [DOI] [PubMed] [Google Scholar]
- 74.Denham JW, O’Brien PC, Dunstan RH, et al. Is there more than one late radiation proctitis syndrome? Radiother Oncol 1999;51(1):43–53. [DOI] [PubMed] [Google Scholar]
- 75.Zaorsky NG, Doyle LA, Hurwitz MD, Dicker AP, Den RB. Do theoretical potential and advanced technology justify the use of high-dose rate brachytherapy as monotherapy for prostate cancer? Expert Rev Anticancer Ther 2014;14(1):39–50. [DOI] [PubMed] [Google Scholar]
- 76.Kleinmann N, Zaorsky NG, Showalter TN, et al. The effect of ethnicity and sexual preference on prostate-cancer-related quality of life. Nature Reviews Urology 2012;9(5):258–65. [DOI] [PubMed] [Google Scholar]
- 77.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007;177(6):2106–31. [DOI] [PubMed] [Google Scholar]
- 78.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 2012;62(3):382–404. [DOI] [PubMed] [Google Scholar]
- 79.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64(6):895–902. [DOI] [PubMed] [Google Scholar]
- 80.Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol 2015;33(8):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


