Abstract
An efficient adsorbent (a quaternary ammonium salt-modified chitosan microsphere, CTA-CSM) was synthesized via an emulsion cross-linking reaction between 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CTA) and chitosan (CS). The adsorption efficiency of the CTA-CSM as an adsorbent was studied using methyl orange dye to evaluate its suitability for wastewater purification. The characterization results showed that the CTA groups were successfully grafted onto the CS microspheres, and the as-prepared CTA-CSM samples exhibited a smooth surface and good dispersibility. The modification of CTA on CTA-CSM significantly improved its ability to remove methyl orange dye. The adsorption process of methyl orange by CTA-CSM was well described by the Langmuir isotherm model and followed the pseudo-second-order kinetic model. Under the optimal conditions, the maximum removal rate (98.9%) and adsorption capacity (131.9 mg/g) of CTA-CSM was higher than those of other previous reports; its removal rate for methyl orange was still up to 87.4% after five recycles. Hence, CTA-CSM is a very promising material for practical dyeing wastewater purification.
1. Introduction
In recent years, with the increasing discharge of dyeing wastewater and causing more pollution to water resources, dyeing wastewater has attracted great attention due to its degradation resistance, strong colority, and high toxicity.1−4 Various methods based on biodegradation,5 adsorption,6−8 oxidation,9 coagulation,10 and other approaches have been widely utilized for dyeing wastewater treatment. Adsorption presents obvious advantages over other approaches because of the simple design, low cost, and high efficiency.11 Until now, many kinds of absorbents have been researched and used in dyeing wastewater treatment, from inorganic materials (clay, zeolite, bauxite, etc.)12−14 to synthetic organic compounds (resin, polyurethane, polypropylene, etc.)15−17 and natural polymer materials (protein, starch, polysaccharide, etc.).18−21 Compared with the first two adsorbents, the natural polymer adsorbents can overcome the disadvantages of high cost, heavy pollution, poor economic efficiency, and the lack of biodegradability. Therefore, there is an urgent need to investigate a new and efficient green natural adsorbent material for the dyeing wastewater treatment.
As a natural biological macromolecule, chitosan (CS) is mainly derived from the outer shell of invertebrates and the cell wall of algae.22 Due to its rich sources and low prices, CS has been universally used in decolorization research23,24 owing to its nontoxicity, harmlessness, high efficiency, biodegradability, and good reusability compared with traditional adsorbents. A large number of free amino and hydroxyl groups exist on its linear macromolecular structure,2 and it can easily coordinate with heavy metal ions. Thus, it can provide favorable conditions for adsorbing various pollutants in dyeing wastewater. However, due to its poor water solubility,25 its application has been seriously limited.
One way to overcome this problem of the CS adsorbent is to modify CS by grafting reactive groups on the CS molecular chain. Recently, the CS-based adsorbents have been considered as one of the most promising functional materials for dyeing wastewater remediation.26 Lee27 reported the use of a series of polyurethane/CS composites for the Acid Violet 48 removal, and the results indicated that the maximum adsorption capacity was 30.0 mg/g at polyurethane/CS composites. Zheng28 successfully synthesized diatomite/CS-Fe(III) composites that were used as an adsorbent for the removal of an anionic azo dye, and the results showed a maximum adsorption capacity of 1250 mg/g. Although quaternary ammonium salt-modified chitosan (CTA-CS) can improve its solubility and adsorption properties for dyeing wastewater adsorbent, the systematic research studies on dealing with the use of CTA-CS for dyeing wastewater adsorbents are scarce.
In this work, a quaternary ammonium salt-modified chitosan microsphere (CTA-CSM) was synthesized by an emulsion cross-linking reaction between 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CTA) and CS. The adsorption performance of CTA-CSM in methyl-orange-simulated dyeing wastewater was investigated. The effects of the dosage of CTA-CSM, temperature, and pH on the adsorption of methyl orange were discussed. The adsorption isotherm models and kinetics of the adsorption process were determined in detail. The adsorption mechanism of CTA-CSM was also proposed based on the observed findings. The recyclability of the CTA-CSM adsorbent was studied. All of the above aims to evaluate the suitability of CTA-CSM to the dyeing wastewater treatment, further providing strong promise for applying this efficient and environmentally friendly adsorbent in wastewater purification.
2. Results and Discussion
2.1. Morphological Analysis
The scanning electron microscopy (SEM) images of CS, CTA-CS, and CTA-CSM and its particle size distribution are shown in Figure 1. It can be found that CS exhibited a network structure and seemed to be slightly wrinkled, while CTA-CS had a distinct wrinkled mass appearance with small particle size. It can be concluded that the appearance of CS was significantly changed after modification by CTA, which was presented from a slightly wrinkled network structure to a random cross-linked structure. Additionally, it can be clearly seen that CTA-CSM showed a regular morphology and great dispersibility. Meantime, the microsphere particles were uniformly distributed, which made the surface of the CTA-CSM relatively smooth. Furthermore, it can be observed that the particle size distribution range of microspheres was calculated as 5–125 μm.
Figure 1.
SEM images of (a) CS, (b) CTA-CS, and (c) CTA-CSM and (d) its particle size distribution.
2.2. Fourier Transform Infrared (FT-IR) Spectra
The FT-IR spectra of all of the samples are shown in Figure 2. The broad band at around 3430 cm–1 was ascribed to the stretching vibration peaks of O–H,29 and the bands of CTA-CS and CTA-CSM were more broadened than that of CS, indicating that the hydroxyl groups formed during the modification. The characteristic bands observed in the FT-IR spectrum at 2920 and 2870 cm–1 were assigned to the stretching vibration peak of aliphatic C–H. In addition, the band at 1600 cm–1 was attributed to the in-plane bending vibration peak of primary amine N–H on the CS molecular chain; it can be found that the peak of CTA-CS was significantly weakened, indicating that the CTA reaction occurred on the amino group of the CS chain. All of the above results confirmed that the CTA groups have been successfully grafted onto the CS chain.
Figure 2.

FT-IR spectra of CS, CTA-CS, and CTA-CSM.
Comparing with the infrared spectra of CTA-CS, it can be seen that a new characteristic band at 1650 cm–1 of CTA-CSM corresponded to the stretching vibration peak of C=N, which was generated by the cross-linking of −CHO and −NH2 on CS, showing that the cross-linking reaction in the preparation process mainly occurred on the amino groups of the CS molecular chains.
2.3. Effects of Conditions on Adsorption
The effect of the dosage of CTA-CSM on decolorization of dyeing wastewater can be observed in Figure 3. It can be seen that there was a rapid adsorption process (L-shaped curve) at the beginning of each adsorption curve, indicating that methyl orange had a high affinity with the CTA-CSM adsorbent surface. Besides, an increase in the dosage of CTA-CSM led to a decrease in the adsorption equilibrium time. The reason was that the number of active sites in the solution increased as the adsorbent dosage increased, resulting in the acceleration of its removal efficiency for methyl orange. Additionally, when the dosage of the adsorbent was more than 15 mg, all of the removal rates for methyl orange achieved the same constant at the adsorption equilibrium time. The removal rate for methyl orange was mainly determined by the adsorption and desorption processes. When the two above processes reached equilibrium, the removal rate would remain stable.30 Thus, the dosage of CTA-CSM in this paper was selected as 15 mg.
Figure 3.

Effect of dosage of CTA-CSM on adsorption (initial of dye concentration = 20 mg/L, T = 35 °C, pH = 9).
The effect of temperature is shown in Figure 4. The removal rate for methyl orange and the adsorption capacity of CTA-CSM increased as the temperature was increased, which indicated that the adsorption process was endothermic. When the temperature was lower, the Brownian motion in the reaction solution was weak, which was not conducive to the occurrence of adsorption. As the temperature was increased, the thermal motion increased significantly, which was beneficial to the improvement of the adsorption process. When the temperature was increased to 35 °C, the adsorption and desorption processes reached an equilibrium state, resulting in the removal rate for methyl orange and the adsorption capacity of CTA-CSM achieved the maximum of 98.0% and 49.1 mg/g, respectively. Hence, the optimal temperature in this case was 35 °C.
Figure 4.

Effect of temperature on adsorption (initial of dye concentration = 20 mg/L, dosage of CTA-CSM = 15 mg, pH = 9).
It can be clearly assayed in Figure 5 that the adsorption efficiency and the pH value related closely. The adsorption efficiency decreased after a prior increase with the increase of the pH value and reached the maximum in a weakly alkaline environment (pH = 9). Under acidic conditions, the large amount of positively charged H+ in the solution interacted with the negatively charged methyl orange by the electric neutralization reaction, which hindered the adsorption of methyl orange. With the increase of pH, the content of H+ in the solution decreased, which greatly weakened its hindering effect on the adsorption of methyl orange, resulting in a gradual increase in the adsorption efficiency of CTA-CSM on methyl orange. Similarly, under weakly alkaline conditions, the hydrogen bonding in this case attracted −OH in the alkaline solution,31 leading the surface of methyl orange to become more negatively charged. Meantime, CTA-CSM itself was positively charged in whether acidic or alkaline solutions.32 Hence, the promotion effect between the two was conducive to the adsorption of methyl orange. If the alkalinity was too strong, the excess OH– in the solution and SO3– in methyl orange showed electrostatic repulsion. Therefore, the pH value was selected to be 9.
Figure 5.

Effect of pH on adsorption (initial of dye concentration = 20 mg/L, dosage of CTA-CSM = 15 mg, T = 35 °C).
A comparison of the removal rates of CS, CTA-CS, and CTA-CSM for methyl orange is investigated in Figure 6. As seen, all of the removal rates of the samples for methyl orange increased first and then remained stable. Furthermore, the final removal rate for methyl orange was CTA-CSM (98.9%) > CTA-CS (90.6%) > CS (22.9%), showing that CTA-CSM had a strong ability to remove the methyl orange dye. Compared with CS, CTA-CS by CTA modification has dramatically improved its removal efficiency for methyl orange. The adsorption of CS on wastewater dye was attributed to the strong chelation and the adsorption ability of −OH and −NH2 on the CS chain,33 whereas the adsorption of CTA-CS even CTA-CSM on wastewater dye was assigned to the electrostatic adsorption and electrical neutralization as a flocculant, which could adsorb small flocs and residual molecules until the adsorption and desorption processes reached equilibrium. Thus, CTA-CS had more cations than CS that can be used in electrical neutralization processes, resulting in better removal efficiency for the methyl orange dye. It also can be seen that CTA-CSM showed better removal efficiency for dyes than CTA-CS. The larger specific surface area of CTA-CSM led to a strong adsorption effect on the dye besides electrostatic adsorption and electrical neutralization of −OH and −NH2, resulting in excellent removal efficiency of CTA-CSM as an adsorbent.
Figure 6.

Removal rates of the dye by CS, CTA-CS, and CTA-CSM (initial of dye concentration = 20 mg/L, dosage of CTA-CSM = 15 mg, T = 35 °C, pH = 9).
2.4. Adsorption Isotherms and Kinetics
Langmuir, Freundlich, and Temkin adsorption isotherms were generated based on the above experiments, which were used to describe the interaction of the dye adsorbed with the adsorbent. The results are shown in Figure 7. The linear forms of the adsorption isotherm equations34,35 and fitting coefficients are summarized in Table 1.
Figure 7.
Adsorption isotherms of MSM for methyl orange (a, Langmuir; b, Freundlich; c, Temkin).
Table 1. Linear Equations of Isotherm Models and Their Fitting Coefficients.
| isotherm model | equation | R2 | |
|---|---|---|---|
| Langmuir | 0.993 | ||
| Freundlich | 0.976 | ||
| Temkin | 0.767 |
It can be seen from Figure 7 that the experimental isotherm
data fitted best with the Langmuir isotherm model, and the fitting
coefficient of CTA-CSM for the adsorption of methyl orange was R2 = 0.993 (Table 1), which was higher than the other two adsorption isotherm
models. Based on the assumption of the Langmuir isotherm model, the
maximum adsorption corresponds to the formation of a monolayer of
adsorbate molecules on the surface of the adsorbent.36 The above indicated that the adsorption process of methyl
orange by CTA-CSM was a monolayer adsorption process. Additionally,
the essential characteristics of the Langmuir isotherm model can be
estimated by the separation factor constant (RL) and determined by equation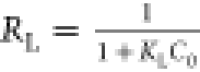 ,34 and a range of 0.04–0.32 is calculated.
According to the value, RL > 1.0, unfavorable; RL = 1.0, linear; 0 < RL < 1.0, favorable; RL = 0,
irreversible. Therefore, the adsorption of methyl orange by CTA-CSM
in this paper can be inferred as favorable adsorption.
,34 and a range of 0.04–0.32 is calculated.
According to the value, RL > 1.0, unfavorable; RL = 1.0, linear; 0 < RL < 1.0, favorable; RL = 0,
irreversible. Therefore, the adsorption of methyl orange by CTA-CSM
in this paper can be inferred as favorable adsorption.
To better understand the adsorption process of methyl orange by CTA-CSM, the kinetic analysis was carried out using a pseudo-first-order model and a pseudo-second-order model, with equations expressed as below37
where Qe (mg/g) is the adsorption capacity at equilibrium, Qt (mg/g) is the adsorption capacity at time t; and K1 and K2 are the equilibrium rate constants of these two models, respectively.
As can be seen from Figure 8, the adsorption process of methyl orange by CTA-CSM could be well described by the pseudo-second-order kinetic model, and its fitting coefficient (R2 = 0.998) was much better than that of the pseudo-first-order kinetic model (R2 = 0.879), indicating that chemical adsorption was the main step in this case. Moreover, the calculated Qe (142.9 mg/g) by the pseudo-second-order kinetic model was close to the experimental Qe (131.9 mg/g), which further confirmed the suitability of the pseudo-second-order kinetic model.
Figure 8.
Adsorption kinetic equation of CTA-CSM for methyl orange.
2.5. Adsorption Mechanism of CTA-CSM
The adsorption process of methyl orange by CTA-CSM had been confirmed to be a monolayer chemical adsorption, and it was favorable for adsorption. The protonation of the amine groups was positively charged, which made the CTA-CSM surface also positively charged. Meantime, methyl orange was ionized in the aqueous solution and negatively charged due to the existence of SO3–. Thus, there was an electrostatic attraction interaction between CTA-CSM and methyl orange during the adsorption process. Subsequently, this kind of synergistic adsorption increased the adsorption efficiency of methyl orange. It has been reported that the electrostatic attraction interaction was the most responsible interaction force for methyl orange adsorption.38 Besides, the surface of CTA-CSM contains a large number of hydroxyl and amine groups, and these functional groups may be combined with the N atom on methyl orange, causing dipole–dipole H-bonding interactions; it may also overlap with methyl orange and form Yoshida H-bonding, which facilitates the adsorption process. Moreover, the possibility of n−π stacking interaction cannot be ignored. The n−π stacking interaction usually occurred from the lone pair of electrons on an oxygen atom to the π orbital of the aromatic ring of dyes.31 Hence, n−π stacking interactions may also occur in this case. The schematic diagram of the interaction between CTA-CSM and methyl orange is shown in Figure 9.
Figure 9.
Schematic diagram of the interaction between CTA-CSM and methyl orange.
2.6. Reusability of CTA-CSM
The recyclability and reusability of the adsorbent is an important factor for its sustainable, low-cost use in industrial applications. Thus, after the adsorption experiment finished, the CTA-CSM was separated, washed, dried, and then directly used to adsorb methyl orange again. The regeneration and reusability of this adsorbent were determined through the removal efficiency after recycling use. The result is shown in Figure 10. It can be seen that although the removal rate gradually decreased with an increase of cycle time, the removal rate still reached 87.4% after five cycles, which was much better than other reported studies,39,40 indicating that the CTA-CSM had great reusability, which was very important and attractive for applications in the adsorption of dyeing wastewater.
Figure 10.

Recycle usability of the CTA-CSM catalyst.
Meanwhile, the adsorption capacity of CTA-CSM was compared with other previously reported adsorbents, and the results are summarized in Table 2. It can be clearly observed that the adsorption capacity of the CTA-CSM adsorbent used in this paper was rather higher compared with those of other studies, with an adsorption capacity of 131.9 mg/g. Hence, CTA-CSM can be used as an efficient adsorbent for treating methyl-orange-containing solutions. The above implied CTA-CSM in this case had promising potential as a sustainable, cost-effective, and high-reusability adsorbent.
Table 2. Comparison of Different Adsorbents for Methyl Orange Removal.
| adsorbent | removal efficiency (%) | adsorption capacity (mg/g) | refs |
|---|---|---|---|
| polyaniline | 98.84 | 75.9 | Tanzifi et al.41 |
| CS/REC/CNT | 80.5 | 41.65 | Chen et al.37 |
| graphene oxide | 16.83 | Robati et al.42 | |
| AHM | 99.60 | 15.56 | Qu et al.40 |
| MnCo2O4 nanospinel | 93 | 100.01 | Tarighi et al.43 |
| GOA | 55.56 | Tu et al.44 | |
| CTA-CSM | 98.9 | 131.9 | this study |
3. Conclusions
In this paper, the CTA-CSM adsorbent based on the natural polymeric material was successfully prepared via an emulsion cross-linking reaction between CTA and CS. The adsorption performances of CTA-CSM on methyl-orange-simulated dyeing wastewater were investigated in detail. The modification was found to greatly improve its ability to remove the methyl orange dye, and the removal rate achieved was 98.9%. The adsorption process followed the Langmuir isotherm model and pseudo-second-order kinetics. The maximum adsorption capacity of CTA-CSM for methyl orange was up to 131.9 mg/g, which was rather higher than those of other previous reports. The removal efficiency for methyl orange still attained 87.4% with great reusability after five recycles. Thus, CTA-CSM can be used as an effective adsorbent for treating methyl-orange-containing solutions, which shows its promising potential of being a sustainable, cost-effective, and highly reusable adsorbent for dyeing wastewater treatment.
4. Experimental Section
4.1. Materials
All of the chemicals used in the experiments were of analytical grade, and the samples were prepared using distilled water. CS, isopropanol, NaOH (40%, wt), methanol, ethanol, acetic acid, paraffin, Span80, formaldehyde, acetone, and methyl orange were obtained from Sinopharm Chemical Reagent Co., Ltd. CTA (60%, wt) was acquired from Aladdin Chemical Reagent Co., Ltd.
4.2. Preparation of CTA-CSM
The steps for preparing the CTA-CSM are as follows. CS (5 g), 80 mL of isopropanol, and a certain amount of NaOH solution were added into a three-necked flask, subsequently stirring at 50 °C for 4 h on a thermostatic magnetic stirrer. Then, CTA was added dropwise, and the mixture was continuously reacted at 70 °C on a thermostatic magnetic stirrer for 7 h. Then, the mixture was washed with the neutral filtrate after cooling to room temperature. Finally, CTA-CS was obtained by drying in a vacuum oven at 80 °C after being washed with methanol and ethanol. All of the reactions are shown in Figure 11.
Figure 11.

Synthesis reaction of CTA-CS.
Subsequently, CTA-CSM was prepared by the emulsion cross-linking method. The previously as-prepared CTA-CS was dissolved in acetic acid to prepare the 3% (w/v) CTA-CS acetic acid solution. Paraffin (80 mL) and 2 mL of Span 80 were added into a three-necked flask. The solution was stirred at 40 °C until it was dispersed homogeneously. Next, CTA-CS acetic acid was added dropwise, subsequently stirring at 40 °C for 2 h. Then, formaldehyde was added and the mixture was centrifuged and washed with acetone and ethanol. Finally, CTA-CSM was obtained by drying in a vacuum oven at 80 °C for 5 h.
4.3. Adsorption Experiments
The CTA-CSM and a certain concentration of methyl orange solution were mixed in a 250 mL flask, following shaking on a constant temperature oscillator of 35 °C. The absorbance of the sample at 464 nm was measured by a UV1800-PC spectrophotometer. Each experiment was performed three times to guarantee experimental stability and accuracy. The relative error between each run was less than 2%. The removal rate R (%) and adsorption capacity q (mg/g) were calculated based on the experiments. The formulas are listed as follows45
where C0 (mg/L) is the initial concentration of methyl orange, Ct (mg/L) is the concentration of methyl orange after adsorption, V (L) is the volume of adsorbate, m (g) is the mass of CTA-CSM, qt (mg/g) is the adsorbed amount at a certain time, and qe (mg/g) is the adsorbed amount at equilibrium.
4.4. Characterization
The morphology and mean diameter of the as-prepared microspheres nanoparticles were observed using scanning electron microscopy (SEM) (Philips XL30, Holland) and a laser light scattering particle size analyzer (SALD 7500, Japan), respectively. Fourier transform infrared spectroscopy (FT-IR) spectra (4000–500 cm–1) were recorded on a Bruker VERTEX 70 FT-IR spectrometer using KBr in the ratio of 1:200. Ultraviolet–visible spectrophotometry was performed using a CARY 300 instrument (Agilent).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21473126).
The authors declare no competing financial interest.
References
- Cai H.; Liang J.; Ning X.; Lai X.; Li Y. Algal toxicity induced by effluents from textile-dyeing wastewater treatment plants. J. Environ. Sci 2020, 91, 199–208. 10.1016/j.jes.2020.01.004. [DOI] [PubMed] [Google Scholar]
- Hui M.; Pu S.; Hou Y.; Zhu R.; Zinchenko A.; Chu W. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018, 345, 556–565. 10.1016/j.cej.2018.03.115. [DOI] [Google Scholar]
- Durruty I.; Fasce D.; Froilan Gonzalez J.; Alejandra Wolski E. A kinetic study of textile dyeing wastewater degradation by Penicillium chrysogenum. Bioprocess Biosyst. Eng. 2015, 38, 1019–1031. 10.1007/s00449-014-1344-9. [DOI] [PubMed] [Google Scholar]
- Hisaindee S.; Meetani M. A.; Rauf M. A. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC, Trends Anal. Chem. 2013, 49, 31–44. 10.1016/j.trac.2013.03.011. [DOI] [Google Scholar]
- Kiriarachchi H. D.; Abouzeid K. M.; Bo L.; El-Shall M. S. Growth Mechanism of Sea Urchin ZnO Nanostructures in Aqueous Solutions and Their Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 14013–14020. 10.1021/acsomega.9b01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C.; Li H.; Yu Y. Homologous-heterogeneous structure control and intelligent adsorption effect of a polycationic gel for super-efficient purification of dyeing wastewater. RSC Adv. 2019, 9, 9421–9434. 10.1039/C9RA00690G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S.; Xia L.; Guo T.; Zhang F.; Cui L.; Su X.; Wang D.; Guo W.; Sun J. Controlled synthesis of flexible graphene aerogels macroscopic monolith as versatile agents for wastewater treatment. Appl. Surf. Sci. 2018, 445, 30–38. 10.1016/j.apsusc.2018.03.132. [DOI] [Google Scholar]
- Gu F.; Geng J.; Li M.; Chang J.; Cui Y. Synthesis of Chitosan-Ignosulfonate Composite as an Adsorbent for Dyes and Metal Ions Removal from Wastewater. ACS Omega 2019, 4, 21421–21430. 10.1021/acsomega.9b03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Wang S.; Kong H.; Liu T.; Xia M. Performance of combined process of anoxic baffled reactor-biological contact oxidation treating printing and dyeing wastewater. Bioresour. Technol. 2007, 98, 1501–1504. 10.1016/j.biortech.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Shen C.; Pan Y.; Wu D.; Liu Y.; Ma C.; Li F.; Ma H.; Zhang Y. A crosslinking-induced precipitation process for the simultaneous removal of poly(vinyl alcohol) and reactive dye: The importance of covalent bond forming and magnesium coagulation. Chem. Eng. J. 2019, 374, 904–913. 10.1016/j.cej.2019.05.203. [DOI] [Google Scholar]
- Kyzas G. Z.; Fu J.; Matis K. A. The Change from Past to Future for Adsorbent Materials in Treatment of Dyeing Wastewaters. Materials 2013, 6, 5131–5158. 10.3390/ma6115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Yao Y.; Yin G.; Kang Y.; Luo Y.; Zhuo L. Preparation of decolorizing ceramsites for printing and dyeing wastewater with acid and base treated clay. Appl. Clay Sci. 2008, 40, 20–26. 10.1016/j.clay.2007.06.009. [DOI] [Google Scholar]
- Zanin E.; Scapinello J.; De Oliveira M.; Rambo C. L.; Franscescon F.; Freitas L.; Muneron De Mello J. M.; Fiori M. A.; Vladimir Oliveira J.; Dal Magro J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. 10.1016/j.psep.2016.11.008. [DOI] [Google Scholar]
- Atasoy A. D.; Bilgic B. Adsorption of Copper and Zinc Ions from Aqueous Solutions Using Montmorillonite and Bauxite as Low-Cost Adsorbents. Mine Water Environ. 2018, 37, 205–210. 10.1007/s10230-017-0464-2. [DOI] [Google Scholar]
- Yang C.; Li L.; Shi J.; Long C.; Li A. Advanced treatment of textile dyeing secondary effluent using magnetic anion exchange resin and its effect on organic fouling in subsequent RO membrane. J. Hazard. Mater. 2015, 284, 50–57. 10.1016/j.jhazmat.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Moawed E. A.; El-Hagrasy M. A.; Senan A. E. A. Application of bio-alkyd resin for the removal of crystal violet and methylene blue dyes from wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 8495–8504. 10.1007/s13762-019-02343-1. [DOI] [Google Scholar]
- Xiao K.; Xu F.; Jiang L.; Duan N.; Zheng S. Resin oxidization phenomenon and its influence factor during chromium(VI) removal from wastewater using gel-type anion exchangers. Chem. Eng. J. 2016, 283, 1349–1356. 10.1016/j.cej.2015.08.084. [DOI] [Google Scholar]
- Xu Z.; Hao N.; Li L.; Zhang Y.; Yu L.; Jiang L.; Sui X. Valorization of Soy Whey Wastewater: How Epigallocatechin-3-gallate Regulates Protein Precipitation. ACS Sustainable Chem. Eng. 2019, 7, 15504–15513. 10.1021/acssuschemeng.9b03208. [DOI] [Google Scholar]
- Wu H.; Liu Z.; Li A.; Yang H. Evaluation of starch-based flocculants for the flocculation of dissolved organic matter from textile dyeing secondary wastewater. Chemosphere 2017, 174, 200–207. 10.1016/j.chemosphere.2017.01.120. [DOI] [PubMed] [Google Scholar]
- Ma W.; Meng M.; Zhang S. F.; Ju B. Z.; Zhang M.. Application Mechanism and Performance of Cationic Native Starch and Cationic Hydrolyzed Starch in Salt-Free Dyeing of Reactive Dyes. In Applied Mechanics and Materials; Trans Tech Publications Ltd., 2012; Vol. 161, pp 212–216. [Google Scholar]
- Ye Z.; Tang M.; Hong X.; Hui K. S. Sustainable composite super absorbents made from polysaccharides. Mater. Lett. 2016, 183, 394–396. 10.1016/j.matlet.2016.07.067. [DOI] [Google Scholar]
- Merz C. R. Physicochemical and Colligative Investigation of alpha (Shrimp Shell)-and beta (Squid Pen)-Chitosan Membranes: Concentration-Gradient-Driven Water Flux and Ion Transport for Salinity Gradient Power and Separation Process Operations. ACS Omega 2019, 4, 21027–21040. 10.1021/acsomega.9b02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balea A.; Concepcion Monte M.; Fuente E.; Luis Sanchez-Salvador J.; Blanco A.; Negro C. Cellulose nanofibers and chitosan to remove flexographic inks from wastewaters. Environ. Sci.: Water Res. Technol. 2019, 5, 1558–1567. 10.1039/C9EW00434C. [DOI] [Google Scholar]
- Guo J.; Chen S.; Liu L.; Li B.; Yang P.; Zhang L.; Feng Y. Adsorption of dye from wastewater using chitosan-CTAB modified bentonites. J. Colloid Interface Sci. 2012, 382, 61–66. 10.1016/j.jcis.2012.05.044. [DOI] [PubMed] [Google Scholar]
- Yan T.; Li C.; Ouyang Q.; Zhang D.; Zhong Q.; Li P.; Li S.; Yang Z.; Wang T.; Zhao Q. Synthesis of gentamicin-grafted-chitosan with improved solubility and antibacterial activity. React. Funct. Polym. 2019, 137, 38–45. 10.1016/j.reactfunctpolym.2019.01.013. [DOI] [Google Scholar]
- Ma H.; Pu S.; Ma J.; Yan C.; Zinchenko A.; Pei X.; Chu W. Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater. Lett. 2018, 211, 91–95. 10.1016/j.matlet.2017.09.091. [DOI] [Google Scholar]
- Lee H. C.; Jeong Y. G.; Min B. G.; Lyoo W. S.; Lee S. C. Preparation and acid dye adsorption behavior of polyurethane/chitosan composite foams. Fibers Polym. 2009, 10, 636–642. 10.1007/s12221-010-0636-1. [DOI] [Google Scholar]
- Zheng L.; Wang C.; Shu Y.; Yan X.; Li L. Utilization of diatomite/chitosan-Fe (III) composite for the removal of anionic azo dyes from wastewater: Equilibrium, kinetics and thermodynamics. Colloids Surf., A 2015, 468, 129–139. 10.1016/j.colsurfa.2014.12.015. [DOI] [Google Scholar]
- Wang W.; Yang X.; Fang Y.; Ding J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid-liquid phase change materials. Appl. Energy 2009, 86, 170–174. 10.1016/j.apenergy.2007.12.003. [DOI] [Google Scholar]
- Gao S.; Luo T.; Zhou Q.; Luo W.; Li H.; Jing L. Surface sodium lignosulphonate-immobilized sawdust particle as an efficient adsorbent for capturing Hg2+ from aqueous solution. J. Colloid Interface Sci. 2018, 517, 9–17. 10.1016/j.jcis.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Jawad A. H.; Mamat N. F. H.; Hameed B. H.; Ismail K. Biofilm of cross-linked Chitosan-Ethylene Glycol Diglycidyl Ether for removal of Reactive Red 120 and Methyl Orange: Adsorption and mechanism studies. J. Environ. Chem. Eng. 2019, 7, 102965 10.1016/j.jece.2019.102965. [DOI] [Google Scholar]
- Liu J.; Ma S.; Zang L. Preparation and characterization of ammonium-functionalized silica nanoparticle as a new adsorbent to remove methyl orange from aqueous solution. Appl. Surf. Sci. 2013, 265, 393–398. 10.1016/j.apsusc.2012.11.019. [DOI] [Google Scholar]
- Herrera-González A. M.; Pelaez-Cid A. A.; Caldera-Villalobos M. Adsorption of textile dyes present in aqueous solution and wastewater using polyelectrolytes derived from chitosan. J. Chem. Technol. Biotechnol. 2017, 92, 1488–1495. 10.1002/jctb.5214. [DOI] [Google Scholar]
- Al-Ghouti M. A.; Da’ana D. A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383 10.1016/j.jhazmat.2020.122383. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Wang X.; Qin D.; Da W.; Hou B.; Hao C.; Wu J. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019, 369, 50–61. 10.1016/j.jhazmat.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Wang X.; Wang G.; Hao C.; Li X.; Li T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Composites, Part B 2019, 169, 45–54. 10.1016/j.compositesb.2019.03.082. [DOI] [Google Scholar]
- Chen J.; Shi X.; Zhan Y.; Qiu X.; Du Y.; Deng H. Construction of horizontal stratum landform-like composite foams and their methyl orange adsorption capacity. Appl. Surf. Sci. 2017, 397, 133–143. 10.1016/j.apsusc.2016.10.211. [DOI] [Google Scholar]
- Bahrudin N. N.; Nawi M. A.; Jawad A. H.; Sabar S. Adsorption Characteristics and Mechanistic Study of Immobilized Chitosan-Montmorillonite Composite for Methyl Orange removal. J. Polym. Environ. 2020, 28, 1901–1913. 10.1007/s10924-020-01734-7. [DOI] [Google Scholar]
- Feng Z.; Danjo T.; Odelius K.; Hakkarainen M.; Iwata T.; Albertsson A. C. Recyclable fully biobased chitosan adsorbents spray-dried in one-pot to microscopic size and enhanced adsorption capacity. Biomacromolecules 2019, 20, 1956–1964. 10.1021/acs.biomac.9b00186. [DOI] [PubMed] [Google Scholar]
- Qu W.; He D.; Huang H.; Guo Y.; Tang Y.; Song R. Characterization of amino-crosslinked hypromellose and its adsorption characteristics for methyl orange from water. J. Mater. Sci. 2020, 55, 7268–7282. 10.1007/s10853-020-04517-6. [DOI] [Google Scholar]
- Tanzifi M.; Hosseini S. H.; Kiadehi A. D.; Olazar M.; Karimipour K.; Rezaiemehr R.; Ali I. Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: Kinetic, isotherm and thermodynamic studies. J. Mol. Liq. 2017, 244, 189–200. 10.1016/j.molliq.2017.08.122. [DOI] [Google Scholar]
- Robati D.; Mirza B.; Rajabi M.; Moradi O.; Tyagi I.; Agarwal S.; Gupta V. K. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng. J. 2016, 284, 687–697. 10.1016/j.cej.2015.08.131. [DOI] [Google Scholar]
- Tarighi S.; Juibari N. M. Green Synthesized Manganese-Cobaltite Nanospinel and Its Dye Removal Characteristics: Isothermal and Kinetic Studies. ChemistrySelect 2019, 4, 6506–6515. 10.1002/slct.201900816. [DOI] [Google Scholar]
- Tu T. H.; Cam P. T. N.; Huy L. V. T.; Phong M. T.; Nam H. M.; Hieu N. H. Synthesis and application of graphene aerogel as an adsorbent for water treatment. Mater. Lett. 2019, 238, 134–137. 10.1016/j.matlet.2018.11.164. [DOI] [Google Scholar]
- Wang X.; Jiang C.; Hou B.; Wang Y.; Hao C.; Wu J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. 10.1016/j.chemosphere.2018.04.183. [DOI] [PubMed] [Google Scholar]







