Abstract
Atomically precise Au25(SG)18 clusters have shown great promise in near-infrared II cerebrovascular imaging, X-ray imaging, and cancer radiotherapy due to their high atomic number, unique molecular-like electronic structure, and renal clearable properties. Therefore, it is important to study the in vivo toxicity of Au25 clusters. Unfortunately, previous toxicological investigations focused on low injected doses (<100 mg kg–1) and routine research methods, such as blood chemistry and biochemistry, which cannot reflect neurotoxicity or tiny changes in neural activity. In this work, in vivo neuroelectrophysiology of Au25 clusters at ultrahigh injected doses (200, 300, and 500 mg kg–1) was investigated. Local field potential showed that the Au25-treated mice showed a spike in delta rhythm and moved to lower frequency over time. The power spectrum showed a 38.3% reduction in the peak value at 10 h post-injection of Au25 clusters compared with 3 h post-injection, which gradually became close to the normal level, indicating no permanent damage to the nervous system. Moreover, no significant structural changes were found in both neurons and glial cells at the histological level. These results of in vivo neuroelectrophysiology will encourage scientists to make more exciting discoveries on nervous system diseases by employing Au25 clusters even at ultrahigh injected doses.
Introduction
Atomically precise Au25(SG)18 clusters have attracted wide interest in the field of biomedicine due to their high atomic number and unique molecular-like electronic structure.1−3 Meanwhile, it possesses ultrasmall hydrodynamic size, rapid kidney clearance, excellent aqueous solubility and photostability.1−9 Thus, Au25 clusters have shown a wide range of applications, including near-infrared II (NIR-II) imaging,1,10−13 X-ray imaging,11 photothermal therapy,2 cancer radiation therapy,3,14−16 and cancer detection.17−21 Particularly, in NIR-II imaging, Au25(SG)18 clusters exhibited high temporal/spatial resolution and deep tissue penetration (>0.6 cm) due to unique emission spectrum (1300–1400 nm) and long stokes shift (670 nm).22 Moreover, our previous studies suggested that Au25 clusters could be applied as promising agents in NIR-II cerebrovascular imaging and primary tumor, vascular, and lymphatic metastases identifying.1,22 Due to ultrahigh injected doses in cancer radiotherapy, X-ray imaging, and NIR-II imaging, it is interesting to study the in vivo toxicity of Au25 clusters at the ultrahigh dose level.
Compared with the toxicology at a low injected dose (<100 mg kg–1), the toxicology of Au25(SG)18 clusters at the ultrahigh injected dose is more important. For NIR-II imaging, Au25 clusters can serve as an efficient NIR-II fluorophore in biological imaging, and to improve the spatial resolution, a high concentration of Au25 clusters was injected to enhance the fluorescence signal for NIR-II cerebrovascular imaging.23,24 Therefore, it is particularly important to study the in vivo toxicity of Au25 clusters at higher doses and conduct extensive research on their performance for its application in the fields of biomedicine and bioimaging. Numerous studies, including our previous studies, have proved that Au25 clusters can be applied as promising agents in NIR-IIa imaging to identify brain hemodynamic distinction of mice with different brain diseases,1 which showed a promising clinical application in dynamic monitoring of cerebral blood flow. Therefore, it is desirable to investigate their biosafety, particularly the neurotoxicity of ultrahigh injected doses of Au25 clusters. In our previous studies, we have widely performed the in vivo toxicity of Au25 clusters by evaluating the whole-body NIR-II imaging, biodistribution, immune response, hematology, and biochemistry. Unfortunately, previous toxicological investigations focused on low injected doses and routine research methods,1,3,25−28 such as blood chemistry and biochemistry, which cannot reflect neurotoxicity or tiny changes in neural activity.1−3,29,30 It is shown that they will play an important role in clinical applications highlighting the necessity of extensive research on their performance. Thus, it is crucial to study neurotoxicity that can estimate tiny changes in the neuroelectrophysiological activity and tissue structure of the central nervous system at ultrahigh injected doses.
In the present study, we analyzed the neurotoxicity of ultrahigh injected doses by in vivo neuroelectrophysiological monitoring. The Au25-treated mice (200, 300, and 500 mg kg–1) showed a spike in delta rhythm and moved to lower frequency over time. The power spectrum showed a 38.3% reduction in the peak value at 10 h post-injection of Au25 clusters compared with 3 h post-injection, which gradually became close to the normal level, indicating no permanent damage to the nervous system. Furthermore, pathologic analyses by hematoxylin and eosin (H&E) staining and immunohistochemistry of Au25-cluster-treated mice at ultrahigh injected doses were evaluated; however, no significant structural changes were found in both neurons and glial cells at the histological level.
Results and Discussion
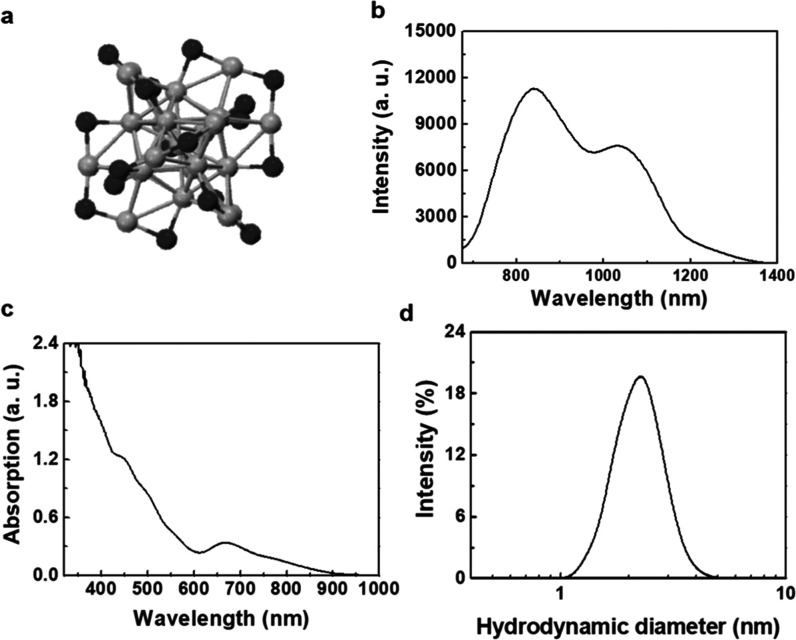
The preparation of Au25 clusters was based on a previously reported method.4,31,32 Several experiments had been carried out to conduct physical property analyses of Au25 clusters in our previous work,1,29 including transmission electron microscopy (TEM) imaging, size distribution, and stability in water. In our previous studies, we have performed in vivo renal clearance and toxicity tests of Au25 clusters protected by different ligands through evaluating biodistribution, immune response, hematology, and biochemistry.1,3,13,22,26,29 Among them, the small-sized GSH-protected Au25 clusters showed great advantage, as they could be effectively eliminated by the kidneys, minimizing any potential side effects caused by the in vivo accumulation of Au25 clusters.29,33 Therefore, the GSH-protected Au25 clusters with great biocompatibility were chosen to further study their impact on the nervous system within the neuroelectrophysiological monitoring method. As shown in Figure 1a, the crystal structure of Au25(SG)18 clusters was composed of 25 gold core atoms and 18 sulfur atoms.1 There were two characteristic emission peaks at the emission spectrum of Au25 clusters in the range of 675–1400 nm under an excitation of 655 nm, revealing their distinct interband transitions (Figure 1b). The mechanism of NIR-II imaging of Au25(SG)18 clusters included a complex electron transfer between surface ligands and gold cores.34,35 The UV–vis absorption spectrum of Au25 clusters recorded in water showed two distinct absorption peaks at 450 and 670 nm (Figure 1c), which corresponded appropriately to the theoretical spectrum of thiolate-protected Au25 clusters.36 The hydrodynamic size of Au25 clusters in water was determined by dynamic light scattering (DLS), and the kinetic diameter was about 2.89 nm (Figure 1d), which was less than the renal filtration threshold of 5.5 nm, resulting in the efficient excretion of Au25 clusters from the body. These results indicated that Au25 clusters were ultrasmall and soluble in water.
Figure 1.
Physical properties of Au25 clusters. (a) Crystal structure of Au25(SG)18 clusters. (b) Emission spectrum of Au25 clusters in the range of 675–1400 nm. Excitation wavelength: 655 nm. (c) UV–vis absorption spectrum of Au25 clusters. (d) Hydrodynamic size of Au25 clusters in water measured by DLS.
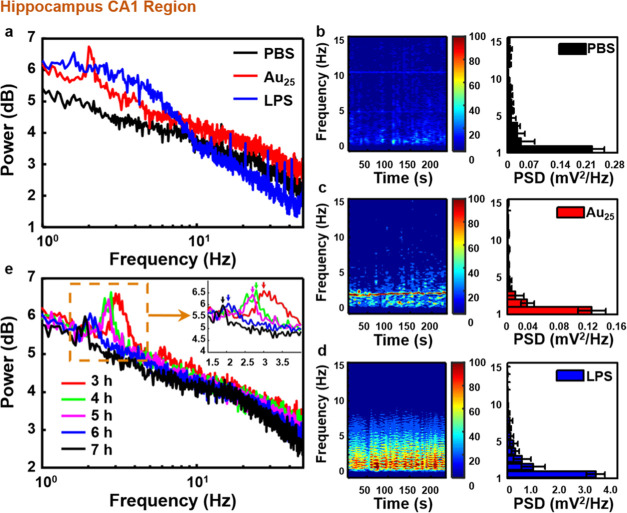
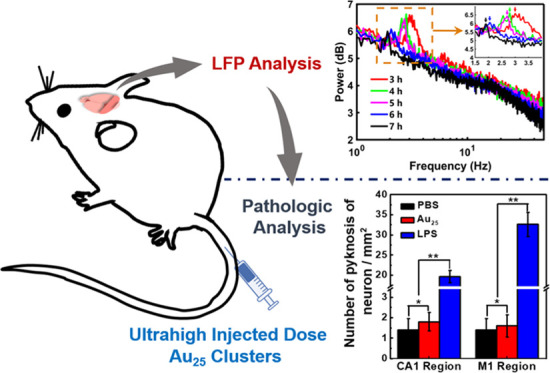
To investigate the neurotoxicity of Au25 clusters, in vivo neuroelectrophysiological experiments were employed to monitor neural activity at different brain regions. The complex network structure of cerebral cortex neurons forms the characteristic information coding of nerve center.37−39 Meanwhile, the monitoring methods of neuroelectrophysiology can also reflect the signal transmission of neurons and synapses. Therefore, research based on the characteristics of biosignals is an important method for the study of brain function and activity. It is worth noting that the hippocampal CA1 region is principal for spatial cognition and the cortex M1 region is one of the crucial brain areas involved in motor function.40−42 In this study, to evaluate the nerve injury of Au25 clusters, local field potential (LFP) signals in CA1 and electrocorticographic (ECoG) signals in M1 of C57 mice injected with ultrahigh dose Au25 clusters by tail vein were recorded simultaneously. Lipopolysaccharides (LPS) is a typical endotoxin that binds to the CD14/TLR4/MD2 receptor complex, which can promote the secretion of proinflammatory factors and induce a toxic reaction in the nervous system.43 Therefore, the LPS-induced acute brain injury model was used as the toxicity control to study the neurotoxicity of Au25 clusters. The mouse injected with Au25 clusters (200 μL, 23 mg mL–1) acted as an experimental mouse, and those injected with phosphate-buffered saline (PBS) and LPS acted as normal control mouse and toxicity control mouse, respectively. There were significant differences at the delta frequency band (1–5 Hz) in the power spectrum analysis of the above three mice in CA1 (Figures 2a and S1). It has been proved that the different frequency bands of neural oscillations are related to various cognitive functions of the brain,44−50 and the delta frequency band is related to memory consolidation in the hippocampal formation,51 which appears only in deep sleep and anesthesia. Once the power in this frequency band has significantly increased compared to the normal control mouse, it indicates that the nervous system has suffered some damage52,53 and caused discomfort in vivo.54 Therefore, the differences between the three mice were carefully compared, and the results of the power spectrum in the delta frequency band were somehow changed by either LPS or Au25 clusters. Compared with the normal control mouse, the experimental mouse showed a spike at 2.25 Hz and the power value was slightly higher, whereas the power of the toxicity control mouse increased sharply over the entire delta rhythm, indicating that LPS had strongly damaged the nerve. Subsequently, the results of the power spectrum were explored quantitatively by time–frequency analyses and power spectrum density (PSD) analyses (Figure 2b–d). Compared with the normal control mouse, the power of the toxicity control mouse was 11.27% higher at 1–5 Hz, while that of the experimental mouse was slightly higher only at about 2 Hz, and the PSDs in the delta band were very close to each other (experimental mouse: 32.91%, normal control mouse: 32.71%). Additionally, the LFP signals of the experimental mouse were recorded for 7 h to analyze the power spectrum results at different time points (Figure 2e). With the passage of time from 3 to 7 h, the position of the power peak moved to a lower frequency and the amplitude decreased gradually (Figure 2e inset). In particular, the value showed a 38.3% reduction after 7 h of injecting Au25 clusters intravenously. Finally, to further reflect the electrophysiological results of mice in the experimental group, the power spectrum results of mice in the normal control group (Figure S2a) and the toxicity control group (Figure S2b) were analyzed for 3–7 h, which is consistent with the conclusions mentioned above. It was proved that the ultrahigh injected dose of Au25 clusters would not cause permanent damage to the nerve and have excellent excretion.
Figure 2.
Changes of power in the hippocampal CA1 region of mice injected with the ultrahigh dose of Au25 clusters. (a) Power spectrum analysis of mice injected with PBS, Au25 clusters, and LPS solution. (b–d) Time–frequency analyses and power spectrum density. (e) Power spectrum analysis of mice injected with Au25 cluster solution for 3–7 h.
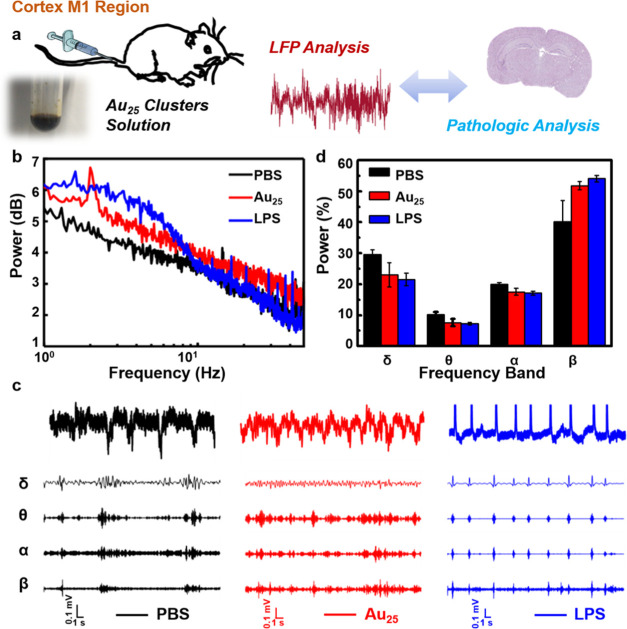
Subsequently, the ECoG signals in M1 were tested simultaneously (Figure 3a). As shown in Figure 3b, the power spectrum in M1 showed similar results to CA1. The power data of various frequency bands, including delta (1–5 Hz), theta (5–8 Hz), α (8–13 Hz), and β (13–30 Hz), were separated, as shown in Figure 3c. The power density of each band (Figure 3d) was quantified to clarify the results of the grand average. Compared with the other two groups of mice, there were significant differences in the toxicity control mouse at delta rhythm (normal control mouse: 23.01%, experimental mouse: 21.52%, and toxicity control mouse: 29.62%) and β rhythm (normal control mouse: 51.81%, experimental mouse: 54.07%, and toxicity control mouse: 40.18%). Some crucial information can be expressed by the value of power in various frequency bands.55,56 It is worth noting that delta rhythm is related to the memory consolidation of the hippocampal structure, and when its PSD is abnormally high, it indicates that the nervous system may be damaged.57,58 Furthermore, β rhythm is closely related to cognitive function, having the right amount of power allows subjects to focus easily and less amount of power may lead to poor cognition.59 Consistent with the results of delta and β frequency bands in CA1, the experimental mouse was similar to the normal control mouse, while the power of the toxicity control mouse was higher in delta and was obviously lower in β than the other two groups of mice. It was further indicated that the ultrahigh injected dose of Au25 clusters hardly caused any damage to the nerve compared with the toxicity group, which was consistent with the conclusion of that in CA1.
Figure 3.
Effects of the ultrahigh dose of Au25 clusters on power in the cortex M1 region. (a) Elementary diagram of the excretion analysis of Au25 clusters using LFP analysis and pathologic analysis. (b) Power spectrum analysis of the three groups. (c) Comparison of signals in delta, theta, α, and β frequency rhythms. (d) Grand average of the power of various rhythms.
In addition, the effects of different concentrations of the ultrahigh injected dose of Au25 clusters on the nervous system were further studied to verify neurotoxicity. The study of excretion of C57 mice with higher doses of Au25 clusters (300 and 500 mg kg–1) injected intravenously was done by power spectrum analyses. The power spectrum in CA1 of mice injected with a dose of 300 mg kg–1 showed a spike at 1.95 Hz (Figure 4a), while it showed a spike at 2.05 Hz with a dose of 500 mg kg–1 (Figure 4b). Moreover, a similar phenomenon was also found in the M1 region (Figure 4c,d). The power spectrum results of higher doses showed two trends, that is, the peak frequency value moved to a lower frequency region and the amplitude decreased gradually over time; specifically, this value showed a decrease of 5.74, 6.51, 1.95, and 3.74%, respectively. The results of neuroelectrophysiology are consistent with those given in Figure 2e, which further proves that Au25 clusters have great biocompatibility and will not cause long-term damage to the nerve at an ultrahigh injected dose.
Figure 4.
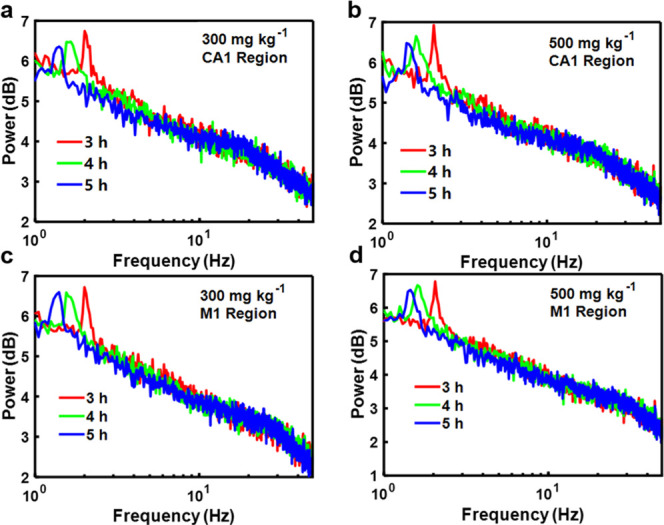
Effects of higher doses of Au25 clusters on excretion of mice. (a–d) Power spectrum analyses at different time points of mice treated with 300 and 500 mg kg–1 of doses of Au25 clusters.
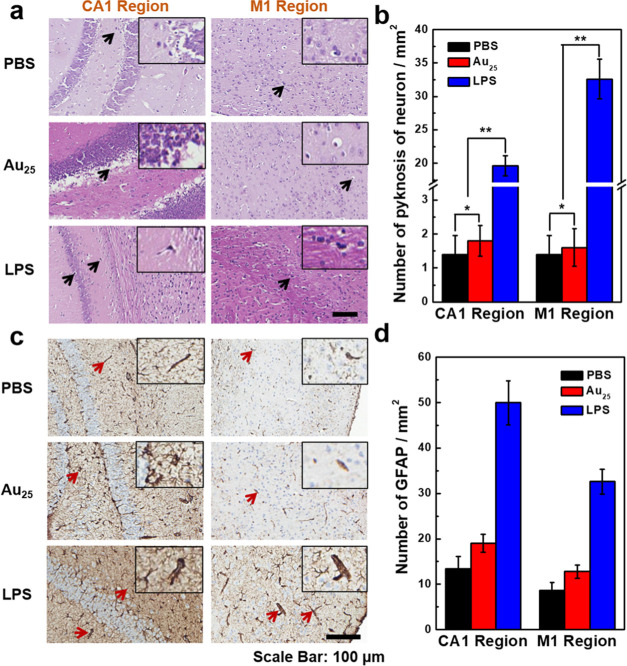
Brain tissue consists of neurons and glial cells, which are highly sensitive to toxicity. Acute poisoning or infection can cause the death of neurons, characterized by neuronal nucleus pyknosis, efforts by the cell body to reduce the deformation, dissolution or disappearance of the nucleus, and reactive hyperplasia of glial cells.60,61 Therefore, the extent of death and hyperplasia of neurons has been regarded as an important indicator of neurotoxicity.62 For further verification, we then used pathologic analysis by H&E staining to evaluate the response state of the neurons of mice injected with Au25 clusters (500 mg kg–1), PBS, and LPS. The mice were sacrificed immediately after conducting the neuroelectrophysiological experiment (10 h post-injection). As shown in Figure 5a, compared with the normal control mouse, the experimental mouse showed no significant difference in brain tissue slice morphology and no obvious abnormalities were observed in the size and morphological structure of the nerve cells in M1. The cells in CA1 were closely arranged and no obvious abnormalities were observed in the size and morphological structure of the pyramidal cells. The results indicated that Au25 clusters had caused no significant damage to the cortex and hippocampus even at the dose of 500 mg kg–1. However, in the brain tissue of the toxicity control mouse, there were obvious lesions, mainly white spaces around some cells in M1 and CA1, which suggested the presence of pyknosis neurons (indicated by black arrows) in the brain tissue of the mouse. In addition, the pyramidal cells in CA1 were disordered in size, morphological structure, and arrangement. Furthermore, pyknosis neurons per unit area were counted under a high-power field of vision (Figure 5b) and the results showed that the pyknosis neurons per unit area in the experimental mouse in CA1 and M1 were 1.33 ± 0.52 and 1.50 ± 0.84, respectively and in the control mouse were 1.17 ± 0.75 and 1.33 ± 0.82, respectively; there was no significant difference (p > 0.05, p > 0.05). However, the toxicity control mouse (33.17 ± 3.66 and 23.33 ± 1.97, respectively) had significantly higher values than the experimental and normal control mice, with a significant difference (p < 0.05, p < 0.05). These results showed that the Au25 clusters at an ultrahigh injected dose did not cause any toxic effects to the neurons of mice.
Figure 5.
Effects of the ultrahigh dose of Au25 clusters on pathology. (a) H&E staining results and (c) immunohistochemical analysis in the hippocampal CA1 and cortical M1 regions of the brain. No significant increase was observed in the number of (b) pyknosis neurons and (d) glial fibrillary acidic protein (GFAP) in the brain tissue of mice injected with Au25 clusters at an ultrahigh dose. * indicates p > 0.05 as compared with the control group, ** indicates p < 0.05 as compared with the control mouse and the experimental mouse (data is analyzed by ANOVA).
When neurons are damaged by toxic effects, glial cells grow to fill the space where neurons die. Therefore, the number of glial cells can be used as an indicator of neurotoxicity.63 In this work, glial fibrillary acidic protein (GFAP) was selected to realize the immunostaining of reactive glial cells after injecting Au25 clusters at a dose of 500 mg kg–1. As shown in Figure 5c, in CA1 and M1, the number of GFAP was significantly increased in the toxicity control mouse, indicating the activity of glial cells (indicated by red arrows). However, the number of GFAP did not increase in the experimental mouse and the normal control mouse. Significantly, the ultrahigh doses of Au25 clusters did not induce glial cell proliferation (Figure 5d). Therefore, the conclusions mentioned above in the neuroelectrophysiological experiments were proved by the results of the histopathologic analyses.
Previous studies have shown that almost no amount of Au25 injected into mice through the tail vein could enter the brain via the blood–brain barrier (BBB),64−66 which may be an important reason why Au25 hardly cause any damage to the nervous system. However, electrodes needed to be implanted into the relevant brain area to collect neuroelectrophysiological signals to monitor the effect of Au25 on the nervous system in this experiment. In particular, there are many studies proving that the implantation of electrodes will cause a certain degree of damage to BBB.67−70 We speculated that this may cause Au25 to be enriched in the brain tissue around the electrode, thereby affecting the nervous system of this part of the brain. Therefore, to further show the influence of Au25 on the nervous system, the brain tissues around the electrode were sliced and performed traditional immunohistochemical processing. Thereafter, the fluorescence imaging of brain slices was evaluated by an immunofluorescence microscope, and the result showed that the red fluorescence of Au25 was emitted at 650 nm under an excitation of 410nm, which proved that Au25 extravasation was enriched around the electrode (Figure S3). Combining the good biocompatibility of Au25 with the neuroelectrophysiological results mentioned above, it is proven that it still does not cause significant damage to the nervous system even if the brain tissues are enriched with Au25.
NIR-II imaging has shown a great advantage in the noninvasive detection of cerebral nervous system diseases, but the nontoxicity of NIR-II agents is the most prerequisite for future clinical applications.13,23,24 Larger gold nanoparticles (usually more than 5 nm) tend to form aggregates in the biological environment and accumulate preferentially in the liver and spleen, hindering clinical applications. Thereafter, we presented Au25 clusters with ultrasmall hydrodynamic size exhibiting molecular-like pharmacokinetics, leading to very rapid clearance, and showing great biocompatibility.1,3 However, the actual in vivo toxicity of functional Au25 clusters could be even more complicated. For example, a small amount of gold clusters stored in a muscle led to the redistribution to other organs in 30 days, which may result in potential toxicity.26 An ultrahigh injected dose of Au25 clusters did not show a toxic reaction to the cerebral nerve at the level of neuroelectrophysiology and histopathology, indicating there was no neurotoxic effect on the central nervous system either functionally or structurally. The results provided the basis to make more exciting discoveries on nervous system diseases.
Conclusions
In summary, we performed neuroelectrophysiological examinations as a toxicity research method to analyze the nerve damage caused by an ultrahigh injected dose of Au25 clusters. The power spectrum of different time points and other neuroelectrophysiological analyses revealed that atomically precise Au25(SG)18 clusters did not cause any permanent damage to the nerve at ultrahigh doses (200, 300, and 500 mg kg–1), which are 2 to 5 times the dose of Au25(SG)18 clusters used in NIR-II imaging.1 Furthermore, no significant structural changes were found in both neurons and glial cells at the histological level. Finally, the present work demonstrated that Au25 clusters did not cause neurotoxicity either in signal transduction function or in tissue structure. These conclusions provide a useful basis for the future application of Au25 clusters in the biomedical field, especially in brain science.
Materials and Methods
Materials and Synthesis
The Au25(SG)18 clusters were synthesized according to a previously reported method.4 The first step was the addition of the aqueous solutions of HAuCl4 (3.75 mL, 40 mM) and glutathione (4.5 mL, 50 mM) under vigorous stirring to ultrapure water (141.75 mL), and then NaOH was added to regulate the PH of the reaction solution to 11.0. Thereafter, the reaction vessel was saturated with CO for 2 min and the reaction product was gently stirred (500 rpm) at room temperature. The reaction product was collected using the rotary evaporator at 24 h, washed with methanol several times, and vacuum-dried. In the last, ∼40 mg of Au25(SG)18 clusters were obtained.
Characterization
The emission spectrum of gold clusters from 675 to 1400 nm was measured by Edinburgh FLS1000 with a measurement range of 400–1700 nm and under an excitation of 655 nm Xenon lamp. The UV–vis absorption spectrum of Au25 clusters was recorded in the range of 300–900 nm using a Cary 6000i UV–vis-NIR spectrophotometer. The hydrodynamic size of Au25 clusters in FBS was determined by dynamic light scattering (DLS) in a 1 cm quartz cuvette using a Brookhaven Instruments 90Plus Particle Size Analyzer.
Animals
All animal experiments were approved according to the guidelines set by the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (IRM, CAMS). Eleven-week-old male C57 mice (IRM Laboratories) were raised under 12 h dark/light circles and allowed ad libitum access to food and water. Mice (n = 9) were stochastically divided into three groups. Mice injected with ultrahigh doses of Au25 clusters (200, 300, and 500 mg kg–1) by tail vein acted as the experimental group, injected with PBS and LPS (2 mg kg–1) acted as the normal control group (n = 3) and the toxicity control group (n = 3), respectively. Mice of these three groups were sacrificed immediately after the neuroelectrophysiological experiment (10 h post-injection). Thereafter, their brain tissues were collected for H&E staining and immunohistochemistry.
Neuroelectrophysiological Experiment
The mice were anesthetized with 30% urethane (4 mL kg–1) by placing them in a stereotaxic frame. After making a 1 cm long of skin incision, all soft tissues from the surface of the skull were removed; two small holes with a diameter of 1 mm were drilled in the skull with an electric cranial drill. Two Ag wire recording electrodes were placed into the hippocampal CA1 region (2.0 mm mediolateral and 2.3 mm posterior to Bregma, depth from the dura, 1.0–1.5 mm) and the cortex M1 region (1.0 mm mediolateral and 0.5 mm anterior to Bregma, depth from the dura, 2.0–2.5 mm), respectively. The LFP signals and ECoG signals were collected for 7 h in the CA1 and M1 regions simultaneously.
LFP Analyses
The LFP and ECoG signals were acquired by Clampfit 10.0 (Molecular Devices, CA) and sampled at 32 kHz. All the treatment of data was analyzed offline using custom-built software in MATLAB (MathWorks). Prior to further analyses, the signal was downsampled to 1000 Hz and filtered to [0.5, 100] Hz using “eegfilt” function in the EEGLAB toolbox.
Power Spectrum Analyses
Power spectra were calculated according to the fast Fourier transform (FFT) algorithm.
PSD Analysis
According to the results of the power spectrum analyses in the CA1 and M1 regions, the ratio of the power value of each frequency within the frequency domain to the total power value (1–15 Hz) was calculated respectively in CA1. Furthermore, the ratio of the power value of different frequency rhythms to the total power value (1–30 Hz) in M1 was calculated, including delta (1–5 Hz), theta (5–8 Hz), α (8–13 Hz), and β (13–30 Hz). The results were calculated using a very simple formula
where f refers to the frequency, f1 → f2 refers
to the frequency domain, and Pf refers to the power amplitude values at a frequency of f Hz 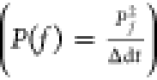 ,
which can also be used as a formula to
calculate the PSD corresponding to each frequency (1–15 Hz),
where Δdt refers to the width of the sampling
window. Meanwhile, the above formula can be used to calculate the
PSD of different rhythms within the frequency range of 1–30
Hz.
,
which can also be used as a formula to
calculate the PSD corresponding to each frequency (1–15 Hz),
where Δdt refers to the width of the sampling
window. Meanwhile, the above formula can be used to calculate the
PSD of different rhythms within the frequency range of 1–30
Hz.
Time–Frequency Analyses
The signal was filtered by 1 Hz high-pass and 20 Hz low-pass filters, and the signal waveforms of different frequency bands were extracted using the wavelet extraction function in the wavelet toolbox from the original signal, with the parameters set as 10 000 Hz time–frequency analysis window length.
Pathology
The brain tissues were collected and fixed in 4% paraformaldehyde (PFA) fix solution for 48 h. Traditional immunohistochemical procedures, such as dehydration, were followed and H&E staining was then performed. After staining, the pathology was observed under a digital optical microscope.
Effect of the Number of Pyknosis Neurons
Five high-power visual fields were extracted from each brain tissue slice. According to the criteria of the pyknosis neurons, the pyknosis neurons were identified and labeled. Then, the number of pyknosis neurons in the core of a high-power visual field and the average value were calculated.
Immunohistochemistry
The tissues were embedded in paraffin and sectioned into 4 μm thick slices. Slices were deparaffinized with xylene and rehydrated in decreasing concentrations of ethanol. After antigen retrieval, slices were then heated in a microwave for 15 min. Slices were incubated at 4 °C overnight in a humid chamber with the following primary antibody GFAP (1:200). After washing 3 times in PBS, the anti-mouse biotinylated secondary antibody was applied to the slices for 20 min and then incubated with HRP-conjugated streptavidin for 20 min at room temperature. Next, slices were dehydrated in decreasing concentrations of ethanol and coverslipped.
If the cell membrane or cytoplasm appeared as a light yellow or brown-yellow stain in the cells, we defined it as positive, and 5 high magnifications (×400) were randomly selected for judgment. The staining intensity criteria of positive cells were as follows: (0) no staining, (1) light yellow staining, (2) brown staining, and (3) dark brown staining. The percentages of positively stained cells were also considered in the evaluations. Thus, a score of 0 was assigned to a sample with no positive cells, samples with <29% of positive cells received a score of 1, those with 30–59% of positive cells received a score of 2, those with ≥60% of positive cells received a score of 3. Finally, these two values (positive cells × staining intensity score) were multiplied to classify the specimens into (−) negative <2 or (+) positive ≥2.
Statistical Analyses
SPSS22.0 statistical software was used for statistical analyses. The statistical data were tested by the chi-square test. All of the data were expressed as the average ± standard deviations (SD) and analyzed using ANOVA; p < 0.05 was considered statistically significant.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 91859101, 81971744, U1932107, and 81471786) and the National Natural Science Foundation of Tianjin (No. 19JCZDJC34000), the Innovation Foundation of Tianjin University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03005.
Power spectrum analysis of other two mice injected with PBS, Au25 clusters, and LPS solution; power spectrum analysis of mice injected with LPS and PBS for 3–7 h; and the distribution of Au25 in the brain tissue around the electrode (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu H.; Hong G.; Luo Z.; Chen J.; Chang J.; Gong M.; He H.; Yang J.; Yuan X.; Li L.; Mu X.; Wang J.; Mi W.; Luo J.; Xie J.; Zhang X.-D. Atomic-Precision Gold Clusters for NIR-II Imaging. Adv. Mater. 2019, 31, 1901015 10.1002/adma.201901015. [DOI] [PubMed] [Google Scholar]
- Katla S. K.; Zhang J.; Castro E.; Bernal R. A.; Li X. Atomically Precise Au25(SG)18 Nanoclusters: Rapid Single-Step Synthesis and Application in Photothermal Therapy. ACS. Appl. Mater. Interfaces 2018, 10, 75–82. 10.1021/acsami.7b12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-D.; Chen J.; Luo Z.; Wu D.; Shen X.; Song S.-S.; Sun Y.-M.; Liu P.-X.; Zhao J.; Huo S.; Fan S.; Fan F.; Liang X.-J.; Xie J. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthcare Mater. 2014, 3, 133–141. 10.1002/adhm.201300189. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Luo Z.; Yu Y.; Lee J. Y.; Xie J. Observation of Cluster Size Growth in CO-Directed Synthesis of Au25(SR)18 Nanoclusters. ACS Nano 2012, 6, 7920–7927. 10.1021/nn3023206. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Wang M.; Yang J.; Zheng X.; Cai W.; Meng G.; Qian H.; Wang H.; Jin R. Well-Defined Nanoclusters as Fluorescent Nanosensors: A Case Study on Au25(SG)18. Small 2012, 8, 2028–2035. 10.1002/smll.201102590. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Jin R. Stability of the Two Au-S Binding Modes in Au25(SG)18 Nanoclusters Probed by NMR and Optical Spectroscopy. ACS Nano 2009, 3, 2036–2042. 10.1021/nn9004999. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Gayathri C.; Gil R. R.; Jin R. Probing the Structure and Charge State of Glutathione-Capped Au25(SG)18 Clusters by NMR and Mass Spectrometry. J. Phys. Chem. A 2009, 113, 6535–6542. 10.1021/ja900386s. [DOI] [PubMed] [Google Scholar]
- Tang S.; Peng C.; Xu J.; Du B.; Wang Q.; Vinluan R. D. III; Yu M.; Kim M. J.; Zheng J. Tailoring Renal Clearance and Tumor Targeting of Ultrasmall Metal Nanoparticles with Particle Density. Angew. Chem., Int. Ed. 2016, 55, 16039–16043. 10.1002/anie.201609043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.; Yuan X.; Yu Y.; Zhang Q.; Leong D. T.; Lee J. Y.; Xie J. From Aggregation-Induced Emission of Au(I)-Thiolate Complexes to Ultrabright Au(0)@Au(I)-Thiolate Core-Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. 10.1021/ja306199p. [DOI] [PubMed] [Google Scholar]
- Antaris A. L.; Chen H.; Cheng K.; Sun Y.; Hong G.; Qu C.; Diao S.; Deng Z.; Hu X.; Zhang B.; Zhang X.; Yaghi O. K.; Alamparambil Z. R.; Hong X.; Cheng Z.; Dai H. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- Xu J.; Yu M.; Carter P.; Hernandez E.; Dang A.; PayalKapur; Hsie J.; Zheng J. In Vivo X-ray Imaging of Transport of Renal Clearable Gold Nanoparticles in the Kidneys. Angew. Chem., Int. Ed. 2017, 56, 13356–13360. 10.1002/anie.201707819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Ma Z.; Wang H.; Zhou B.; Zhu S.; Zhong Y.; Wang J.; Wan H.; Antaris A.; Ma R.; Zhang X.; Yang J.; Zhang X.; Sun H.; Liu W.; Liang Y.; Dai H. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29, 1605497 10.1002/adma.201605497. [DOI] [PubMed] [Google Scholar]
- Wang H.; Mu X.; Yang J.; Liang Y.; Zhang X.-D.; Ming D. Brain imaging with near-infrared fluorophores. Coord. Chem. Rev. 2019, 380, 550–571. 10.1016/j.ccr.2018.11.003. [DOI] [Google Scholar]
- Liu J.; Yu M.; Zhou C.; Yang S.; Ning X.; Zheng J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Zhou C.; Liu L.; Zhang S.; Sun S.; Hankins J. D.; Sun X.; Zheng J. Interactions of Renal-Clearable Gold Nanoparticles with Tumor Microenvironments: Vasculature and Acidity Effects. Angew. Chem., Int. Ed. 2017, 56, 4314–4319. 10.1002/anie.201612647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.; Wang X.; Zeng S.; Ramamurthy G.; Burda C.; Basilion J. P. Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer. Small 2019, 15, 1900968 10.1002/smll.201900968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.; Gao X.; Xu J.; Du B.; Ning X.; Tang S.; Bachoo R. M.; Yu M.; Ge W.-P.; Zheng J. Targeting orthotopic gliomas with renal-clearable luminescent gold nanoparticles. Nano Res. 2017, 10, 1366–1376. 10.1007/s12274-017-1472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Zheng J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh Y. R.; Yu P.; Wen X.; Tang J. The enhancement of electron-phonon coupling in glutathione-protected Au25 clusters. J. Colloid Interface Sci. 2013, 402, 86–89. 10.1016/j.jcis.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Stamplecoskie K. G.; Kamat P. V. Size-dependent excited state behavior of glutathione-capped gold clusters and their light-harvesting capacity. J. Am. Chem. Soc. 2014, 136, 11093–11099. 10.1021/ja505361n. [DOI] [PubMed] [Google Scholar]
- Li Y. Z.; Ganguly R.; Hong K. Y.; Li Y.; Tessensohn M. E.; Webster R.; Leong W. K. Stibine-protected Au13 nanoclusters: syntheses, properties and facile conversion to GSH-protected Au25 nanocluster. Chem. Sci. 2018, 9, 8723–8730. 10.1039/C8SC03132K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Mu X.; Zhang X.-D.; Ming D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjugate Chem. 2020, 31, 260–275. 10.1021/acs.bioconjchem.9b00610. [DOI] [PubMed] [Google Scholar]
- Perrault S. D.; Walkey C.; Jennings T.; Fischer H. C.; Chan W. C. W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- Chou L. Y. T.; Chan W. C. W. Fluorescence-tagged gold nanoparticles for rapidly characterizing the size-dependent biodistribution in tumor models. Adv. Healthcare Mater. 2012, 1, 714–721. 10.1002/adhm.201200084. [DOI] [PubMed] [Google Scholar]
- Xu J.; Yu M.; Peng C.; Carter P.; Tian J.; Ning X.; Zhou Q.; Tu Q.; Zhang G.; Dao A.; Jiang X.; PayalKapur; Hsieh J.-T.; Zhao X.; Liu P.; Zheng J. Dose Dependencies and Biocompatibility of Renal Clearable Gold Nanoparticles: From Mice to Non-human Primates. Angew. Chem., Int. Ed. 2018, 57, 266–271. 10.1002/anie.201710584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-D.; Luo Z.; Chen J.; Wang H.; Song S.-S.; Shen X.; Long W.; Sun Y.-M.; Fan S.; Zheng K.; Leong D. T.; Xie J. Storage of gold nanoclusters in muscle leads to their biphasic in vivo clearance. Small 2015, 11, 1683–1690. 10.1002/smll.201402233. [DOI] [PubMed] [Google Scholar]
- Yan R.; Sun S.; Yang J.; Long W.; Wang J.; Mu X.; Li Q.; Hao W.; Zhang S.; Liu H.; Gao Y.; Ouyang L.; Chen J.; Liu S.; Zhang X. D.; Ming D. Nanozyme-Based Bandage with Single-Atom Catalysis for Brain Trauma. ACS Nano 2019, 13, 11552–11560. 10.1021/acsnano.9b05075. [DOI] [PubMed] [Google Scholar]
- Mu X.; Wang J.; Li Y.; Xu F.; Long W.; Ouyang L.; Liu H.; Jing Y.; Wang J.; Dai H.; Liu Q.; Sun Y.; Liu C.; Zhang X.-D. Redox Trimetallic Nanozyme with Neutral Environment Preference for Brain Injury. ACS Nano 2019, 13, 1870–1884. 10.1021/acsnano.8b08045. [DOI] [PubMed] [Google Scholar]
- Zhang X.-D.; Wu D.; Shen X.; Liu P.-X.; Fan F.-Y.; Fan S.-J. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 2012, 33, 4628–4638. 10.1016/j.biomaterials.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Mu X.; He H.; Wang J.; Long W.; Li Q.; Liu H.; Gao Y.; Ouyang L.; Ren Q.; Sun S.; Wang J.; Yang J.; Liu Q.; Sun Y.; Liu C.; Zhang X.-D.; Hu W. Carbogenic Nanozyme with Ultrahigh Reactive Nitrogen Species Selectivity for Traumatic Brain Injury. Nano Lett. 2019, 19, 4527–4534. 10.1021/acs.nanolett.9b01333. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Nachammai V.; Zhang B.; Yan N.; Leong D. T.; Jiang D. E.; Xie J. Toward understanding the growth mechanism: tracing all stable intermediate species from reduction of Au(I)-thiolate complexes to evolution of Au25 nanoclusters. J. Am. Chem. Soc. 2014, 136, 10577–10580. 10.1021/ja505429f. [DOI] [PubMed] [Google Scholar]
- Yuan X.; Zhang B.; Luo Z.; Yao Q.; Leong D. T.; Yan N.; Xie J. Balancing the rate of cluster growth and etching for gram-scale synthesis of thiolate-protected Au25 nanoclusters with atomic precision. Angew. Chem., Int. Ed. 2014, 53, 4623–4627. 10.1002/anie.201311177. [DOI] [PubMed] [Google Scholar]
- Shichibu Y.; Negishi Y.; Tsunoyama H.; Kanehara M.; Teranishi T.; Tsukuda T. Extremely high stability of glutathionate-protected Au25 clusters against core etching. Small 2007, 3, 835–839. 10.1002/smll.200600611. [DOI] [PubMed] [Google Scholar]
- Swanick K. N.; Hesari M.; Workentin M. S.; Ding Z. Interrogating Near-Infrared Electrogenerated Chemiluminescence of Au25(SC2H4Ph)18+ Clusters. J. Am. Chem. Soc. 2012, 134, 15205–15208. 10.1021/ja306047u. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Zeng C.; Sfeir M. Y.; Cotlet M.; Iida K.; Nobusada K.; Jin R. Evolution of Excited-State Dynamics in Periodic Au28, Au36, Au44, and Au52 Nanoclusters. J. Phys. Chem. Lett. 2017, 8, 4023–4030. 10.1021/acs.jpclett.7b01597. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Suhan J.; Jin R. One-pot synthesis of atomically monodisperse, thiol-functionalized Au25 nanoclusters. J. Mater. Chem. 2009, 19, 622–626. 10.1039/B815983A. [DOI] [Google Scholar]
- Rutishauser U.; Ross I. B.; Mamelak A. N.; Schuman E. M. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 2010, 464, 903–907. 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Hassouna I.; Ott C.; Wustefeld L.; Offen N.; Neher R. A.; Mitkovski M.; Winkler D.; Sperling S.; Fries L.; Goebbels S.; Vreja I. C.; Hagemeyer N.; Dittrich M.; Rossetti M. F.; Krohnert K.; Hannke K.; Boretius S.; Zeug A.; Hoschen C.; Dandekar T.; Dere E.; Neher E.; Rizzoli S. O.; Nave K. A.; Siren A. L.; Ehrenreich H. Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus. Mol. Psychiatry 2016, 21, 1752–1767. 10.1038/mp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N.; Yasuda H.; Hanamura K.; Ishizuka Y.; Sekino Y.; Shirao T. Drebrin a regulates hippocampal LTP and hippocampus-dependent fear learning in adult mice. Neuroscience 2016, 324, 218–226. 10.1016/j.neuroscience.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Yang J.; Wang L.; Wang F.; Tang X.; Zhou P.; Liang R.; Zheng C.; Ming D. Low-Frequency Pulsed Magnetic Field Improves Depression-Like Behaviors and Cognitive Impairments in Depressive Rats Mainly via Modulating Synaptic Function. Front. Neurosci. 2019, 13, 820. 10.3389/fnins.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D. M.; Sprengel R.; Sanderson D. J.; McHugh S. B.; Rawlins J. N. P.; Monyer H.; Seeburg P. H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Barker A. T.; Jalinous R.; Freeston I. L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. H.; Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wang X.-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010, 90, 1195–1268. 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou M.; Cogno S. G.; Gigg J.; Samengo I.; Elijah D. H.; Kropff E.; Montemurro M. A. Bursting Neurons in the Hippocampal Formation Encode Features of LFP Rhythms. Front. Comput. Neurosc. 2016, 10, 133 10.3389/fncom.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauviere L.; Rafrafi N.; Thinus-Blanc C.; Bartolomei F.; Esclapez M.; Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J. Neurosci. 2009, 29, 5402–5410. 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman R. S.; Gandal M. J.; Maxwell C. R.; Lazarewicz M. T.; Finkel L. H.; Contreras D.; Turetsky B. I.; Siegel S. J. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience 2009, 158, 705–712. 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 1978, 201, 160–163. 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Senkowski D.; Gallinat J. Dysfunctional Prefrontal Gamma-Band Oscillations Reflect Working Memory and Other Cognitive Deficits in Schizophrenia. Bio. Psychiatry 2015, 77, 1010–1019. 10.1016/j.biopsych.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal Sharp Wave-Ripple: A Cognitive Biomarker for Episodic Memory and Planning. Hippocampus 2015, 25, 1073–1188. 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M.; Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog. Brain Res. 2011, 193, 93–110. 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Rasch B.; Born J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsam T.; Monazzam M. R.; Haghdoost A. A.; Ghotbi M. R.; Dehghan S. F. Effect of extremely low frequency electromagnetic field exposure on sleep quality in high voltage substations. Iran. J. Environ. Healt. 2012, 9, 15 10.1186/1735-2746-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardo F.; Vinding M. C.; Allen M.; Jensen T. S.; Finnerup N. B. Delta and gamma oscillations in operculo-insular cortex underlie innocuous cold thermosensation. J. Neurophysiol. 2017, 117, 1959–1968. 10.1152/jn.00843.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartur G.; Pratt H.; Soroker N. Changes in mu and beta amplitude of the EEG during upper limb movement correlate with motor impairment and structural damage in subacute stroke. Clin. Neurophysiol. 2019, 130, 1644–1651. 10.1016/j.clinph.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Pierre Carpentier A. F. Frederic Dorandeu, Guy Lallement, Delta activity as an early indicator for soman-induced brain damage: A review. NeuroToxicology 2001, 22, 299–315. 10.1016/S0161-813X(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Bonfiglio L.; Olcese U.; Rossi B.; Frisoli A.; Arrighi P.; Greco G.; Carozzo S.; Andre P.; Bergamasco M.; Carboncini M. C. Cortical source of blink-related delta oscillations and their correlation with levels of consciousness. Hum. Brain Mapp. 2013, 34, 2178–2189. 10.1002/hbm.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh F.; Ibrahim F.; Rashid R.; Shafiabady N.; Seghatoleslam T.; Habil H. Acute effects of methadone on EEG power spectrum and event-related potentials among heroin dependents. Psychopharmacology 2018, 235, 3273–3288. 10.1007/s00213-018-5035-0. [DOI] [PubMed] [Google Scholar]
- Rodier P. M. Developing brain as a target of toxicity. Environ. Health Perspect. 1995, 103, 73–76. 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P. J.; Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Hamilton B. F.; Gould D. H. Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: a type of hypoxic (energy deficient) brain damage. Acta Neuropathol. 1987, 72, 286–297. 10.1007/BF00691103. [DOI] [PubMed] [Google Scholar]
- Yoo D.; Magsam A. W.; Kelly A. M.; Stayton P. S.; Kievit F. M.; Convertine A. J. Core-Cross-Linked Nanoparticles Reduce Neuroinflammation and Improve Outcome in a Mouse Model of Traumatic Brain Injury. ACS Nano 2017, 11, 8600–8611. 10.1021/acsnano.7b03426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan D.; Ye D.; Heo G. S.; Zhang X.; Luehmann H.; Yue Y.; Detering L.; Komarov S.; Taylor S.; Tai Y. C.; Rubin J. B.; Chen H.; Liu Y. Focused Ultrasound Enabled Trans-Blood Brain Barrier Delivery of Gold Nanoclusters: Effect of Surface Charges and Quantification Using Positron Emission Tomography. Small 2018, 14, 1703115 10.1002/smll.201703115. [DOI] [PubMed] [Google Scholar]
- Ye D.; Sultan D.; Zhang X.; Yue Y.; Heo G. S.; Kothapalli S. V. V. N.; Luehmann H.; Tai Y.-c.; Rubin J. B.; Liu Y.; Chen H. Focused ultrasound-enabled delivery of radiolabeled nanoclusters to the pons. J. Controlled Release 2018, 283, 143–150. 10.1016/j.jconrel.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonavane G.; Tomoda K.; Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf., B 2008, 66, 274–280. 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Bennett C.; Mohammed F.; Alvarez-Ciara A.; Nguyen M. A.; Dietrich W. D.; Rajguru S. M.; Streit W. J.; Prasad A. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials 2019, 188, 144–159. 10.1016/j.biomaterials.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.; Samikkannu M.; Mohammed F.; Dietrich W. D.; Rajguru S. M.; Prasad A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials 2018, 164, 1–10. 10.1016/j.biomaterials.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena T.; Karumbaiah L.; Gaupp E. A.; Patkar R.; Patil K.; Betancur M.; Stanley G. B.; Bellamkonda R. V. The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials 2013, 34, 4703–4713. 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Potter K. A.; Buck A. C.; Self W. K.; Capadona J. R. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J. Neural Eng. 2012, 9, 046020 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.