Abstract
Sorafenib (SORA), a multi kinase inhibitor, is the standard first-line targeted therapy approved by the Food and Drug Administration for advanced hepatocellular carcinoma (HCC). However, emerging evidence from clinical practice indicates that SORA alone has only moderate antitumor effects and could not completely inhibit the progression of the disease. Therefore, it is very necessary and urgent to develop novel combination therapy to improve the clinical outcomes of SORA. The pharmacological study on the chemosensitizing effects of natural products has become a hotspot in recent years, which is commonly thought to be a potential way to improve the effectiveness of drugs in clinical use. Berbamine (BBM) has potential sensitizing effects in multiple chemotherapies and target therapy. However, it remains unclarified whether the combination of BBM and SORA as a treatment could exert a synergistic effect on HCC cell lines. In this study, we first investigated whether BBM can increase the sensitivity of HCC cell lines to SORA. The results revealed that the combination of BBM and SORA could synergistically inhibit the growth of two HCC cell lines and promoted their apoptosis. Mechanistically, our results showed that BBM exerted a dose-dependent inhibitory effect on the basal and IL-6-induced STAT3 activation of HCC cell lines. In addition, the combined treatment of BBM and SORA synergistically suppressed STAT3 phosphorylation at Tyr705 and knockdown of STAT3 abolished the sensitization effect of BBM, indicating that BBM’s sensitization effect is mainly mediated by its inhibition of STAT3. These findings identify a new type of natural STAT3 inhibitor and provide a novel approach to the enhancement of SORA efficacy by blocking the activation of STAT3.
1. Introduction
Liver cancer ranks as the seventh most commonly diagnosed cancer globally and is the second leading cause of cancer-related mortalities worldwide in 2018, with a 5 year survival rate of 18%.1,2 Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers and is characterized as a highly therapy-resistant cancer type.3 The multitarget tyrosine kinase inhibitor (TKI) sorafenib (SORA) is the first systemic therapy approved by the Food and Drug Administration that targets the treatment of HCC and is also the standard of care for frontline therapy.4 Although SORA treatment has a 2–3 month survival advantage in terms of median overall survival (mOS), there is increasing evidence that only a very small number of patients actually reap long-term benefits from this therapy.5 Due to the development of primary and acquired resistance, a substantial proportion of HCC patients is rapidly refractory to SORA treatment within 6 months.6 Therefore, there is an urgent need to elucidate the mechanism underlying this resistance and to find a solution that can improve the clinical outcomes of SORA.
In recent studies, researchers have made many attempts to overcome the problem of SORA resistance. In general, there are two major approaches in this direction: using alternative drugs as second-line treatment in HCC patients who are not sensitive to SORA or using SORA in combination with other anticancer drugs. Regorafenib, one of SORA derivatives, is currently the mainstream second-line therapy for HCC patients who have progressed after SORA treatment. According to the results of the phase 3 trial named RESORCE, compared to the placebo group, regorafenib significantly improved the patients’ mOS rate and progression-free survival (PFS), and exhibited a similar safety profile to SORA.7 In addition, a great many efforts have been undertaken to study whether the combination therapy can delay the emergence of SORA resistance and improve the efficiency of SORA. Several clinical trials have been designed to combine SORA with other promising anti-HCC drugs (everolimus and adriamycin), with some success.8,9 However, compared with SORA monotherapy, all clinical trials of this type of combination therapy have not shown advantages in overall survival or toxicity. Fortunately, more and more results indicate that in preclinical models, the combination of natural products and SORA may be a feasible method to effectively improve the sensitivity of SORA.10 For example, Saraswati et al. revealed that dihydrochalcone flavonoid phloretin effectively potentiated the antitumor effect of SORA in two SORA-resistant HCC cell lines by enhancing SHP-1-mediated STAT3 inactivation.11 Besides, two independent research groups successively reported that several well-known natural-derived products such as luteolin, wogonin, and artesunate also effectively promoted the efficacy of SORA in HCC cells.12−14
Berbamine (BBM) is one of the major bioactive components of traditional Chinese herbal medicine Berberis amurensis.15 In Chinese medicine, there is a well-documented history of BBM being used in clinical practice for treating a variety of diseases, such as autoimmune disease, cancer, and inflammation.16−18 Numerous studies have revealed that BBM exerts favorable inhibitory activity on HCC cells in vitro and in vivo. The anti-HCC effects of BBM are involved in the inactivation of various critical protumorigenic signaling pathways, such as Ca2+/calmodulin-dependent protein kinase II γ (CaMKII γ), p53, and Fas signals.19−21 Furthermore, a number of recent research findings intriguingly suggested that BBM also displayed unexpected synergy with multiple chemotherapies and target therapy. Jin et al. and Wang et al., respectively, identified that BBM effectively improved the effects of gemcitabine, celecoxib, and trichostatin A on pancreatic cancer and breast cancer cells through the regulation of the TGF-β/Smad signaling pathway and Bcl-2 family protein expression.22,23 Moreover, Hu et al. discovered that BBM could synergize with gefitinib to inhibit the growth of pancreatic cancer cells via suppression of STAT3 signaling.24 However, to the best of our knowledge, no study has considered the potential and the underlying mechanisms of BBM in sensitizing HCC cells to other targeted therapies (such as SORA).
Herein, this study aims to investigate the synergistic effects of BBM and SORA on the two HCC cell lines PRF-PLC5 and HCC-Lm3. The results showed that BBM significantly improved the SORA sensitivity of HCC cells. In addition, the results of western blot analysis indicated that BBM may render HCC cell lines sensitive to SORA by synergistically inhibiting STAT3.
2. Results
2.1. BBM Enhanced the Antigrowth Effects of SORA on PRF-PLC-5 and HCC-Lm3 Cells
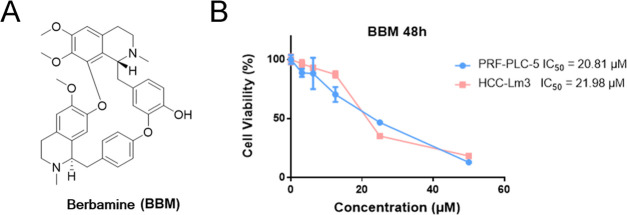
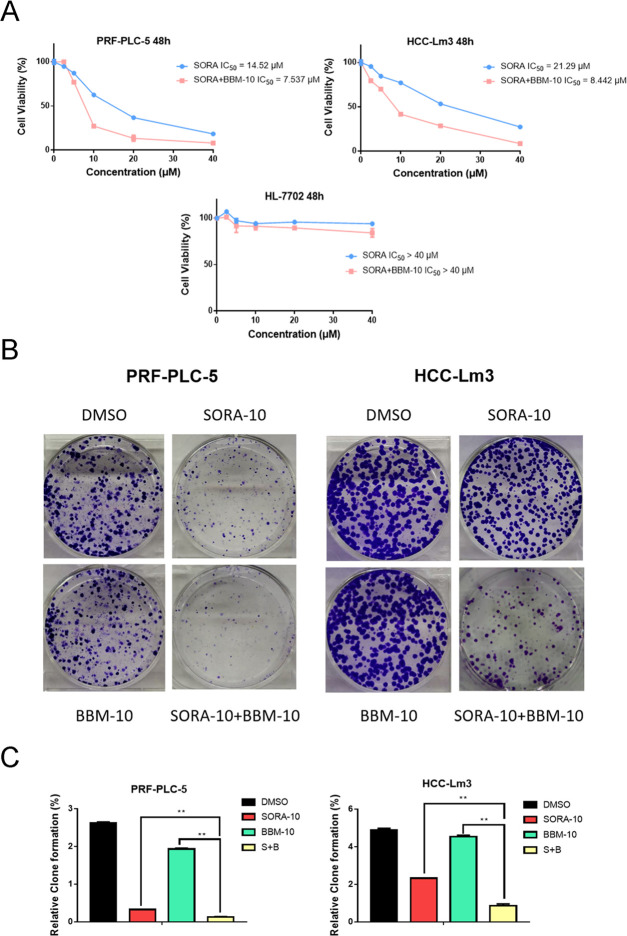
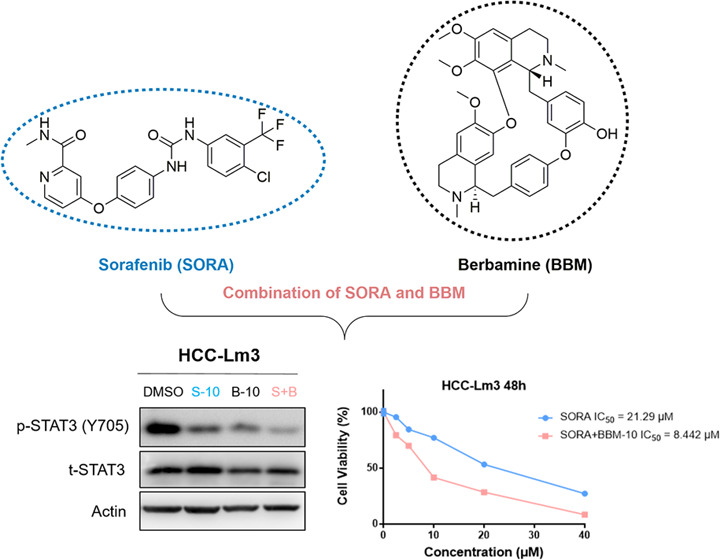
To investigate whether BBM (Figure 1A) could synergize with SORA to have an effect on HCC cell lines, the proliferative activities of PRF-PLC-5 and HCC-Lm3 cells were determined by MTS colorimetric assay. As shown in Figure 1B, BBM dose-dependently suppressed the proliferation of PRF-PLC-5 and HCC-Lm3 cells with an IC50 value of 20.81 and 21.98 μM, respectively. Next, we further evaluated the potential role of BBM in sensitizing HCC cells to SORA by comparing the antiproliferative effects of SORA when it was administered alone and in combination with BBM. To justify the use of the combination therapy, the concentration of BBM was set to half of its IC50 values, about 10 μM, which should minimize the BBM-induced cytotoxicity to PRF-PLC-5 and HCC-Lm3 cells. As shown in Figure 2A, SORA treatment alone moderately decreased the viability of HCC cell lines, while the combination of SORA with 10 μM BBM notably enhanced the proliferation inhibitory effect of SORA, leading to increased sensitivity of both PRF-PLC-5 and HCC-Lm3 cells to SORA (IC50 value of SORA versus SORA plus 10 μM BBM: 14.52–7.537 μM for PRF-PLC-5, and 21.29–8.442 μM for HCC-Lm3, respectively). Noticeably, we observed that when the concentration of SORA was set to 10 μM, the combination of SORA and BBM achieved the greatest synergy. Therefore, based on this observation, the concentrations of SORA and BBM were both set to 10 μM in the following combination treatment. Furthermore, to preliminarily explore whether combining SORA with BBM will augment the cytotoxicity on normal human cells, the proliferative activity of human liver cell line HL-7702 was also determined. Our results (Figure 2A) showed that combining SORA with BBM only exerts a very slight synergetic effect on the proliferation of HL-7702 cells when compared to the effect on cancerous liver cells, indicating that BBM could be a safe and efficient SORA sensitizer.
Figure 1.
(A) Chemical structures of berbamine (BBM). (B) Antiproliferative effects of BBM on PRF-PLC-5 and HCC-Lm3 cells. The cells were treated with compounds at different concentrations (50, 25, 12.5, 6.25, and 3.125 μM) for 48 h, and then MTS assay was performed to calculate the survival rate for each group of cells. Data were compiled from three independent experiments.
Figure 2.
Combined use of BBM and SORA can act synergistically to inhibit HCC cell growth. (A) Effect of combining SORA with BBM on the cell viability of PRF-PLC-5, HCC-Lm3, and HL-7702 cells. MTS assay was performed to measure cell viability. (B) Effect of combining SORA with BBM on the formation of PRF-PLC-5 and HCC-Lm3 cell clones. Cells in each group were exposed variably for 10–14 days, after which they were photographed. The data were compiled from three independent experiments that were replicated in triplicate. Representative photos are shown. (C) Quantitative results of the colony formation were analyzed through dissolving the crystal violet dye in 33% aqueous acetic acid and reading the absorbance at 570 nm (OD570). **P < 0.01 compared to the negative control.
To answer the question of whether the combination of SORA and BBM could exert a synergetic effect on the long-term growth of HCC cells, we further tested the clonogenic capacity of HCC cells in different groups. On days 10–14 after the cells were differentially treated for 24 h, the cell colonies were subjected to staining with crystal violet and photographs were taken. Our results demonstrated that the combination of SORA with BBM as a treatment significantly reduced the colony formation of PRF-PLC-5 and HCC-Lm3 cells (Figure 2B), compared to the group treated with each compound (10 μM SORA or 10 μM BBM) separately. In addition, the staining results were quantified by dissolving crystal violet in 33% acetic acid aqueous solution, which provided a more intuitive demonstration of the sensitization effect of BBM on SORA-induced growth inhibition (Figure 2C).
2.2. Combined Treatment of SORA and BBM Synergistically Arrests HCC Cells at G0/G1 Phase
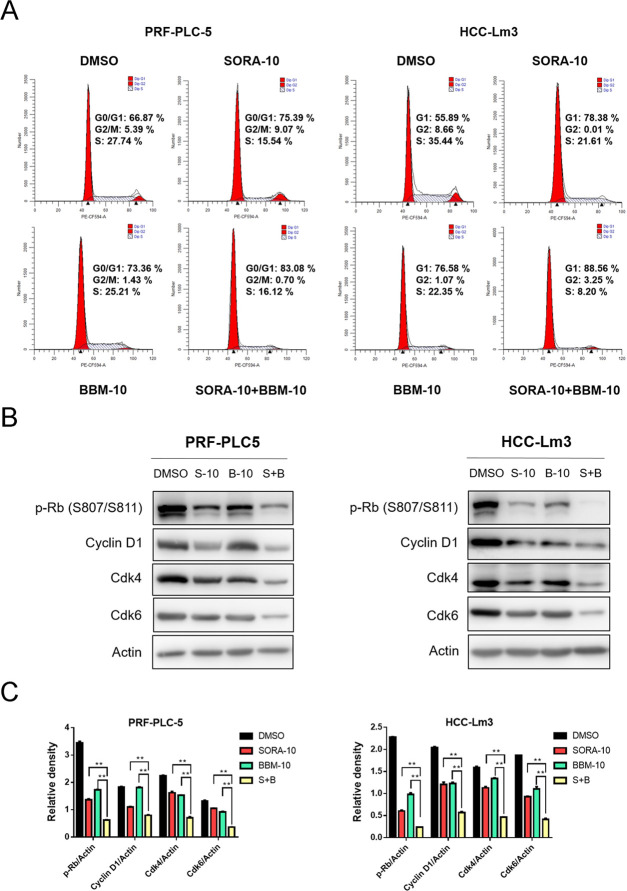
To explore how BBM can synergistically enhance the inhibitory effect of SORA on HCC cell growth, flow cytometry was used to analyze the cell cycle distribution of each treatment group. The results demonstrated that the combined use of SORA and BBM markedly promoted the G0/G1 phase arrest of HCC cells (Figure 3A). In addition, the expression of key signaling molecules responsible for the G1/S phase transition, such as cyclin D1, Cdk4, Cdk6, and phosphorylated Rb, was determined by western blot analysis. Consistently, the results suggested that BBM and SORA exerted a synergistic inhibitory effect on the expression of the aforementioned cell cycle-related proteins (Figure 3B,C). Overall, our results indicate that BBM and SORA act synergistically to arrest cells in the G0/G1 phase by downregulating the expression of G1/S phase transition-related proteins.
Figure 3.
BBM and SORA synergistically induce HCC cell cycle arrest. (A) Single-agent treatment with BBM (10 μM), SORA (10 μM) alone, or combined treatment with BBM (10 μM) and SORA (10 μM) for 48 h. Cell cycle distribution was assessed using cytometry (Becton Dickinson Fascalibor, BD Biosciences, Franklin Lakes, NJ). For cell cycle distribution, a representative histogram is provided. Data were compiled from three independent experiments and reported in the form of mean ± SD. All of the experiments were performed in triplicate. (B) Western blot analysis of G0/G1 phase-related protein phosphorylated Rb (p-Rb), cyclin D1, Cdk4, and Cdk6. Actin was shown as the control of equal loading. (C) Quantification of the relative expression of cell cycle-related proteins (p-Rb/actin, cyclin D1/actin, Cdk4/actin, and Cdk6/actin) using ImageJ software and analysis with Graphpad prism 7. **P < 0.01 compared to the negative control.
2.3. Combined Treatment of SORA and BBM Synergistically Induces HCC Cells Apoptosis
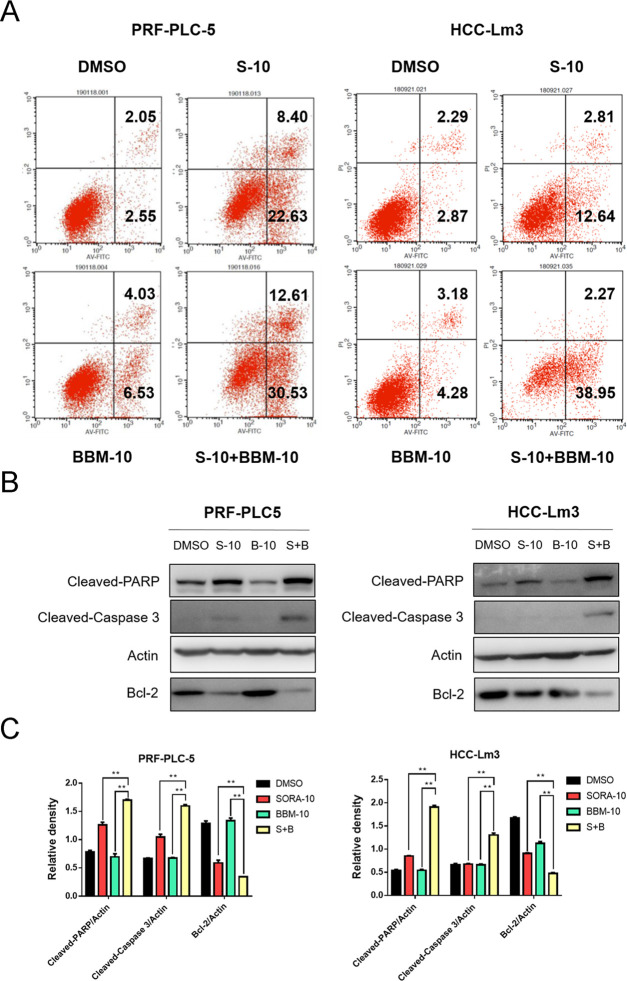
To investigate whether the combination of SORA and BBM has a synergetic effect on the induction of HCC cells apoptosis, the degree of cell apoptosis was analyzed through Annexin V-FITC and PI double staining assay and counted by flow cytometry. Our results showed that the combined use of SORA and BBM significantly promoted HCC cell apoptosis compared with the single-agent treatment group (Figure 4A). Furthermore, to uncover the molecular mechanisms underlying apoptosis sensitization induced by BBM, western blot analysis was performed to determine the expression levels of the apoptosis-related proteins, including Bcl-2, cleaved caspase 3, and cleaved PARP. The results showed a significant upregulation in the expression of proapoptotic molecules in the combined treatment group, including cleaved PARP and cleaved caspase 3, whereas there was a downregulation in the expression of the antiapoptotic molecule Bcl-2 (Figure 4B,C). Noticeably, although BBM treatment alone exerts negligible effects on the expression of cleaved caspase 3 and cleaved PARP, it produces certain proapoptosis effects based on the flow cytometry results. The most possible reason is that BBM could promote HCC cells apoptosis through other signaling pathways such as Fas- and p53-mediated apoptosis-related pathways, which has been discovered in HepG2 and SMMC-7721 cells in previous studies.20,21
Figure 4.
BBM and SORA synergistically induce HCC cell apoptosis. (A) Single-agent treatment with BBM (10 μM), SORA (10 μM) alone, or combined treatment with BBM (10 μM) and SORA (10 μM) for 48 h. Apoptosis was analyzed using Annexin V/propidium iodide (PI) staining. Data were compiled from three independent experiments and reported in the form of mean ± SD. All of the experiments were performed in triplicate. (B) Extraction of total protein and detection of the expression of cleaved PARP, cleaved caspase 3, and Bcl-2 using western blotting. Actin was shown as the control of equal loading. (C) Quantification of the relative expression of apoptosis-related proteins (cleaved PARP/actin, cleaved caspase 3/actin, and Bcl-2/actin) using ImageJ software and analysis with Graphpad prism 7. **P < 0.01 compared to the negative control.
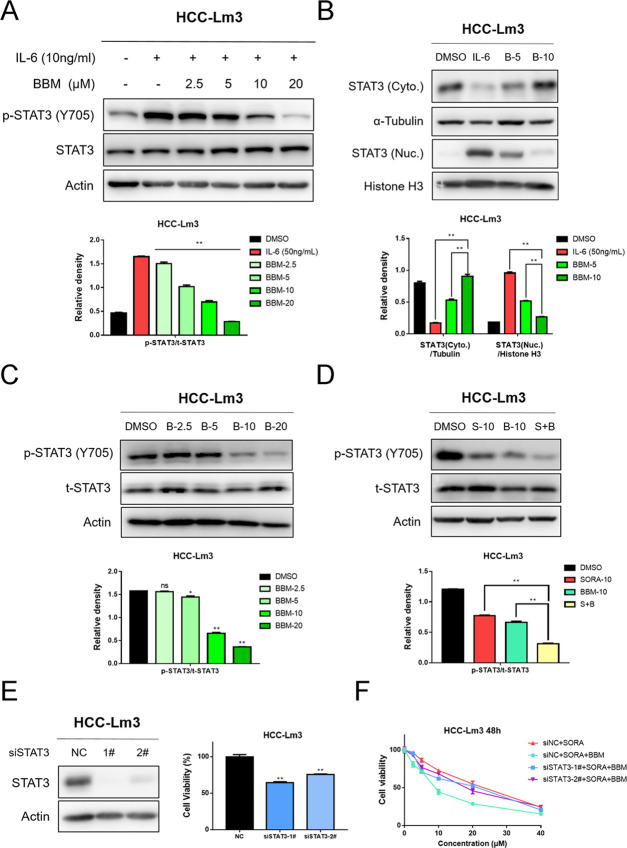
2.4. BBM Suppresses the Activation of STAT3 in HCC Cells and Potentiates STAT3 Inhibition of SORA
As a downstream molecule of various oncogenic tyrosine kinase receptors (RTKs), signal transducer and activator of transcription 3 (STAT3) is widely overexpressed in a variety of solid tumors and plays a crucial role in the occurrence and development of cancer.25 Additionally, a growing number of studies have pointed out that the reactivation of STAT3 signal is closely associated with the emergence of resistance to several targeted therapies, such as EGFR inhibitor erlotinib and angiogenesis inhibitor SORA.26,27 Intriguingly, Hu et al. reported in their recent study that BBM could sensitize pancreatic cancer cells to gefitinib through the suppression of STAT3 signaling.24 Based on their finding, we hypothesized in the current study that the sensitization of SORA to BBM might also be mediated by STAT3 inhibition. First, western blot analysis was performed to verify the STAT3 inhibitory activity of BBM on HCC cells. As shown in Figure 5A,B, the result demonstrated that BBM not only dose-dependently suppressed the phosphorylation of STAT3 at tyrosine 705 (Y705) induced by IL-6 but also decreased the nuclear translocation of STAT3. Apart from that, our results suggested that BBM also effectively downregulated the basal phosphorylation of STAT3 Y705 in HCC-Lm3 cells (Figure 5C). Next, to explain whether the SORA sensitization effects of BBM are mediated by STAT3 inhibition, the level of p-STAT3 after treatment with SORA, BBM, and SORA plus BBM was determined by western blotting. The results (Figure 5D) demonstrated that the combination of SORA and BBM completely blocked the activation of STAT3 in HCC cells. Moreover, the SORA sensitization effects of BBM on STAT3-knockdown cells were also evaluated. As shown in Figure 5E,F, our results demonstrated that knockdown of STAT3 expression displayed certain inhibitory effects on the cell viability of HCC-Lm3 cells and greatly impaired the sensitization effects of BBM, suggesting that BBM sensitizes SORA in a STAT3 level-dependent manner. Overall, these results confirm that the synergy between BBM and SORA inactivates STAT3 signaling in HCC cells, which might be the main cause for the synergy between SORA and BBM.
Figure 5.
Combined use of BBM and SORA activities in synergy to suppress STAT3 activation in HCC cells, and knockdown of STAT3 abolished the sensitization effect of BBM. (A) HCC-Lm3 cells were pretreated with 2.5, 5, 10, and 20 μM BBM for 2 h, and then stimulated with IL-6 (10 ng/mL) for 30 min. Western blot analysis was performed to determine STAT3 phosphorylation. Relative STAT3-phosphorylated (Y705) expression (p-STAT3/t-STAT3) was quantified. (B) After HCC-Lm3 cells were treated with IL-6 (10 ng/mL) with or without BBM (5 and 10 μM) for 2 h, western blotting was used to determine the levels of STAT3 in the nucleus and the cytoplasm. (C) HCC-Lm3 cells were treated with BBM at an indicated concentration for 24 h, and then western blot analysis was used to determine the phosphorylated and total STAT3 proteins. (D) Treatment of HCC-Lm3 cells with SORA alone (10 μM), BBM alone (10 μM), or in combination with SORA (10 μM) and BBM (10 μM) for 24 h. Western blotting was used to determine the phosphorylated and total STAT3. The relative expression of all bands in (A)–(D) was quantified using ImageJ software and analysis with Graphpad prism 7. **P < 0.01 compared to the negative control. ns means no significance. (E) STAT3 expression level and cell viability of HCC-Lm3 cells with STAT3 knocking down were examined by western blot analysis and MTS assay. **P < 0.01 compared to the negative control. (F) siNC or siSTAT3 transfected cells were treated with SORA (10 μM) or SORA (10 μM) plus BBM (10 μM) for 48 h, and then the cell viability was measured by MTS assay.
3. Discussion
In the past few years, the global incidence of HCC has been on the rise. Although surgical techniques and screening procedures for early detection of HCC are constantly progressing, the diagnosis and prognosis of HCC remain extremely unsatisfactory.28 For most patients with advanced HCC, SORA is still the preferred first-line treatment. As a multitarget tyrosine kinase inhibitor, SORA effectively inhibits tumor angiogenesis and proliferation of HCC via suppression of a wide variety of receptor tyrosine kinases and serine/threonine kinases, including KDR, c-Kit, Flt3, Raf-1, and B-Raf.4 However, it is inevitable that the acquired drug resistance of SORA will be increased in clinical applications. Therefore, a better understanding of the molecular mechanisms underlying the acquired resistance of SORA is required to overcome SORA resistance and improve the clinical effect of SORA therapy. In recent studies, abnormal activation of certain molecular pathways is considered to be the main cause of SORA resistance, including EGFR activation, abnormal activation of PI3K/Akt, and JAK/STAT3 pathways, epithelial–mesenchymal transition (EMT), autophagy, and hypoxic microenvironment.29 Based on the above findings, researchers have tried out a great many combination regimens to reasonably sensitize HCC cells to SORA. For example, Shi et al. found that the combined treatment of SORA and autophagy inhibitor chloroquine (CQ) produced a significant synergistic effect in HCC cells both in vitro and in vivo.30 Moreover, Li et al. reported that the dual PI3K/mTOR inhibitor BEZ235 efficiently increased the inhibitory effect of SORA on both parental and SORA-resistant HepG2 cells (HepG2 and HepG2R) via suppression of the PI3K/AKT/mTOR pathway.31 All of these results indicate that the discovery of effective SORA sensitizers may be a promising method to overcome SORA resistance.
A large number of recent studies have shown that natural products show great potential to combat the development of multidrug resistance in cancer.32 A typical example is the safe and effective anticancer natural product BBM, which has been widely used clinically to resist imatinib-induced neutropenia in China and Japan. In addition to its broad antitumor activity, the sensitization effect of BBM on certain chemotherapy (gemcitabine) and targeted therapy (EGFR inhibitor gefitinib) has also attracted the attention of many researchers.22,24 However, it remains unclear whether BBM could exert sensibilization effects on HCC cells to other targeted therapy like SORA. Herein, we first reported that the combined treatment with BBM significantly enhanced the antiproliferative, cell cycle arrest, and proapoptotic effects of SORA in HCC cells. In addition, despite some previous studies showing that BBM has good antiproliferative activity against HCC cell lines, there is insufficient evidence for the main molecular mechanism of BBM. In this study, by determining the effects of STAT3 signaling after single-agent (BBM) treatment or combined treatment (BBM plus SORA), we confirmed that the anti-HCC activity and synergistic effect of BBM was primarily mediated by the inhibition of STAT3 signaling. It is worth noting that in a recent study, Huang et al. reported that berberine, another isoquinoline alkaloid, also synergistically sensitizes HCC cells to SORA, indicating that isoquinoline might be a favorable structural framework for the design of SORA sensitizers.33 Moreover, another recent study by Hu et al. demonstrated that BBM effectively enhances the efficacy of gefitinib on pancreatic cancer cells through its STAT3 inhibition. Consistently, our results found that the inhibition of BBM on STAT3 signaling in HCC cells also was involved with the sensitization of SORA.
In recent years, STAT3 has been widely regarded as a hub of oncogenic signals because it acts as a key mediator of many signaling pathways triggered by cytokines (e.g., IL-6, IL-22, and TNF-α), growth factors (e.g., EGF, PDGF, and VEGF), and carcinogens (e.g., nicotine, hepatitis C virus core protein, and lipopolysaccharide).25 During STAT3 activation, Tyr705 phosphorylation indicates the initiation of STAT3 activation, which leads to the gradual transformation of STAT3 into an active conformation, dimerization, and nuclear translocation. Upon activation, STAT3 switches on the expression of antiapoptotic (e.g., Bcl-2, Mcl-2, and survivin), proproliferative (e.g., cyclin D1, cyclin B, cdc2), and angiogenesis-related genes (e.g., VEGF, bFGF, and HIF-1α).34 Growing evidence suggests that the dysregulation of STAT3 signal transduction may be involved in a variety of carcinogenic processes, including the initiation, progression, and drug resistance development of HCC.34 Interestingly, although SORA could directly inactivate STAT3 through a kinase-independent mechanism, some recent studies have reported that in SORA-resistant HCC cells,35 the activity of STAT3 and its downstream oncogenes Mcl-1 and Cyclin D1 was upregulated, indicating that the negative feedback regulation of STAT3 activity may be closely associated with the acquired SORA resistance.36 With the concerted efforts by many researchers, a large number of natural STAT3 inhibitors, such as β-caryophyllene oxide, capillarisin, CIMO, etc., has been identified in recent years, which displayed great potential in the negative regulation of the growth of various human cancer cell lines.37−41 To investigate whether this new approach could improve the efficacy of SORA on HCC cells, the combined effect of SORA with several natural STAT3 inhibitors (including phloretin, ursodeoxycholic acid, and silibinin) was evaluated and showed favorable synergistic effects.11,42,43 However, the lack of STAT3 inhibitors approved for clinical use has largely limited the clinical application prospects of this combination therapy. Some potent STAT3 inhibitors, such as OPB-31121, TTI-101, and S31-201, are still in early clinical studies or preclinical studies. Unlike these molecules, BBM has been approved for clinical use for a long time, which will provide great convenience for the rapid development of a combination therapy that includes SORA and BBM.
4. Conclusions
In conclusion, our study shows that BBM could enhance the inhibitory effect of SORA on PRF-PLC-5 and HCC-Lm3 cells, thereby inhibiting proliferation, preventing cell cycle, and promoting cell apoptosis. Mechanistically, our results indicate that BBM exhibits strong cellular STAT3 inhibitory activity and can act synergistically with SORA to inactivate STAT3 signaling in HCC cells. These results provide a theoretical basis for the combined use of BBM and SORA as a new combination therapy for HCC.
5. Material and Methods
5.1. Cell Lines and Reagents
Human HCC cell lines PRF-PLC5 and HCC-Lm3, and human liver cell line HL-7702 were purchased from the American Type Culture Collection (Manassas). Both of these cell lines were embedded in a DMEM medium (Gibco, Grand Island, NY), which was supplemented with 10% fetal bovine serum (FBS, Gibco) and 100 units/mL penicillin/streptomycin. The cell lines were maintained in a 5% CO2-humidified incubator at 37 °C. CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay Kit was obtained from Promega (Promega, Medison). The first antibodies used in this study, including phosphorylated Rb (Ser801/Ser811), cyclin D1, Cdk4, Cdk6, cleaved PARP, cleaved caspase 3, Bcl-2, phosphorylated-STAT3 (Tyr705), STAT3, α-tubulin, histone H3, and β-actin were all procured from Cell Signaling Technology (Danvers, MA). The Goat anti-rabbit and Goat anti-Mouse IgG-HRP secondary antibody were procured from Santa Cruz Biotechnology (Santa Cruz, CA). The negative control small-interfering RNA (siNC) and two siRNA targeting STAT3 were obtained from Genepharma (Shanghai, China). A lipofectamine RNAiMAX transfection reagent was purchased from Invitrogen Corporation (California). SORA and BBM were obtained from MCE (Shanghai, China), and the purity of SORA and BBM detected by HPLC exceeds 99%.
5.2. Cell Viability Assay
In accordance with the manufacturer’s operating procedures, cell viability assay was performed along with the MTS assay. First, PRF-PLC-5, HCC-Lm3, or HL-7702 cells were seeded for a brief time period in a 96-well plate at a density of 5 × 103 cells per well in 100 μL of a fresh DMEM or RPMI-1640 medium. Subsequently, the cells were subjected to treatment with compounds at varying concentrations for 48 h. After 30 min of treatment with the MTS solution, a microplate reader was used to record the absorbance of each well at 490 nm. Each experimental step was repeated three times.
5.3. Clonogenic Assay
PRF-PLC5 (800 cells per well) and HCC-Lm3 cells (1000 cells per well) were seeded in a 6-well plate. The next day, the medium was replaced with a fresh DMEM medium containing DMSO, BBM (10 μM), SORA (10 μM), or SORA (10 μM) plus BBM (10 μM). After incubation with different compounds for 24 h, the cells were then transferred to a fresh medium, in which they were allowed to grow for 10–14 days. Subsequently, the colonies were rinsed twice with PBS, fixed with methanol for 15 min, and then stained with 0.1% crystal violet solution and photographed. Finally, to quantify the results of the colony formation analysis, the crystal violet dye in each well was dissolved in 33% aqueous acetic acid, and the absorbance of each well was recorded at 570 nm.
5.4. Cell Apoptosis Analysis
PRF-PLC-5 or HCC-Lm3 cell lines were grown in a 6-well plate overnight, after which they were subjected to treatment with DMSO, BBM (10 μM), SORA (10 μM), or SORA (10 μM) plus BBM (10 μM) for 48 h. Then, the cells were collected with trypsin and washed twice with PBS, and resuspended in 1× binding buffer to achieve a concentration of 1 × 106 cells/mL. Then, the cell suspension was stained with 5 μL of FITC Annexin V and 1 μL of PI at room temperature for 15 min. After that, the FACS Calibur flow cytometer (BD) was used to determine the number of apoptotic cells.
5.5. Cell Cycle Analysis
PRF-PLC-5 or HCC-Lm3 cells were grown in a 6-well plate overnight and then treated with DMSO, BBM (10 μM), SORA (10 μM), or SORA (10 μM) plus BBM (10 μM) for 48 h. The cells were trypsinized, collected, washed twice with ice-cold PBS, and fixed in 75% ice-cold ethanol at 4 °C overnight. After centrifugation, the cells were washed twice with ice-cold PBS and then stained with 500 μL of PBS containing 50 μg/mL propidium iodide (PI) for 30 min at room temperature. The FACS Calibur instrument (Becton Dickinson FACSCalibor, BD Biosciences, NJ) was utilized to calculate the percentage of cells in the G0/G1, S, and G2/M phases.
5.6. Western Blot
After treating the cells with the indicated compounds for 24 h, the cells were washed and lysed with PBS and radioimmunoprecipitation assay (RIPA) buffer, respectively. The BCA protein assay kit (Beyotime Biotechnology, Shanghai, China) was used to quantify cell lysates. Then, equal amounts of protein extracts were separated by SDS-PAGE and transferred to a PVDF membrane. Next, the membranes were blocked with 5% skim milk TBST solution at room temperature, incubated with the specific primary antibody at 4 °C overnight, and then incubated with the corresponding secondary antibody at room temperature for 1 h. Finally, according to the manufacturer’s operating procedures, the immunoreactive bands were detected using the ECL detection kit in the Amersham Imager 600 system (GE Healthcare Life Sciences, Shanghai, China). The results were analyzed using ImageJ software to quantify the relative band density ratio.
5.7. Cytoplasmic and Nuclear Protein Extraction
In accordance with the manufacturer’s operating procedures, the Thermo Scientific NE-PER Nuclear and Cytoplasmic Extraction Kit was utilized to extract the nuclear and cytoplasmic proteins. Cells (3 × 105) were briefly seeded in a 6-well plate overnight and then subjected to incubation with compounds at indicated concentrations for 24 h. Cells were collected by trypsinization and then centrifuged at 500g for 5 min. In compliance with the given assay instructions, the cytoplasmic extraction reagents and nuclear extraction reagents were gradually added to the cell pellet. Afterward, the cytoplasmic and nuclear proteins were collected separately.
5.8. siRNA Transfection
HCC-Lm3 cells were seeded in a 6-well plate at a density of 2 × 105 cells per well overnight and transfected using Lipofectamine RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s instructions. After 24 h of transfection, the cells were trypsinized, counted, and seeded into new 6-well and 96-well plates for western blot assay and MTS assay, respectively. For MTS assay, the transfected cells were treated with SORA (10 μM) or SORA (10 μM) plus BBM (10 μM) for another 48 h.
5.9. Statistical Analysis
The results are reported in the form of mean ± standard errors of the mean (SEM). To compare the differences between groups, one-way analysis of variance (ANOVA) was adopted as the data analysis method and conducted using GraphPad Pro (GraphPad, San Diego, CA). The significance level was set at 0.05, with a P value smaller than 0.05 (P < 0.05), indicating a statistically significant result. All of the experiments were replicated for a minimum of three times.
Acknowledgments
We are thankful for funding from the Zhejiang Provincial Natural Science Foundation of China (approval ID: LY20H160014, LGF19H18005).
The authors declare no competing financial interest.
References
- Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68, 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Llovet J. M.; Montal R.; Sia D.; Finn R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta-Caldarola G.; Divella R.; Mazzocca A.; Infusino S.; Ferraro E.; Filippelli G.; Daniele A.; Sabba C.; Abbate I.; Brandi M. Sorafenib: the gold standard therapy in advanced hepatocellular carcinoma and beyond. Future Oncol. 2015, 11, 2263–2266. 10.2217/fon.15.161. [DOI] [PubMed] [Google Scholar]
- Fujiwara N.; Friedman S. L.; Goossens N.; Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Jin R.; Zhao J.; Liu J.; Ying H.; Yan H.; Zhou S.; Liang Y.; Huang D.; Liang X.; Yu H.; Lin H.; Cai X. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015, 367, 1–11. 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Bruix J.; Qin S.; Merle P.; Granito A.; Huang Y. H.; Bodoky G.; Pracht M.; Yokosuka O.; Rosmorduc O.; Breder V.; Gerolami R.; Masi G.; Ross P. J.; Song T.; Bronowicki J. P.; Ollivier-Hourmand I.; Kudo M.; Cheng A. L.; Llovet J. M.; Finn R. S.; LeBerre M. A.; Baumhauer A.; Meinhardt G.; Han G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- Koeberle D.; Dufour J. F.; Demeter G.; Li Q.; Ribi K.; Samaras P.; Saletti P.; Roth A. D.; Horber D.; Buehlmann M.; Wagner A. D.; Montemurro M.; Lakatos G.; Feilchenfeldt J.; Peck-Radosavljevic M.; Rauch D.; Tschanz B.; Bodoky G. Swiss Group for Clinical Cancer, R. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- Abou-Alfa G. K.; Shi Q.; Knox J. J.; Kaubisch A.; Niedzwiecki D.; Posey J.; Tan B. R. Jr.; Kavan P.; Goel R.; Lammers P. E.; Bekaii-Saab T. S.; Tam V. C.; Rajdev L.; Kelley R. K.; El Dika I.; Zemla T.; Potaracke R. I.; Balletti J.; El-Khoueiry A. B.; Harding J. H.; Suga J. M.; Schwartz L. H.; Goldberg R. M.; Bertagnolli M. M.; Meyerhardt J.; O’Reilly E. M.; Venook A. P. Assessment of Treatment With Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1582–1588. 10.1001/jamaoncol.2019.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Zhang G.; Wu J.; Jia M. Adjuvant therapy for hepatocellular carcinoma: current situation and prospect. Drug Discoveries Ther. 2013, 7, 137–143. 10.5582/ddt.2013.v7.4.137. [DOI] [PubMed] [Google Scholar]
- Saraswati S.; Alhaider A.; Abdelgadir A. M.; Tanwer P.; Korashy H. M. Phloretin attenuates STAT-3 activity and overcomes sorafenib resistance targeting SHP-1-mediated inhibition of STAT3 and Akt/VEGFR2 pathway in hepatocellular carcinoma. Cell Commun. Signaling 2019, 17, 127. 10.1186/s12964-019-0430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X. Q.; Rong L. W.; Wang R. X.; Zheng X. L.; Zhang L.; Zhang L.; Lin Y.; Wang X.; Li Z. P. Luteolin and sorafenib combination kills human hepatocellular carcinoma cells through apoptosis potentiation and JNK activation. Oncol. Lett. 2018, 16, 648–653. 10.3892/ol.2018.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong L. W.; Wang R. X.; Zheng X. L.; Feng X. Q.; Zhang L.; Zhang L.; Lin Y.; Li Z. P.; Wang X. Combination of wogonin and sorafenib effectively kills human hepatocellular carcinoma cells through apoptosis potentiation and autophagy inhibition. Oncol. Lett. 2017, 13, 5028–5034. 10.3892/ol.2017.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W.; Shuo L.; Yingru X.; Min M.; Runpeng Z.; Jun X.; Dong H. Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2019, 519, 41–45. 10.1016/j.bbrc.2019.08.115. [DOI] [PubMed] [Google Scholar]
- Firouzi S.; Malekahmadi M.; Ghayour-Mobarhan M.; Ferns G.; Rahimi H. R. Barberry in the treatment of obesity and metabolic syndrome: possible mechanisms of action. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2018, 11, 699–705. 10.2147/DMSO.S181572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Y.; Ling L. H.; Teh B. S.; Seow W. K.; Thong Y. H. Anti-inflammatory and immunosuppressive properties of the bis-benzylisoquinolines: in vitro comparisons of tetrandrine and berbamine. Int. J. Immunopharmacol. 1989, 11, 395–401. 10.1016/0192-0561(89)90086-6. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Lu L.; Guo T. B.; Qiu J.; Yang Y.; Liu A.; Zhang J. Z. Novel immunomodulatory properties of berbamine through selective down-regulation of STAT4 and action of IFN-gamma in experimental autoimmune encephalomyelitis. J. Immunol. 2008, 181, 1491–1498. 10.4049/jimmunol.181.2.1491. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Tan Y.; Wu G.; Liu L.; Wang Y.; Luo Y.; Shi J.; Huang H. Berbamine overcomes imatinib-induced neutropenia and permits cytogenetic responses in Chinese patients with chronic-phase chronic myeloid leukemia. Int. J. Hematol. 2011, 94, 156–162. 10.1007/s12185-011-0887-7. [DOI] [PubMed] [Google Scholar]
- Meng Z.; Li T.; Ma X.; Wang X.; Van Ness C.; Gan Y.; Zhou H.; Tang J.; Lou G.; Wang Y.; Wu J.; Yen Y.; Xu R.; Huang W. Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca(2)(+)/calmodulin-dependent protein kinase II. Mol. Cancer Ther. 2013, 12, 2067–2077. 10.1158/1535-7163.MCT-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Cao J.; Yu B.; Wang S.; Liu L.; Tao L.; Sun W. Berbamine induces SMMC-7721 cell apoptosis via upregulating p53, downregulating survivin expression and activating mitochondria signaling pathway. Exp. Ther. Med. 2018, 15, 1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. Y.; Lv Q. H.; Dong Q.; Xu R. Z.; Dong Q. H. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J. Asian Nat. Prod. Res. 2009, 11, 219–228. 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- Jin X.; Wu Y. Berbamine enhances the antineoplastic activity of gemcitabine in pancreatic cancer cells by activating transforming growth factor-beta/Smad signaling. Anat. Rec. 2014, 297, 802–809. 10.1002/ar.22897. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu Q.; Zhang Y.; Liu K.; Yu P.; Liu K.; Luan J.; Duan H.; Lu Z.; Wang F.; Wu E.; Yagasaki K.; Zhang G. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol. Cancer 2009, 8, 81. 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.; Cai H.; Yang S.; Tu J.; Huang X.; Chen G. Berbamine Enhances the Efficacy of Gefitinib by Suppressing STAT3 Signaling in Pancreatic Cancer Cells. OncoTargets Ther. 2019, 12, 11437–11451. 10.2147/OTT.S223242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A.; Shanmugam M. K.; Perumal E.; Li F.; Nachiyappan A.; Dai X.; Swamy S. N.; Ahn K. S.; Kumar A. P.; Tan B. K.; Hui K. M.; Sethi G. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta, Rev. Cancer 2013, 1835, 46–60. 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Lee H. J.; Zhuang G.; Cao Y.; Du P.; Kim H. J.; Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 2014, 26, 207–221. 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Xie L.; Zeng Y.; Dai Z.; He W.; Ke H.; Lin Q.; Chen Y.; Bu J.; Lin D.; Zheng M. Chemical and genetic inhibition of STAT3 sensitizes hepatocellular carcinoma cells to sorafenib induced cell death. Int. J. Biol. Sci. 2018, 14, 577–585. 10.7150/ijbs.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L.; El-Serag H. B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L.; Liu L.; Yang S.; Ren J.; Lai P. B. S.; Chen G. G. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim. Biophys. Acta, Rev. Cancer 2017, 1868, 564–570. 10.1016/j.bbcan.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Shi Y. H.; Ding Z. B.; Zhou J.; Hui B.; Shi G. M.; Ke A. W.; Wang X. Y.; Dai Z.; Peng Y. F.; Gu C. Y.; Qiu S. J.; Fan J. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 2011, 7, 1159–1172. 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- Li A.; Zhang R.; Zhang Y.; Liu X.; Wang R.; Liu J.; Liu X.; Xie Y.; Cao W.; Xu R.; Ma Y.; Cai W.; Wu B.; Cai S.; Tang X. BEZ235 increases sorafenib inhibition of hepatocellular carcinoma cells by suppressing the PI3K/AKT/mTOR pathway. Am. J. Transl. Res. 2019, 11, 5573–5585. [PMC free article] [PubMed] [Google Scholar]
- Dinic J.; Podolski-Renic A.; Stankovic T.; Bankovic J.; Pesic M. New Approaches With Natural Product Drugs for Overcoming Multidrug Resistance in Cancer. Curr. Pharm. Des. 2015, 21, 5589–5604. 10.2174/1381612821666151002113546. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Wang K.; Gu C.; Yu G.; Zhao D.; Mai W.; Zhong Y.; Liu S.; Nie Y.; Yang H. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol. Rep. 2018, 40, 1525–1532. 10.3892/or.2018.6552. [DOI] [PubMed] [Google Scholar]
- Siveen K. S.; Sikka S.; Surana R.; Dai X.; Zhang J.; Kumar A. P.; Tan B. K.; Sethi G.; Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim. Biophys. Acta, Rev. Cancer 2014, 1845, 136–154. 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Hung M. H.; Tai W. T.; Shiau C. W.; Chen K. F. Downregulation of signal transducer and activator of transcription 3 by sorafenib: a novel mechanism for hepatocellular carcinoma therapy. World J. Gastroenterol. 2014, 20, 15269–15274. 10.3748/wjg.v20.i41.15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. F.; Tai W. T.; Hsu C. Y.; Huang J. W.; Liu C. Y.; Chen P. J.; Kim I.; Shiau C. W. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur. J. Med. Chem. 2012, 55, 220–227. 10.1016/j.ejmech.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Loh C. Y.; Arya A.; Naema A. F.; Wong W. F.; Sethi G.; Looi C. Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. 10.3389/fonc.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.; Cho S. K.; Kapoor S.; Kumar A.; Vali S.; Abbasi T.; Kim S. H.; Sethi G.; Ahn K. S. beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. 10.1002/mc.22035. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Chiang S. Y.; Nam D.; Chung W. S.; Lee J.; Na Y. S.; Sethi G.; Ahn K. S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014, 345, 140–148. 10.1016/j.canlet.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Mohan C. D.; Bharathkumar H.; Bulusu K. C.; Pandey V.; Rangappa S.; Fuchs J. E.; Shanmugam M. K.; Dai X.; Li F.; Deivasigamani A.; Hui K. M.; Kumar A. P.; Lobie P. E.; Bender A.; Basappa; Sethi G.; Rangappa K. S. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. 10.1074/jbc.M114.601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Kim C.; Sethi G.; Ahn K. S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. 10.18632/oncotarget.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Cho Y. Y.; Cho E. J.; Yu S. J.; Lee J. H.; Yoon J. H.; Kim Y. J. Synergistic effect of ursodeoxycholic acid on the antitumor activity of sorafenib in hepatocellular carcinoma cells via modulation of STAT3 and ERK. Int. J. Mol. Med. 2018, 42, 2551–2559. 10.3892/ijmm.2018.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Yang H.; Cui T.; Pan P.; Kabir N.; Chen D.; Ma J.; Chen X.; Chen Y.; Yang Y. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur. J. Pharmacol. 2018, 832, 39–49. 10.1016/j.ejphar.2018.05.027. [DOI] [PubMed] [Google Scholar]