Abstract
Excessive water production from natural gas reservoirs is a main challenge facing the industry nowadays. Polymeric gelants have been widely applied to seal the water production zones, leading to a more feasible production operation. Nevertheless, conventional treatments fail in reservoirs characterized with the presence of sour gases. In this paper, aluminum-based salts are investigated as potential replacement for the conventional chromium acetate as crosslinkers for polyacrylamide (PAM), where aluminum has the advantage of being more environment-friendly besides its abundance. The investigation covers the whole pH range and examines the rheological behavior of the mature gels in the temperature range between 25 and 100 °C. While chromium acetate was proven to be sensitive to the presence of sour gases, namely, CO2 and H2S, because of the inability to produce a stable gel at the acidic conditions, this paper presents aluminum-based crosslinkers that are more tolerable toward high acidity. Unlike the conventional crosslinkers, the gelation rate in aluminum acetate and aluminum aminoacetate systems was found to decrease with the increase in pH. Both the crosslinkers succeeded in producing a strong gel with a storage modulus of more than 2000 Pa. Moreover, this study relates the physical stability of the colloidal aluminum crosslinkers with the viscoelastic behavior of the mature gel. The results reveal that aluminum acetate, among the screened salts, has a controllable gelation time at pH conditions between 3.5 and 8.5 and is the most stable in the temperature range 25–100 °C. PAM/AlAc system has a gelation time of around 50 min at 75 °C making it suitable for near-wellbore treatments, while the gelation time increased to 80 min upon increasing the pH of the system from 4.1 to 4.6. Moreover, the system showed good stability in saline conditions with NaCl concentration of up to 50,000 ppm. Scanning electron microscopy of freeze-dried samples proved the uniform distribution of colloidal crosslinkers and showed sheets wrapping around the colloidal particles. The performance of the new crosslinker is compared with available commercial crosslinkers.
1. Introduction
Excessive water production from oil and gas wells is considered as one of the main challenges that influences the feasibility of production. It is estimated that the daily global production of water from oil reservoirs is around 220 million barrels, confining to a global average of 3:1 water to oil ratio.1 The same problem also extends to natural gas wells where many remediation activities have failed .2 Processing this unwanted water, from separation to deoiling and pumping, can be a lengthy and costly process that will increase the worldwide annual water handling cost to more than 40 billion dollars.3 Besides the economic factor, the presence of water in the pipes and midstream facilities induces other problems including, but not limited to, scale formation, corrosion, and microbial growth.4,5 Reducing the quantity of produced water not only impacts the cost of operation but also enhances the lifetime of the well and increases the recovery factor.6 Therefore, given the prevailing high water production and its associated problems as well as the strict environmental constrains of disposing it, there have been growing appeals for reducing the produced quantities to maintain an environment-friendly and a profitable operation.7
Zhu et al. (2017) categorized the root causes of the problem to two reasons; the first is because of leaking behind the casing of the drilled well, while the second is because of the viscous fingering in which water mobility is favored over the oil in the porous media.6 A whole range of different approaches to the problem are proposed and applied in oil and gas reservoirs. Several industries have proposed the use of mechanical treatments such as the use of packers and inflow control devices, which selectively pass the oil or gas over other fluids.8,9 Nevertheless, chemical treatments have been shown to cope with the problem more efficiently.10 In situ crosslinkable polymers have been widely used for water shut-off where the formulation is designed to be injected as a liquid and the gelation is to be complete in the designated area, leading to plugging the water-producing zone.11 Moreover, gelling system has wide flexibility, allowing for alternative designs for the differently characterized reservoirs.12 Recently, in situ gelants have also been proposed to be used for other applications in oilfields such as wellbore strengthening and as loss circulation materials giving them the advantage of broad applicability.13,14
Polyacrylamide (PAM) is the most abundantly used base polymer for water control purposes because of the low cost as well as the commercial availability.15 Over the last three decades, researchers have successfully investigated many crosslinkers, which can be classified generally into organic and inorganic crosslinkers. Organic crosslinkers, such as polyethyleneimine, hydroquinone, and hexamethylenetetramine, have achieved many successes in conformance control applications.16,17 Nonetheless, organic crosslinkers are not favored in reservoirs characterized with moderate to low temperatures (<80 °C) because of the long gelation time they have at these conditions.10 On the other hand, inorganic crosslinkers are best suited for such reservoirs where the temperature allows for a controllable gelation time.7,18 Inorganic crosslinkers form a colloidal dispersion in the polymeric solution; they are described by having a multivalent metallic base (Mn+) associated with the ligand where the crosslinking is achieved by the initiation of coordination bonds between the metal and the functional groups on the base polymer.19
The use of inorganic crosslinkers has been investigated broadly, nominating chromium acetate to be the best in terms of controllability.19−21 The presence of acetate provides a better gelation control since it has a structure similar to the carboxylate groups on PAM, making the crosslinking reaction slow.7 One of the main drawbacks of using chromium as a crosslinker is that the higher oxidation state (Cr6+) is very toxic. Although the used Cr3+ has an acceptable toxicity limit, it has a tendency under some conditions to oxidize, as well as Cr3+ itself poses a destructive risk on the marine life when disposed even at low concentrations.10 Therefore, aluminum, the third most abundant element in the earth’s crust,22 can be of less environmental impact, which motivated some researchers for proposing it as an alternative to chromium.23
Gelation parameters, gel strength and gelation time, are highly affected by the surrounding conditions. Although some researchers reported the ability of PAM/Cr(III) system to produce a gel at high pH values of up to 12.5,24 other researchers noted that the stability of chromium colloids decreases in aqueous solutions when the pH exceeds 5.5, compromising the feasibility of the operation.25 Additionally, te Nijenhuis et al. (2003) reported that the PAM/Cr(III) system can only crosslink at pH conditions between 5 and 9,26 while Karimi et al. suggested that the pH range between 5.5 and 7.5 produces the most stable gel.27 Hence, the behavior of inorganic complexes in aqueous solutions is not yet well understood. Another factor that affects the stability of colloidal crosslinkers is the type of additives in the system where recent studies showed that silica nanoparticles can enhance both the thermal as well as physical stabilities of micro-sized particles in the polymeric solutions.28,29 Apart from the stability of particles, crosslinking of the inorganic crosslinkers was found to be pH-triggered, having the gelation time decreased as the pH increases.30 The effect of pH is believed to result from the hydrolysis of PAM that is increased at high pH, leading to the conversion of more amide groups to carboxylate groups resulting in more crosslinkable sites.31,32 Moreover, the crosslinking reaction follows Arrhenius’s equation where the increase in the temperature increases the crosslinking rate.33,34 While pH and temperature have the most significant effect on the gelation time, other factors influence the equilibrium gel strength such as the salt type and content and the polymer to crosslinker ratio.27,33,35
Although research on aluminum as a crosslinking agent for in situ water shut-off treatments is limited, it has been extensively investigated in colloidal dispersion gel (CDG) systems. CDGs are systems with low polymers and crosslinker concentration, used for in-depth treatments and enhanced oil recovery, that produce a viscous flowing gel after crosslinking.36 Aluminum citrate is the most common crosslinker in CDGs where its ability in penetrating porous media and reducing permeability has been proven.37−39 PAM/Al-citrate CDG system has also been successfully applied in more than 30 cases in Asia, North America, and Latin America.40 Nevertheless, aluminum has rarely been used to produce a rigid gel.
Based on the physical stability of the system as well as the rheological performance, different types of aluminum-based crosslinkers are screened and investigated in this study. While Cai and Huang (2001) claimed that the PAM/Al-citrate system is only stable at low pH and low temperature conditions,41 this paper presents an investigation on PAM/aluminum systems with different associated ligands, namely, acetate, amino-acetate, nitrate, and lactate, to examine their tolerance toward the change in the surrounding environment. Previous studies have focused on evaluating the gelation performance of such systems. Given that inorganic crosslinkers are poorly soluble in aqueous solutions, physical stability is an essential parameter that defines the completion of the gelation reaction. The objectives of this study are to (i) investigate the ability of the different screened aluminum crosslinkers to produce a strong gel, (ii) evaluate the physical and thermal stabilities of the polymer/inorganic colloidal systems, and (iii) asses the rheological and viscoelastic behaviors of the mature gels.
2. Materials and Methods
2.1. Materials
PAM samples were supplied by SNF Floerger, France, having a molecular weight of 700,000 Da. Nanosilica (NS) was purchased from NYACOL Inc., USA, as a liquid dispersion with an average particle size of 50 nm. Sodium chloride and ammonium chloride salts are from Research Lab, India, with purities of >99%. The aluminum complexes used are summarized in Table 1 below.
Table 1. Types of Aluminum-Based Crosslinkers Used in the Study.
2.2. Experimental Procedures
The gelling solutions were prepared at room temperature. Deionized water was used in the preparation of all solutions. The prepared solutions were stirred for 10 min followed by sonication in a water bath for another 10 min. PAM concentration was fixed at 9% in all experiments, which has been proven to provide the optimum gel strength with poylethylenimine (PEI).42 Highly concentrated solutions of HCl (39 M) and KOH (20 wt %) were used to adjust the pH when needed. The solutions were then poured in GL18 Duran test tubes and placed in an oil bath with a predetermined temperature for 24 h. Some separation was observed is some samples leaving a part of the sample to be poorly crosslinked; therefore, the percentage of the separated phase is reported to reflect the stability of the system after being aged for 24 h at 75 °C (Figure S1).
2.3. Rheological Testing
Viscoelastic behavior is a major part that defines the successfulness of the gel treatments. The most important parameter is the storage modulus (G′), which reflects the solidlike behavior and the strength of the mature gel. After the curing is complete in the oil bath, a gel sample is then retrieved from the gel phase to be tested in the rheometer. An Anton Paar MCR 302 rheometer was used to conduct all experiments. Plate–plate geometry with 25 mm diameter and 2 mm gap was used to test the mature gel samples. Frequency sweep tests were performed in the range between 1 and 100 Hz at a fixed strain of 10% (within the linear viscoelastic region). For dynamic tests, 10 Hz was obtained to be the optimum frequency where all tested samples are within the linear viscoelastic range in the chosen strain; therefore, storage modulus values at 10 Hz were used for comparison between the different samples.
2.4. Zeta Potential
Zeta potential reflects the electric double layer surrounding the particles in the suspended system, where the higher the magnitude, the more stable the colloidal system. To assess the physical stability of the colloidal crosslinkers in the aqueous solutions, the zeta potential of the inorganic particles was tested. Zeta potential tests were performed in a Malvern Nanosizer at room temperature. A solution of 3 mg/L particles in water was prepared, and then the pH of the system was adjusted from low to high pH values. Zeta potential experiments were conducted at ambient conditions.
2.5. Thermogravimetric Analysis
The thermal stability of crosslinkers is measured through the thermogravimetric analysis (TGA) technique using a PerkinElmer Pyris 1 TGA equipment. Nitrogen gas was used as the purging gas at a rate of 35 mL/min. The samples were heated from 25 to 105 °C at a rate of 10 °C/min and then kept at 105 °C for 1 more hour to ensure the evaporation of all volatile compounds.
2.6. Scanning Electron Microscopy
Scanning electron microscopy (SEM) has been proven to be an effective imaging technique used to examine the microstructure of the hydrogels. As the conventional SEM does not accept a humid sample, the tested hydrogel was dried in the following procedure: (1) The sample was dried in a regular freezer at a temperature of −20 °C for 48 h. (2) The sample was then frozen at −40 °C under vacuum conditions using a VirTis freeze drying equipment. The SEM tests were then conducted using a Nova Nano SEM 450 microscope from FEI.
2.7. Sealability Test
To test the plugging efficiency of the aluminum-based polymeric gel, an API certified high pressure/high temperature permeability plugging tester (PPT) was used. The sealing tests were conducted using the PPT equipped with an aluminum slotted disc with artificially made microfracture of 1 in. length and 1000 μm width. Two tests were conducted at two different temperatures (50 and 130 °C), and these temperatures were selected as an example of low and elevated reservoir temperatures. The cumulative filtrate volume and sealing pressure are used as performance indicators.
3. Results and Discussion
3.1. Effect of Crosslinker Concentration
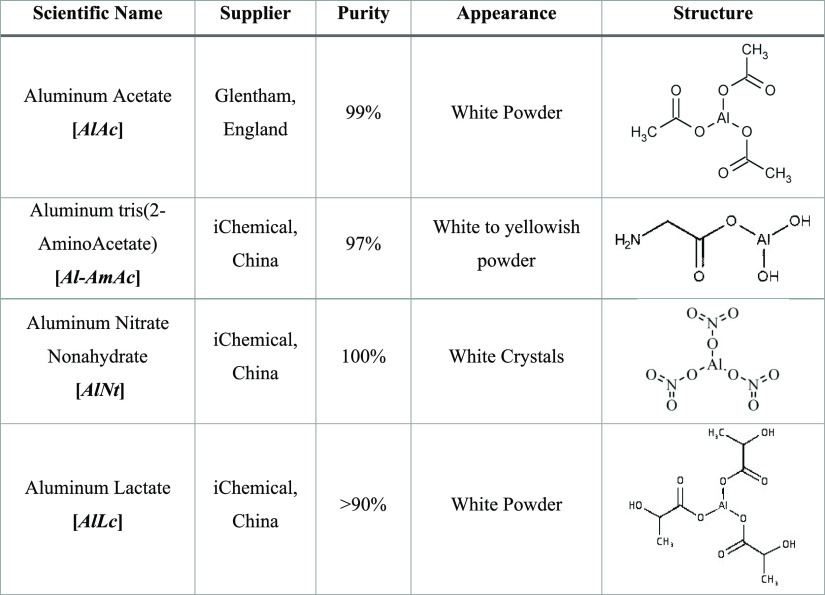
Screening process of the chosen aluminum-based crosslinkers starts by varying the polymer to crosslinker ratio. In the current study, PAM’s concentration is fixed at 9% to benchmark with previous studies where PAM was crosslinked with the organic crosslinker PEI and the inorganic crosslinker chromium acetate using the same polymer concentration.13,43Figure 1 illustrates the final gel strength of the produced gels with the different crosslinkers as a function of concentration at a fixed temperature of 75 °C without pH modifications. Aluminum lactate was screened out as it failed to crosslink with PAM where no gel is produced when a concentration of up to 5% was used at varying acidic conditions between 2.1 and 11.3 and temperatures between 25 and 100 °C. Moreover, mixing PAM with AlNt produced a rapid strong gel, which made it difficult to be transferred to the test tube. Therefore, 1 wt % ammonium chloride has been used with all AlNt experiments throughout the study to slow down the reaction as it has been previously reported to be an effective retarder.42 The change in the storage modulus (∼300 Pa), for both AlAc and AlNt, was insignificant after 3 wt %. Hence, this concentration has been used as the optimum concentration in this investigation. Yet, Al-AmAc failed to produce a gel at concentrations of less than 3 wt %; the storage modulus kept increasing in the range between 3 and 5 wt %, reaching a maximum value of 125 Pa, where the concentration of 5 wt % was considered as the optimum.
Figure 1.
Storage modulus of the mature gel as a function of the crosslinker concentration.
3.2. Effect of pH
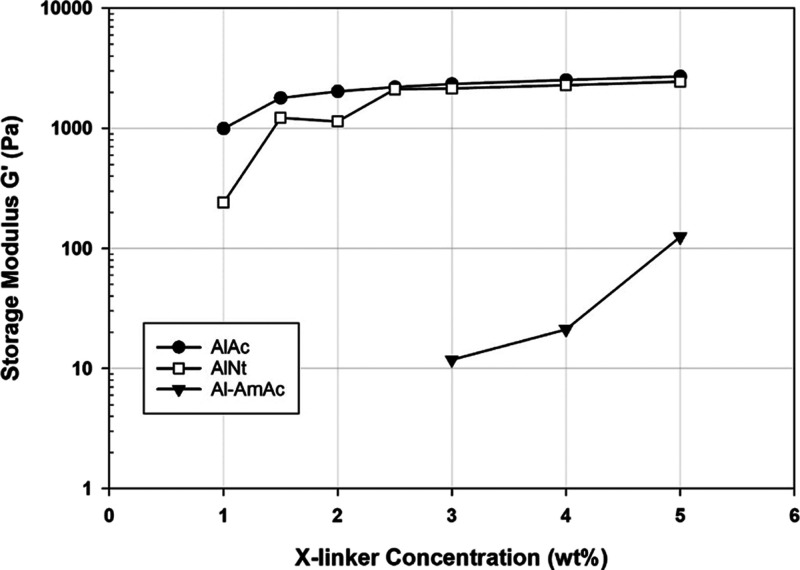
Acidity of the system is a key parameter that controls the gelation process in crosslinkable polymeric systems. The gelation can be very fast at some conditions to produce a rapid gel, while it fails to produce a gel at some other conditions. Among the three screened crosslinkers, two of them exhibited colloidal behavior: AlAc and Al-AmAc, where the physical stability and the suspension of the system affect the degree of success. On the other side, AlNt is soluble in aqueous media, within the studied range of parameters, where the degree of suspension is not an issue. The stability of both colloidal crosslinkers in aqueous solutions was studied through zeta potential (Figure 2). For AlAc, it is clear that the stability decreases at higher pH conditions. Al-AmAc, however, showed the least stability in the pH range between 8 and 10. The internal mechanism of the dissociation of aluminum salts given by eqs 1 and 2 can justify this behavior; the intensity of the positively charged surfaces increases at low pH conditions, while it decreases at high pH environments.
| 1 |
| 2 |
Figure 2.
Zeta Potential of the colloidal crosslinkers in aqueous solutions.
The settling behavior of the gelants crosslinked with colloidal crosslinkers (AlAc and Al-AmAc) was in agreement with the zeta potential results as more separation was observed at higher pH conditions. Moreover, both the gelants reach a pH value where no more gel is produced because of the high settling rate, reducing the contact time between the polymer and the crosslinker. The sole system in both AlAc and Al-AmAc failed to produce a gel at pH conditions of more than 6.2 and 8.7, respectively. This behavior can be attributed to be resulting from two combined effects; the first is that the crosslinking reaction rate decreases with the increase in pH, unlike the behavior of chromium acetate which exhibited a higher crosslinking rate in the high pH range.31 The other effect is because the stability of such particles decreases in the alkaline conditions where the settling rate becomes faster, allowing less contact time between the polymer and the crosslinker. To test this theory, NS was added to the system in an attempt to increase the stability of the colloidal system and decrease the settling rate since NS was successful in providing stability to another colloidal crosslinker in a previous study.28 As presented in Table 2, NS succeeded in physically stabilizing these systems where a gel was produced at conditions where the gel was not producible in the absence of NS. In addition, it has successfully decreased the separation phase at lower pH conditions. Hence, the results provide evidence that the destabilization of the system is the main factor that leads to the failure of inorganic colloidal particles to crosslink with PAM.
Table 2. Degree of Suspension and Physical Stability of Colloidal Crosslinkers at Different pH Conditions.
| system | pH | separation phase % (sole system) | separation phase % (with NS) | notes |
|---|---|---|---|---|
| PAM 9 wt % + AlAc 3 wt % | 2.0 ± 0.5 | 0 | 0 | rapid gel |
| 4.0 ± 0.5 | 18.4 | 12.17 | more stability with NS | |
| 6.0 ± 0.5 | 20.8 | 15.7 | more stability with NS | |
| 8.0 ± 0.5 | 100 | 30.8 | no gel without NS | |
| 10.0 ± 0.5 | 100 | 100 | no gel | |
| PAM 9 wt % + Al-AmAc 5 wt % | 2.0 ± 0.5 | 0 | 0 | rapid gel |
| 4.0 ± 0.5 | 0 | 0 | rapid gel | |
| 6.0 ± 0.5 | 0 | 0 | stable with and without NS | |
| 8.0 ± 0.5 | 23 | 0 | more stability with NS | |
| 10.0 ± 0.5 | 100 | 7.2 | no gel without NS |
Figure 3 shows the viscoelastic behavior for the systems crosslinked with AlAc and Al-AmAc, where the sole systems are plotted with triangles and the systems with NS are plotted with circular points. Both systems behaved in a similar manner where the high decrease in acidity produces a rapid gel and no gel is produced at high pH conditions. Moreover, the separation phase was observed to increase as the pH increases. Therefore, a conclusion can be drawn that the gelation rate decreases with the decrease of pH for these two colloidal crosslinkers. The strengthening effect of NS can also be observed in PAM/Al-AmAc as higher strength was attained at similar pH conditions.
Figure 3.
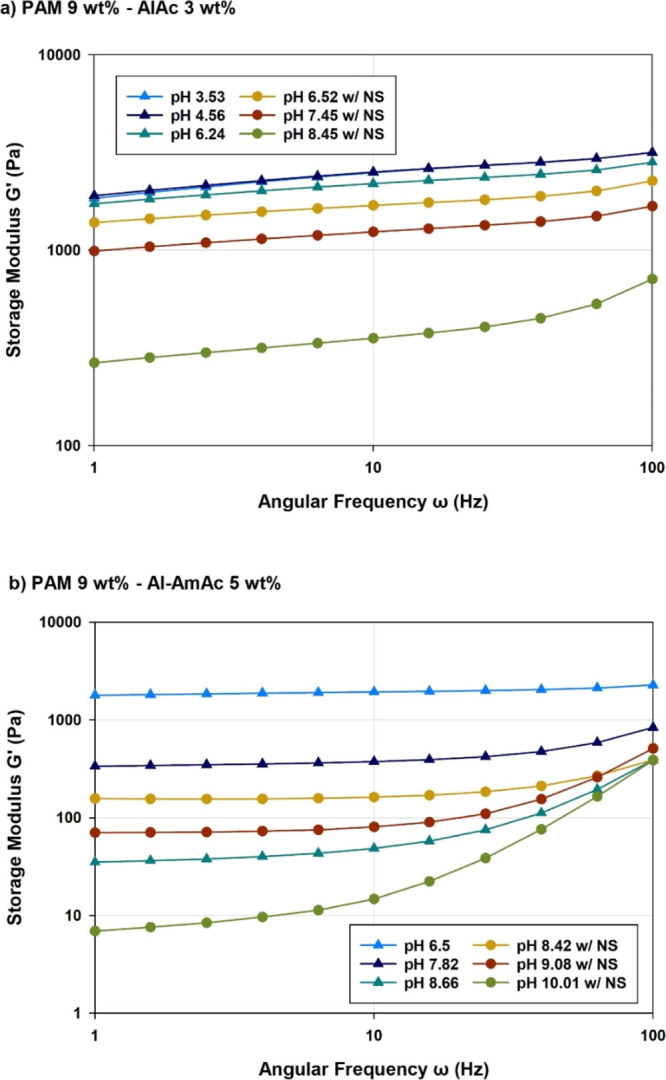
Frequency sweep of PAM crosslinked with (a) AlAc and (b) Al-AmAc at varying pH conditions.
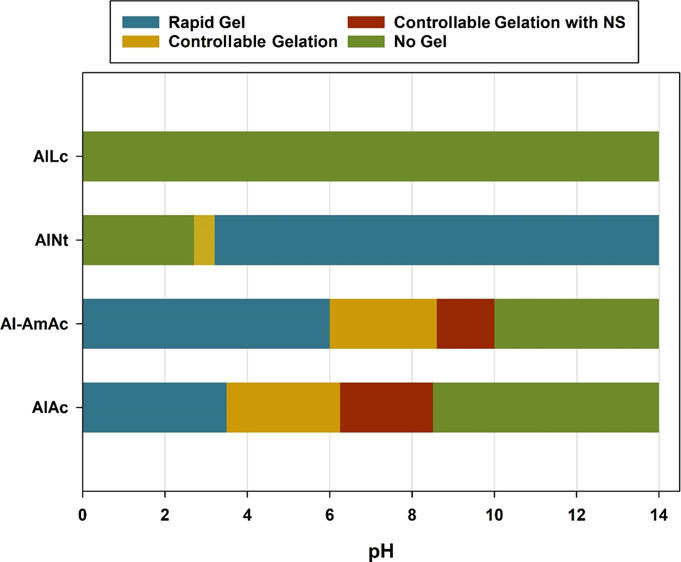
Even with the addition of ammonium chloride as a retarder, AlNt had a very narrow window of controllable gelation. The solution remained viscous at the region between 2.8 and 3.2, where increasing the pH more than that produces a rapid gel, while the system does not produce a gel, even at elevated temperatures, when the pH is decreased below that range. Figure 4 summarizes the behavior of all examined gelant systems in the whole pH range. Contrary to the behavior of chromium acetate, AlAc and Al-AmAc showed an increase in the gelation time with the increase in pH, where the system forms a rapid gel at low pH conditions. Consequently, aluminum-based crosslinkers can be superior in the presence of acid gases (such as H2S and CO2), which were proven to limit the application of chromium acetate and weaken the gel produced.15
Figure 4.
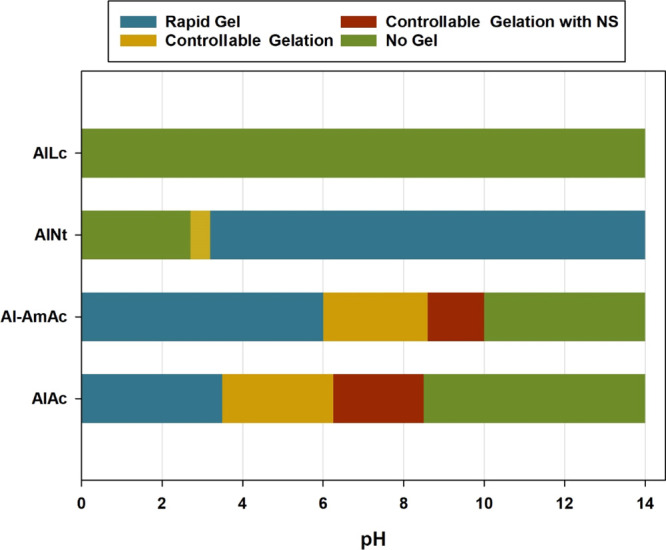
Gelation behavior of the different aluminum-based crosslinked PAM at the whole pH range.
3.3. Effect of Aging Temperature and Thermal Stability
Temperature plays an important role in the gelation process. The effect of temperature on the equilibrium gel strength is described in Figure 5 in the range between 25 and 100 °C representing most of the oil reservoirs. The effect of temperature on the gelation system is complex as it can be an interaction of several consequences. On the one hand, the increase in the temperature is known to increase the crosslinking rate,26 which will hinder the particles from further settling, leading to more crosslinking intensity. On the other hand, PAM exhibits thermal-thinning behavior,44 suggesting that the settling velocity of the colloids will increase with temperature. Furthermore, the degree of hydrolysis in PAM was found to increase at elevated temperatures, causing more amide groups to turn into carboxylates.45 While some researchers described the carboxylate groups as the crosslinking sites,31 the increase in temperature can increase the crosslinking density in the bulk of the system. Moreover, thermal stresses in general are known to weaken the strength of materials. Therefore, the combined effect of all of the above led to the behavior shown in Figure 5. The strength of the AlAc system had a directly proportional relation with the temperature within the studied scale, while both AlNt and Al-AmAc had polynomial behavior, where a peak was reached followed by a decrease in the strength. Similar polynomial like behavior was reported for the PAM/chromium-acetate gelant in the literature.26
Figure 5.
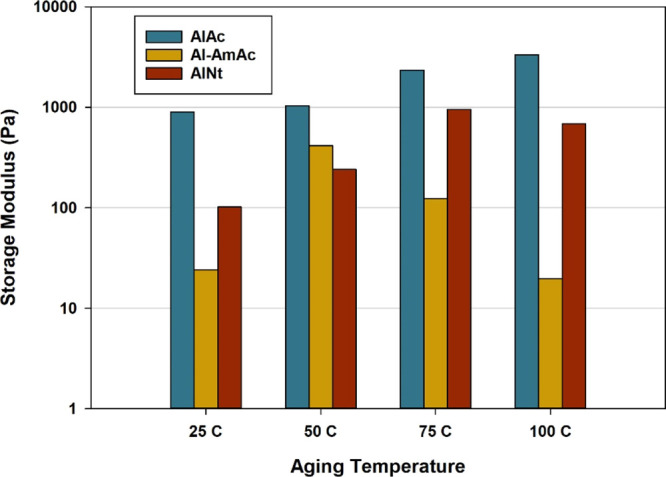
Effect of aging temperature on the final gel strength of the different gelling systems.
To better understand the thermal stability of the systems, TGA was carried out for the screened crosslinkers (Figure 6). AlAc and Al-AmAc revealed a good thermal stability to a temperature of up to 105 °C. Weight loss of less than 10% was observed in both systems, which can mostly be attributed to the loss of moisture content. On the contrary, AlNt showed poor behavior, where around 67% of the net weight was lost by exposing it to a temperature of 105 °C. AlNt holds nine water molecules per each molecule of aluminum nitrate (Al(NO3)3·9H2O) naturally. However, calculating the water content reveals that it only accounts for around 43% of the net mass in the system. The remaining lost 24% can be explained by the chemical properties of the compound. AlNt has a melting point and a boiling point of 72.8 and 135 °C, respectively. Thus, at a temperature of 105 °C, AlNt has some vapor pressure that is swept away by the purging nitrogen gas, leading to a continuous decrease in the net weight. As a result of this behavior, although the gel samples that crosslinked with AlNt produced a good strength, it was observed that some syneresis started to develop after only 24 h of aging. Therefore, AlNt can only produce a stable gel in low-temperature reservoirs.
Figure 6.
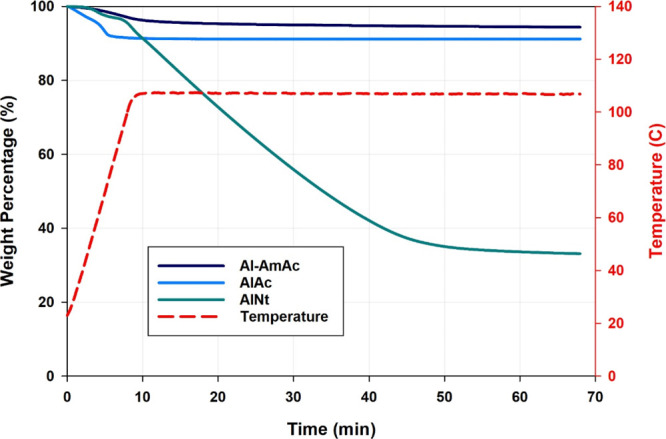
TGA for the screened crosslinkers.
3.4. Effect of Salinity
In some oil reservoirs, especially in the offshore, access to fresh water may be limited. Therefore, it is essential to examine the compatibility of these systems with the salinity content. While the salt content had a positive impact on stabilizing the colloidal system by decreasing the separated phase, generally, it impacted negatively the equilibrium strength of the gel phase (in the salinity range from 0 to 50,000 ppm) (Figure 7). The main effect in the saline conditions is believed to be caused by the main polymer chain, not the colloidal crosslinkers. Salts have been reported to have a shielding effect on acrylamide chains where the cations adsorb the negatively charged carboxylic group, leading to a decrease in the available crosslinking sites.46 Moreover, salts cause the chains of PAM to shrink and encounter compaction more entanglements;47 hence, the accessibility of the crosslinkers to the crosslinking sites becomes limited, and the probability of reaction decreases according to the collision theory. Moreover, it has been reported that the presence of salts reverses the flocculation process using PAM in clay suspensions, suggesting that salts give a better stability in the system similar to the behavior of aluminum as shown in Figure 7.48 Data from Figure 7 fits the Arrhenius relation (G′ = 386.9·e14152/C), where G′ is the storage modulus in Pa and C is the concentration of NaCl in ppm with R2 = 0.9035.
Figure 7.
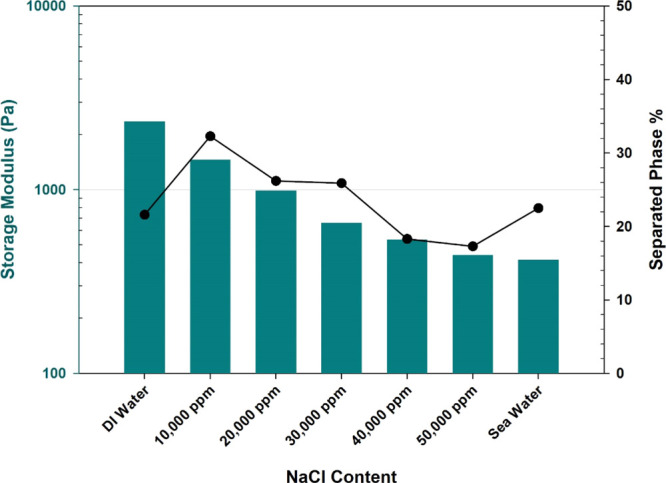
Salinity effect on the storage modulus of the gel and physical stability of the PAM/AlAc gelant.
3.5. Gelation Time
The course of gelation is shown in Figure 8 for the PAM/AlAc system. The experiment was designed to mimic the injection process where the temperature was increased at a rate of 1 °C/min and the pressure was kept at 500 psi.49 The gelation time is described as the time where the storage and loss moduli become equal where the solidlike behavior dominates beyond that point. The gelation time for PAM/AlAc without pH modifications (4.1 pH) is around 50 min making it suitable for near-wellbore treatments. After 5 h of exposing the sample to a temperature of 75 °C, the storage modulus was still increasing at a very low rate. The bottle test for the same sample reveals the equilibrium storage modulus after 24 h of aging as around 2340 Pa compared to 1025 Pa after 5 h, as presented in Figure 8. This can be attributed to the slow rate of conversion as the system approaches the equilibrium where the crosslinking is not 100% complete after 5 h. Moreover, the samples from the test tubes were measured at ambient conditions where the thermal stresses were released, which may have resulted in a higher strength. Figure 8 also provides evidence of the reversed pH triggering where the slight increase of pH from 4.1 to 4.6 delayed the gelation from 50 to 80 min.
Figure 8.
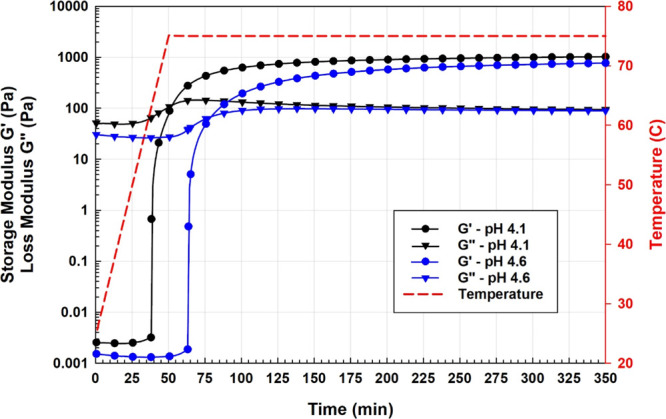
Gelation profile of PAM/AlAc system at pH of 4.1 (black) and 4.6 (blue).
3.6. Sealing-Ability Testing
The permeability plugging test with the slotted aluminum disc is used to simulate the microfractures that usually exist in the formation. The result of PPT experiments showed that the PAM/AlAc gel of 9 wt.% PAM, 1% NS, and 3% Al/Ac have completely sealed the microfracture (as shown in Figure 9). The polymeric gel held a sealing pressure up to 700 psi and more than 2000 psi with no flow when experiments were conducted at 50 and 130 °C, respectively. This test provides a preliminary proof that such a formulation can be suitable for large-scale conformance control application in low- as well as high-temperature reservoirs.
Figure 9.

Aluminum fractured disc (1″ × 1000 μm) completely sealed with PAM/AlAc (9:3).
3.7. Crosslinking Mechanism
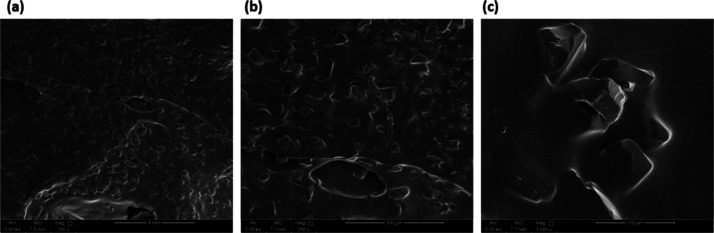
SEM imaging was used to understand the nature of the colloid–polymer interaction on the microlevel (Figure 10). The images show the uniform distribution of the particles throughout the sample revealing the importance of physical stability for such systems. The 3D structure shows the behavior of polymer chains where they took the shape of sheets wrapping around the colloidal particles that provide the crosslinking.
Figure 10.
SEM images of PAM/AlAc colloidal gel at a scale of (a) 1 mm, (b) 500, and (c) 100 μm.
Similar to the other inorganic crosslinkers, it is believed that the crosslinking between aluminum colloidal crosslinkers and PAM is through coordination bonds. Moreover, the outcome of screening the different aluminum-based crosslinkers implies the high dependence of the associated ligands on the gelation behavior. The presence of the acetate functional group in both AlAc and Al-AmAc slowed down the crosslinking as the similarity between acetate and the carboxylic group on PAM shifted the equilibrium and had a retardation effect.
3.8. Aluminum-Based Crosslinkers Versus Other Crosslinkers
Evaluating the new proposed crosslinkers would not be complete without benchmarking with the currently available commercial crosslinkers. The screening process in this study nominates AlAc as the best aluminum-based crosslinker as it provided the strongest gel, has a good thermal and physical stability, and has a wide range of applicability. Recent studies by our group have examined the rheological behavior of PEI and chromium acetate as the most commonly used crosslinkers, which will be used to benchmark.13,43 PEI has been successfully applied in many high-temperature reservoirs, proving to be able to reduce the water-cut in the producers.16 Compared to PEI, AlAc prospered in terms of gel strength where a storage modulus of up to 2340 Pa was achieved in the unmodified system compared to 1670 Pa achieved by PEI using the same polymer concentration. AlAc also provided a better strength compared to PAtBA/chromium acetate system that had a gel strength of 1283 Pa. Thus, aluminum crosslinkers can be an environment-friendly alternative to replace chromium acetate for water shut-off applications.
4. Conclusions
In conclusion, this work examines the ability of different aluminum-based crosslinkers to provide a strong gel to be used for water shut-off applications. Four different ligands associated with aluminum were investigated, namely, acetate, aminoacetate, nitrate, and lactate. The findings of this study can be summarized as follows:
All examined crosslinkers were able to crosslink PAM to produce an inflowing gel, except aluminum lactate.
AlNt has a very narrow pH window of applicability between 2.8 and 3.2 where increasing the pH more produces a rapid gel, while the system fails to produce a gel when the pH is reduced to below that range. On the contrary, AlAc and Al-AmAc respond differently to the change in pH compared to the conventional crosslinkers where the gelation rate decreases with the increase in pH.
AlAc has the widest pH window of applicability where it can crosslink PAM in the range between 3.5 and 8.5 with the help of NS to provide better stability for the colloids in the aqueous solution.
AlAc, which was nominated as the best crosslinker among the screened ones, showed some compatibility with the saline conditions as the gel was produced even at very high salinities. Nevertheless, the salinity compromises the strength of the gel as it was reduced by 82% from freshwater to seawater.
The system of PAM/AlAc has a gelation time of around 50 min at a temperature of 75 °C, making it suitable for near-wellbore plugging, while the gelation was delayed to 80 min when the pH was increased from 4.1 to 4.6.
The fracture sealing experiments showed perfect sealings with no flow after the gel is formed inside the fracture with 700 and 2000 psi sealing pressure at 50 and 130 °C, respectively.
PAM/AlAc system produced a comparable gel strength to the conventional gelants, nominating the aluminum-based crosslinkers as a greener replacement to chromium acetate.
Acknowledgments
The authors would like to acknowledge the support of Qatar National Research Fund (a member of Qatar Foundation) through grant # NPRP10-0125-170240. The findings achieved herein are solely the responsibility of the authors. The authors would also like to thank with gratitude SNF Floerger Company for supplying the PAM samples. The acknowledgment is also extended to the Central Laboratories Unit in Qatar University for carrying out the SEM tests and to the University of Oklahoma for supporting this research.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02967.
Illustration on the separation that takes place in the colloidal-gel systems where two distinguished regions exist (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Halliburton . Oilfield Water Management. https://www.halliburton.com/en-US/ps/solutions/clean-energy/oilfield-water-management/default.html (accessed Feb 15, 2020).
- Fayzullin M.; Tippel P.; Gonzalez J.; Egger S.. Understanding Excessive Water Production in Highly Faulted Mature Gas Condensate Field: From Well Operations to Revival of Integrated History Matching. IADC/SPE Asia Pacific Drilling Technology Conference; Society of Petroleum Engineers, 2014.
- Bailey B.; Crabtree M.; Tyrie J.; Elphick J.; Kuchuk F.; Romano C.; Roodhart L. Water Control. Oilfield Rev. 2000, 12, 30–51. [Google Scholar]
- Kamal M. S.; Hussein I.; Mahmoud M.; Sultan A. S.; Saad M. A. S. Oilfield Scale Formation and Chemical Removal: A Review. J. Pet. Sci. Eng. 2018, 171, 127–139. 10.1016/j.petrol.2018.07.037. [DOI] [Google Scholar]
- Machuca L. L.; Lepkova K.; Petroski A. Corrosion of Carbon Steel in the Presence of Oilfield Deposit and Thiosulphate-Reducing Bacteria in CO2 Environment. Corros. Sci. 2017, 129, 16–25. 10.1016/j.corsci.2017.09.011. [DOI] [Google Scholar]
- Zhu D.; Bai B.; Hou J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. 10.1021/acs.energyfuels.7b02897. [DOI] [Google Scholar]
- Borling D.; Chan K.; Hughes T.; Sydansk R. Pushing out the Oil with Conformance Control. Oilfield Rev. 1994, 6, 44–58. [Google Scholar]
- Hernandez J.; Lai B. H.; Bahabanian O.; Goyallon D.; Nordin A. A. A.; Nwafor C. E.; Taleballah E. Y.; Semin L.. Openhole Mechanical Packer with Eccentric Shunt-Tube Gravel Pack Assembly Reduces Capex and Accelerates Production in Kaombo Deepwater Development, Angola. SPE International Conference and Exhibition on Formation Damage Control; Society of Petroleum Engineers, 2018.
- Andrade A.; Chango M.; Atahualpa G.; Correa R.; Corona G.; Calvopina B.; Pico J.. Production Performance of Multiple Completion Designs: Openhole, Slotted Liner, ICD, and AICD: A Case Study for Water Control in Villano Field, Ecuador. SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers, 2018.
- Hamza A.; Shamlooh M.; Hussein I. A.; Nasser M.; Salehi S. Polymeric Formulations Used for Loss Circulation Materials and Wellbore Strengthening Applications in Oil and Gas Wells: A Review. J. Pet. Sci. Eng. 2019, 180, 197–214. 10.1016/j.petrol.2019.05.022. [DOI] [Google Scholar]
- El-karsani K. S. M.; Al-Muntasheri G. A.; Hussein I. A. Polymer Systems for Water Shutoff and Profile Modification: A Review Over the Last Decade. SPE J. 2014, 19, 135–149. 10.2118/163100-pa. [DOI] [Google Scholar]
- Hajilary N.; Vafaie Sefti M.; Dadvand Koohi A. Experimental Study of Water Shutoff Gel System Field Parameters in Multi-Zone Unfractured Gas-Condensate Reservoirs. J. Nat. Gas Sci. Eng. 2015, 27, 926–933. 10.1016/j.jngse.2015.09.038. [DOI] [Google Scholar]
- Shamlooh M.; Hamza A.; Hussein I. A.; Nasser M. S.; Magzoub M.; Salehi S. Investigation of the Rheological Properties of Nanosilica-Reinforced Polyacrylamide/Polyethyleneimine Gels for Wellbore Strengthening at High Reservoir Temperatures. Energy Fuels 2019, 33, 6829–6836. 10.1021/acs.energyfuels.9b00974. [DOI] [Google Scholar]
- Magzoub M. I.; Salehi S.; Hussein I. A.; Nasser M. S. Loss Circulation in Drilling and Well Construction: The Significance of Applications of Crosslinked Polymers in Wellbore Strengthening: A Review. J. Pet. Sci. Eng. 2020, 185, 106653. 10.1016/j.petrol.2019.106653. [DOI] [Google Scholar]
- Bai B.; Zhou J.; Yin M. A Comprehensive Review of Polyacrylamide Polymer Gels for Conformance Control. Pet. Explor. Dev. 2015, 42, 525–532. 10.1016/s1876-3804(15)30045-8. [DOI] [Google Scholar]
- Al-Muntasheri G. A.; Sierra L.; Garzon F. O.; Lynn J. D.; Izquierdo G. A.. Water Shut-off with Polymer Gels in A High Temperature Horizontal Gas Well: A Success Story. SPE Improved Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, Oklahoma, USA, 2010; p 24.
- Liu Y.; Dai C.; Wang K.; Zhao M.; Zhao G.; Yang S.; Yan Z.; You Q. New Insights into the Hydroquinone (HQ)–Hexamethylenetetramine (HMTA) Gel System for Water Shut-off Treatment in High Temperature Reservoirs. J. Ind. Eng. Chem. 2016, 35, 20–28. 10.1016/j.jiec.2015.09.032. [DOI] [Google Scholar]
- Sun F.; Lin M.; Dong Z.; Zhang G. Delayed-Crosslink Hydrogel for Improving Oil Recovery in Differential Heterogeneous Reservoirs. ACS Omega 2020, 5, 228–235. 10.1021/acsomega.9b02545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. R.; Eoff L.; Dalrymple E. D.; Black K.; Brown D.; Rietjens M. A Natural Polymer-Based Cross-Linker System for Conformance Gel Systems. SPE J. 2003, 8, 99–106. 10.2118/84937-pa. [DOI] [Google Scholar]
- Vargas-Vasquez S. M.; Romero-Zerón L. B. A Review of the Partly Hydrolyzed Polyacrylamide Cr(III) Acetate Polymer Gels. Pet. Sci. Technol. 2008, 26, 481–498. 10.1080/10916460701204594. [DOI] [Google Scholar]
- Karimi S.; Esmaeilzadeh F.; Mowla D. Identification and Selection of a Stable Gel Polymer to Control or Reduce Water Production in Gas Condensate Fields. J. Nat. Gas Sci. Eng. 2014, 21, 940–950. 10.1016/j.jngse.2014.10.026. [DOI] [Google Scholar]
- Yaroshevsky A. A. Abundances of Chemical Elements in the Earth’s Crust. Geochem. Int. 2006, 44, 48–55. 10.1134/s001670290601006x. [DOI] [Google Scholar]
- Stavland A.; Jonsbraten H. C.. New Insight into Aluminium Citrate/Polyacrylamide Gels for Fluid Control. SPE/DOE Improved Oil Recovery Symposium; Society of Petroleum Engineers, 1996.
- Sydansk R. D. Newly Developed Chromium(III) Gel Technology. SPE Reservoir Eng. 1990, 5, 346–352. 10.2118/19308-pa. [DOI] [Google Scholar]
- Jain R.; McCool C. S.; Green D. W.; Willhite G. P.; Michnick M. J. Reaction Kinetics of the Uptake of Chrromium(III) Acetate by Polyacrylamide. SPE J. 2005, 10, 247–255. 10.2118/89399-pa. [DOI] [Google Scholar]
- te Nijenhuis K.; Mensert A.; Zitha P. L. J. Viscoelastic Behaviour of Partly Hydrolysed Polyacrylamide/Chromium(III) Gels. Rheol. Acta 2003, 42, 132–141. 10.1007/s00397-002-0264-9. [DOI] [Google Scholar]
- Karimi S.; Kazemi S.; Kazemi N. Syneresis Measurement of the HPAM-Cr (III) Gel Polymer at Different Conditions: An Experimental Investigation. J. Nat. Gas Sci. Eng. 2016, 34, 1027–1033. 10.1016/j.jngse.2016.08.011. [DOI] [Google Scholar]
- Hamza A.; Shamlooh M.; Hussein I. A.; Nasser M. S.; Salehi S. Rheology of Triamine Functionalized Silica Reinforced Polymeric Gels Developed for Conformance Control Applications. Energy Fuels 2020, 34, 1093. 10.1021/acs.energyfuels.9b03625. [DOI] [Google Scholar]
- Maurya N. K.; Mandal A. Studies on Behavior of Suspension of Silica Nanoparticle in Aqueous Polyacrylamide Solution for Application in Enhanced Oil Recovery. Pet. Sci. Technol. 2016, 34, 429–436. 10.1080/10916466.2016.1145693. [DOI] [Google Scholar]
- Koohi A. D.; Moghaddam A. Z.; Sefti M. V.; Moghadam A. M. Swelling and Gelation Time Behavior of Sulfonated Polyacrylamide/Chromium Triacetate Hydrogels. J. Macromol. Sci., Part B: Phys. 2011, 50, 1905–1920. 10.1080/00222348.2010.549419. [DOI] [Google Scholar]
- Sun F.; Lin M.; Dong Z.; Zhu D.; Wang S. L.; Yang J. Effect of Composition of HPAM/Chromium(III) Acetate Gels on Delayed Gelation Time. J. Dispersion Sci. Technol. 2016, 37, 753–759. 10.1080/01932691.2015.1041034. [DOI] [Google Scholar]
- El-Karsani K. S. M.; Al-Muntasheri G. A.; Sultan A. S.; Hussein I. A. Gelation of a Water-Shutoff Gel at High Pressure and High Temperature: Rheological Investigation. SPE J. 2015, 20, 1103–1112. 10.2118/173185-pa. [DOI] [Google Scholar]
- Broseta D.; Marquer O.; Blin N.; Zaitoun A.. Rheological Screening of Low-Molecular-Weight Polyacrylamide/Chromium(III) Acetate Water Shutoff Gels. SPE/DOE Improved Oil Recovery Symposium; Society of Petroleum Engineers, 2000.
- He H.; Wang Y.; Qi Z.; Sun X. Gelation Performance and Feasibility Study of an Environmental Friendly Improved Inorganic Aluminum Gel for Conformance Control Under Harsh Reservoir Conditions. J. Energy Resour. Technol. 2017, 139, 012911. 10.1115/1.4035512. [DOI] [Google Scholar]
- Zhang L.; Khan N.; Pu C. Influence of Salinity on the Properties of the Different HPAM/Al 3+ Systems. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 2019, 74, 37. 10.2516/ogst/2019011. [DOI] [Google Scholar]
- Bjørsvik M.; Høiland H.; Skauge A. Formation of Colloidal Dispersion Gels from Aqueous Polyacrylamide Solutions. Colloids Surf., A 2008, 317, 504–511. 10.1016/j.colsurfa.2007.11.025. [DOI] [Google Scholar]
- Al-Assi A. A.; Willhite G. P.; Green D. W.; McCool C. S. Formation and Propagation of Gel Aggregates Using Partially Hydrolyzed Polyacrylamide and Aluminum Citrate. SPE J. 2009, 14, 450–461. 10.2118/100049-pa. [DOI] [Google Scholar]
- Smith J. E.Performance of 18 Polymers in Aluminum Citrate Colloidal Dispersion Gels. SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers, 1995.
- Mack J. C.; Smith J. E.. In-Depth Colloidal Dispersion Gels Improve Oil Recovery Efficiency. SPE/DOE Improved Oil Recovery Symposium; Society of Petroleum Engineers, 1994.
- Manrique E.; Reyes S.; Romero J.; Aye N.; Kiani M.; North W.; Thomas C.; Kazempour M.; Izadi M.; Roostapour A.; Muniz G.; Cabrera F.; Lantz M.; Norman C.. Colloidal Dispersion Gels (CDG): Field Projects Review. SPE EOR Conference at Oil and Gas West Asia; Society of Petroleum Engineers, 2014.
- Cai W.; Huang R. Study on Gelation of Partially Hydrolyzed Polyacrylamide with Titanium(IV). Eur. Polym. J. 2001, 37, 1553–1559. 10.1016/s0014-3057(01)00041-6. [DOI] [Google Scholar]
- El Karsani K. S. M.; Al-Muntasheri G. A.; Sultan A. S.; Hussein I. A. Impact of Salts on Polyacrylamide Hydrolysis and Gelation: New Insights. J. Appl. Polym. Sci. 2014, 131, 41185. 10.1002/app.41185. [DOI] [Google Scholar]
- Shamlooh M.; Hamza A.; Hussein I. A.; Nasser M.; Salehi S.. Reinforcement of Polyacrylamide-Co-Tert-Butyl Acrylate Base Gel Using Nanosilica for Conformance Control at Low and High Reservoir Temperatures. SPE International Conference and Exhibition on Formation Damage Control; Society of Petroleum Engineers: Louisiana: USA, 2020.
- Magzoub M. I.; Salehi S.; Hussein I. A.; Nasser M. S.. A Comprehensive Rheological Study for a Flowing Polymer-Based Drilling Fluid Used for Wellbore Strengthening. SPE International Conference and Exhibition on Formation Damage Control; Society of Petroleum Engineers, 2020.
- Al-Muntasheri G. A.; Nasr-El-Din H. A.; Peters J.; Zitha P. L. J. Investigation of a High Temperature Organic Water Shutoff Gel: Reaction Mechanisms. SPE J. 2006, 11, 497–504. 10.2118/97530-pa. [DOI] [Google Scholar]
- Livney Y. D.; Portnaya I.; Faupin B.; Ramon O.; Cohen Y.; Cogan U.; Mizrahi S. Interactions between Inorganic Salts and Polyacrylamide in Aqueous Solutions and Gels. J. Polym. Sci., Part B: Polym. Phys. 2003, 41, 508–519. 10.1002/polb.10406. [DOI] [Google Scholar]
- Xiong B.; Loss R. D.; Shields D.; Pawlik T.; Hochreiter R.; Zydney A. L.; Kumar M. Polyacrylamide Degradation and Its Implications in Environmental Systems. npj Clean Water 2018, 1, 1. 10.1038/s41545-018-0016-8. [DOI] [Google Scholar]
- Shaikh S. M. R.; Nasser M. S.; Magzoub M.; Benamor A.; Hussein I. A.; El-Naas M. H.; Qiblawey H. Effect of Electrolytes on Electrokinetics and Flocculation Behavior of Bentonite-Polyacrylamide Dispersions. Appl. Clay Sci. 2018, 158, 46–54. 10.1016/j.clay.2018.03.017. [DOI] [Google Scholar]
- El-Karsani K. S. M.; Al-Muntasheri G. A.; Sultan A. S.; Hussein I. A. Gelation Kinetics of PAM/PEI System. J. Therm. Anal. Calorim. 2014, 116, 1409–1415. 10.1007/s10973-014-3754-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.