Abstract
Fabricating large, high-crystalline-quality single-crystal samples of hexagonal ferrite Ba(Fe1–xScx)12O19 is the first important step to elucidating its helimagnetic structure and developing it for further applications. In this study, single crystals of Ba(Fe1–xScx)12O19 of various Sc concentrations x were successfully grown by the spontaneous crystallization method using Na2O–Fe2O3 flux. We determined the optimal starting composition of reagents for Ba(Fe1–xScx)12O19 growth as a function of x. In situ monitoring of the crystal nucleus generation accelerated the success of crystal growth. The obtained crystals comprised black and lamellate structures with a size of 13 mm × 8 mm × 2 mm and a surface of {001} orientation. X-ray diffraction and elemental analysis revealed that the obtained crystals were composed of single-phase Ba(Fe1–xScx)12O19 of high crystalline quality. The lattice constants a and c increased linearly with increasing x, thereby following Vegard’s law. The temperature dependence of magnetization and the magnetization curves at 77 K of the x = 0.128 crystal exhibited behavior characteristics of helimagnetism. Neutron diffraction measurements of the x = 0.128 crystal exhibited magnetic satellite reflection peaks below 211 K, providing evidence that Ba(Fe1–xScx)12O19 behaves as a helimagnetic material.
1. Introduction
Hexagonal ferrites are one of the most important magnetic oxides owing to their practical applications. Several types of hexagonal ferrites are classified by their chemical composition, such as M-(MFe12O19), W-(MMe2Fe16O27), Y-(M2Me2Fe12O22), and Z-type (M3Me2Fe24O41) (where M: Ba, Sr, Pb, etc. and Me: Mg, Co, Ni, Zn, etc.).1 They are widely used in industrial fields, including electronic devices and communication and information technology, for example, as permanent magnetic components for DC motors and speakers, magnetic recording media for audio and magnetic cards, and so on. Since the crystallographic study of Braun2 and the magnetic study of Gorter,3 considerable efforts have been made in realizing their importance. Recently, hexagonal ferrites have attracted significant attention as electromagnetic wave-absorbing materials for 5G mobile communication systems. Another cutting-edge property of hexagonal ferrites is helimagnetism. In the hexagonal ferrite system, the helimagnetic components behave as multiferroic materials that exhibit ferroelectricity, while retaining ferrimagnetism in a weak magnetic field. Various hexagonal ferrites, including (Ba0.5Sr0.5)2Zn2Fe12O22, Ba2Mg2Fe12O22, and Ba0.52Sr2.48Co2Fe24O41, have been studied as a multiferroic system.4−15
It is known that helimagnetism also appears in the Ba(Fe1–xScx)12O19 system, where the nonmagnetic Sc3+ ion is added to the most popular M-type hexagonal ferrite BaFe12O19 (space group, P63/mmc (no. 194)), with x being the Sc concentration. The crystal structure of Ba(Fe1–xScx)12O19 is shown in Figure S1. O2– and Ba2+ of large ionic radii take a close-packed structure along the c-axis. Fe3+ and Sc3+ of small ionic radii occupy the interlayer tetrahedral, octahedral, and bipyramid sites.1,2 Perekalina et al. conducted torque and magnetization measurements on single Ba(Fe1–xScx)12O19 crystals and found anomalous magnetic behavior depending on the temperature and anomalous magnetic anisotropy in Sc-rich crystals.16 Aleshko-Ozhevskiĭ et al. demonstrated that Sc-rich Ba(Fe1–xScx)12O19 crystals exhibit helimagnetism by observing magnetic satellite reflections in neutron diffraction patterns.17 Several Mössbauer spectroscopic studies have been conducted to investigate the distribution of Sc3+ within the lattice structure and their influence on the magnetic properties.18,19 The magnetic and magnetoelectric properties of the material system have also been studied to verify their potential use as multiferroic materials.20 The magnetic, magnetoelastic, magnetoelectric, and transport properties of polycrystalline Ba(Fe1–xScx)12O19 compounds have been recently reported by Gupta et al.21,22 According to their reports, polycrystalline Ba(Fe1–xScx)12O19 compounds possess a longitudinal conical magnetic structure, coexisting with a spin glass-like phase, and exhibit ferroelectricity even in zero magnetic field. Directions of magnetization for all of the atoms have also been elucidated. As described above, a lot of research has been conducted on the Ba(Fe1–xScx)12O19 system; however, its single-crystal growth method has never been reported in detail before. The property of single crystals is different from polycrystalline compounds, and the detailed helimagnetic structure of single crystals, such as the position, size, and direction of each magnetic moment, still remains to be examined. Additionally, the mechanism behind the helimagnetic behavior of the Ba(Fe1–xScx)12O19 system is yet to be clarified.
Our previous studies have been focused on the helimagnetism occurring in the Ba2(Zn1–xMgx)2Fe12O22 and (Ba1–xSrx)2Zn2Fe12O22 systems, where the nonmagnetic Mg2+ or Sr2+ ion is substituted into the Y-type hexagonal ferrite Ba2Zn2Fe12O22.23−30 Initially, Momozawa et al. had successfully grown large single Ba2(Zn1–xMgx)2Fe12O22 and (Ba1–xSrx)2Zn2Fe12O22 crystals of high crystalline quality by the flux method using the Na2O–Fe2O3 flux.23−25 Neutron diffraction and magnetization measurements were conducted on the obtained single crystals to reveal their helimagnetic structure and the occurrence of helimagnetism in those systems.26−28 The redistribution of Fe3+, Zn2+, and Mg2+ cations occurred due to the Mg substitution. Local lattice deformation around the Sr2+ cation was caused by the Sr substitution. Consequently, a competition between the three superexchange interactions was induced and results in a distorted helimagnetic structure, consisting of large and small ferrimagnetic groups. We have also estimated the exchange integrals of the three competing superexchange interactions at low temperatures.29,30 One of the valuable outcomes in the research of these two systems is the ability to grow large, high quality single crystals, enabling a detailed magnetic analysis by neutron diffraction. By obtaining single crystals with various Mg or Sr concentrations, analysis was systematically performed to essentially understand the appearance mechanism of helimagnetism and the superexchange interaction.
Since Ba(Fe1–xScx)12O19 is a relatively simple system, where the nonmagnetic trivalent Sc3+ cations are substituted for magnetic trivalent Fe3+ cations, it is ideal for investigating its magnetic behavior. It is possible to understand the essential mechanisms behind the helimagnetism and the superexchange interactions in the most versatile M-type hexagonal ferrite by applying the above-mentioned argument to the Ba(Fe1–xScx)12O19 system. As more than 50 years have passed since the discovery of helimagnetism in Ba(Fe1–xScx)12O19, the technology for analysis of the magnetic structure has advanced significantly. Neutron diffraction is the best method for the analysis of a microscopic magnetic structure, in which case, the large single crystals of high crystalline quality are indispensable. No detailed reports have been published regarding the single-crystal growth of Ba(Fe1–xScx)12O19. We have found a superior single-crystal growth technique for hexagonal ferrites, and if it is possible to grow Ba(Fe1–xScx)12O19 with various Sc concentrations, the systematic study described above will lead to an essential understanding of the M-type hexagonal ferrite Ba(Fe1–xScx)12O19. In this paper, we report a successful growth of single Ba(Fe1–xScx)12O19 crystals using the flux method, based on previously reported investigations.23−25
2. Results and Discussion
2.1. Crystal Growth and Characterization
The advantage of our single-crystal growth method is that the formation of a crystal nucleus can be confirmed by the in situ observation. As shown in Figure 1a, we can clearly distinguish the crystallization of a hexagonal crystal and its nucleation temperature. If no crystal nuclei are crystallized or many crystal nuclei are crystallized, the growth procedure can be modified appropriately by adding reagents or reheating the furnace, which saves time during fabrication. As a result, in situ process monitoring has accelerated the success of the Ba(Fe1–xScx)12O19 crystal growth. Figure 1b shows the nucleation temperature of Ba(Fe1–xScx)12O19 as a function of the Sc concentration x′ of the starting composition, where we denote the Sc concentration of the starting composition as x′, which is different from x, the Sc concentration of the single crystals. In Figure 1b, the solid line indicates a guide showing the nucleation temperature increase and decrease. The nucleation temperature exhibits a unique behavior, namely, it increases up to x′ = 0.08 and exhibits a decrease above x′ = 0.08. This behavior is caused by the following two factors. One is a decrease in the amount of Na2O, as shown in Figure 9, and the other is an increase in the concentration of Sc3+, which has a larger ionic radius than Fe3+. The former factor causes a rise in the nucleation temperature due to the reduction in the flux as a solvent. The latter factor causes difficulty in the nucleation process due to local lattice distortion, thus resulting in a decrease in the nucleation temperature.
Figure 1.

(a) Photo of a hexagonal crystal on the surface of a molten solution (x′ = 0.04 run). (b) Nucleation temperature as a function of x′.
Figure 9.
Starting composition of reagents for single-crystal growth of Ba(Fe1–xScx)12O19 depending on x′. The x′ values, where the crystal growth was performed, are indicated in the plot.
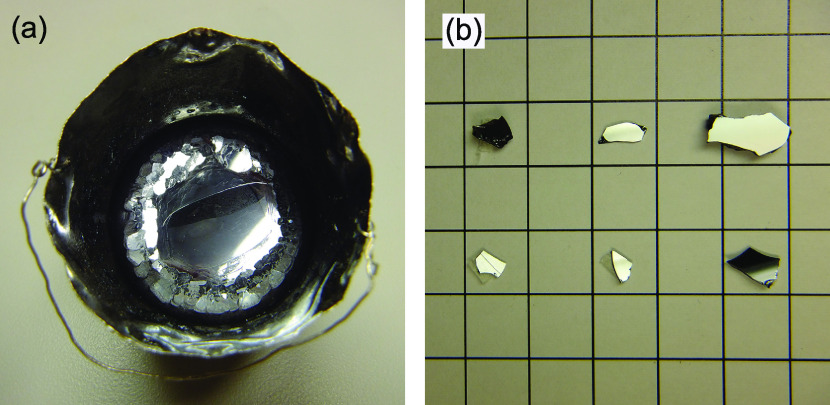
Figure 2a shows as-grown crystals in a platinum crucible, obtained from the x′ = 0.05 run. A large hexagonal crystal, of diameter 25 mm, with a highly distinct {001} plane orientation is visible in the center of the crucible, which is surrounded by small crystals. Figure 2b shows crystals obtained after washing the flux medium with hot diluted nitric acid. They consist of black, lamellate structures with a size of up to 13 mm × 8 mm × 2 mm. Because they have a well-developed {001} mirror-like surface, they appear white in Figure 2.
Figure 2.
(a) Photo of the fabricated crystals in a platinum crucible with a diameter of 40 mm obtained from x′ = 0.05. (b) Photo of the fabricated crystals obtained after washing the flux medium with hot diluted nitric acid (scale: 1 × 1 cm2).
An energy dispersive X-ray spectroscopy (EDS) spectrum of the powdered x′ = 0.08 crystal is exemplarily depicted in Figure S2, and the experimentally determined elemental analysis results for the obtained crystal are listed in Table S1. The atomic ratio of (Fe + Sc) to Ba is close to 1:12, which is consistent with the stoichiometry of the M-type hexagonal ferrite. The Sc concentration of single crystals, x, is also determined from the elemental EDS analysis, listed in Table S2. Figure 3 shows a comparison of x with x′. The Sc concentration value of the fabricated crystals, x, is greater than the Sc concentration of the starting composition, x′, and saturates around x = 0.19. Single crystals of high crystalline quality could not be obtained at Sc concentrations higher than x′ = 0.24, since the lattice deformation would become too large to maintain the crystal structure of the M-type hexagonal ferrite. Trace amounts of Si and Al were detected by EDS as atomic impurities with concentrations ranging from 0.13 to 2.6 atom %, which came from the surroundings such as electric furnace blocks and ceramic tubes. Al3+ can substitute Fe3+, which may affect the crystal structure and magnetic properties. However, the effect of Sc-substitution is more dominant than that of Al-substitution in our fabricated single crystals. This is because the lattice constants, a and c, of the fabricated single crystals increase uniformly with increase in Sc concentrations x, which can be seen from Figure 5 described later. Since the ionic radius of Al3+ (0.054 nm for 6 coordination) is smaller than that of Fe3+ (0.064 nm for 6 coordination), Al-substitution results in a decrease in lattice constants.
Figure 3.
Sc concentration of the obtained crystals, x, as a function of the Sc concentration of the starting composition, x′.
Figure 5.
Lattice constants a and c as a function of x, obtained at 296 K.
Figure 4 shows the (00l) X-ray diffraction (XRD) pattern of the single x = 0.128 crystal. Owing to the symmetry of the space group P63/mmc (no. 194) for Ba(Fe1–xScx)12O19, only the diffraction peaks indexed as l = 2n are observed. Sharp diffraction peaks and the splitting of Cu Kα1 and Cu Kα2 lines are indicative of high crystalline quality. From the position (2θ) of the (00 28) diffraction peak, the lattice constant c is calculated to be c = 23.55(7) Å for the x = 0.128 crystal. The XRD pattern for the powdered sample of the same x = 0.128 crystal is shown in Figure S3. Only diffraction peaks assigned to Ba(Fe1–xScx)12O19 are observed, and no additional peaks, due to impurities, are observed. The lattice constant a for the x = 0.128 crystal is calculated to be a = 5.92(9) Å from the (220) diffraction peak. From the results of elemental analysis and XRD, we conclude that the obtained crystals are the single phase of Ba(Fe1–xScx)12O19. Figure 5 shows the lattice constants a and c as a function of x, obtained at 296 K. The lattice constant c exhibits a significant increase, while the lattice constant a increases slightly with the increase in x. This is due to the ionic radius of Sc3+ (0.074 nm for 6 coordination) being larger than that of Fe3+ (0.064 nm for 6 coordination). The linear relationship between c and x is expressed by
| 1 |
Similarly, the linear relationship between a and x is expressed by
| 2 |
Although both parameters, c and a, obey Vegard’s law with the increase in x, c is more sensitive to x since Ba(Fe1–xScx)12O19 consists of a close-packed structure along the c-axis.
Figure 4.
(00l) XRD pattern of the single x = 0.128 crystal measured at 296 K, which was prepared from the x′ = 0.08 run. Cu Kα radiation was used.
2.2. Magnetic Properties and Evidence of Helimagnetism
Figure 6 shows the magnetization σ of the x = 0 and 0.128 crystals under an external magnetic field of 5 kOe, applied in the direction parallel to the c-axis of the sample, as a function of temperature, ranging from 2 to 880 K. With the increase in temperature, σ of the x = 0 crystal remains nearly constant up to 50 K and then exhibits a nearly linear decrease down to 0 emu/g at the Curie point of 744 K. This is because the Sc-free BaFe12O19 crystal exhibits ferrimagnetism in the measured temperature range below the Curie point. On the other hand, σ of the crystal with Sc concentration of x = 0.128 initially increases with the increase in temperature, reaches the maximum value of 55.6 emu/g at 213 K, and consequently decreases linearly down to 0 emu/g at 541 K. Thus, the x = 0.128 crystal is expected to have a magnetic order, which is different from the characteristic ferrimagnetic behavior of the x = 0 crystal below 213 K. We expect that the temperature at which σ of the x = 0.128 crystal has a maximum value is the magnetic phase transition temperature between ferrimagnetism and helimagnetism, considering the previously published literature.16,17
Figure 6.
Magnetization, σ, of the x = 0 and 0.128 crystals as a function of temperature, measured by (a) SQUID and (b) VSM. An external magnetic field of 5 kOe was applied in the direction parallel to the c-axis of the sample.
This prediction is supported by the magnetization curves of the x = 0 and 0.128 crystals measured at 296 and 77 K, which are shown in Figure 7. The x = 0 crystal exhibits a strong magnetic anisotropy characteristic of a hard ferrite material. When the magnetic field is applied parallel to the c-axis of the sample, σ is saturated at the demagnetization field of 5 kOe at both temperatures. However, if the magnetic field is applied along the c-plane of the sample, σ is saturated at 18 kOe at 296 K, while saturation magnetization is not achieved at 77 K. In contrast to the x = 0 crystal, the x = 0.128 crystal shows almost no magnetic anisotropy. Magnetization processes of the x = 0.128 crystal at 296 K almost coincide with each other when the magnetic field is applied along the direction of the c-axis and the c-plane of the sample. It should be noted that an anomalous magnetization behavior is observed and is more prominent at 77 K in the Sc-rich crystals. Specifically, σ exhibits a gradual increase and a sharp bending point at 1.7 kOe at relatively low magnetic fields. The above-mentioned behavior is characteristic of the helimagnetic order in the hexagonal ferrite system.31,32
Figure 7.
Magnetization curves, measured at T = 296 K (a, b) and 77 K (c, d), with a magnetic field of H = 5 kOe, applied parallel to the c-axis and the c-plane of the x = 0 (blue line) (a, c) and 0.128 (red line) (b, d) crystal samples.
To confirm the magnetic phase transition and the appearance of helimagnetic order in the x = 0.128 crystal, neutron diffraction experiments were performed at various temperatures. Figure 8a shows (00l) neutron diffraction patterns as a function of Miller index l, measured on the x = 0.128 crystal. Nuclear scattering peaks are indexed as l = 2n (n is an integer), which are coincident with the XRD pattern of the single crystal, as shown in Figure 4. The indices and intensities of the nuclear scattering peaks show nearly no dependence on the temperature. Magnetic satellite reflection peaks, indexed as (00 2(n ± δ)), are also observed on both sides of the nuclear scattering peaks below 211 K, where δ (0 < δ < 0.5) represents the incommensurability of magnetic satellite reflections. They are related to the helimagnetic order with the propagation vector, oriented along the direction of the c-axis of the sample. Figure 8b shows δ as a function of temperature, which exhibits a decrease with an increase in temperature. The intensities of the magnetic satellite peaks also exhibit a decrease with an increase in the temperature. The sensitivity of the indices and intensities of the satellite peaks to the temperature indicates that the helimagnetic structure changes with temperature. Based on the temperature dependence of the magnetic satellite peaks, we estimate that the helimagnetic order in the x = 0.128 crystal appears below 211 K. This shows a good correlation to the temperature at which the magnetization σ exhibits a maximum value. It is concluded that the x = 0.128 crystal undergoes a magnetic phase transition at 211 K and demonstrates the helimagnetic behavior below 211 K. A detailed investigation of the magnetic structure is currently in progress.
Figure 8.

(a) (00l) neutron diffraction patterns of the x = 0.128 crystal measured at various temperatures. (b) Incommensurability δ of magnetic satellite reflections as a function of temperature.
3. Conclusions
Fabrication of hexagonal ferrite Ba(Fe1–xScx)12O19 single crystal was successfully conducted by the spontaneous crystallization method using Na2O–Fe2O3 flux. The best starting composition was determined by trial and error as (4.300x′ + 10.53)BaCO3, (26.00x′ + 63.17)(1 – x′)Fe2O3, (26.00x′ + 63.17)x′Sc2O3 ((∵26.00x′ + 63.17)(Fe2O3 + Sc2O3)), and (−30.30x′ + 26.30)Na2CO3, with values given in mol %. We succeeded in obtaining optically black and lamellate-structured single crystals with a distinct plane of {001} orientation, with a size of up to 13 mm × 8 mm × 2 mm. In situ monitoring of the crystal nucleation was effectively used to improve the success of crystal growth. XRD and EDS characterization revealed that the obtained crystals consisted of a single-phase Ba(Fe1–xScx)12O19. The lattice constants a and c increased with increasing Sc concentration x in accordance with Vegard’s law. The magnetization σ as a function of temperature exhibited a maximum value at low temperatures, and a gradual increase as well as a bending point was exhibited by the x = 0.128 crystal at 77 K and at low applied magnetic fields. This suggests the occurrence of helimagnetic order at low temperatures. The neutron diffraction pattern of the x = 0.128 crystal demonstrated the temperature dependence of the magnetic satellite reflection peaks below 211 K, which is characteristic of a phase transition between helimagnetic and ferrimagnetic orders in the hexagonal ferrite system. Neutron diffraction and magnetization measurements were conducted on a series of Ba(Fe1–xScx)12O19 single crystals, and interesting results regarding their magnetic phase diagram and helimagnetic structures will be presented in our future work, which will be useful in the future research in this field.
4. Experimental Section
4.1. Single-Crystal Growth
Single crystals of Ba(Fe1–xScx)12O19 were grown by the spontaneous crystallization method from the BaO–Fe2O3–Sc2O3–Na2O subsystem using Na2O–Fe2O3 flux. The starting reagents were powdered BaCO3 (Wako Pure Chemical Co.), Fe2O3 (Kanto Chemical Co., Inc.), Sc2O3 (Shin-Etsu Chemical Co., Ltd.), and Na2CO3 (Wako Pure Chemical Co.). The Sc concentration x′ of the starting composition ranged from 0 to 0.240. Single crystals of high crystalline quality were not obtained at x′ ≥ 0.240 because the crystal structure would become unstable due to the significant deformation in the crystal lattice, as described in the Results and Discussion section. The optimal starting composition of the reagents was determined by trial and error. Figure 9 shows the best starting composition for the crystal growth as a function of x′. The slight modifications were based on the composition for the Sc-free BaFe12O19, reported by Gambino et al.,33 namely, (4.300x′ + 10.53)BaCO3, (26.00x′ + 63.17)(1 – x′)Fe2O3, (26.00x′ + 63.17)x′Sc2O3 ((∵26.00x′ + 63.17)(Fe2O3+Sc2O3)), and (−30.30x′ + 26.30)Na2CO3 in units of mol %.
Each reagent was appropriately weighed to follow the intended ratio with the maximum deviation of 1 mg, while the total mass was set to 50 g. The weighed reagents were mixed by shaking in a plastic container to prevent the contamination of metal components and packed into an uncovered platinum crucible. The platinum crucible, filled with the reagents, was placed into the electric furnace, as shown in Figure S4. Briefly, the features of this electric furnace are as follows. (1) Four layers of SiC heating elements in the shape of parallel crosses were constructed. By controlling the power applied to each layer, it was possible to achieve a temperature gradient in the vertical direction of the furnace.23 Thereby, the reagents were supplied to a crystal nucleus on the surface of the solution. (2) A window, at the top of the furnace, was opened for the in situ observations. (3) An R-type thermocouple was inserted from the bottom to monitor and control the furnace temperature using a proportional-integral-differential (PID) program. The temperature of mixture was increased to the maximum temperature of 1693 K and kept constant for a duration of 20 h to sufficiently dissolve the reagents in the flux. Consequently, the temperature was gradually decreased with the presence of a temperature gradient, where the upper part of the furnace was about 10 K lower than the top. The crystal nuclei were then observed through the furnace window. After the crystallization of hexagonal crystals was confirmed by the in situ observations, the temperature was further decreased to 1373 K at a rate of 0.5 K/h. This was followed by the cooling of the furnace to temperatures below 773 K at a rate of 1 K/min. Hot diluted nitric acid was used to separate the obtained crystals from the flux medium. All crystal growth processes were performed under an air atmosphere.
4.2. Characterization
The crystals, obtained from the preparation batches, were characterized by means of XRD and EDS. XRD (Rigaku, MiniFlexII) experiments were conducted at 296 K on the crystals and the powdered samples, which were prepared by grinding a portion of each crystal to clarify the crystal phase and lattice constants, a and c. Cu Kα irradiation was used, and the lattice constants, a and c, were calculated from the XRD reflection patterns of the (220) peak in the powdered samples and the (00 28) peak in the crystal samples, respectively. Elemental analysis was performed by EDS (JEOL, JED-2300(S)) to investigate the composition and the Sc concentration x of each crystal. The average value of the composition and the Sc concentration x was calculated from measurements at 10 points on each sample.
Magnetization properties of the x = 0 and 0.128 crystals were determined by measuring a disk-shaped piece, diameter of about 1 mm, of each sample. The temperature-dependent magnetization, σ, was measured from 2 to 300 K using a SQUID magnetometer (Quantum Design, MPMS3) and from 300 to 880 K using a vibrating sample magnetometer (VSM; Tamagawa Co., Ltd., TM-VSM2430-HGC). An external magnetic field of 5 kOe was applied parallel to the direction of the c-axis of the sample. Magnetization curves at 77 and 296 K were obtained by VSM in directions parallel to the c-axis and the c-plane of the sample. Time-of-flight (TOF) Laue single-crystal neutron diffraction measurements were carried out on the x = 0.128 crystal at various temperatures using the single crystal neutron diffractometer, SENJU, installed at the BL18 of the Materials and Life Science Experimental Facility (MLF) of J-PARC.34 The wavelength of the applied neutron source ranged from 0.4 to 8.8 Å.
Acknowledgments
The neutron diffraction experiments at the Materials and Life Science Experimental Facility of the J-PARC were performed under a user program (Proposal Nos. 2018B0073 and 2019A0211). The authors would like to thank Editage (www.editage.com) for English language editing.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03671.
Crystal structure of Ba(Fe1–xScx)12O19 (Figure S1); an exemplary EDS spectrum (Figure S2); elemental analysis results (Table S1); list of Sc concentrations, chemical compositions, and lattice constants of obtained single crystals (Table S2); XRD pattern for the x = 0.128 powdered crystal (Figure S3); and schematic representation of the electric furnace cross section (Figure S4) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Smit J.; Wijn H. P. J.. Ferrites; Philips Technical Library: Eindhoven, 1959. [Google Scholar]
- Braun P. B. The Crystal Structures of a New Group of Ferromagnetic Compounds. Philips Res. Rep. 1957, 12, 491–548. [Google Scholar]
- Gorter E. W. Saturation Magnetization of Some Ferrimagnetic Oxides with Hexagonal Crystal Structures. Proc. IEE 1957, 104, 255–260. 10.1049/pi-b-1.1957.0042. [DOI] [Google Scholar]
- Kimura T.; Lawes G.; Ramirez A. P. Electric Polarization Rotation in a Hexaferrite with Long-Wavelength Magnetic Structures. Phys. Rev. Lett. 2005, 94, 137201 10.1103/PhysRevLett.94.137201. [DOI] [PubMed] [Google Scholar]
- Ishiwata S.; Taguchi Y.; Murakawa H.; Onose Y.; Tokura Y. Low-Magnetic-Field Control of Electric Polarization Vector in a Helimagnet. Science 2008, 319, 1643–1646. 10.1126/science.1154507. [DOI] [PubMed] [Google Scholar]
- Sagayama H.; Taniguchi K.; Abe N.; Arima T.-h.; Nishikawa Y.; Yano S.-i.; Kousaka Y.; Akimitsu J.; Matsuura M.; Hirota K. Two Distinct Ferroelectric Phases in the Multiferroic Y-type Hexaferrite Ba2Mg2Fe12O22. Phys. Rev. B 2009, 80, 180419 10.1103/PhysRevB.80.180419. [DOI] [Google Scholar]
- Ishiwata S.; Okuyama D.; Kakurai K.; Nishi M.; Taguchi Y.; Tokura Y. Neutron Diffraction Studies on the Multiferroic Conical Magnet Ba2Mg2Fe12O22. Phys. Rev. B 2010, 81, 174418 10.1103/PhysRevB.81.174418. [DOI] [Google Scholar]
- Lee H. B.; Song Y.-S.; Chung J.-H.; Chun S. H.; Chai Y. S.; Kim K. H.; Reehuis M.; Prokeš K.; Mat’aš S. Field-Induced Incommensurate-to-Commensurate Phase Transition in the Magnetoelectric Hexaferrite Ba0.5Sr1.5Zn2(Fe1–xAlx)12O22. Phys. Rev. B 2011, 83, 144425 10.1103/PhysRevB.83.144425. [DOI] [Google Scholar]
- Chun S. H.; Chai Y. S.; Jeon B.-G.; Kim H. J.; Oh Y. S.; Kim I.; Kim H.; Jeon B. J.; Haam S. Y.; Park J.-Y.; Lee S. H.; Chung J.-H.; Park J.-H.; Kim K. H. Electric Field Control of Nonvolatile Four-State Magnetization at Room Temperature. Phys. Rev. Lett. 2012, 108, 177201 10.1103/PhysRevLett.108.177201. [DOI] [PubMed] [Google Scholar]
- Chang H.; Lee H. B.; Song Y.-S.; Chung J.-H.; Kim S. A.; Oh I. H.; Reehuis M.; Schefer J. Al Doping Effect on Magnetic Phase Transitions of Magnetoelectric Hexaferrite Ba0.7Sr1.3Zn2(Fe1–xAlx)12O22. Phys. Rev. B 2012, 85, 064402 10.1103/PhysRevB.85.064402. [DOI] [Google Scholar]
- Lee H. B.; Chun S. H.; Shin K. W.; Jeon B.-G.; Chai Y. S.; Kim K. H.; Schefer J.; Chang H.; Yun S.-N.; Joung T.-Y.; Chung J.-H. Heliconical Magnetic Order and Field-Induced Multiferroicity of the Co2Y-type Hexaferrite Ba0.3Sr1.7Co2Fe12O22. Phys. Rev. B 2012, 86, 094435 10.1103/PhysRevB.86.094435. [DOI] [Google Scholar]
- Song Y. Q.; Fang Y.; Wang L. Y.; Zhou W. P.; Cao Q. Q.; Wang D. H.; Du Y. W. Spin Reorientation Transition and Near Room-Temperature Multiferroic Properties in a W-type Hexaferrite SrZn1.15Co0.85Fe16O27. J. Appl. Phys. 2014, 115, 093905 10.1063/1.4867370. [DOI] [Google Scholar]
- Hirose S.; Haruki K.; Ando A.; Kimura T. Mutual Control of Magnetization and Electrical Polarization by Electric and Magnetic Fields at Room Temperature in Y-type BaSrCo2–xZnxFe11AlO22 Ceramics. Appl. Phys. Lett. 2014, 104, 022907 10.1063/1.4862432. [DOI] [Google Scholar]
- Nakajima T.; Tokunaga Y.; Matsuda M.; Dissanayake S.; Fernandez-Baca J.; Kakurai K.; Taguchi Y.; Tokura Y.; Arima T.-h. Magnetic Structures and Excitations in a Multiferroic Y-type Hexaferrite BaSrCo2Fe11AlO22. Phys. Rev. B 2016, 94, 195154 10.1103/PhysRevB.94.195154. [DOI] [Google Scholar]
- Kocsis V.; Nakajima T.; Matsuda M.; Kikkawa A.; Kaneko Y.; Takashima J.; Kakurai K.; Arima T.; Kagawa F.; Tokunaga Y.; Tokura Y.; Taguchi Y. Magnetization-Polarization Cross-Control Near Room Temperature in Hexaferrite Single Crystals. Nat. Commun. 2019, 10, 1247 10.1038/s41467-019-09205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perekalina T. M.; Cheparin V. P. Ferrimagnetism of Hexagonal Ferrites. Sov. Phys. – JETP 1968, 9, 2524–2526. [Google Scholar]
- Aleshko-Ozhevskiǐ O. P.; Sizov R. A.; Yamzin I. I.; Lubimtsev V. A. Helicoidal Antiphase Spin Ordering in Hexagonal Ferrites of the BaScxFe12–xO19 (M) System. Sov. Phys. – JETP 1969, 28, 425–430. [Google Scholar]
- Kamzin A. S.; Rozenbaum V. L.; Ol’khovik L. P. Mössbauer Studies of the Surface and Bulk Magnetic Structure of Scandium-Substituted Ba-M-type Hexaferrites. Phys. Solid State 1999, 41, 433–439. 10.1134/1.1130797. [DOI] [Google Scholar]
- Korovushkin V. V.; Shipko M. N.; Kostishin V. G.; Isaev I. M.; Mironovich A. Y.; Trukhanov S. V.; Trukhanov A. V. Structural and Magnetic Properties of a BaScxFe12–xO19 Substituted Hexagonal Ferrite. Inorg. Mater. 2019, 55, 1007–1013. 10.1134/S0020168519100066. [DOI] [Google Scholar]
- Tokunaga Y.; Kaneko Y.; Okuyama D.; Ishiwata S.; Arima T.; Wakimoto S.; Kakurai K.; Taguchi Y.; Tokura Y. Multiferroic M-Type Hexaferrites with a Room-Temperature Conical State and Magnetically Controllable Spin Helicity. Phys. Rev. Lett. 2010, 105, 257201 10.1103/PhysRevLett.105.257201. [DOI] [PubMed] [Google Scholar]
- Gupta S.; Upadhyay S. K.; Siruguri V.; Sathe V. G.; Sampathkumaran E. V. Observation of Magnetoelastic and Magnetoelectric Coupling in Sc Doped BaFe12O19 Due to Spin-Glass-Like Phase. J. Phys.: Condens. Matter 2019, 31, 295701 10.1088/1361-648X/ab1798. [DOI] [PubMed] [Google Scholar]
- Gupta S.; Deshpande S.; Sathe V.; Siruguri V. Effect of Scandium Substitution on Magnetic and Transport Properties of the M-type Barium Hexaferrites. J. Alloys Compd. 2020, 815, 152467 10.1016/j.jallcom.2019.152467. [DOI] [Google Scholar]
- Momozawa N.; Mita M.; Takei H. Single Crystal Growth of (Ba1–xSrx)2Zn2Fe12O22 from High Temperature Solution. J. Cryst. Growth 1987, 83, 403–409. 10.1016/0022-0248(87)90303-4. [DOI] [Google Scholar]
- Momozawa N.; Taguchi Y.; Sakai H.; Abe M.; Nishiyama K. Magnetic Properties of Ba2(Zn1–xMgx)2Fe12O22. I. Single Crystal Growth. Mater. Tech. 1996, 14, 16–20. [Google Scholar]
- Momozawa N.; Taguchi Y.; Sakai H.; Abe M.; Nishiyama K. Magnetic Properties of Ba2(Zn1–xMgx)2Fe12O22. II. Characterization of Single Crystals. Mater. Tech. 1996, 14, 42–46. [Google Scholar]
- Momozawa N.; Yamaguchi Y.; Takei H.; Mita M. Magnetic Structure of (Ba1–xSrx)2Zn2Fe12O22 (x = 0-1.0). J. Phys. Soc. Jpn. 1985, 54, 771–780. 10.1143/JPSJ.54.771. [DOI] [Google Scholar]
- Momozawa N.; Yamaguchi Y.; Mita M. Magnetic Structure Change in Ba2Mg2Fe12O22. J. Phys. Soc. Jpn. 1986, 55, 1350–1358. 10.1143/JPSJ.55.1350. [DOI] [Google Scholar]
- Momozawa N. Neutron Diffraction Study of Helimagnet (Ba1–xSrx)2Zn2Fe12O22. J. Phys. Soc. Jpn. 1986, 55, 4007–4013. 10.1143/JPSJ.55.4007. [DOI] [Google Scholar]
- Momozawa N.; Nagao Y.; Utsumi S.; Abe M.; Yamaguchi Y. Cation Distribution and Helimagnetic Structure of the Ba2(Zn1–xMgx)2Fe12O22 System as Revealed by Magnetization Measurements and Neutron Diffraction. J. Phys. Soc. Jpn. 2001, 70, 2724–2732. 10.1143/JPSJ.70.2724. [DOI] [Google Scholar]
- Utsumi S.; Yoshiba D.; Momozawa N. Superexchange Interactions of (Ba1–xSrx)2Zn2Fe12O22 System Studied by Neutron Diffraction. J. Phys. Soc. Jpn. 2007, 76, 034704 10.1143/JPSJ.76.034704. [DOI] [Google Scholar]
- Momozawa N.; Yamaguchi Y.; Takei H.; Mita M. Modification of Helix in (Ba1–xSrx)2Zn2Fe12O22 Due to Applied Magnetic Field. J. Phys. Soc. Jpn. 1985, 54, 3895–3903. 10.1143/JPSJ.54.3895. [DOI] [Google Scholar]
- Momozawa N.; Yamaguchi Y. Field-Induced Commensurate Intermediate Phases in Helimagnet (Ba1–xSrx)2Zn2Fe12O22 (x = 0.748). J. Phys. Soc. Jpn. 1993, 62, 1292–1304. 10.1143/JPSJ.62.1292. [DOI] [Google Scholar]
- Gambino R. J.; Leonhard F. Growth of Barium Ferrite Single Crystals. J. Am. Ceram. Soc. 1961, 44, 221–224. 10.1111/j.1151-2916.1961.tb15364.x. [DOI] [Google Scholar]
- Ohhara T.; Kiyanagi R.; Oikawa K.; Kaneko K.; Kawasaki T.; Tamura I.; Nakao A.; Hanashima T.; Munakata K.; Moyoshi T.; Kuroda T.; Kimura H.; Sakakura T.; Lee C.-H.; Takahashi M.; Ohshima K.-i.; Kiyotani T.; Noda Y.; Arai M. SENJU: a New Time-of-Flight Single-Crystal Neutron Diffractometer at J-PARC. J. Appl. Crystallogr. 2016, 49, 120–127. 10.1107/S1600576715022943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.