Abstract
Gas explosions are destructive disasters in coal mines. Coal mine gas is a multi-component gas mixture, with methane (CH4) being the dominant constituent. Understanding the process and mechanism of mine gas explosions is of critical importance to the safety of mining operations. In this work, three flammable gases (CO, C2H6, and H2) which are commonly present in coal mines were selected to explore how they affect a methane explosion. The explosion characteristics of the flammable gases were investigated in a 20 L spherical closed vessel. Experiments on binary- (CH4/CO, CH4/C2H6, and CH4/H2) and multicomponent (CH4/CO/C2H6/H2) mixtures indicated that the explosion of such mixtures is more dangerous and destructive than that of methane alone in air, as measured by the explosion pressure. Furthermore, a self-promoting microcirculation reaction network is proposed to help analyze the chemical reactions involved in the multicomponent (CH4/CO/C2H6/H2) gas explosion. This work will contribute to a better understanding of the explosion mechanism of gas mixtures in coal mines and provide a useful reference for determining the safety limits in practice.
1. Introduction
Gas explosions are ruinous disasters in coal mines.1−4 Explosions of fuel–air mixtures are characterized by specific parameters, such as explosive limits, maximum explosion pressure, maximum rate of pressure rise, and time to reach the maximum explosion pressure. These parameters reflect explosion intensity and destructiveness. A number of experimental studies on methane (CH4) explosion characteristics can be found in the literature in the last decades.5−9 Coward and Jones10 and Zabetakis11 investigated the flammability characteristics of combustible gases and vapors under a variety of environmental conditions. It is worth noting that the experimental results depend on certain factors of the investigated process, such as the size and type of explosion chambers, energy and type of ignition source, initial pressure and temperature, and flammable mixture flow.12,13 The explosion characteristic tests of the flammable gases were conducted from the mid-1980s through the 1990s at the Pittsburgh Research Laboratory (PRL) in different volume chambers (8, 20, 120, 25, and 500 L).14−17 The experimental data reported include lower explosive limit (LEL), upper explosive limit (UEL), peak explosion pressures, and the maximum rate of pressure rise. The tests were performed at ambient temperature and pressure under both quiescent and turbulent conditions. Initial temperature and pressure have tremendous effect on explosion parameters.18−22 The experimental results show that the explosive limits of methane/natural gas can be significantly extended at high temperatures and high pressures, and the UEL is more sensitive than the LEL as pressure and temperature increase.23 The peak explosion pressure is slightly reduced at high-temperature conditions and gradually increases with the initial pressure.23,24 Moreover, many scholars have investigated the influence of initial ignition energy and initial turbulence on the explosion behavior of methane/air mixtures.16,17,25,26 The scholars proved that the level of initial ignition energy significantly impacts the flame and explosion characteristics and also extends the explosive limits of methane.27 The gas flow turbulence increases the maximum explosion pressure and burning velocity.28 Because of the great catastrophe caused by coal dust explosions, many researchers have made great efforts to explore the mechanism of CH4/coal dust mixtures in recent years. The researchers found that the presence of coal dust with methane not only increases the explosion pressure but also accelerates the time of the explosion.29 The explosion risk of hybrid CH4/coal dust is much higher than that of pure coal dust explosion.30 Furthermore, coal mine gas is a multicomponent gas mixture, with methane being the dominant constituent. However, most reported studies treated the mine gas as pure methane without considering carbon monoxide, hydrocarbons, hydrogen, and other flammable gases; as a consequence, the results may significantly deviate from the reality in coal mines.
Hydrogen (H2), although appearing in small amounts in coal mine gas, has a wide explosion range with a low minimum ignition energy, thus posing a high explosion risk.31−34 Several studies have reported the effects of H2 addition on the explosion characteristics of hydrocarbon fuel streams or natural gas, in particular an increase in the laminar burning velocity35−38 and also a decrease in the laminar flame thickness.36 Jackson et al.39 carried out a combined experimental and numerical investigation on the effects of H2 addition to lean premixed CH4 flames. The results indicate a significant enhancement of lean flammability limits for relatively small amounts of H2. The effects of hydrogen concentration on spherically propagating laminar hydrogen/methane/air flames were studied at different equivalence ratios at atmospheric pressure by Okafor et al.40 The results showed that an increase in hydrogen concentration in the binary fuel led to an increase in laminar burning velocity. Yu et al.41 investigated the effects of hydrogen addition on the propagation characteristics of methane/air premixed flames at different equivalence ratios in a venting duct. The results indicated that the tendency toward flame instability increased with the fraction of hydrogen, and the premixed hydrogen/methane flame underwent a complex shape change with the increasing hydrogen fraction. Using a standard 20 L spherical explosion vessel, the explosion characteristics of H2/CH4/air and CH4/coal dust/air mixtures were investigated by Li et al.42 The results showed that the presence of molecular hydrogen would significantly increase the maximum explosion pressure and the rate of pressure rise of H2/CH4/air mixtures.
Some research was performed on the explosion characteristics of binary mixtures such as CO/CH4, C2H4/CH4, and C2H6/CH443−45 or ternary systems such as H2/CH4/CO in the context of the nitrogenous fertilizer industry.46 Experimental results revealed that the flammability limits of the mixture gas are related to many factors, such as the inherent properties of the flammable gases, the state of the mixture gas (temperature, pressure, and composition), ignition energy, size and geometry of the container, and the flame spread direction. Zheng et al.47 proposed a BP neural network model to predict the minimum and maximum explosive limits of a flammable gas mixture containing H2, CH4, and CO based on experimental data. It is worth noting that the flammable gas mixtures investigated belong to rich H2/lean CH4 fuel, which is quite different from the coal mine gas.
With respect to the explosion characteristics of coal mine gas, existing results are still largely based on single- or binary-component flammable gases. Beyond binary mixtures, few studies were conducted with gas compositions that are of interest to the chemical industry but not necessarily relevant to coal mines. In coal mines, the composition of the flammable gases varies according to the conditions in the coal mine. Besides CH4, CO is the main product of low-temperature oxidation of coal, just like C2H6, C3H8, C2H4, and other hydrocarbon gases or the product of degradation and cracking of coal after the temperature reaches a certain threshold.48,49 C2H6 is a gaseous hydrocarbon produced during the low-temperature oxidation of coal and accounts for a large proportion, especially in the coalbed gas of kerosene symbiotic mines.49 H2 is mainly the product of degradation and cracking of coal after the temperature reaches the threshold value.49 The H2 content is generally small, but H2 has the highest explosion risk in coal mine gas. Therefore, the content and composition of the flammable gases are not fixed but vary with time and location.
As mentioned above, the explosion characteristics of multicomponent flammable gases including CH4, CO, C2H6, and H2 have been reported in the literature. However, existing studies are either focused on binary mixtures, for example, CH4/C2H4,43 CH4/CO,43,44 and CH4/C2H6,45 or the mixture composition is very different from that of typical gases in coal mines, for example, Zheng et al.47 studied a CH4/H2/CO mixture relating to the rich H2/lean CH4 fuel. Against this background, the purpose of the present study is to help bridge this knowledge gap by experimentally investigating the effect of multicomponent flammable gases on the explosion characteristics of methane in the context of coal mines. Therefore, three flammable gases (CO, C2H6, and H2) were selected and their individual explosion properties were studied, prior to the exploration of the binary- (CH4/CO, CH4/C2H6, and CH4/H2) and multicomponent (CH4/CO/C2H6/H2) mixtures. The novelty of our work is thus about the explosion characteristics of CH4/CO/C2H6/H2 mixtures, with the compositions relevant to the situations in coal mines. The quantitative results of the explosive limits and pressures of such mixtures have not been reported before. Furthermore, a self-promoting microcirculation reaction network was proposed to help analyze the chemical reactions involved in the multicomponent (CH4/CO/C2H6/H2) gas explosion. The reaction network suggests that chain initiation and chain-branching reactions in CH4/CO/C2H6/H2/air mixtures could happen more easily and faster than in CH4/air mixtures, which could help further study the explosion reaction mechanism of multicomponent flammable gases.
2. Results and Discussion
2.1. Parameters for Assessing Explosion Intensity
The explosion experiments at particular gas concentrations will generate pressure–time curves, which normally show the pressure increases to a peak value before it falls. This peak pressure is designated the maximum explosion pressure (Pmax). Pmax is specific to the concentration of the flammable gas, and thus we can obtain the extreme explosion pressure (MaxPmax), that is, MaxPmax = Max{Pmax1, Pmax2, Pmax3, ..., Pmaxn}, where 1, 2, 3, ..., n are the different gas concentrations investigated in the experiments. The corresponding gas concentration at MaxPmaxis the most dangerous concentration (Cm), which is normally slightly greater than the stoichiometric concentration for this gas–air reaction.
Another measure used is the explosion risk degree (F), proposed by Kondou and Rock:50F = 1 – (L/U)0.5, where U and L are the UEL and LEL, respectively, of a particular flammable gas.
2.2. Explosive Limits of CH4, CO, C2H6, and H2
The explosive limits of CH4, CO, C2H6, and H2 were measured and given in Table 1, which also show that the explosion risk degree of CO, C2H6, and H2 is higher than that of methane. Therefore, it is necessary and significant to study how the presence of a small amount of CO, C2H6, or H2 affects the explosion characteristics of methane in the context of coal mines.
Table 1. Explosive Limits of Monocomponent Flammable Gas.
| experimental
data |
|||
|---|---|---|---|
| flammable gas | LEL/% | UEL/% | explosion risk degree F |
| CH4 | 5.05 | 15.35 | 0.426 |
| CO | 12.95 | 70.15 | 0.570 |
| C2H6 | 3.15 | 12.55 | 0.499 |
| H2 | 4.55 | 71.85 | 0.748 |
2.3. Impact of CO, C2H6, and H2 on Methane Explosion Characteristics
In typical coal mine gases, the CO concentration is no more than 3.0% and the C2H6 and H2 concentrations less than 2.0%. Therefore, CO of four different concentrations (0.5, 1.0, 2.0, and 3.0%) and C2H6 and H2 of four different concentrations (0.5, 1.0, 1.5, and 2.0%) were added to CH4/air mixtures for experimentation, respectively.
The explosive limits of flammable gas have been well investigated by Coward, Hughes and Raybould, and Zabetakis.5,10,11 Le Chatelier’s formula is widely used to determine the explosive limits of the flammable gas mixtures. For hydrocarbon–air mixture, the prediction of Le Chatelier’s formula is relatively accurate, but for the gas mixture containing H2 or CO, this does not fit.10,43,47,51,52 The explosive limits of the binary mixed gases CH4 + CO and CH4 + C2H4 were determined by Deng et al.43 The results show that there is a certain gap between the value calculated by Le Chatelier’s formula and the experimental data. Furthermore, the UELs of binary fuel mixtures of hydrogen with methane, ethylene, and propane in air were determined experimentally at elevated temperatures by Wierzba and Ale.52 It was shown then that the experimental limits of hydrogen–methane mixtures deviate slightly from those calculated using Le Chatelier’s rule and the UELs of hydrogen–ethylene mixtures deviate significantly from those calculated using Le Chatelier’s rule over the range of temperatures tested and at a residence time of 10 min. It was suggested that the narrowing of the UEL is due to surface reactions on the stainless steel wall during the waiting time that tends to change the mixture composition just prior to spark ignition. The explosive limits of these mixtures exposed to longer residence times do not obey Le Chatelier’s rule. Hence, the explosive limits of CH4/CO, CH4/C2H6, or CH4/H2 are measured. The impacts of CO, C2H6, and H2 on the explosive limits of methane are presented in Table 2. The experimental data show that the addition of CO, C2H6, and H2 tends to reduce the LEL of CH4 in varying degrees. This is especially the case for C2H6: when the added C2H6 reaches 2.0%, the LEL of CH4 significantly reduces from 5.05 to 2.25%. Adding CO and C2H6 decreases the UEL of CH4, whereas adding H2 increases the UEL of CH4 first before it reduces it.
Table 2. Impact of CO, C2H6, or H2 Addition on the Explosive Limits of CH4.
| explosive
limits of CH4 |
|||
|---|---|---|---|
| flammable gas | added concentration/% | LEL/% | UEL/% |
| CO | 0.5 | 5.05 | 15.25 |
| 1.0 | 4.95 | 14.95 | |
| 2.0 | 4.75 | 14.45 | |
| 3.0 | 4.25 | 13.95 | |
| C2H6 | 0.5 | 4.55 | 14.35 |
| 1.0 | 3.95 | 13.45 | |
| 1.5 | 3.25 | 12.75 | |
| 2.0 | 2.25 | 12.15 | |
| H2 | 0.5 | 4.75 | 15.85 |
| 1.0 | 4.15 | 15.55 | |
| 1.5 | 3.55 | 15.25 | |
| 2.0 | 2.85 | 15.05 | |
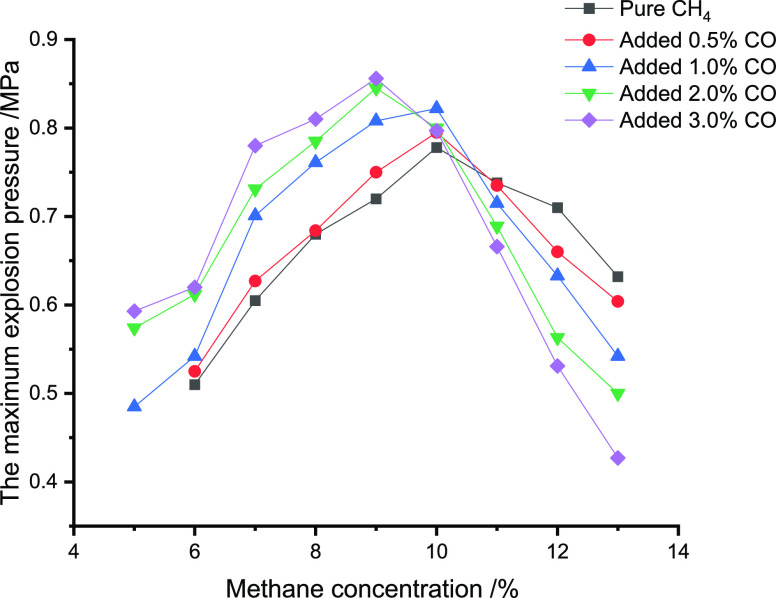
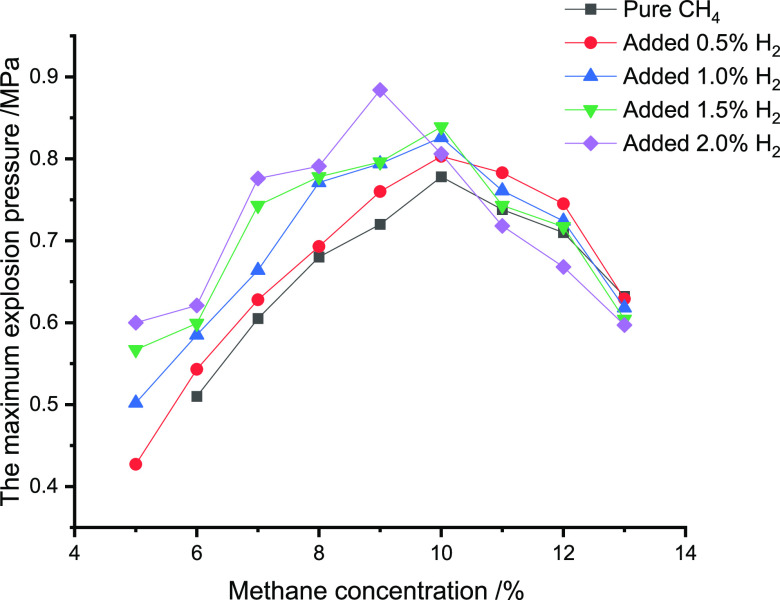
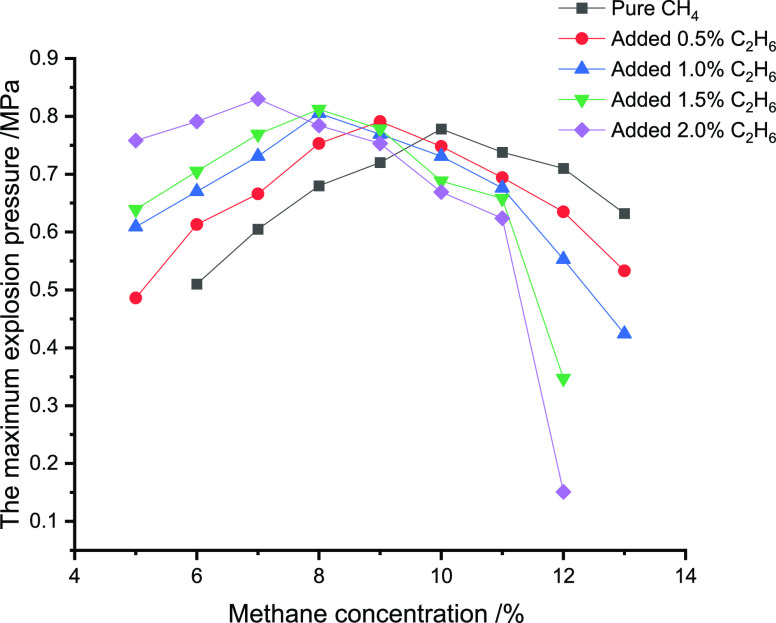
Tables 3–5 and Figures 1–3 illustrate the impact of CO, C2H6,
and H2 on Pmax for different
CH4 concentrations, whereas the results on MaxPmax and Cm are shown in Table 6. It should be noted that Cm of pure
CH4 is ∼10%. After adding CO, C2H6, or H2, the Cm value
of CH4 moves toward LEL at different levels with an increased
amount of the other gas added; this effect is particularly pronounced
for C2H6. With the same amount of the other
gas added, the impact on Cm of binary-component
mixtures is as follows: 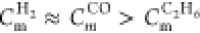 . The Pmax value
of binary mixtures (CH4/CO, CH4/C2H6, CH4/H2) is higher than that
of pure CH4 when the concentration of CH4 is
between LEL and Cm; on the other hand,
when the CH4 concentration is between Cm and UEL, Pmax of binary
mixtures is lower than that of pure CH4.
. The Pmax value
of binary mixtures (CH4/CO, CH4/C2H6, CH4/H2) is higher than that
of pure CH4 when the concentration of CH4 is
between LEL and Cm; on the other hand,
when the CH4 concentration is between Cm and UEL, Pmax of binary
mixtures is lower than that of pure CH4.
Table 3. Impact of CO Addition on Pmax for Different Concentrations of CH4.
| concentration
of CH4/% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Pmax of different concentrations of CH4/CO/MPa |
|||||||||
| concentration of CO/% | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 0 | 0.510 | 0.605 | 0.680 | 0.720 | 0.778 | 0.738 | 0.710 | 0.632 | |
| 0.5 | 0.525 | 0.627 | 0.684 | 0.750 | 0.795 | 0.735 | 0.660 | 0.604 | |
| 1.0 | 0.485 | 0.542 | 0.701 | 0.761 | 0.808 | 0.822 | 0.715 | 0.633 | 0.542 |
| 2.0 | 0.574 | 0.612 | 0.731 | 0.785 | 0.845 | 0.800 | 0.689 | 0.563 | 0.500 |
| 3.0 | 0.593 | 0.620 | 0.780 | 0.810 | 0.856 | 0.797 | 0.666 | 0.531 | 0.427 |
Table 5. Impact of H2 Addition on Pmax of Different Concentrations of CH4.
| concentration
of CH4/% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Pmax of different concentrations of CH4/H2/MPa |
|||||||||
| concentration of H2/% | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 0 | 0.510 | 0.605 | 0.680 | 0.720 | 0.778 | 0.738 | 0.710 | 0.632 | |
| 0.5 | 0.427 | 0.543 | 0.628 | 0.693 | 0.760 | 0.803 | 0.783 | 0.745 | 0.629 |
| 1.0 | 0.502 | 0.585 | 0.664 | 0.771 | 0.794 | 0.826 | 0.761 | 0.724 | 0.618 |
| 1.5 | 0.567 | 0.599 | 0.743 | 0.778 | 0.796 | 0.839 | 0.743 | 0.717 | 0.604 |
| 2.0 | 0.600 | 0.621 | 0.776 | 0.791 | 0.884 | 0.806 | 0.718 | 0.668 | 0.597 |
Figure 1.
Impact of CO addition on Pmax for different concentrations of CH4.
Figure 3.
Impact of H2 addition on Pmax for different concentrations of CH4.
Table 6. Comparison of the Impact of CO, C2H6, and H2 Added Respectively on MaxPmax and Cm of the Gas Mixtures.
| added concentration/% | added flammable gas | MaxPmax of gas mixtures/MPa | Cm of the gas mixtures/% | concentration of CH4/% |
|---|---|---|---|---|
| 0.0 | 0.778 | 10.0 | 10.0 | |
| 0.5 | C2H6 | 0.791 | 9.5 | 9.0 |
| CO | 0.795 | 10.5 | 10.0 | |
| H2 | 0.803 | 10.5 | 10.0 | |
| 1.0 | C2H6 | 0.805 | 9.0 | 8.0 |
| CO | 0.822 | 11.0 | 10.0 | |
| H2 | 0.826 | 11.0 | 10.0 | |
| 2.0 | C2H6 | 0.830 | 9.0 | 7.0 |
| CO | 0.845 | 11.0 | 9.0 | |
| H2 | 0.884 | 11.0 | 9.0 |
Figure 2.
Impact of C2H6 addition on Pmax for different concentrations of CH4.
Table 4. Impact of C2H6 Addition on Pmax for Different Concentrations of CH4.
| concentration
of CH4/% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Pmax of different concentrations of CH4/C2H6/MPa |
|||||||||
| concentration of C2H6/% | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 0 | 0.510 | 0.605 | 0.680 | 0.720 | 0.778 | 0.738 | 0.710 | 0.632 | |
| 0.5 | 0.486 | 0.613 | 0.666 | 0.753 | 0.791 | 0.748 | 0.694 | 0.635 | 0.533 |
| 1.0 | 0.609 | 0.670 | 0.731 | 0.805 | 0.769 | 0.731 | 0.676 | 0.553 | 0.424 |
| 1.5 | 0.639 | 0.705 | 0.769 | 0.812 | 0.778 | 0.688 | 0.658 | 0.347 | |
| 2.0 | 0.758 | 0.791 | 0.830 | 0.784 | 0.753 | 0.669 | 0.624 | 0.151 | |
Furthermore, after adding CO, C2H6, or H2, the MaxPmax value
of the binary-component mixtures is higher than that of pure CH4. Increasing the amount of the added CO, C2H6, or H2 leads to a more significant rise of MaxPmax. With the same amount added,
the impact on MaxPmax of binary-component
mixtures is as follows: 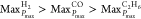 . In summary, the explosion intensity and
destructive power of binary-component mixtures (CH4/CO,
CH4/C2H6, and CH4/H2) are significantly higher than that of pure CH4.
. In summary, the explosion intensity and
destructive power of binary-component mixtures (CH4/CO,
CH4/C2H6, and CH4/H2) are significantly higher than that of pure CH4.
Normally, the explosions in low gaseous mines belong to oxygen-rich explosion, and 5.0% has been viewed as the critical value for CH4 concentration as it is approximately the LEL. However, our experimental results show that the LEL of CH4 will be significantly reduced in the presence of CO, C2H6, or H2, exposing the increasing risk even at a low CH4 concentration. Meanwhile, the most dangerous concentration (Cm) for CH4, producing the highest explosion overpressure, also reduces in the presence of CO, C2H6, or H2. Therefore, the effects of CO, C2H6, or H2 on the explosive limits and Cm of CH4 must be fully considered, and the alarm threshold of CH4 needs to be lowered accordingly in coal mine gas monitoring and early alarm system.
2.4. Impact of CO/C2H6/H2 Mixtures on Methane Explosion Characteristics
Usually CO, C2H6, H2, and CH4 coexist in coal mines. To gain further understanding of the explosion behavior of multicomponent flammable gases, CO, C2H6, and H2 were added to CH4/air mixtures with four different ratios, as given in Table 7. The results are shown in Tables 7 and 8 and Figure 4.
Table 7. Impact of CO/C2H6/H2 Added Simultaneously on the Explosive Limits of CH4.
| explosive
limits of CH4 |
|||||
|---|---|---|---|---|---|
| no. | CO/% | C2H6/% | H2% | LEL/% | UEL/% |
| 1 | 0 | 0 | 0 | 5.05 | 15.35 |
| 2 | 0.8 | 0.3 | 0.1 | 4.25 | 14.05 |
| 3 | 1.0 | 0.5 | 0.2 | 3.85 | 13.45 |
| 4 | 1.5 | 0.8 | 0.5 | 3.25 | 13.15 |
| 5 | 2.0 | 1.0 | 0.5 | 2.65 | 12.95 |
Table 8. Impact of CO/C2H6/H2 Added Simultaneously on Pmax for Different Concentrations of CH4.
| concentration
of CH4/% |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Pmax of different
concentrations of CH4/C2H6/CO/H2 mixtures/MPa |
||||||||||
| no. | CO/% | C2H6/% | H2/% | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 1 | 0 | 0 | 0 | 0.510 | 0.605 | 0.680 | 0.720 | 0.778 | 0.738 | 0.710 |
| 2 | 0.8 | 0.3 | 0.1 | 0.626 | 0.717 | 0.750 | 0.790 | 0.806 | 0.734 | 0.651 |
| 3 | 1.0 | 0.5 | 0.2 | 0.649 | 0.755 | 0.770 | 0.833 | 0.780 | 0.696 | 0.633 |
| 4 | 1.5 | 0.8 | 0.5 | 0.725 | 0.768 | 0.805 | 0.870 | 0.766 | 0.672 | 0.386 |
| 5 | 2.0 | 1.0 | 0.5 | 0.773 | 0.791 | 0.883 | 0.813 | 0.746 | 0.627 | 0.174 |
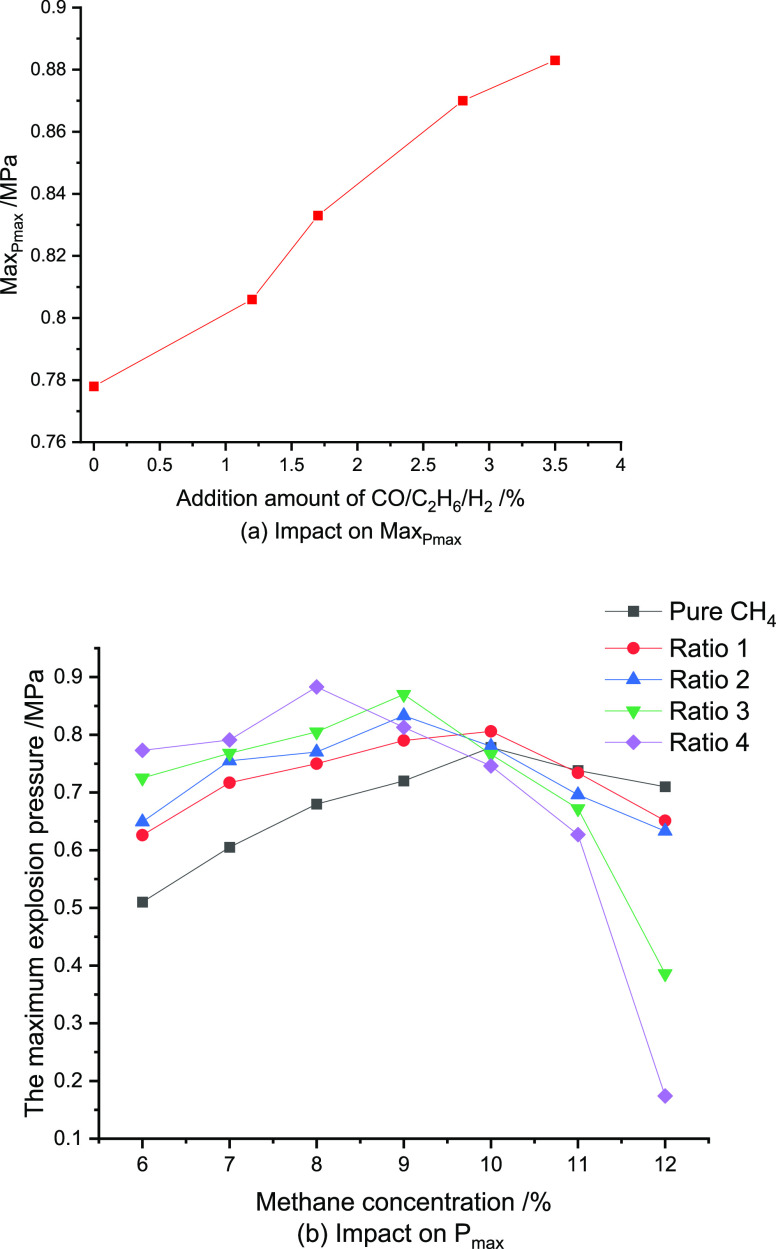
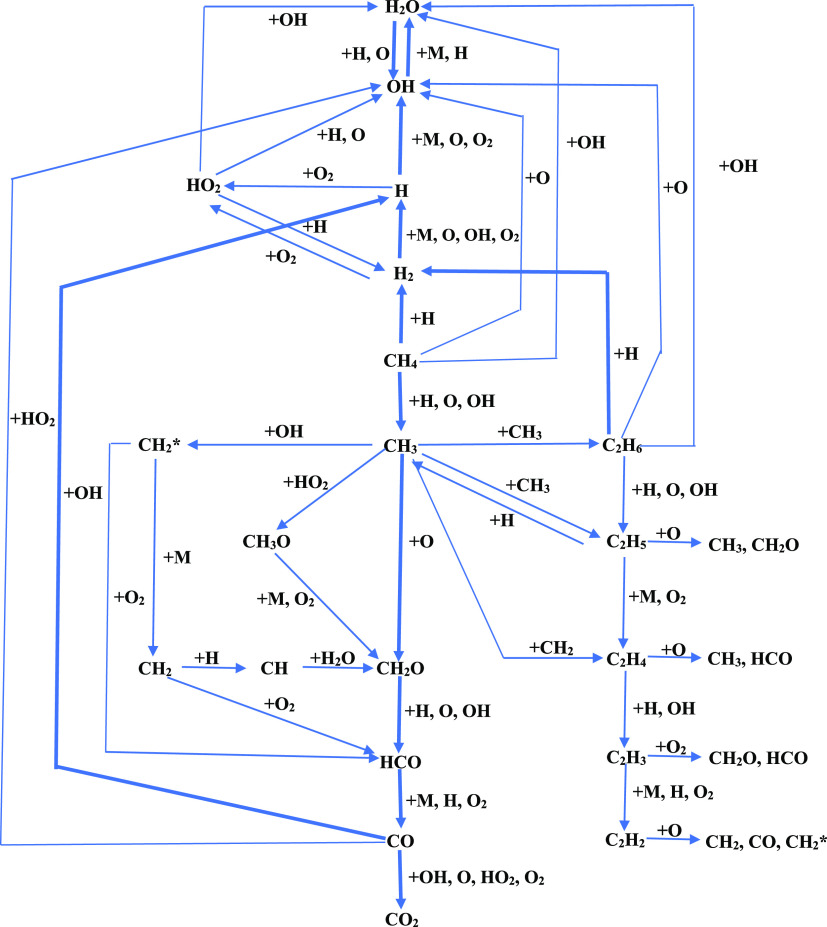
Figure 4.
Impact of CO/C2H6/H2 addition on Pmax and MaxPmax of CH4/CO/C2H6/H2 mixtures.
When CO, C2H6, and H2 were added together to CH4/air mixtures, both the LEL and UEL of CH4 decreased. Note that the Cm value of pure CH4 is approximately 10%; this Cm of CH4 shifts toward the LEL with the increase of the CO/C2H6/H2 amount. With regard to the maximum explosion pressure, when the CH4 concentration is between LEL and 9.0%, Pmax of the CH4/CO/C2H6/H2 mixtures is higher than that of pure CH4, whereas for the CH4 concentration between 11.0% and UEL, Pmax of the CH4/CO/C2H6/H2 mixtures is lower than that of pure CH4. With the increase of the added CO/C2H6/H2, the experimental results also indicate a more significant rise in MaxPmaxof the CH4/CO/C2H6/H2 mixtures.
The reaction mechanism of multicomponent flammable gases is complicated in coal mines. Other flammable gases (CO, C2H6, and H2) with a slight change in concentration could make a significant impact on the explosion characteristics of CH4. Moreover, if a gas explosion occurs, a certain amount of H2, CO, and other flammable gases may be produced. This is likely to trigger the second explosion which would be more dangerous than the first one.53 Therefore, the likelihood and risk of the second gas explosion should be fully assessed in the emergency rescue system, in particular, with regard to a small change of other flammable gases.
Furthermore, the explosion characteristic parameters of multicomponent flammable gases such as explosive limits, maximum explosion pressure, and Cm may not be obtained by simply superimposing the values from single- or binary-component flammable gases.
2.5. Theoretical Analysis of the Impact of CO, C2H6, and H2 on the Explosion Characteristics of CH4
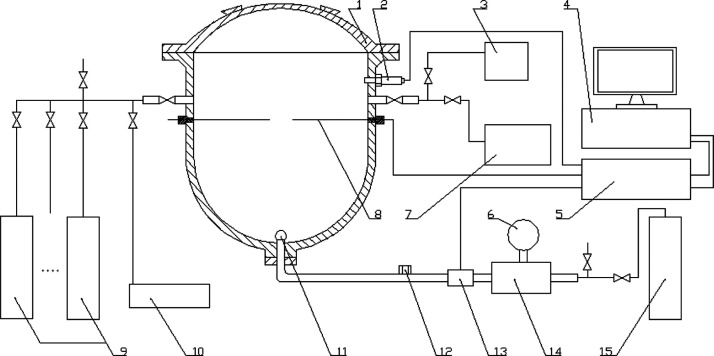
From the perspective of the chemical reaction kinetics, we proposed a self-promoting microcirculation reaction network of the multicomponent flammable gases (Figure 5)
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
Figure 5.
Self-promoting microcirculation reaction network of the multicomponent flammable gases.
Reaction 1 would be the primary chain initiation reaction in the initial reaction period, as reaction 1 is easier to be triggered than reaction 2 according to the reaction activation energy.54 With the reaction progressing, the temperature rises, which would make reaction 2 the main chain initiation reaction. Reactions 3 and 4 are the main branching chain reactions, and reactions 5–11 are the main elementary reactions in the multicomponent flammable gas reaction system. Either reaction 1 or 2 provides H• radicals that develop a radical pool of OH•, O•, and H• by the chain reactions 3 and 4. The reactions 1–4 are of great importance in the oxidation reaction mechanisms of hydrocarbon in that they provide the essential chain-branching and propagating steps as well as the radical pool for fast reactions. Moreover, the reactions 5–11 will produce new H2, H•, and OH• radicals which further accelerate the rates of reactions 1–11. Thus, as illustrated in Figure 5, the above reactions may lead to a self-promoting microcirculation system and a positive feedback mechanism. With the progress of the reaction, the reaction rate, the heat release, and the pressure will increase constantly until the explosion pressure reaches the maximum value. It is important to realize that any high-temperature hydrocarbon mechanism involves H2 and CO oxidation kinetics and that most, if not all, of CO2 that is formed results from reaction 9. However, experimental evidence indicates that the oxidation of CO to CO2 comes late in the reaction scheme7 because reaction 9 is slower than the reaction 6 or 11. Hence, the chain initiation of H2 can produce highly active H• and OH• radicals, and CO may be mainly involved in the later reaction of CH4, which will further accelerate the reaction speed of the main reactant CH4. In reaction 2, M is the usual third body. CO may participate in molecular collisions as the usual third body to help produce H• radicals. Therefore, CO may increase the collision frequency and make the chain initiation reaction of CH4/CO/C2H6/H2/air mixtures easier than CH4/air mixtures. It is worth to note that the chain initiating reaction of methane/air mixtures is difficult and slow. However, in the presence of OH, O, and H radicals, the reactions 5–7 that involved methane are all fast. Furthermore, the positive feedback mechanism of the self-promoting microcirculation may make the reaction rate of CH4/CO/C2H6/H2/air mixtures faster than CH4/air mixtures, resulting in more heat release and higher explosion pressure. C2H6 oxidizes much more slower than hydrogen, and very small quantities of hydrogen will increase the rate of CO oxidation substantially.7 Therefore, the influence of C2H6 on the explosion process is likely smaller than that of CO and H2. Chain initiation and chain branching reactions initiated by H2 in multicomponent flammable gas mixtures are easier and faster than that in the methane/air mixture. CO and C2H6 also may accelerate the chain initiation reaction as the third body. Thus, the rate of CH4 oxidation is substantially faster than that of the pure methane reaction system. The above theoretical analysis gives further support to the observations in the experiments that binary- (CH4/CO, CH4/C2H6, and CH4/H2) and multicomponent (CH4/CO/C2H6/H2) mixtures are more dangerous, and the resulting explosion is more destructive than that by pure CH4.
3. Conclusions
Three representative gases, CO, C2H6, and H2, were selected to investigate the impact of their presence on CH4 explosion characteristics. The explosion strength and explosion destructive power are higher for binary- (CH4/CO, CH4/C2H6, and CH4/H2) and multicomponent mixtures (CH4/CO/C2H6/H2) than for pure CH4 by measuring the explosion pressure Pmax and MaxPmax. Because of the decrease of the LEL and Cm of CH4 in the presence of CO, C2H6, and H2, the impact of other flammable gases on the explosion characteristics of CH4 must be fully considered, and the alarm threshold of CH4 needs to be lowered accordingly in coal mine gas monitoring and early alarm system. Meanwhile, other flammable gases (CO, C2H6, and H2) with a slight change in concentration could make a significant impact on the explosion characteristics of CH4. Experimental results indicate that the characteristic explosion parameters of multicomponent flammable gases such as explosive limits, maximum explosion pressure, and Cm may not be obtained by simply superimposing the values from single- or binary-component flammable gases. Experiment is still the primary way to obtain these parameters. The experimental data will also potentially provide guidance for the further study of the reaction mechanism of multicomponent gas explosion. Furthermore, a self-promoting microcirculation reaction network of the multicomponent flammable gases (CH4/CO/C2H6/H2) was proposed, combining the theory analysis with experimental data. This reaction network reflects the impact of CO, C2H6, and H2 on the explosion characteristics of CH4 and aids to reasonably infer the explosion reaction mechanism of multicomponent flammable gases.
For multicomponent flammable gases, the dynamics of the reaction and the interactions between components can become quite complex. The investigation on the explosion microscopic reaction mechanism of multicomponent flammable gases and the influence of temperature, pressure, ignition energy, and turbulence on multicomponent flammable gas characteristics will be conducted in future work. Furthermore, the scale of the experiments is comparatively small in relation to large industrial scales, and advanced computational tools combined with experiments should indeed be welcomed.
4. Experimental Methods
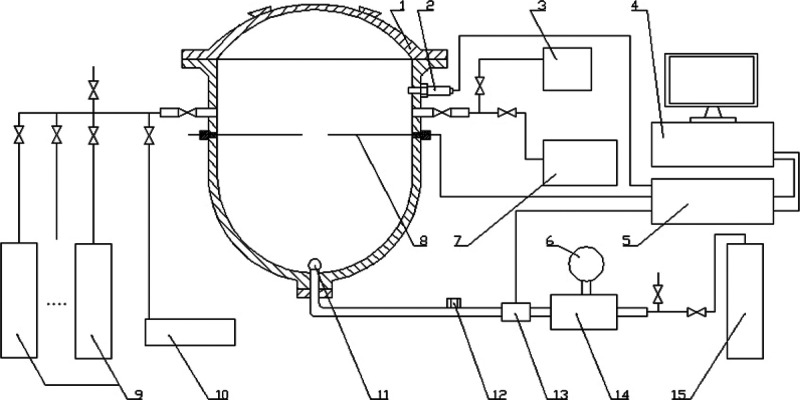
Experiments were performed in a 20 L spherical closed vessel which consists of an explosion vessel, a gas distribution system, an ignition system, and a measurement system, as shown in Figure 6. The explosion vessel (designed and produced by the Chongqing Branch of China Coal Research Institute, China), which can withstand a maximum pressure of 3.0 MPa, is made of stainless steel and is nearly spherical. The approximate dimensions are 34 cm in height, 30 cm in diameter, and 19,900 cm3 in effective volume. The gas distribution system is composed of bottles of pure CH4, CO, C2H6, and H2, an air compressor, a vacuum pump, and a pressure gauge. The flammable gases used in this experiment were provided by the Shanghai Pujing Gas company. The purity of each flammable gas was higher than 99.99%. The partial pressure method was used for mixture preparation, with a high-accuracy sensitive pressure transducer. The ignition source for the experimental setup was a detonating pyrotechnic ignition device (supplied by Liuyang Wenchi Electric Ignition Co., China) with a calorimetric energy of 5 J. The ignition position is in the center of the vessel. For the measurement of the static pressure, an NTS-2A precise digital pressure gauge (produced by NTS Co., Japan) was used. The measurement of the dynamic explosion pressure was achieved using a CY-DB 1303-type pressure sensor (produced by Baoji Huarui Sensor Institute, China) and a multifunction explosive reaction controller (produced by the Chongqing Branch of China Coal Research Institute).
Figure 6.
Schematic diagram of the experimental setup. 1 Explosion reactor; 2 pressure transducer; 3 air compressor; 4 computer; 5 controller; 6 manometer; 7 vacuum pump; 8 ignition electrode; 9 gas steel bottle group; 10 precision digital pressure gauge; 11 dispersion nozzle; 12 powder addition mouth; 13 electromagnetic valve; 14 compressed air storage room; 15 compressed air steel bottle.
The explosion characteristics were determined at ambient temperature and pressure. The electric igniter was placed at the center of the reactor, and the explosion vessel was evacuated and purged with fresh air three times. Then, the required mixture of flammable gases and air was injected into the vessel using the partial pressure method, waiting for at least 5 min to allow the gas to fully mix in the reactor. Afterward, the igniter was ignited by the ignition controller, and the pressure data were recorded and saved into the computer. Both the data acquisition instrument and ignition controller were connected with a synchronizer trigger to ensure the synchronization of ignition and data acquisition. A minimum of three experiments were performed for each initial condition of the flammable mixtures. The maximum explosion pressure listed in the tables is the maximum value among the three experiments.
Acknowledgments
This research is partially funded by the UK EPSRC (no. EP/R001588/1) and the Foundation for Outstanding Young Scientist of Shandong Province (no. BS2013NJ003). This research was conducted in the key laboratory of Western Mine Exploitation and Hazard Prevention, Xi’an University of Science and Technology. The authors are indebted to their support.
The authors declare no competing financial interest.
References
- Tutak M.; Brodny J. Analysis of the Impact of Auxiliary Ventilation Equipment on the Distribution and Concentration of Methane in the Tailgate. Energies 2018, 11, 3076. 10.3390/en11113076. [DOI] [Google Scholar]
- Brune J. F.; Saki S. A. Prevention of gob ignitions and explosions in longwall mining using dynamic seals. Int. J. Min. Sci. Technol. 2017, 27, 999–1003. 10.1016/j.ijmst.2017.06.026. [DOI] [Google Scholar]
- Tutak M.; Brodny J. Forecasting Methane Emissions from Hard Coal Mines Including the Methane Drainage Process. Energies 2019, 12, 3840. 10.3390/en12203840. [DOI] [Google Scholar]
- Tutak M.; Brodny J. Predicting methane concentration in longwall regions using artificial neural networks. Int. J. Environ. Res. Public Health 2019, 16, 1406. 10.3390/ijerph16081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. J.; Raybould W. E. The rapid determination of the explosibility of mine fire gases. Min. Eng. 1960, 120, 37–53. [Google Scholar]
- Moen I. O.; Lee J. H. S.; Hjertager B. H.; Fuhre K.; Eckhoff R. K. Pressure development clue to turbulent flame propagation in large-scale methane-air explosions. Combust. Flame 1982, 47, 31–52. 10.1016/0010-2180(82)90087-6. [DOI] [Google Scholar]
- Glassman I.; Yetter R. A.; Glumac N. G.. Combustion; Academic Press, 2014. [Google Scholar]
- Janovsky B.; Selesovsky P.; Horkel J.; Vejsa L. Vented confined explosions in Stramberk experimental mine and AutoReaGas simulation. J. Loss Prev. Process Ind. 2006, 19, 280–287. 10.1016/j.jlp.2005.06.038. [DOI] [Google Scholar]
- Checkel M. D.; Ting D. S.-K.; Bushe W. K. Flammability limits and burning velocities of ammonia/nitric oxide mixtures. J. Loss Prev. Process Ind. 1995, 8, 215–220. 10.1016/0950-4230(95)00027-x. [DOI] [Google Scholar]
- Coward H. F.; Jones G. W.. Limits of Flammability of Gases and Vapors; US Government Printing Office, 1952. [Google Scholar]
- Zabetakis M. G.Flammability Characteristics of Combustible Gases and Vapors, No. BULL-627; Bureau of Mines: Washington DC, 1965. [Google Scholar]
- Shebeko Y. N.; Tsarichenko S. G.; Korolchenko A. Y.; Trunev A. V.; Navzenya V. Y.; Papkov S. N.; Zaitzev A. A. Burning velocities and flammability limits of gaseous mixtures at elevated temperatures and pressures. Combust. Flame 1995, 102, 427–437. 10.1016/0010-2180(95)00002-n. [DOI] [Google Scholar]
- Mittal M. Explosion pressure measurement of methane-air mixtures in different sizes of confinement. J. Loss Prev. Process Ind. 2017, 46, 200–208. 10.1016/j.jlp.2017.02.022. [DOI] [Google Scholar]
- Hertzberg M.The Theory of Flammability Limits: Radiative Losses and Selective Diffusional Demixing; US Department of the Interior, Bureau of Mines, 1982. [Google Scholar]
- Hertzberg M. Selective diffusional demixing: occurrence and size of cellular flames. Prog. Energy Combust. Sci. 1989, 15, 203–239. 10.1016/0360-1285(89)90009-9. [DOI] [Google Scholar]
- Hertzberg M.; Cashdollar K. L.; Zlochower I. A.. Flammability limit measurements for dusts and gases: ignition energy requirements and pressure dependences. Symposium (International) on Combustion; Elsevier, 1988; Vol. 21, pp 303–313. [Google Scholar]
- Cashdollar K. L.; Zlochower I. A.; Green G. M.; Thomas R. A.; Hertzberg M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. 10.1016/s0950-4230(99)00037-6. [DOI] [Google Scholar]
- Kundu S.; Zanganeh J.; Moghtaderi B. A review on understanding explosions from methane-air mixture. J. Loss Prev. Process Ind. 2016, 40, 507–523. 10.1016/j.jlp.2016.02.004. [DOI] [Google Scholar]
- Vanderstraeten B.; Tuerlinckx D.; Berghmans J.; Vliegen S.; Van’t Oost E.; Smit B. Experimental study of the pressure and temperature dependence on the upper flammability limit of methane/air mixtures. J. Hazard. Mater. 1997, 56, 237–246. 10.1016/s0304-3894(97)00045-9. [DOI] [Google Scholar]
- Egolfopoulos F. N.; Cho P.; Law C. K. Laminar flame speeds of methane-air mixtures under reduced and elevated pressures. Combust. Flame 1989, 76, 375–391. 10.1016/0010-2180(89)90119-3. [DOI] [Google Scholar]
- Chen J.-R.; Tsai H.-Y.; Chien J.-H.; Pan H.-J. Flow and flame visualization near the upper flammability limits of methane/air and propane/air mixtures at elevated pressures. J. Loss Prev. Process Ind. 2011, 24, 662–670. 10.1016/j.jlp.2011.05.012. [DOI] [Google Scholar]
- Bunev V. A.; Bolshova T. A.; Babkin V. S. The nature of the upper laminar flammability limit in methane-air mixtures at high pressures. Dokl. Phys. Chem. 2013, 452, 197–199. 10.1134/s001250161307004x. [DOI] [Google Scholar]
- Huang L.; Pei S.; Wang Y.; Zhang L.; Ren S.; Zhang Z.; Xiao Y. Assessment of flammability and explosion risks of natural gas-air mixtures at high pressure and high temperature. Fuel 2019, 247, 47–56. 10.1016/j.fuel.2019.03.023. [DOI] [Google Scholar]
- Gieras M.; Klemens R.; Rarata G.; Wolański P. Determination of explosion parameters of methane-air mixtures in the chamber of 40dm3 at normal and elevated temperature. J. Loss Prev. Process Ind. 2006, 19, 263–270. 10.1016/j.jlp.2005.05.004. [DOI] [Google Scholar]
- Dobashi R. Experimental study on gas explosion behavior in enclosure. J. Loss Prev. Process Ind. 1997, 10, 83–89. 10.1016/s0950-4230(96)00050-2. [DOI] [Google Scholar]
- Scheid M.; Geißler A.; Krause U. Experiments on the influence of pre-ignition turbulence on vented gas and dust explosions. J. Loss Prev. Process Ind. 2006, 19, 194–199. 10.1016/j.jlp.2005.04.005. [DOI] [Google Scholar]
- Ajrash M. J.; Zanganeh J.; Moghtaderi B. Influences of the initial ignition energy on methane explosion in a flame deflagration tube. Energy Fuels 2017, 31, 6422–6434. 10.1021/acs.energyfuels.6b03375. [DOI] [Google Scholar]
- Kundu S. K.; Zanganeh J.; Eschebach D.; Badat Y.; Moghtaderi B. Confined explosion of methane-air mixtures under turbulence. Fuel 2018, 220, 471–480. 10.1016/j.fuel.2018.02.043. [DOI] [Google Scholar]
- Ajrash M. J.; Zanganeh J.; Moghtaderi B. Methane-coal dust hybrid fuel explosion properties in a large scale cylindrical explosion chamber. J. Loss Prev. Process Ind. 2016, 40, 317–328. 10.1016/j.jlp.2016.01.009. [DOI] [Google Scholar]
- Song S.-x.; Cheng Y.-f.; Meng X.-r.; Ma H.-h.; Dai H.-y.; Kan J.-t.; Shen Z.-w. Hybrid CH4/coal dust explosions in a 20-L spherical vessel. Process Saf. Environ. Prot. 2019, 122, 281–287. 10.1016/j.psep.2018.12.023. [DOI] [Google Scholar]
- Yu G.; Law C. K.; Wu C. K. Laminar flame speeds of hydrocarbon air mixtures with hydrogen addition. Combust. Flame 1986, 63, 339–347. 10.1016/0010-2180(86)90003-9. [DOI] [Google Scholar]
- Emami S. D.; Rajabi M.; Che Hassan C. R.; Hamid M. D. A.; Kasmani R. M.; Mazangi M. Experimental study on premixed hydrogen/air and hydrogen-methane/air mixtures explosion in 90 degree bend pipeline. Int. J. Hydrogen Energy 2013, 38, 14115–14120. 10.1016/j.ijhydene.2013.08.056. [DOI] [Google Scholar]
- Ma Q.; Zhang Q.; Pang L.; Huang Y.; Chen J. Effects of hydrogen addition on the confined and vented explosion behavior of methane in air. J. Loss Prev. Process Ind. 2014, 27, 65–73. 10.1016/j.jlp.2013.11.007. [DOI] [Google Scholar]
- Sun Z.-Y. Experimental studies on the explosion indices in turbulent stoichiometric H2/CH4/air mixtures. Int. J. Hydrogen Energy 2019, 44, 469–476. 10.1016/j.ijhydene.2018.02.094. [DOI] [Google Scholar]
- Ren J.-Y.; Qin W.; Egolfopoulos F. N.; Tsotsis T. T. Strain-rate effects on hydrogen-enhanced lean premixed combustion. Combust. Flame 2001, 124, 717–720. 10.1016/s0010-2180(00)00205-4. [DOI] [Google Scholar]
- Halter F.; Chauveau C.; Djebaïli-Chaumeix N.; Gökalp I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane-hydrogen-air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. 10.1016/j.proci.2004.08.195. [DOI] [Google Scholar]
- Di Sarli V.; Benedetto A. D. Laminar burning velocity of hydrogen-methane/air premixed flames. Int. J. Hydrogen Energy 2007, 32, 637–646. 10.1016/j.ijhydene.2006.05.016. [DOI] [Google Scholar]
- Tahtouh T.; Halter F.; Samson E.; Mounaïm-Rousselle C. Effects of hydrogen addition and nitrogen dilution on the laminar flame characteristics of premixed methane-air flames. Int. J. Hydrogen Energy 2009, 34, 8329–8338. 10.1016/j.ijhydene.2009.07.071. [DOI] [Google Scholar]
- Jackson G. S.; Sai R.; Plaia J. M.; Boggs C. M.; Kiger K. T. Influence of H2 on the response of lean premixed CH4 flames to high strained flows. Combust. Flame 2003, 132, 503–511. 10.1016/s0010-2180(02)00496-0. [DOI] [Google Scholar]
- Okafor E. C.; Hayakawa A.; Nagano Y.; Kitagawa T. Effects of hydrogen concentration on premixed laminar flames of hydrogen-methane-air. Int. J. Hydrogen Energy 2014, 39, 2409–2417. 10.1016/j.ijhydene.2013.11.128. [DOI] [Google Scholar]
- Yu M.; Zheng K.; Zheng L.; Chu T.; Guo P. Effects of hydrogen addition on propagation characteristics of premixed methane/air flames. J. Loss Prev. Process Ind. 2015, 34, 1–9. 10.1016/j.jlp.2015.01.017. [DOI] [Google Scholar]
- Li Q.; Lin B.; Dai H.; Zhao S. Explosion characteristics of H2/CH4/air and CH4/coal dust/air mixtures. Powder Technol. 2012, 229, 222–228. 10.1016/j.powtec.2012.06.036. [DOI] [Google Scholar]
- Deng J.; Luo Z.; Wu X.; Hu Y. Explosive limits of mixed gases containing CH4, CO and C2H4 in the goaf area. Min. Sci. Technol. 2010, 20, 557–562. 10.1016/s1674-5264(09)60243-x. [DOI] [Google Scholar]
- Deng J.; Cheng F.; Song Y.; Luo Z.; Zhang Y. Experimental and simulation studies on the influence of carbon monoxide on explosion characteristics of methane. J. Loss Prev. Process Ind. 2015, 36, 45–53. 10.1016/j.jlp.2015.05.002. [DOI] [Google Scholar]
- Luo Z.; Hao Q.; Wang T.; Li R.; Cheng F.; Deng J. Experimental study on the deflagration characteristics of methane-ethane mixtures in a closed duct. Fuel 2020, 259, 116295. 10.1016/j.fuel.2019.116295. [DOI] [Google Scholar]
- Hu Y.; Zhou B.; Yang Y. The limit and container factors of H2, CH4 and CO multiple explosive mixed gas. Sci. China, Ser. B: Chem. 2002, 1, 35–39. [Google Scholar]
- Zheng L. G.; Fan S. J.; Yu M. G.; Yu S.; Zuo Q. Predictive model on explosion limits of explosive gas mixture containing H2, CH4 and CO based on neural network. Int. Symp. Saf. Sci. Technol. 2006, 1227–1231. [Google Scholar]
- Li Q.-W.; Xiao Y.; Zhong K.-Q.; Shu C.-M.; Lü H.-F.; Deng J.; Wu S. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981. 10.1016/j.fuel.2020.117981. [DOI] [Google Scholar]
- Luo Z.; Li D.; Su B.; Wang T.; Li K.; Li Q.; Deng J. Thermodynamic effects of the generation of H*/OH*/CH2O* on flammable gas explosion. Fuel 2020, 280, 118679. 10.1016/j.fuel.2020.118679. [DOI] [Google Scholar]
- Wang H.Research on experiment and numerical simulation of explosion characteristics for multi-component flammable gases in coal mine. Ph.D. Thesis, Xi’an University of Science and Technology, June 2009. [Google Scholar]
- Wierzba I.; Karim G. A.; Cheng H. The flammability of rich gaseous fuel mixtures including those containing propane in air. J. Hazard. Mater. 1988, 20, 303–312. 10.1016/0304-3894(88)87019-5. [DOI] [Google Scholar]
- Wierzba I.; Ale B. B. Rich flammability limits of fuel mixtures involving hydrogen at elevated temperatures. Int. J. Hydrogen Energy 2000, 25, 75–80. 10.1016/s0360-3199(99)00009-9. [DOI] [Google Scholar]
- Brady D.; Cliff D.. Opportunity for re-entry into a coal mine immediately following an explosion. 12th Coal Operators Conference; University of Wollongong & The Australasian Institute of Mining and Metallurgy, 2012; pp 335–339.
- Zhou B. Z.; Wei Y. S.; Pan Z. W.; Guo Z.; Hu Y. Y. Preliminary study on the mechanism of branched chain reaction for gas-air mixture. J. Qinghai Normal Univ. 1995, 1, 34–38. [Google Scholar]