Abstract
Background
The People’s Republic of China (P. R. China) has made significant progress on schistosomiasis control. Among the 12 provincial-level administrative divisions (PLADs) with schistosomiasis endemic in P. R. China, Guangdong, Shanghai, Fujian, Guangxi and Zhejiang PLADs (following as five PLADs) had successively eliminated schistosomiasis during 1985–1995. However, consolidation of the schistosomiasis elimination in these five PLADs remains challenging. In the current study, we sought to understand the epidemic situation in these post-elimination areas and their surveillance capabilities on schistosomiasis.
Methods
Annual data reflecting the interventions and surveillance on human beings, cattle and snails based on county level from 2005 to 2016 were collected through the national schistosomiasis reporting system and the data were analyzed to understand the epidemic status of schistosomiasis in the five PLADs. A standardized score sheet was designed to assess the surveillance capacity for schistosomiasis of selected disease control agencies in five PLADs and ten counties. Assessment on surveillance capacity including schistosomiasis diagnostic skills, identification of snails’ living and infection status and knowledge about schistosomiasis and its control were made. Descriptive analysis was used to analyze the epidemic status and evaluation results on surveillance capacities.
Results
The assessments showed that no local cases in humans and cattle or infected snail were found in these five PLADs since 2005. However, from 2005 to 2016, a total of 221 imported cases were detected in Zhejiang, Shanghai and Fujian, and 11.98 hm2 of new snail habitats were found in Zhejiang, Shanghai and Guangxi. In addition, snail infestation reoccurred in 247.55 hm2 of former snail habitats since 2011. For the surveillance capacity assessment, the accuracy rate of IHA and MHT were 100 and 89.3%, respectively. All participants could judge the living status of snails accurately and 98.1% on the infection status of snails. The accuracy rate of the questionnaire survey was 98.0%.
Conclusions
Elimination of schistosomiasis was consolidated successfully in five PLADs of P. R. China due to effective and strong post-elimination surveillance. Comprehensive consolidation strategies should be focused on the elimination of residual snails and the prevention of imported infection sources to consolidate the achievements of schistosomiasis control.
Keywords: Schistosomiasis, China, Elimination, Surveillance, Evaluation
Background
Schistosomiasis, caused by parasitic trematode blood-dwelling flukes called Schistosoma, is one of the most important neglected tropical diseases in the world in terms of public health impacts [1, 2]. According to the report of World Health Organization (WHO), schistosomiasis is transmitted in 78 countries around the world. In 2017, at least 220.8 million people needed prophylactic treatment for schistosomiasis [3]. In the past decade, with the increased quantity of donation of praziquantel by international organizations and companies and increased willingness to give priority for schistosomiasis control or elimination by governments of endemic countries, great progress had been obtained in many countries. Meanwhile, elimination of schistosomiasis is regarded as an achievable goal in endemic regions or countries if continuous interventions and adequate resources are provided.
Schistosomiasis japonica is the only disease caused by schistosomes for human beings and livestock in P. R. China, distributed in 12 provinces along and south of Yangtze River with a long history. Investigations conducted in the 1950s proved that there were 380 endemic counties within 12 provinces, with about 12 million people and 1.2 million cattle infected with schistosomes, and over 100 million people at risk of infection. In addition, the total habitat area of Oncomelania hupensis, the only intermediate host of S. japonicum, was approximately 14.5 billion m2 [4–7]. Following three decades of unremitting efforts with control strategies shifted from snails control to morbidity control, Guangdong (1985), Shanghai (1985), Fujian (1987), Guangxi (1989) and Zhejiang (1995) eliminated schistosomiasis successively.
Being a zoonotic parasitic disease, the transmission of schistosomiasis japonica is influenced by biological, natural and social factors. Multiple studies proved that schistosomiasis easily rebounded or spread to new areas due to weakened interventions, ecological changes caused by flooding, construction of water conservancy projects, increased migration of goods or human resources etc., without a sensitive surveillance and response system [8–11]. As Shanghai, Guangdong, Fujian, Guangxi and Zhejiang had eliminated schistosomiasis at least 20 years before, we evaluated the epidemic situation and the surveillance capabilities on schistosomiasis among the five PLADs, to facilitate the consolidation of elimination achievements in post elimination era and provide reference for other regions where schistosomiasis had been eliminated or will be eliminated.
Methods
Study sites and research design
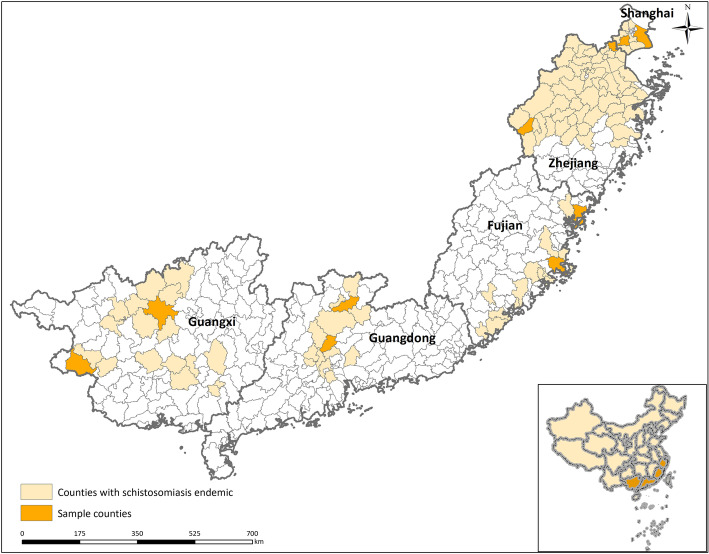
Among the five PLADs, Shanghai and Zhejiang are located in the Yangtze River Delta, in the east of China, Fujian, Guangdong and Guangxi are located in the south of China. According to the epidemiological characteristics, the endemic areas in Shanghai belong to waterway-network regions, while the endemic areas in Zhejiang, Guangdong, Guangxi and Fujian are mainly hilly and mountainous regions (Fig. 1).
Fig. 1.
The location of research settings
The study consisted of two parts: (i) Data reflecting the schistosomiasis intervention and surveillance conducted in Shanghai, Guangdong, Fujian, Guangxi and Zhejiang PLADs based on county level were collected and analyzed to understand the epidemic status of schistosomiasis. (ii) A standardized score sheet was designed to assess the surveillance capacity for schistosomiasis of selected disease control agencies.
Retrospective data collections
A comprehensive surveillance strategy focused on clearing the internal snail habitats and infection source, preventing imported snails and cases from other provinces with ongoing schistosomiasis transmission was conducted in the five PLADs. Annual data reflecting the interventions and surveillance on human beings, cattle and snails based on county level from 2005 to 2016 were collected through the national schistosomiasis reporting system. Variables could be split into three categories: variables reflecting serological tests and stool examination for schistosomiasis (people with serum positive would do stool examinations if available and some floating people with higher infection risk from other epidemic provinces may get stool examination directly) on humans; variables on serological tests and stool examinations for schistosomiasis on cattle, and variables reflecting the distribution of Oncomelania snails including total habitats, new infested areas, etc.
Assessment on surveillance capacity
A standardized score sheet was developed to assess the surveillance capacity after consulting experienced experts in the field of schistosomiasis control. The score sheet included two parts: the first part focused on the capacity of testing skills including schistosomiasis diagnostic skills, identification of snails’ living and infection status among the professionals; the second part was to assess the knowledge level about schistosomiasis and its control among the professionals. The assessment was implemented during December 2015–March 2016 in a blind manner.
Assessment on schistosomiasis testing skills
Laboratory testing skills of schistosomiasis were performed at provincial and county level among laboratory professionals. Two counties were selected in each province. The sample counties were selected according to their endemic situation before elimination. Counties with more schistosomiasis patients or higher density of snails were selected to assess their surveillance capacity. The assessed testing skills included: (i) Diagnostic skills included the indirect hameagglutination assay (IHA) and the miracidia hatching technique (MHT), which are most widely used in low endemic areas with light infection intensity for population screening and confirmation of schistosome infection respectively. (ii) Snails dissection and microscopic method to identify the snails’ living and infection status.
Preparation of reference panels for assessment
To ensure the consistence and comparability of the assessed results among different provinces, reference panels for testing methods were prepared and coded by National Institute of Parasitic Diseases, Chinese Center for Disease Prevention and Control (NIDP, China CDC). Each panel for IHA contained five serum samples (four from schistosomiasis cases and one from healthy persons), while panels for MHT included two samples containing mature eggs of schistosome obtained from the liver of infected rabbits and three negative samples with boiled-water treated eggs. Each snail panel consisted of three dead Oncomelania snails and seven living snails, while three of living snails were infected by schistosomes confirmed by shedding method. The test results were determined by technicians from NIPD, China CDC and were regarded as gold standard. The same patch of diagnostic kits were provided to technicians from provinces and counties.
Laboratory testing and score
The technicians in selected agencies were asked to perform the IHA and MHT tests according to the protocol of each method [12–14] and judge the results. The results were documented and reported to NIPD, China CDC within the given time. The total score of testing skills was 20, five points for IHA and MHT respectively and 10 points for snail identification. The detailed evaluation rules are provided in Additional file 1: Table S1.
Assessment on basic knowledge on schistosomiasis control
A questionnaire was designed by the professionals and experts on schistosomiasis prevention and control to assess the basic knowledge of medical staff responsible for schistosomiasis surveillance. The questionnaire was composed of three parts: knowledge of epidemiological and transmission characteristics of schistosomiasis japonica, diagnosis and treatment, case reporting and management. Each part consisted of five questions. The full questionnaire is provided in Additional file 2. Three professionals from CDC at provincial level and county level and two medical agencies at county level attended the assessment respectively. The assessment adopted the form of closed-book examination. The total score for the questionnaire was 10. One point would be deducted per error answer until the score was decreased to zero (Additional file 1: Table S1).
Data management and statistical analysis
All data were transferred to Microsoft Excel software, version 2013 (Microsoft Office, CA, USA) for data compilation. The positive rate of serological test (number of antibody positives/number of serum samples examined) and the infection rate of cattle in different years and provinces, number of cases with stool examination positive, number of cattle with stool examination positive, and areas of newly detected snails in each county from 2005 to 2016 were calculated to analyze the epidemic situation. The results of assessments on diagnostic skills, snail identification and questionnaire survey were determined by the accuracy rate (number of correctly tested samples/number of total samples or number of correct answers/number of questions). 95% confidence intervals (CI) were calculated using standard formulae based on the binomial distribution. Microsoft Excel software, version 2013 (Microsoft Office, CA, USA) and the statistical software SPSS, version 23.0 (SPSS Inc., Chicago, USA) were used to analyze the epidemic trend based on descriptive analysis.
Results
Surveillance results of schistosomiasis
Surveillance results on population
From 2005 to 2016, a total of 3 569 509 serological tests were conducted for schistosomiasis screening and 24 978 blood samples were determined as antibody positives in the five PLADs (Table 1). The annual positive rate of serological tests was 0.56–0.90%, with a slight fluctuation from 2005 to 2010, and a sustained downward trend from 2011.
Table 1.
Surveillance results of schistosomiasis on human beings in five PLADs during 2005–2016
| Year | Serological tests | Stool examination | |||
|---|---|---|---|---|---|
| No. serum samples examined | No. antibody positives | Positive rate (%, 95 CI) | No. fecal examinations | No. stool positives | |
| 2005 | 428 800 | 3479 | 0.81 (0.78–0.84) | 4565 | 44 |
| 2006 | 475 182 | 2681 | 0.56 (0.54–0.59) | 4740 | 36 |
| 2007 | 476 201 | 3348 | 0.70 (0.68–0.73) | 5876 | 27 |
| 2008 | 391 047 | 2647 | 0.68 (0.65–0.70) | 3549 | 17 |
| 2009 | 339 583 | 3062 | 0.90 (0.87–0.93) | 4145 | 21 |
| 2010 | 345 645 | 3057 | 0.88 (0.85–0.92) | 3993 | 12 |
| 2011 | 240 121 | 1488 | 0.62 (0.59–0.65) | 5167 | 12 |
| 2012 | 223 487 | 1332 | 0.60 (0.56–0.63) | 3720 | 9 |
| 2013 | 221 765 | 1324 | 0.60 (0.57–0.63) | 2570 | 14 |
| 2014 | 150 182 | 973 | 0.65 (0.61–0.69) | 3520 | 10 |
| 2015 | 143 617 | 842 | 0.59 (0.55–0.63) | 2563 | 8 |
| 2016 | 133 879 | 745 | 0.56 (0.52–0.60) | 1589 | 11 |
| Total | 3 569 509 | 24 978 | – | 45 997 | 221 |
No. Number of. – means not applicable
Totally 45 997 stool examination were performed during the 12 years in five PLADs and 221 stool positives were detected (Table 1). Among the stool positives, Zhejiang, Shanghai and Fujian accounted for 87.33% (193/221), 11.31% (25/221) and 1.36% (3/221), respectively, while no stool positive cases were found in Guangdong or Guangxi. All stool positives were imported cases who got infection from other endemic areas.
Surveillance results on cattle
From 2005 to 2016, a total of 82 858 serological tests were conducted for surveillance on cattle in the five PLADs and 19 serological positives were found in Zhejiang Province, with 11 and 8 positive in 2014 and 2015, respectively (Table 2). Meanwhile, a total of 42 645 stool examination were performed, and no infected cattle was detected.
Table 2.
Surveillance data of cattle in the five PLADs from 2005 to 2016
| Year | No. serological tests | No. positive serological tests | No. stool examinations | No. positive stool examinations |
|---|---|---|---|---|
| 2005 | 12 869 | 0 | 4812 | 0 |
| 2006 | 8846 | 0 | 4231 | 0 |
| 2007 | 8827 | 0 | 4027 | 0 |
| 2008 | 7525 | 0 | 2700 | 0 |
| 2009 | 7601 | 0 | 2593 | 0 |
| 2010 | 5730 | 0 | 2362 | 0 |
| 2011 | 6706 | 0 | 3109 | 0 |
| 2012 | 6068 | 0 | 2882 | 0 |
| 2013 | 5917 | 0 | 3031 | 0 |
| 2014 | 4878 | 11 | 3299 | 0 |
| 2015 | 4859 | 8 | 4368 | 0 |
| 2016 | 3032 | 0 | 5231 | 0 |
| Total | 82 858 | 19 | 42 645 | 0 |
No. Number of
Data on snail survey
Snail surveys were conducted in 126 837.75 hm2 of areas from 2005 to 2016 in the five PLADs. The area infested with living Oncomelania hupensis presented a descending trend, decreased from 112.70 hm2 in 2005 to 52.81 hm2 in 2016 (Table 3). Among the five PLADs, Guangdong maintained the status without snails’ infestation since 1992. The area of snail habitats in Zhejiang Province always accounted for the largest percentage, but presented a decrease trend from 80.72 hm2 in 2005 to 44.41 hm2 in 2016. However, no infected snail was found through dissection method in the five PLADs.
Table 3.
Results of snail survey in the five PLADs during 2005–2016
| Year | Areas conducted snail survey (hm2) | Areas with living snails (hm2) | Area of new snail habitats (hm2) | Area with recurrent snails (hm2) | Areas with mollusciciding (hm2) | Areas with environmental modification (hm2) |
|---|---|---|---|---|---|---|
| 2005 | 13 181.83 | 112.70 | 1.26 | a | 261.58 | 9.06 |
| 2006 | 11 190.36 | 116.12 | 0.00 | a | 306.71 | 26.36 |
| 2007 | 11 059.95 | 126.90 | 4.66 | a | 239.83 | 23.34 |
| 2008 | 11 243.00 | 95.90 | 0.00 | a | 184.40 | 24.02 |
| 2009 | 10 735.92 | 100.26 | 0.00 | a | 110.07 | 15.12 |
| 2010 | 11 059.37 | 71.06 | 0.60 | a | 117.55 | 5.16 |
| 2011 | 10 046.77 | 58.52 | 0.82 | 51.74 | 118.06 | 4.64 |
| 2012 | 9161.18 | 79.15 | 0.61 | 61.52 | 320.61 | 2.77 |
| 2013 | 9326.08 | 62.52 | 0.43 | 35.64 | 123.77 | 16.33 |
| 2014 | 9441.76 | 48.46 | 1.18 | 28.90 | 123.29 | 3.43 |
| 2015 | 9833.01 | 47.52 | 0.43 | 32.60 | 103.23 | 3.88 |
| 2016 | 10 558.53 | 52.81 | 1.99 | 37.15 | 102.79 | 5.34 |
| Total | 126 837.75 | – | 11.98 | 247.55 | 2111.89 | 139.45 |
ameans not available. – means not applicable
During the 12 years, 11.98 hm2 of new snail habitats (environments with no snails initially) were found in Zhejiang (6.53 hm2), Shanghai (4.19 hm2) and Guangxi (1.26 hm2). In addition, snail infestation reoccurred in 247.55 hm2 of former snail habitats since 2011, mainly distributed in Zhejiang (224.62 hm2), Guangxi (11.48 hm2), Fujian (10.73 hm2) and Shanghai (0.72 hm2).
Mollusciciding and extended mollusciciding were implemented among areas with snails. During the 12 years, mollusciciding were done with the area of 2111.89 hm2 and environmental modifications were done among some appropriate environments with the area of 139.45 hm2.
Comprehensive assessments of surveillance capacity
Capacity for testing skills and snail detection
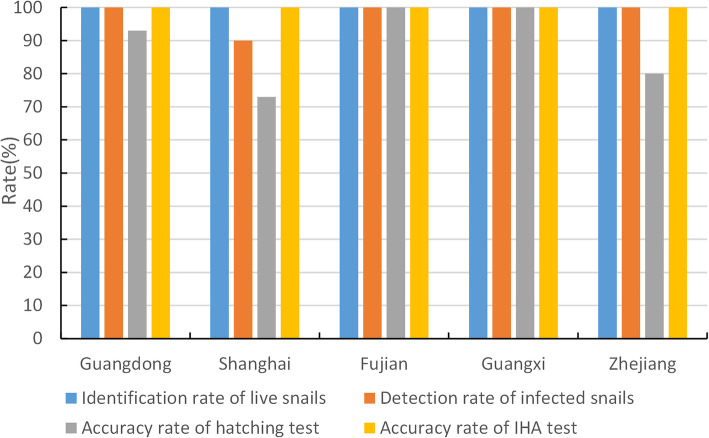
Totally 15 disease prevention and control agencies attended the assessment on testing skills and snails detection. For IHA, all professionals from the 15 agencies could perform and judge the results of five serum samples accurately, the accuracy rate was 100% (75/75). For MHT, the average accuracy rate was 89.3% (67/75, 95% CI: 82.2–96.5%), while eight wrong judgment results were all occurred in four CDC laboratories at county level.
For snail identification, all agencies preformed excellent capacity to identify the snails’ living status with the accuracy rate of 100% (150/150) (Fig. 2). The average accuracy rate of identifying infection status of snails was 98.1% (103/105, 95% CI: 95.4–100.8%). And two wrong judgment results occurred in one CDC at county level.
Fig. 2.
Evaluation results of schistosomiasis diagnosis and snail detection among fifteen institutions in the five PLADs
Questionnaires survey on basic knowledge of schistosomiasis
Total of 108 medical staffs in 15 disease prevention and control agencies attended the questionnaire survey (Table 4). The results showed that the average accuracy rate of all respondents was 98.0% (529/540, 95% CI: 96.8–99.2%). Among five PLADs, respondents from Guangdong and Guangxi PLADs answered all questions correctly. The wrong-answered questions were mainly about knowledge on national comprehensive schistosomiasis control strategies and diagnosis of schistosomiasis.
Table 4.
Scores of questionnaires on schistosomiasis control knowledge among professionals from 15 institutions in 5 PLADs and 10 counties
| Province | Level of each agency | No. respondents | No. questions | No. correct answers | Accuracy rate (%) |
|---|---|---|---|---|---|
| Guangdong | Province | 9 | 45 | 45 | 100.0 |
| Qingcheng | 9 | 45 | 45 | 100.0 | |
| Qujiang | 9 | 45 | 45 | 100.0 | |
| Subtotal | 27 | 135 | 135 | 100.0 | |
| Shanghai | Municipality | 3 | 15 | 15 | 100.0 |
| Pudong | 9 | 45 | 45 | 100.0 | |
| Songjiang | 9 | 45 | 39 | 86.7 | |
| Subtotal | 18 | 90 | 84 | 93.3 | |
| Fujian | Province | 3 | 15 | 14 | 93.3 |
| Xiapu | 9 | 45 | 45 | 100.0 | |
| Fuqing | 9 | 45 | 44 | 97.8 | |
| Subtotal | 21 | 105 | 103 | 98.1 | |
| Guangxi | Autonomous region | 3 | 15 | 15 | 100.0 |
| Jingxi | 9 | 45 | 45 | 100.0 | |
| Yizhou | 9 | 45 | 45 | 100.0 | |
| Subtotal | 21 | 105 | 105 | 100.0 | |
| Zhejiang | Province | 3 | 15 | 15 | 100.0 |
| Jiahsan | 9 | 45 | 45 | 100.0 | |
| Changshan | 9 | 45 | 42 | 93.3 | |
| Subtotal | 21 | 105 | 102 | 97.2 | |
| Total | 108 | 540 | 529 | 98.0 |
No. Number of
The total scores of the surveillance assessment
Based on the results of testing skills and snail detection and questionnaire surveys, except for Fujian scored 29 points, the scores of the other four PLADs were all 30 points (Table 5). In ten counties, the scores ranged from 26 to 30 points.
Table 5.
Comprehensive assessment scores of schistosomiasis surveillance capabilities in five PLADs and ten counties
| Province | Level of each agency | Diagnostic skills | Snail identification | Questionnaire survey | Total |
|---|---|---|---|---|---|
| Guangdong | Province | 10 | 10 | 10 | 30 |
| Qingcheng | 9 | 10 | 10 | 29 | |
| Qujiang | 10 | 10 | 10 | 30 | |
| Shanghai | Municipality | 10 | 10 | 10 | 30 |
| Pudong | 10 | 9 | 10 | 29 | |
| Songjiang | 6 | 10 | 4 | 20 | |
| Fujian | Province | 10 | 10 | 9 | 29 |
| Xiapu | 10 | 10 | 10 | 30 | |
| Fuqing | 10 | 10 | 9 | 29 | |
| Guangxi | Autonomous region | 10 | 10 | 10 | 30 |
| Jingxi | 10 | 10 | 10 | 30 | |
| Yizhou | 10 | 10 | 10 | 30 | |
| Zhejiang | Province | 10 | 10 | 10 | 30 |
| Jiahsan | 8 | 10 | 10 | 28 | |
| Changshan | 9 | 10 | 7 | 26 |
Discussion
Although the definition of schistosomiasis elimination was announced by WHO in recent years and the debate of how to prove elimination is still going on, Guangdong (1985), Shanghai (1985), Fujian (1987), Guangxi (1988) and Zhejiang (1995) were announced that schistosomiasis was eliminated successively according to the criteria issued by Chinese government at that time. Then the five PLADs transferred to post-elimination surveillance with main tasks to find and eliminate local residual infectious sources and remaining snail habitats, and prevent the import of infectious sources and snails from other endemic areas [15–17]. Before our study, except one foci with two new cases reemerged in a farm of Guangdong Province in 1992 but rapidly was under controlled [17, 18], no new infections occurred in other PLADs [19].
In our study, no local infection in reservoir hosts and intermediate host was detected in the five PLADs during 2005–2016, proving the successful consolidation of schistosomiasis elimination. And to improve the surveillance effectiveness on schistosomiasis in China, the sensitivity and specificity of the diagnosis reagents were improved and validated [20]. The comprehensive assessment results showed that the staffs have mastered the basic knowledge of schistosomiasis prevention and presented good capacity for schistosomiasis detection. All of these are elementary components of a sensitive and rapid surveillance platform. However, we noticed that there were some samples misdiagnosed and wrong answers for questionnaire answered by staff at county level. Capacity building should be strengthened focusing on schistosomiasis control and diagnosis through continued training and practices to consolidate local achievements on schistosomiasis control.
However, risks of the re-emergence and resurgence of schistosomiasis still existed in the five PLADs through our study. Over the past 12 years, a total of 221 imported cases were found in the five PLADs, mainly in Zhejiang Province, neighbored with Jiangxi and Anhui provinces where the transmission of schistosomiasis is still going on. Studies showed that most of the imported cases in Zhejiang Province are farmers, migrant workers and merchants from schistosomiasis endemic areas, primarily from Anhui, Jiangxi, Hubei [21–23]. In recent years, owing to urbanization and economic development, the number of the floating population from domestic epidemic areas has an increasing trend. At the same time, with the escalation of international trade and entry-exit personnels, the risk of importing cases from abroad infected with S. haematotium or S. mansoni is also increasing [24–26]. In view of this situation, on the one hand, supervision and treatment should be strengthened for imported definite cases to eliminate the transmission potential; on the other hand, health education should be promoted for the floating population from schistosomiasis endemic areas to spread the knowledge of schistosomiasis prevention and control [27].
The surveillance data proved that the area of snail habitats kept a low level during 2005–2016, compared with 95 900.70 hm2 reported by Wu [17]. The remaining snail habitats mainly distributed in the places that the ecological environments are complicated or water level is unstable, where molluscaciding approach doesn’t work well. In addition, several articles published also presented the evidence that the rebound or spread of snails habitats were occurred in Shanghai, Fujian, Guangxi and Zhejiang [17, 28–30]. It is worth noting that the connection of water systems or the transplanting of seedlings and aquatic plants from the snail habitat areas may also lead to the possibility of snail importation and spread [31, 32]. The development of new snail habitats and snails reappeared in former snail habitats in four provinces except Guangdong Province, providing evidence that eliminating Oncomelania snails completely was quite difficult. Although Guangdong Province kept the achievement with no Oncomelania snails detected, a new challenge for Guangdong Province is the invasion and spread of Biomphalaria straminea [33].
Considering the potential risks of schistosomiasis still existed in the five PLADs, snail control through environmental modification and surveillance focused on eliminating remaining snails and preventing imported infection sources should be continued and strengthened, to prevent the re-emergence of schistosomiasis, and consolidate the achievements of schistosomiasis elimination. Risk assessment should be conducted timely if there were large water conservancy projects or importing plants or animals from endemic areas, etc. [34, 35]. Specifically, the monitoring of the environments where snails infested previously or connected with snail habitats should be strengthened through multi ways [10, 36]. Meanwhile, the floating people and livestock from the areas where the transmission of schistosomiasis has not been interrupted or the epidemic situation of schistosomiasis is recovering should be inspected emphatically, and the patients should be treated in time if they are found [37–39].
Several limitations of this study should be noted. One is that data collection on surveillance results is based on the retrospective data collection on human, cattle and snails, some detailed information especially personal information on human with serum positive couldn’t be available. And some deep analysis on human couldn’t be implemented. The other one is only two counties were selected from each PLADs to evaluate the surveillance capacities, and there were 112 counties with schistosomiasis endemic in this five PLADs, so there may be a selection bias in the results of assessments on the surveillance capacities.
Conclusions
Elimination of schistosomiasis was consolidated successfully in five PLADs of P. R. China due to effective and strong post-elimination surveillance. Being a zoonotic parasitic diseases, challenges still exist to maintain the achievements as imported cases and snail habitats were detected during 2005–2016. Continuous surveillance should be strengthened through capacity building for staff responsible for schistosomiasis surveillance, providing adequate funding and resources.
Supplementary information
Additional file 1: Table S1. Detailed grading rules for the assessment on surveillance capacity in the five provinces.
Additional file 2. The questionnaire on basic knowledge of schistosomiasis control.
Acknowledgements
We thank all the staff from provincial schistosomiasis control institutes and county-level schistosomiasis control stations in Guangdong, Shanghai, Fujian, Guangxi and Zhejiang for participating in this study.
Abbreviations
- CDC
Center for Disease Control
- CI
Confidence intervals
- MHT
Miracidia Hatching technique
- IHA
Indirect hemagglutination assay
- NIPD
National Institute of Parasitic Diseases
- P. R. China
People’s Republic of China
- WHO
World Health Organization
Authors’ contributions
Xiao-Nong Zhou, Shi-Zhu Li, Jing Xu, Shan Lv and Chun-Li Cao designed and guided this study, Jing-Yi Guo and Li-Juan Zhang wrote the main manuscript text. All authors reviewed the manuscript and approved the final manuscript for publication.
Funding
This study was financially supported by the National Special Science and Technology Project for Major Infectious Diseases of China (No. 2018ZX10101002–002).
Availability of data and materials
All data generated or analyzed during this study are kept confidential by NIPD, China CDC. The datasets are available from the corresponding author on a reasonable request.
Ethics approval and consent to participate
This article was based on analysis of routine surveillance data from NIPD, China CDC. The project leaders and staff led the review, analysis and interpretation of the data. No personal information was disclosed.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
Xiao-Nong is an Editor-in-Chief of the journal Infectious Diseases of Poverty. He was not involved in the peer-review or handling of the manuscript. The authors have no other competing interests to disclose.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40249-020-00758-4.
References
- 1.Lewis FA, Tucker MS. Schistosomiasis. Adv Exp Med Biol. 2014;766:47–75. doi: 10.1007/978-1-4939-0915-5_3. [DOI] [PubMed] [Google Scholar]
- 2.Elliott DE. Schistosomiasis: pathophysiology, diagnosis, and treatment. Gastroenterol Clin N Am. 1996;25(3):599–625. doi: 10.1016/S0889-8553(05)70265-X. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Schistosomiasis. https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. Accessed 2 Mar 2020.
- 4.Li SZ, Utzinger J, Bergquist R, Zhou XN. Preface: elimination of schistosomiasis japonica in the People’s Republic of China: the last leg. Adv Parasitol. 2016;92:xix–xxii. doi: 10.1016/S0065-308X(16)30052-5. [DOI] [Google Scholar]
- 5.Mao CP. A review of the epidemiology of schistosomiasis japonica in China. Am J Trop Med Hyg. 1948;28(5):659–672. doi: 10.4269/ajtmh.1948.s1-28.659. [DOI] [PubMed] [Google Scholar]
- 6.Mao SP, Shao BR. Schistosomiasis control in the People’s Republic of China. Am J Trop Med Hyg. 1982;31(1):92–99. doi: 10.4269/ajtmh.1982.31.92. [DOI] [PubMed] [Google Scholar]
- 7.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, et al. The public health significance and control of schistosomiasis in China--then and now. Acta Trop. 2005;96(2–3):97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96(2–3):69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Yang GJ, Liu L, Zhu HR, Griffiths SM, Tanner M, Bergquist R, et al. China’s sustained drive to eliminate neglected tropical diseases. Lancet Infect Dis. 2014;14(9):881–892. doi: 10.1016/S1473-3099(14)70727-3. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Yang K, Li SZ, Zhou XN. Surveillance system after transmission control of schistosomiasis in P.R. China. Chin J Schisto Control. 2014;26(1):1–5. [PubMed] [Google Scholar]
- 11.Zhang LJ, Li SZ, Wen LY, Lin DD, Abe EM, Zhu R, et al. The establishment and function of schistosomiasis surveillance system towards elimination in The People’s Republic of China. Adv Parasitol. 2016;92:117–141. doi: 10.1016/bs.apar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Deol AK, Fleming FM, Calvo-Urbano B, Walker M, Bucumi V, Gnandou I, et al. Schistosomiasis - assessing progress toward the 2020 and 2025 global goals. N Engl J Med. 2019;381(26):2519–2528. doi: 10.1056/NEJMoa1812165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhou XN, Jiang QW, Wu XH, Zhao GM, Lin DD, Zhang SQ, et al. The function and evolution of the criteria for control and elimination of schistosomiasis in China. Chin J Schisto Control. 2007;19(1):1–4.
- 15.Wen LY. Schistosomiasis surveillance manual. Beijing: People’s Medical Publishing House; 2014. [Google Scholar]
- 16.Health Mo . Handbook of schistosomiasis control. 3. Shanghai: Shanghai Science and Technology Press; 2000. [Google Scholar]
- 17.Wu XH, Chen MG, Zheng J. Surveillance of schistosomiasis in five provinces of China which have reached the national criteria for elimination of the disease. Acta Trop. 2005;96(2–3):276–281. doi: 10.1016/j.actatropica.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Huang SY, Rong SM, Liu JS, Ou ZY, Shi XC, Ma WJ. Results and analysis of schistosomiasis surveillance in Guangdong province. Chin J Parasit Dis Control. 1997;10(3):173–175. [Google Scholar]
- 19.Xu J, Steinman P, Maybe D, Zhou XN, Lv S, Li SZ, et al. Evolution of the national schistosomiasis control programmes in the People’s Republic of China. Adv Parasitol. 2016;92:1–38. doi: 10.1016/bs.apar.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Peeling RW, Chen JX, Wu XH, Wu ZD, Wang SP, et al. Evaluation of immunoassays for the diagnosis of Schistosoma japonicum infection using archived sera. PLoS Negl Trop Dis. 2011;5(1):e949. doi: 10.1371/journal.pntd.0000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JF, Yan XL, Wen LY, Zhang X, Yu LL, Du HJ, et al. Epidemiological analysis on the imported cases of schistosomiasis in Zhejiang Province during 1996-2017. Chin J Parasitol Parasit Dis. 2018;36(6):586–592. [Google Scholar]
- 22.Zhang JF, Yan XL, Du HJ, Yu LL, Sun F, Lin LJ, et al. Analysis of surveillance and control implications on schistosomiasis endemics in Zhejiang province from 2012 to 2014. Chin J Vector Biol Control. 2016;27(1):9–13. [Google Scholar]
- 23.Wen LY, Zhu MD, Yan XL, Chen JH, Zhang JF, Tao HQ. Report on surveillance of schistosomiasis in Zhejiang province from 1996 to 2005. Chin J Zoonoses. 2007;23(6):605–607. [Google Scholar]
- 24.Dai SM, Guan Z, Zhang LJ, Lv S, Cao CL, Li SZ, et al. Imported schistosomiasis, China, 2010-2018. Emerg Infect Dis. 2020;26(1):179–180. doi: 10.3201/eid2601.191250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JF, Wen LY, Xu J, Liang YS, Yan XL, Ren GH, et al. Current status and transmission risks of oversea imported schistosomiasis in China. Chin J Schisto Control. 2019;31(1):26–32. doi: 10.16250/j.32.1374.2019021. [DOI] [PubMed] [Google Scholar]
- 26.Guan Z, Lv S, Li SZ, Xu J. Endemic status of schistosomiasis in floating population and challenges in schistosomiasis control in China. Chin J Parasitol Parasit Dis. 2017;35(6):598–603. [Google Scholar]
- 27.Guan Z, Lv S, Li SZ, Dang H, Zhang LJ, Xu J. Analysis on the situation of schistosome infections in floating population in national schistosomiasis surveillance sites of China. Chin J Schisto Control. 2018;30(2):124–130. doi: 10.16250/j.32.1374.2017246. [DOI] [PubMed] [Google Scholar]
- 28.Li LS, Zhang RY, Cheng YZ, Lin CX, Chen BJ, Li YR, et al. Schistosomiasis endemic situation in Fujian Province from 2003 to 2008. Chin J Schisto Control. 2009;21(6):525–527. [Google Scholar]
- 29.Cheng YZ, Yu DL, Li LS, Lin CX, Chen ZL. Increase and spread of survival Oncomelania snails and future control strategy in Fujian Province. J Trop Dis Parasitol. 2006;4(3):143–146. [Google Scholar]
- 30.Ruan YQ, Li XM, Zhang HM, Tan YG, Huang FM, Lin R, et al. Surveillance of schistosomiasis in Guangxi Zhuang Autonomous Region, 2002--2007. Chin J Schisto Control. 2008;20(4):293–295. [Google Scholar]
- 31.Xie L, Gu WL, Fu XF. Investigation and analysis of Oncomelania hupensis snails in Jiaxing city, 2016. Pract Prev Med. 2019;26(11):1352–1355. [Google Scholar]
- 32.Jiang XJ, Wang KT, Jin FX, He TC, Huang DS. Investigations on snails’ distribution and ecology in rivers of Shanghai suburbs. Chin J Schisto Control. 2003;15(6):456–458. [Google Scholar]
- 33.Yang Y, Huang SY, Pei FQ, Chen Y, Jiang QW, Deng ZH, et al. Spatial distribution and habitat suitability of Biomphalaria straminea, intermediate host of Schistosoma mansoni, in Guangdong, China. Infect Dis Poverty. 2018;7(1):109. doi: 10.1186/s40249-018-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang YS, Wang W, Li HJ, Shen XH, Xu YL, Dai JR. The South-to-North Water Diversion Project: effect of the water diversion pattern on transmission of Oncomelania hupensis, the intermediate host of Schistosoma japonicum in China. Parasit Vectors. 2012;5:52. doi: 10.1186/1756-3305-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai SM, Edwards J, Guan Z, Lv S, Li SZ, Zhang LJ, et al. Change patterns of Oncomelania snail burden in areas within the Yangtze River drainage after the three gorges dam operated. Infect Dis Poverty. 2019;8(1):48. doi: 10.1186/s40249-019-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SL, Li YL, Zhang LJ, Lv S, Xu J. Thinking on schistosomiasis control under the strategy of China’s Yangtze River Economic Belt. Chin J Schisto Control. 2019;31(5):459–473. doi: 10.16250/j.32.1374.2019225. [DOI] [PubMed] [Google Scholar]
- 37.Zhou XN. Implementation of precision control to achieve the goal of schistosomiasis elimination in China. Chin J Schisto Control. 2016;28(1):1–4. [PubMed] [Google Scholar]
- 38.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 39.Abe EM, Tambo E, Xue J, Xu J, Ekpo UF, Rollinson D, et al. Approaches in scaling up schistosomiasis intervention towards transmission elimination in Africa: leveraging from the Chinese experience and lessons. Acta Trop. 2020;208:105379. doi: 10.1016/j.actatropica.2020.105379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Detailed grading rules for the assessment on surveillance capacity in the five provinces.
Additional file 2. The questionnaire on basic knowledge of schistosomiasis control.
Data Availability Statement
All data generated or analyzed during this study are kept confidential by NIPD, China CDC. The datasets are available from the corresponding author on a reasonable request.