Abstract
Streptococcus spp. are a source of morbidity and mortality in captive non-human primate populations. However, little is known about the lesions associated with naturally occurring streptococcal infections in baboons (Papio spp.). The pathology database of the Southwest National Primate Research Center was searched for all baboon autopsies from 1988 to 2018 in which Streptococcus spp. were cultured. Baboons on experimental protocol were excluded. The gross autopsy and histopathology reports were reviewed. Archived specimens were retrieved and reviewed as needed for confirmation or clarification. Fifty-six cultures were positive for Streptococcus spp. in 54 baboons with evidence of bacterial infection. Associated gross lesions included purulent exudate, fibrinous to fibrous adhesions, hemorrhage, mucosal thickening, organomegaly, and abscessation. Histologic lesions included suppurative inflammation, abscessation, necrosis, hemorrhage, fibrin accumulation, and thrombosis. Lungs and pleura (n=31) were the most commonly infected organ followed by the central nervous system (n=16), spleen (n=15), soft tissues (n=12), air sacs, liver, peritoneum, adrenal glands, heart, lymph nodes, uterus, kidneys, biliary system, bones, ears, umbilical structures, mammary glands, pancreas, placenta, and salivary glands. Infections by non-beta-hemolytic Streptococcus spp. predominated in the lungs and air sacs; the most common isolate was Streptococcus pneumoniae. Infections by beta-hemolytic Streptococcus spp. predominated in the soft tissues and reproductive tract. Naturally occurring beta-hemolytic and non-beta-hemolytic Streptococcus spp. infections cause morbidity and mortality in captive baboon populations. The lesions associated with streptococcal infection are similar to those reported in human infection. Thus, the baboon may represent an underutilized model for studying Streptococcus spp. as pathogens.
Keywords: baboon, bacteria, infection, meningitis, pneumonia, primate, splenitis, Streptococcus
Streptococcus spp. cause morbidity and mortality in captive non-human primates (NHP). Spontaneous streptococcal infections have been associated with upper respiratory disease, pneumonia, meningitis, sepsis, otitis, ophthalmitis, hepatitis, endometritis, and air sacculitis in captive NHP populations.3,4,12,19,22–24,26–28,32,34,39,44,46,51,54,57,58,62–64 In a 23-year study on spontaneous death in captive baboons, Streptococcus spp. were a leading cause of pneumonia, sepsis, and meningoencephalitis.16 However, the detailed description of the lesions of spontaneous streptococcal infections in baboons dates back more than 45 years and includes only 2 cases of infant baboons with polyarthritis and meningoencephalitis.7
Pathogenic strains and commensal Streptococcus spp. are a significant cause of human disease, including meningitis, pneumonia, cellulitis, necrotizing fasciitis, otitis, wound infection, and bacteremia9,14,18,29,36,60 Continued research is needed for the development of new vaccines, antibiotics, and treatment regimens for human streptococcal infections. Baboons are utilized as a translational model of various Streptococcus spp. infections due to their similarity to humans in terms of susceptibility to infection and the clinical course of disease.2,25,38,47,49,55,59 The value of baboons as an experimental model and the paucity of literature on spontaneous streptococcal infections emphasize the need for more studies on Streptococcus spp. infections in captive baboon populations. Here we present a 31-year retrospective study of streptococcal infection associated lesions in 54 baboons housed at the housed at the Southwest National Primate Research Center (SNPRC), Texas Biomedical Research Institute, San Antonio, TX.
Materials and Methods
Animals
Baboons were housed in two open-top 6-acre metal and concrete corrals with dirt floors, gang cages with concrete floors, or individual metal cages if special handling was required. Animals were fed commercial monkey chow (Purina 5 LEO Monkey Diet, Purina Mills, St. Louis, MO) and supplemented with grains, fruits, and vegetables. Water was available ad libitum. All procedures were approved by the SNPRC Institutional Animal Care and Use Committee. Autopsy and histopathology of baboons that died or were euthanized were performed by board-certified veterinary pathologists. Tissues were fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, sectioned at 5 μm, stained with hematoxylin and eosin (HE), and in some cases, Gram stain. Bacterial culture samples were taken at autopsy or antemortem within 7 days of autopsy. Cultures were performed by in-house or referral laboratories.
Case selection
The SNPRC pathology database for all baboon autopsies between 1988–2018 was searched for cases in which a Streptococcus sp. was cultured. Baboons on experimental protocol were excluded. The gross autopsy and histopathology reports were individually reviewed. Archived specimens were reviewed as needed for confirmation or clarification.
Bacterial nomenclature
Bacterial culture results (Supplemental Table S1) are presented as reported by the in-house or referral laboratories at autopsy or within the 7-day period prior to death. The data gathered during this 31-year-period overlap a time of major changes in Streptococcus spp. taxonomy and nomenclature. The current classification system relies on technologies that were not readily available in the past.17 In order to maintain the integrity and clarity of the data, the bacterial isolates were designated in the following manner: “non-beta Streptococcus spp.” include culture reports with S. pneumoniae, S. bovis, viridans streptococcus, microaerophilic streptococcus, and alpha-hemolytic Streptococcus spp. not otherwise specified. Isolates designated “beta Streptococcus spp.” include beta-hemolytic Streptococcus species with Lancefield antigen group A, B, C, G, or F, S. pyogenes, S. agalactiae, and beta-hemolytic Streptococcus spp. with group not otherwise specified. In cases in which the hemolytic activity was not indicated on the culture reports, the isolates were designated as “undetermined.” The current classification of Enterococcus spp. and Lactococcus spp. as separate from Streptococcus spp. was observed.
Lesion etiology
Lesions were attributed to streptococcal infection if a Streptococcus sp. was cultured from that tissue. Other tissues in a Streptococcus spp.-positive animal were deemed infected with Streptococcus spp. if neutrophilic inflammation was present in the tissue and there was no evidence of a separate cause.
Results
Over the 31-year (1988–2018) period of this study, 54 baboons had Streptococcus spp. infection detected at autopsy (Supplemental Table S1). In two cases (Cases 26 and 38), two different categories of Streptococcus spp. (non-beta Streptococcus spp. and beta Streptococcus spp.) were isolated from two separate culture locations resulting in 56 cultures positive for Streptococcus spp. Baboons ranged from 4 days to 23.4 years of age; 23 (43%) were female, and 31 (57%) were male. Twenty-seven baboons were found dead in their cages, 24 baboons were euthanized due to clinical signs, and 3 baboons (Cases 17, 43, and 49) were euthanized for colony management purposes.
Culture results
Streptococcus spp. culture results are summarized in Supplemental Table S1. In two baboons (Cases 35 and 47), beta Streptococcus spp. and non-beta Streptococcus spp. were isolated from the same site. In five baboons (Cases 11, 23, 36, 42, and 54), the same category (non-beta Streptococcus spp. versus beta Streptococcus spp.) of Streptococcus spp. were cultured from two different sites. In six baboons (Cases 2, 32, 38, 44, 46, and 52), the presence of multiple Lancefield group antigens from a single culture site indicated infection with multiple beta Streptococcus spp.
Of the 56 cultures positive for a streptococcal agent, Streptococcus spp. were the only isolates in 33 (59%) cases. The remaining 23 (41%) cultures were additionally positive for bacteria of other genera including Pasteurella multocida (n=7), Staphylococcus aureus (n=6), Escherichia coli (n=5), Klebsiella pneumoniae (n=3), Enterococcus spp. (n=2), Morganella morganii (n=2), Haemophilus spp. (n=1), and Lactobacillus spp. (n=1).
Cultured Streptococcus spp. were categorized as non-beta Streptococcus spp., beta Streptococcus spp., mixed beta and non-beta Streptococcus spp., or hemolytic activity not reported (undetermined). Tissue distribution and categorization of streptococcal isolates is summarized in Figure 1.
Figure 1:
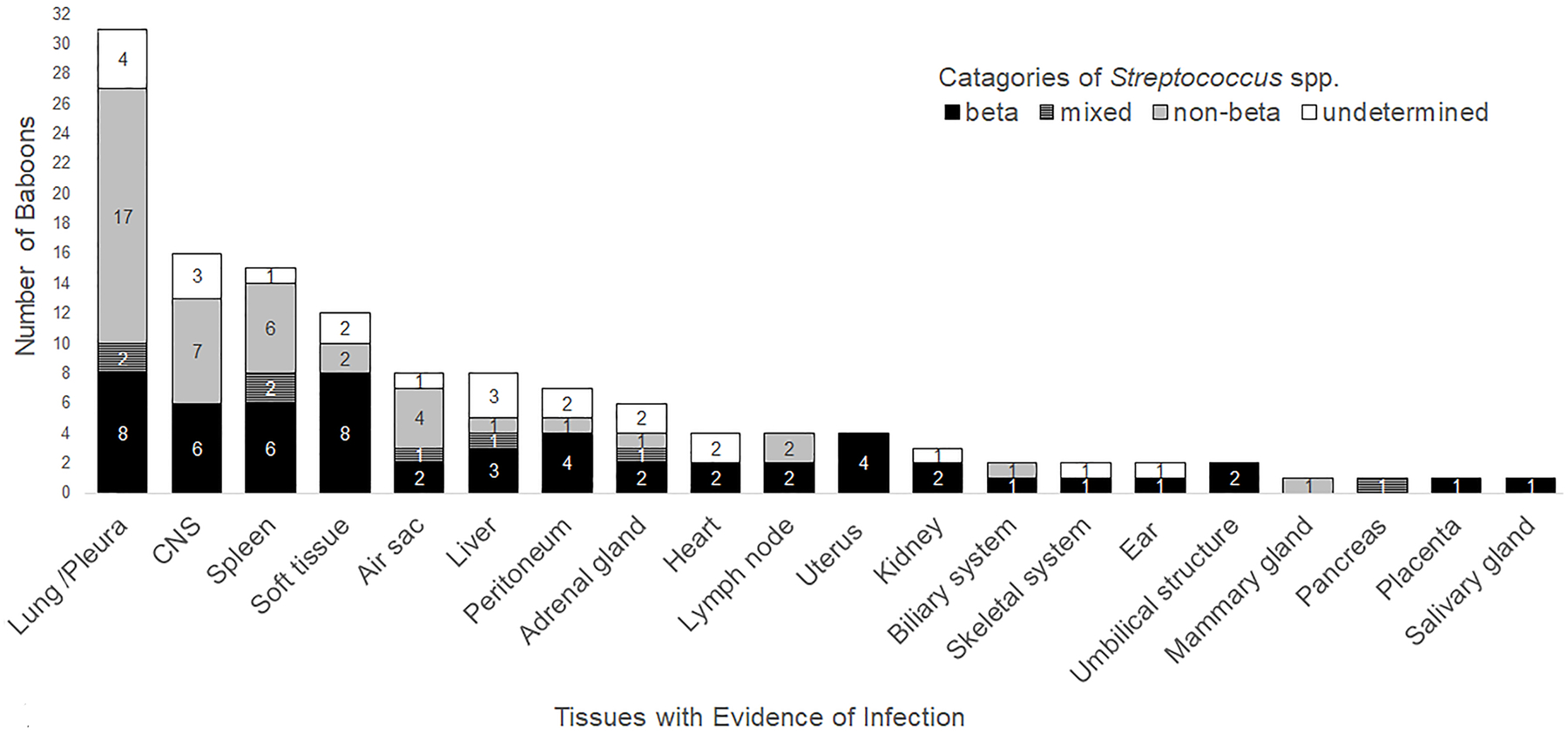
Streptococcus spp. categories and infected tissues. Tissues with evidence of Streptococcus spp. infection in 54 baboons are sorted by prevalence and category of Streptococcus spp. cultured. Beta includes beta Streptococcus spp. and is represented by the black bar, non-beta includes non-beta Streptococcus spp. and is represented by the grey bar, mixed includes beta and non-beta Streptococcus spp. and is represented by the striped grey and black bar, and undetermined includes Streptococcus spp. with hemolytic activity not reported and is represented by the white bar. Soft tissue includes skin, subcutis, and skeletal muscle. Biliary system includes biliary duct and gall bladder. CNS, central nervous system.
Pathology
All baboons in this study had gross lesions at autopsy that included purulent exudate, fibrinous to fibrous adhesions, hemorrhage, mucosal thickening, organomegaly, and abscessation (Figures 2–6). All tissues that cultured positive for Streptococcus spp. had suppurative inflammation histologically. Major histologic diagnoses/findings in tissues infected with Streptococcus spp. included abscessation, necrosis, hemorrhage, fibrin accumulation, and thrombosis (Figures 7–10). Thrombosis of small and medium-sized vessels within areas of intense inflammation and/or necrosis was detected in 18 (33%) baboons. Gram stain was available for some cases. Chains of gram-positive cocci, consistent with Streptococcus spp., were associated with regions of intense inflammation in Cases 2, 14, 21, 22, 25, 27, 29, 36, 42, and 44. The presence of gram-positive cocci could not be confirmed with Gram stain in Cases 9, 16, 31, 32, 35, 38, and 49.
Figure 2:
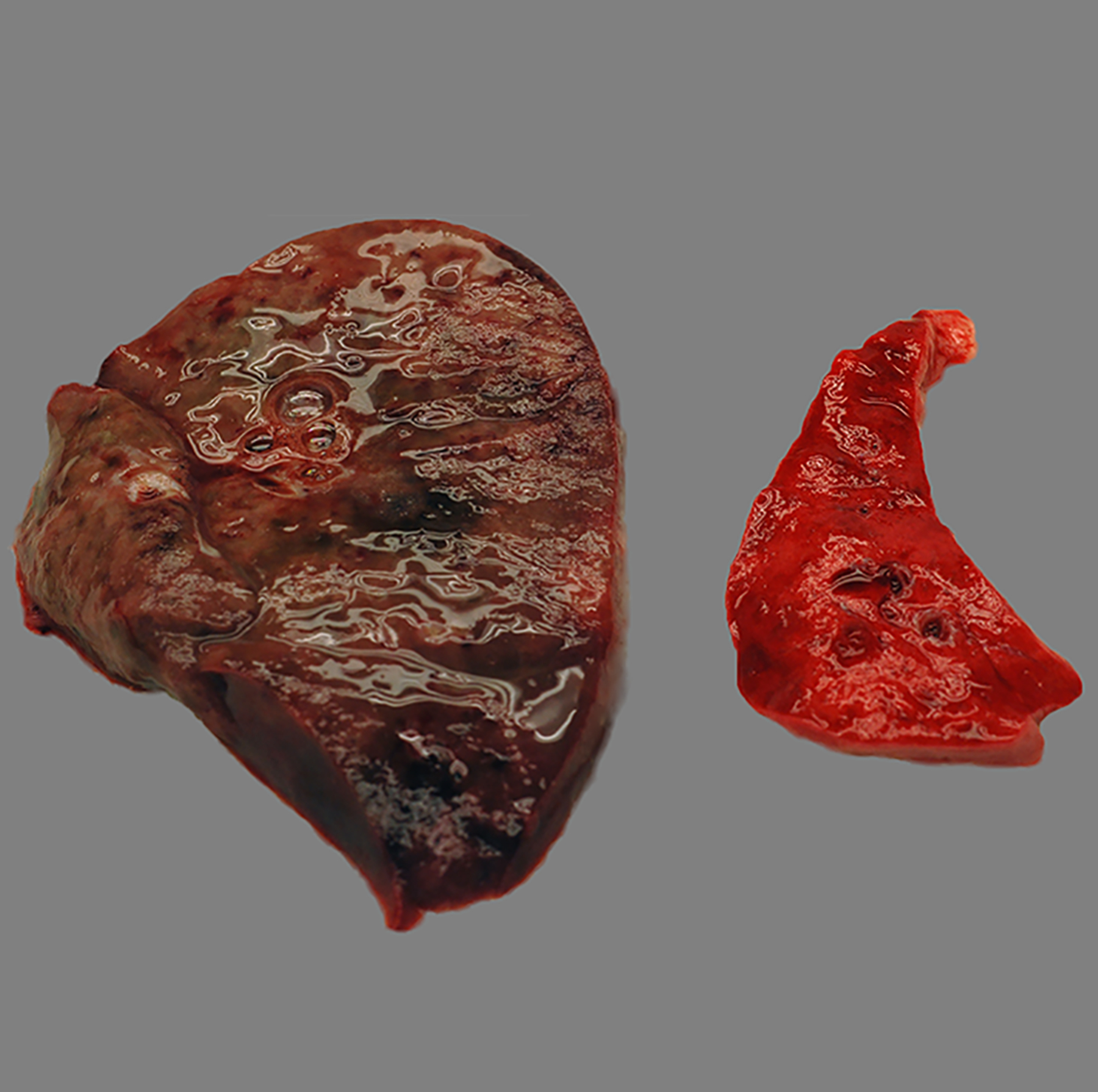
Purulent lobar bronchopneumonia, lung, baboon, case 24. The infected lung at left is swollen, wet, and dark red to black compared to the non-infected lung at right. The culture yielded Streptococcus pneumoniae.
Figure 6:
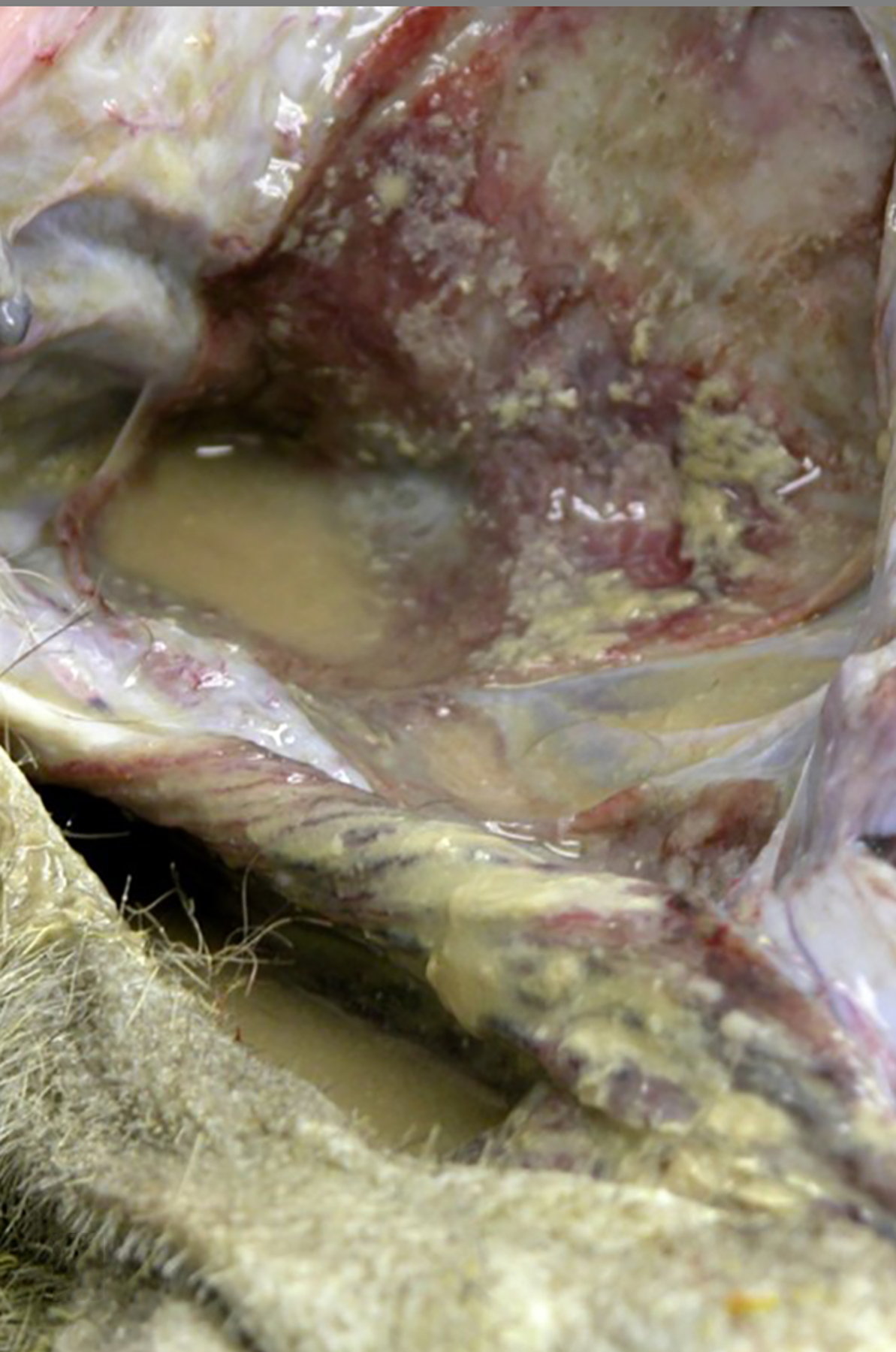
Purulent air sacculitis, air sacs, baboon , case 27. Thick, tan exudate is adhered to the mucosa with pooling of purulent, tan fluid in the air sacs bilaterally. The culture yielded non-beta-hemolytic Streptococcus spp. and Escherichia coli.
Figure 7:
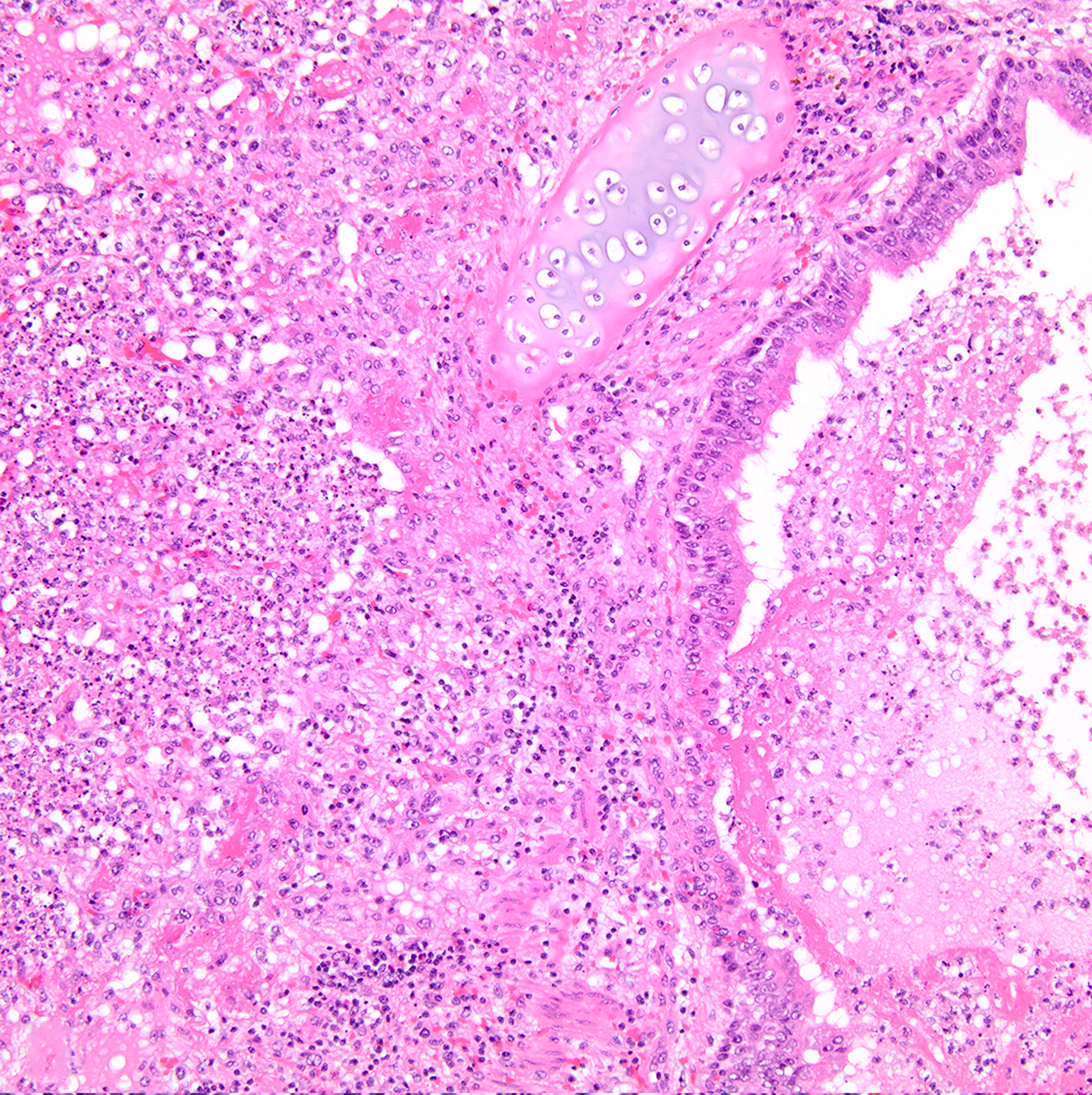
Necrosuppurative bronchopneumonia, lung, baboon , case 24. Airways and alveoli are filled with inflammatory infiltrates, necrotic cellular debris, and fibrin. The culture yielded Streptococcus pneumoniae. Hematoxylin and eosin (HE).
Figure 10:
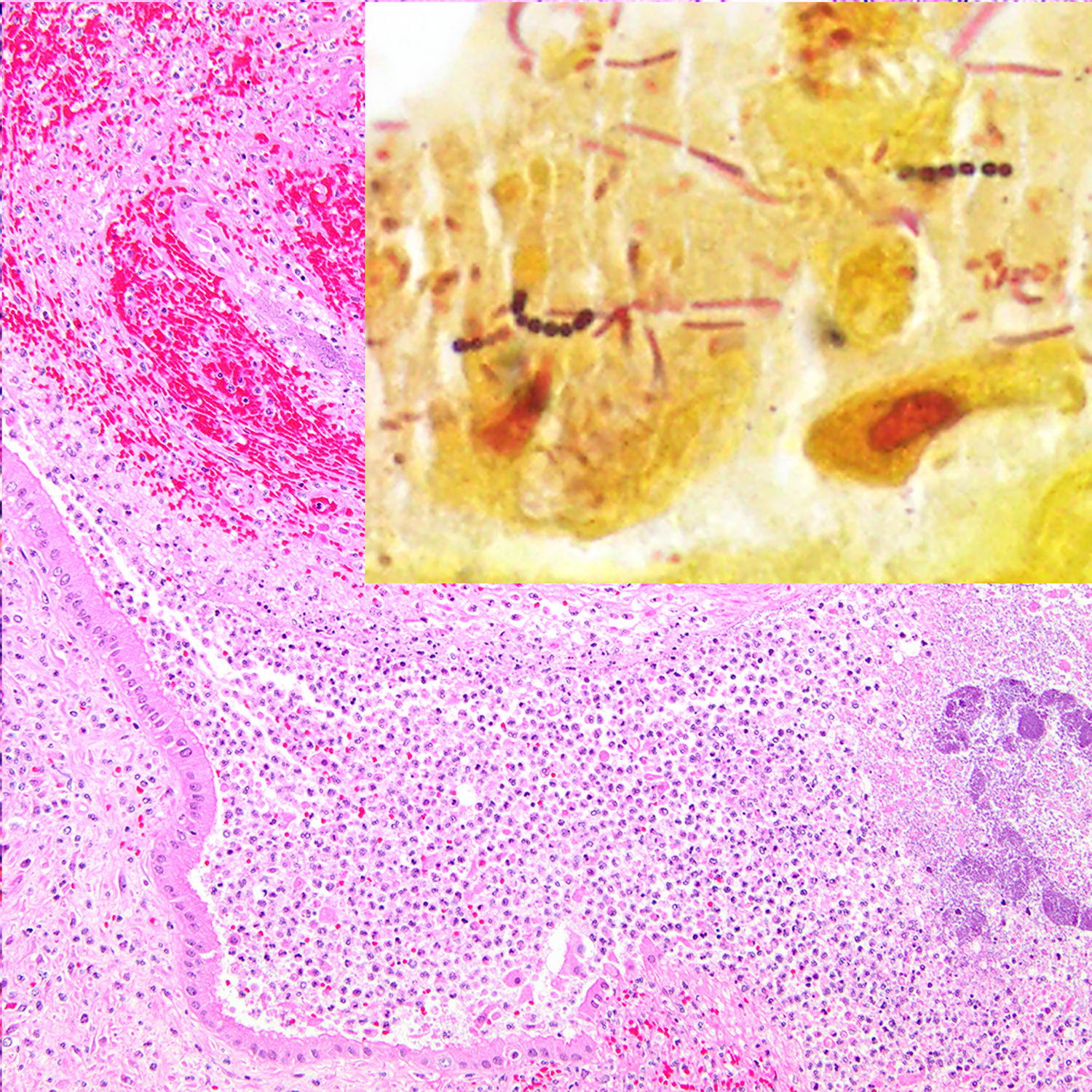
Suppurative metritis, uterus, baboon , case 42. The lumen of the uterus contains intense inflammatory infiltrates, hemorrhage, fibrin, and colonies of basophilic bacteria. The culture yielded beta-hemolytic Streptococcus spp. HE. Inset: chains of gram-positive cocci are associated with the epithelium. Pleomorphic gram-negative bacteria are also present. Gram stain.
Pneumonia was the most common diagnosis with 31 baboons affected. Gross evidence of pneumonia was present in 21 baboons and included lungs which were dark red, firm, and non-collapsing, and areas of necrosis or abscessation were variably present. Grossly visible pleuritis was present in 7 baboons with concurrent pneumonia and was identified by the presence of purulent material in the thorax and/or fibrinous adhesions between lung lobes and the parietal pleura. Non-beta Streptococcus spp. were cultured in 19 (61%) baboons and beta Streptococcus spp. were cultured in 10 (32%) baboons with pneumonia. There were 14 baboons in which the only bacterial lesion was pneumonia and/or pleuritis. Of these, 12 (86%) baboons were positive for a non-beta Streptococcus spp., the most common being S. pneumoniae (n=10).
There were 16 baboons with evidence of Streptococcus spp. infection of the CNS. Of these, 8 (50%) were less than 4 months of age and 8 (50%) were ages 1 year to 23 years. All baboons with infection of the CNS had gross evidence of disease which included cloudy meninges, hemorrhage, purulent material in the calvarium, malacia, and/or abscessation. Microscopic diagnoses included meningoencephalitis, meningitis, hemorrhage, thrombosis, encephalitis, and/or myelitis. Non-beta Streptococcus spp. were cultured in 7 (44%) baboons and beta Streptococcus spp. were cultured in 6 (38%) baboons with CNS lesions. Two animals (Cases 33 and 50) diagnosed with otitis were also diagnosed with meningitis.
Splenitis was diagnosed in 15 baboons. Gross lesions were present in 4 cases and included splenomegaly and/or abscessation. In all baboons with splenitis, two or more tissues were infected with Streptococcus spp. and the most commonly co-infected tissue was the brain (n=10). Non-beta Streptococcus spp. were cultured in 8 (53%) baboons and beta Streptococcus spp. were cultured in 8 (53%) baboons with splenitis.
Soft tissue infection which included the skin, muscle, and/or subcutaneous tissues was diagnosed in 12 baboons. Gross evidence of traumatic skin injury such as laceration, bite wounds, and/or draining tracts were present in 8 baboons, 6 of which cultured positive for beta Streptococcus spp. Overall, non-beta Streptococcus spp. were associated with 2 (17%) baboons and beta Streptococcus spp. were associated with 8 (67%) baboons with soft tissue lesions.
Air sacculitis was identified in 8 male baboons. Gross evidence of air sacculitis included purulent material in the air sac, thickening of the membranes, hemorrhage, and/or necrosis. Two baboons with air sacculitis were euthanized for colony management purposes and air sacculitis was an incidental finding (Cases 31 and 43). Five cases of air sacculitis had concurrent pneumonia. Non-beta Streptococcus spp. were associated with 5 baboons, and beta Streptococcus spp. were associated with 3 baboons with air sacculitis.
Four baboons were diagnosed with metritis and all cultured beta Streptococcus spp. Two baboons were post-partum and had evidence of retained placenta either grossly or microscopically (Cases 42 and 44). In case 46, a mummified fetus was removed by cesarean section before the animal was euthanized. In case 48, endometriosis was diagnosed in the uterus, ovary, colon, urinary bladder, and lung.
In conclusion, the most common tissues with evidence of Streptococcus sp. infection in 54 baboons were the lung and pleura (n=31), CNS (n=16), spleen (n=15), and soft tissue including skin, subcutis, and the muscle (n=12). Other sites of infection included the air sacs (n=8), liver (n=8), peritoneum (n=7), adrenal glands (n=6), heart (n=4), lymph nodes (n=4), uterus (n=4), kidneys (n=3), biliary system (n=2), bone (n=2), ear (n=2), umbilical structures (n=2), mammary gland (n=1), pancreas (n=1), placenta (n=1), and salivary gland (n=1).
Discussion
Between 1988–2018, 8,567 colony baboons (non-experimental) were autopsied at the SNPRC and Streptococcus spp. were isolated postmortem from 54 baboons. Only animals with gross evidence of bacterial infection are cultured at autopsy, therefore, the true prevalence of Streptococcus spp. infections at the SNPRC is likely higher and represents an important cause of morbidity and mortality in captive baboon populations.
Over the past 50 years, reports of spontaneous Streptococcus spp. infection in captive NHPs have been limited to sporadic outbreaks and individual cases.3,4,12,19,22–24,26–28,32,34,39,44,46,51,54,57,58,63–65 Culture results were often reported as Streptococcus spp. isolates with few reports providing speciation or further characterization. Furthermore, autopsy findings or histologic descriptions have been inconsistently reported, so current understanding of the pathology of streptococcal infection in NHPs is limited.
Literature on beta-hemolytic Streptococcus spp. infections in NHPs is particularly limited and the description of associated lesions is based on isolated case reports of infection with Group A Streptococcus spp., S. equi sub spp. zooepidemicus, beta-hemolytic Streptococcus spp., beta-hemolytic Streptococcus sp. not defined, and an outbreak of S. zooepidemicus contaminated food source.39,40,44,51,63 In this study, 23 baboons had beta-hemolytic Streptococcus spp. infection. Species and groups identified in this study include S. agalactiae, S. pyogenes, Group F Streptococcus, and cultures with Groups A, B, C, and G Streptococcus spp. We demonstrate the potential for these beta Streptococcus spp. to cause disease in the baboon.
It is important to note that 41% of the bacterial cultures were also positive for bacteria of other genera. We understand the dilemma this may cause in the interpretation of bacterial culture results. Establishing primary versus secondary infections with Streptococcus spp. and determining the role of bacteria as either commensal, synergistic, or opportunistic in the disease process cannot be determined with confidence since the normal resident bacterial flora in the upper respiratory tract, skin and other tissue compartments of our baboon population has not been characterized. Additionally, bacterial flora will vary depending on the water source, food, housing setting, captivity status, and between research institutions.10 Our intention was to present bacterial cultures as reported at the time of collection. We also recognize that the extent of bacterial isolation and characterization was variable among the different testing laboratories utilized over the period of this study; therefore, mixed bacterial isolates may be underreported in some cases.
Gram stain demonstrated chains of gram-positive cocci bacteria consistent with Streptococcus spp. in some, but not all cases in which Gram stain was performed. We found that correlation of active inflammatory disease of known bacterial origin (positive culture results) with presence or absence of bacteria on Gram stain was variable and, in some occasions, non-contributory. This is similar to other previously described studies.48
S. pneumoniae is one of the most prevalent pneumonia-causing bacterial pathogens in captive NHPs.52 We identified 21 baboons with S. pneumoniae infection; 14 of these baboons developed pneumonia. The predominance of S. pneumoniae in this and previous studies could reflect higher prevalence and pathogenicity; however, our findings suggest that beta Streptococcus spp. infections can cause similar lesions, are readily cultured, and result in mortality. The ability to reliably and easily speciate S. pneumoniae based on optochin sensitivity and bile solubility since the mid 1900’s might explain the more frequent reports of this streptococcal species as compared to others.6 However, misidentification of S. pneumoniae can occur with laboratory error, optochin resistance, and bile insolubility in some strains.62 Furthermore, Streptococcus spp. have undergone a number of reclassifications, nomenclature changes, and identification methods in the last 50 years which limits the interpretation of historic literature and laboratory culture results that lack exact methodology.17,31 Regardless of nomenclature conventions, Streptococcus spp. infections in NHPs and humans share common pathologic features that will be the focus of the remainder of the discussion with applicable correlations to specific Streptococcus spp. or groups.
Streptococcus spp. are well recognized as causes of human respiratory disease. The Centers for Disease Control and Prevention reports that up to 3 in 10 children and 1 in 10 adults with a sore throat have a Group A Streptococcus spp. infection. In young children with pneumonia, S. pneumoniae is the most common isolate.9 Human streptococcal infections typically cause upper respiratory disease that can progress to pneumonia.33 We identified 31 baboons with Streptococcus spp.-associated pneumonia. At the SNPRC, the nasal passages, tonsils and pharynx are not routinely evaluated in NHP diagnostic cases, so it is unknown if there was an upper respiratory component to any of the reported cases. Isolates from these cases included S. pneumoniae, beta Streptococcus spp., and/or non-beta Streptococcus spp.
Streptococcus spp. are important causes of meningitis in humans. Specifically, S. pneumoniae, S. agalactiae, S. pyogenes, and S. suis have been implicated as important pathogens with S. pneumoniae accounting for over half of all bacterial meningitis cases in humans of all ages in the United States.8 In the current study, 16 baboons of all ages had streptococcal meningitis and two of these cases were likely secondary to otitis. As of 2000, human infants and children in the United States are routinely vaccinated against pneumococcus, which has dramatically reduced the incidence of childhood meningitis. However, in countries where vaccination is not common, S. pneumoniae continues to be a significant cause of infant and childhood meningitis.30,36,61 In humans, S. pneumoniae causes bacteremia and meningitis by first colonizing the upper airways and causing upper and/or lower respiratory disease.33 We suspect a similar pathogenesis in spontaneous baboon cases of S. pneumoniae meningitis because eight baboons with meningitis also had concurrent lower respiratory disease. Whether S. pneumoniae can spontaneously colonize the upper airways in baboons is unknown; however, experimental colonization of the pharynx of healthy baboons persisted up to 6 weeks.2,25
Group B Streptococcus is also a primary cause of meningitis in children, especially in infants up to 3 months of age. Group B Streptococcus infection in infants can result from in utero infection, vaginal exposure during birth, or nosocomial exposure.1,30 We identified cases of S. pneumoniae and beta Streptococcus spp. in immature baboons, with baboons less than 4 months of age being over represented in cases of meningitis (8/16, 50%). Infant baboons at the SNPRC are not vaccinated against pneumococcus and deceased infants without obvious gross lesions are rarely cultured. Spontaneous streptococcal meningitis may be under-reported in baboon colonies and may represent an under-utilized model of infant meningitis.
In women, Group B Streptococcus is a part of normal vaginal flora but has the potential to result in ascending uterine infections in some pregnant women.1,30 The 4 baboons with beta Streptococcus spp. uterine infection presented with the following comorbidities: 2 animals with retained placenta, 1 with a mummified fetus, and 1 with endometriosis. Compromised uterine lining would likely have disrupted normal pathogen clearance and reproductive flora, possibly predisposing the animals to ascending infection.35 Streptococcus spp. have been identified as normal vaginal flora in healthy baboons; however, typing and species identification was not performed.45,53
It is well established that the spleen is important in the clearance of invasive streptococcal infections. Splenectomized or asplenic humans have 50-fold increased risk of developing a fulminant septic infection with encapsulated bacteria and suffer higher morbidity rates than patients with a spleen.15,43 In a study of invasive S. pneumoniae infection, small-dose intravenous inoculation of mice with S. pneumoniae resulted in a fivefold preferential sequestration by the spleen compared with the lung and liver within 60 min. This dramatic splenic sequestration was not observed in similar experiments with S. aureus, E. coli, or P. aeruginosa.13,37 Phagocytosis by resident splenic macrophages and neutrophils is important in S. pneumoniae clearance within the first 24 hours of infection. Furthermore, a dramatic influx of circulating neutrophils to the spleen of mice was observed 24 and 48 hours after inoculation with S. pneumoniae.13 In the current study, neutrophilic splenitis was evident in 15 baboons, all of which had more than one tissue site infected and could be classified as having an invasive Streptococcus spp. infection.
Streptococcus spp. in humans are associated with skin lesions including pyoderma, abscesses, surgical site infections, and type II necrotizing fasciitis.18,20,21,60 Traumatic skin lesions and/or cellulitis with Streptococcus spp. infection was observed in 12 baboons. Beta Streptococcus spp. have been cultured from the skin, eyes, throat, nares, and gastrointestinal tract of healthy baboons.5,11,41 Although the specific species and pathogenicity of these isolates were not explored, this suggests that some beta Streptococcus spp. may represent normal flora in baboons, with the ability to be opportunistic pathogens.
Bacterial species, strain, and virulence factors can have major implications for streptococcal pathogenicity and virulence in humans.1,8,29,42 Human streptococcal isolates are well-defined via molecular characterization and genetic sequencing resulting in multiple levels of sub-speciation and serological characterization.17 The advanced sequencing and typing techniques that are routine in human cases are rarely if ever performed at NHP breeding facilities or zoos. More information regarding the pathogenicity of specific Streptococcus spp. in NHP populations may become available as advanced diagnostic techniques become more readily available and affordable in the diagnostic setting. Regardless of specific species and serotypes, a number of similarities in the lesions in NHP and human streptococcal infections are apparent and highlight the relevance of the baboon as a model for vaccine development.50,56 Furthermore, we demonstrate the pathogenicity and potential for disseminated disease in multiple different Streptococcus spp. in captive baboons.
Supplementary Material
Figure 3:
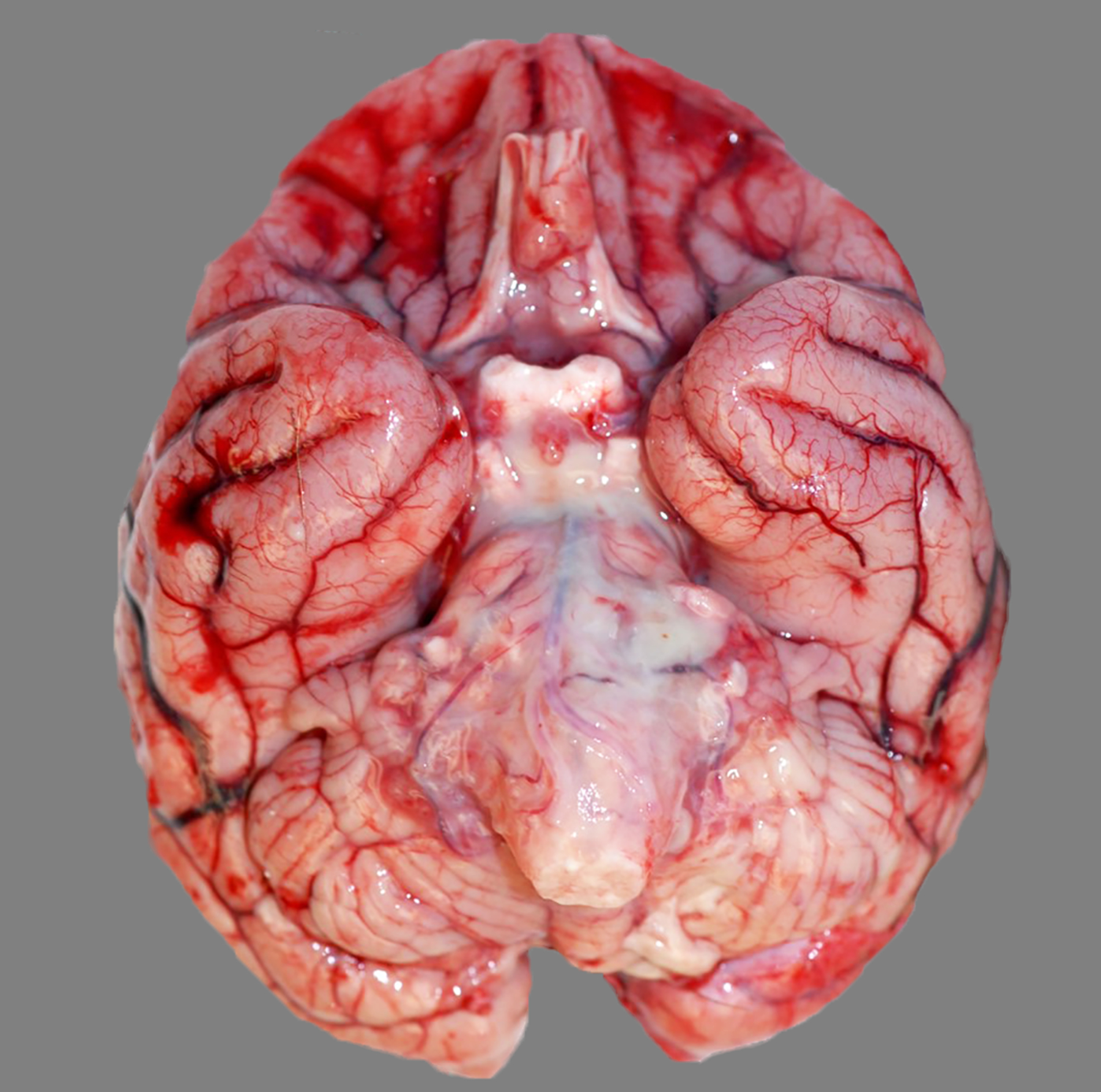
Purulent meningoencephalitis, brain, baboon, case 33. The meninges of the ventral brain stem are expanded by cloudy tan to white, purulent exudate. The culture yielded beta-hemolytic Streptococcus spp.
Figure 4:
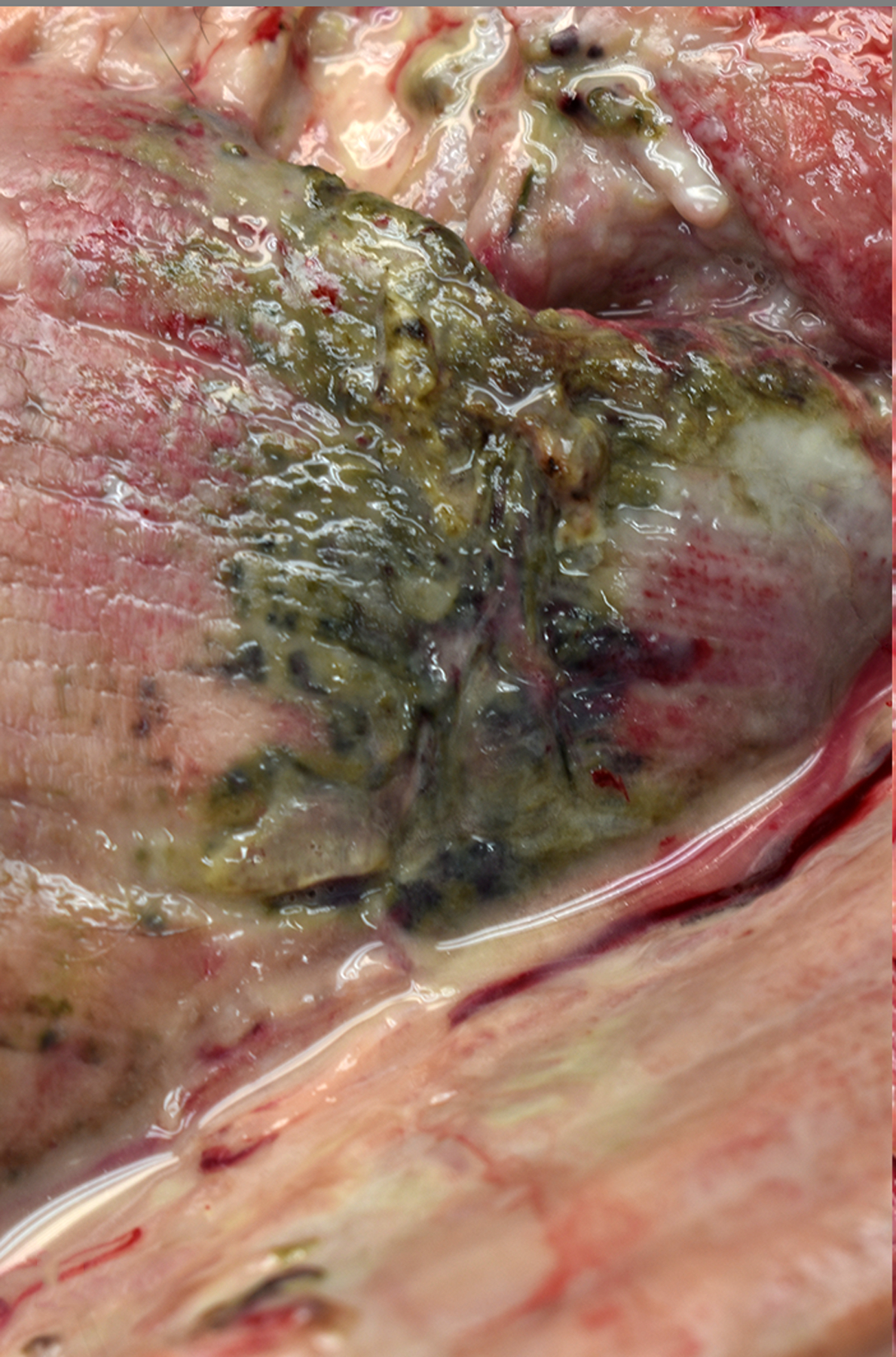
Purulent myositis, skeletal muscle, baboon , case 26. Skeletal muscle of the ventral left thigh is soft, green to black, and oozes cloudy, purulent fluid on section. The culture yielded beta-hemolytic Streptococcus spp.
Figure 5:
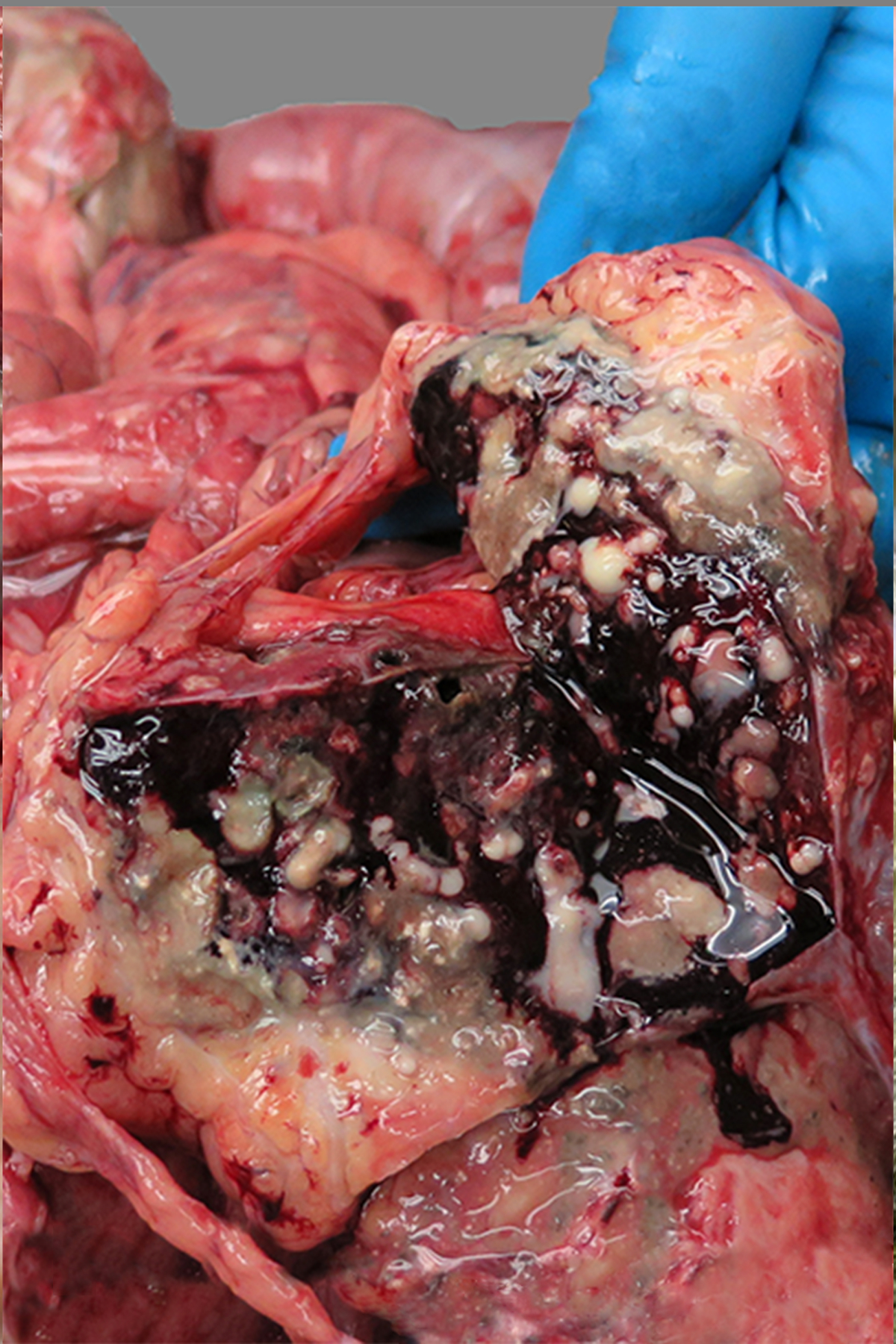
Abscesses, spleen, baboon , case 36. The spleen is enlarged. On cut section, multifocal to coalescing nodules exude white to green purulent fluid. The culture yielded beta-hemolytic Streptococcus spp. and Pasteurella multocida.
Figure 8:
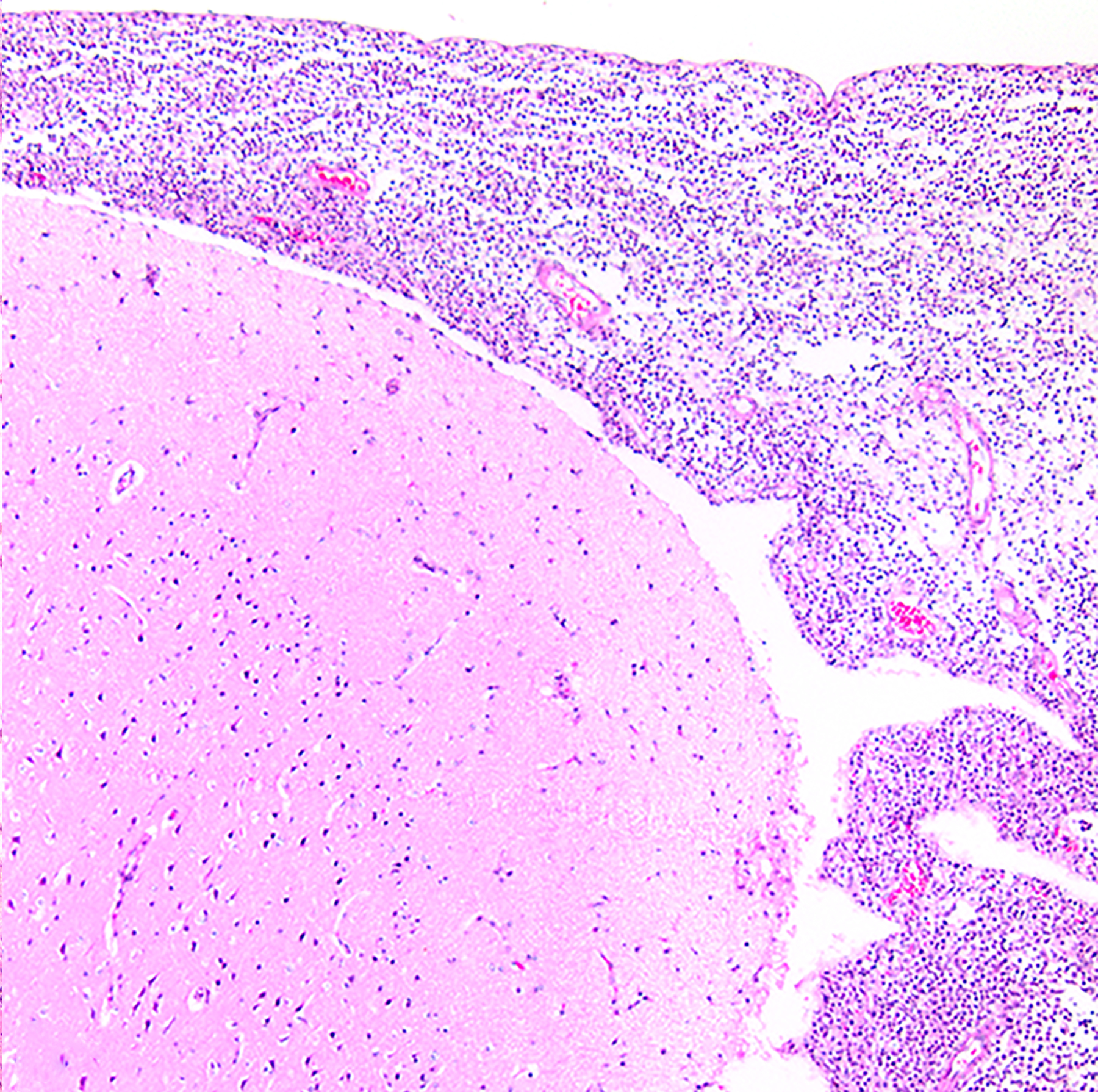
Suppurative meningoencephalitis, brain, baboon , case 11. The meninges are markedly expanded by intense inflammatory infiltrates. The culture yielded Streptococcus pneumoniae. HE.
Figure 9:
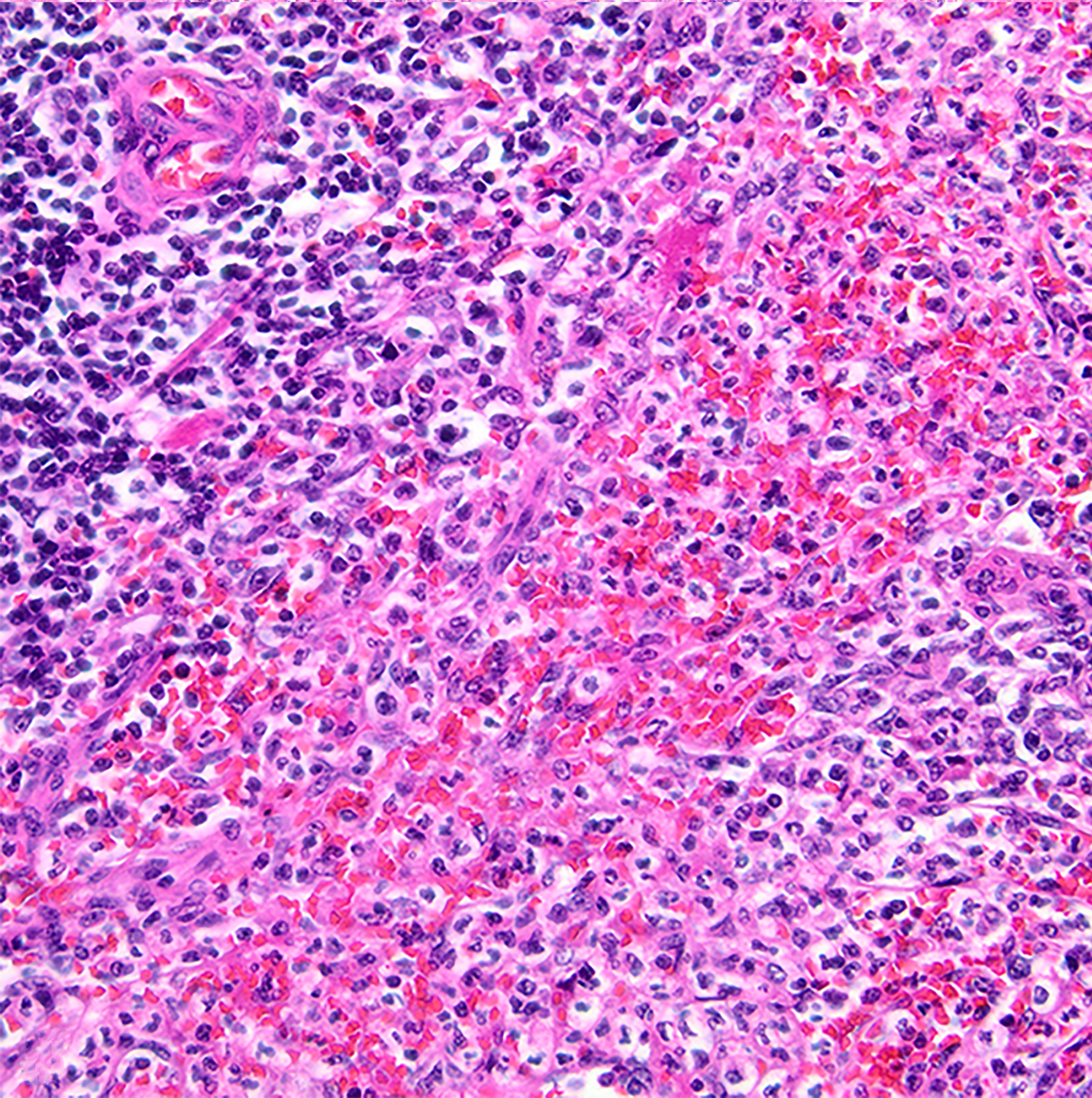
Suppurative splenitis, spleen, baboon , case 12. Neutrophils infiltrate the splenic red pulp. The culture yielded Streptococcus pneumoniae. HE.
Acknowledgements
The authors would like to thank Sarah Pennington, Colin Chuba, Jesse Martinez, and Renee Escalona for their anatomic pathology support and the clinical support staff of the Texas Biomed. Additionally, the authors would like to thank Margaret Miller for her assistance in preparing this manuscript for publication. Dr. Davis is a Molecular Pathology Fellow in the Comparative Biomedical Scientist Training Program supported by the National Cancer Institute in partnership with Purdue University.
Funding
This investigation used resources that were supported by the Southwest National Primate Research Center grant P51 OD011133 from the Office of Research Infrastructure Programs, National Institutes of Health. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number C06 RR-14578 and C06 RR015456.
Footnotes
Declaration of Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Katelin L Davis, Department of Comparative Pathobiology, Purdue University, West Lafayette, IN; Comparative Biomedical Scientist Training Program, National Cancer Institute, Bethesda, MD.
Olga Gonzalez, Southwest National Primate Research Center, San Antonio, TX.
Shyamesh Kumar, Southwest National Primate Research Center, San Antonio, TX.
Edward J Dick, Jr., Southwest National Primate Research Center, San Antonio, TX
References
- 1.Armistead B, Oler E, Adams Waldorf K, Rajagopal L. The double life of group B Streptococcus: asymptomatic colonizer and potent pathogen. J Mol Biol. 2019. 26;431:2914–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashbaugh CD, Moser TJ, Shearer MH, White GL, Kennedy RC, Wessels MR. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell Microbiol. 2000;2:283–292. [DOI] [PubMed] [Google Scholar]
- 3.Berendt RF, McDonough WE, Walker JS. Persistence of diplococcus pneumoniae after influenza virus infection in Macaca mulatta. Infect Immun. 1974;10:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleyer M, Kunze M, Gruber-Dujardin E, Mätz-Rensing K. Spontaneous lung pathology in a captive common marmoset colony (Callithrix jacchus). Primate Biol. 2017;4:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boncyk L Normal and pathogenic bacteria isolated from conditioned, non-conditioned and wild baboons (Papio Cynocephalus). Theses Diss. 1973. [Google Scholar]
- 6.Bowers EF, Jeffries LR. Optochin in the identification of Str. pneumoniae. J Clin Pathol. 1955;8:58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack M, Boncyk L, Moore G, Kalter S. Bacterial meningo-encephalitis in newborn baboons (Papio cynocephalus). Contemp Primatol. 1974;493–501. [Google Scholar]
- 8.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National center for immunization and respiratory diseases. Division of bacterial diseases. 2019. [Google Scholar]
- 10.Clayton JB, Gomez J, Amato K, et al. The gut microbiome of nonhuman primates: Lessons in ecology and evolution. Am J Primatol. 2018;80:e22867. [DOI] [PubMed] [Google Scholar]
- 11.Council SE, Savage AM, Urban JM, et al. Diversity and evolution of the primate skin microbiome. Proc Biol Sci. 2016;283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullin CO, Colgin LMA, Lewis AD. Air sacculitis in three rhesus macaques (Macaca mulatta) and one Japanese macaque (M. fuscata). J Med Primatol. 2017;46:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deniset JF, Surewaard BG, Lee W-Y, Kubes P. Splenic Ly6Ghigh mature and Ly6Gint immature neutrophils contribute to eradication of S. pneumoniae. J Exp Med. 2017;214:1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deutscher M, Lewis M, Zell ER, et al. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;53:114–123. [DOI] [PubMed] [Google Scholar]
- 15.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet Lond Engl. 2011;378:86–97. [DOI] [PubMed] [Google Scholar]
- 16.Dick EJ, Owston MA, David JM, Sharp RM, Rouse S, Hubbard GB. Mortality in captive baboons (Papio spp.): a 23 year study. J Med Primatol. 2014;43:169–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facklam R What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazili T, Riddell S, Kiska D, et al. Streptococcus anginosus Group Bacterial Infections. Am J Med Sci. 2017;354:257–261. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG, Wikse SE. Bacterial meningoencephalitis in rhesus monkeys: clinical and pathological features. Lab Anim Sci. 1971;21:558–563. [PubMed] [Google Scholar]
- 20.Frick S, Cerny A. Necrotizing fasciitis due to Streptococcus pneumoniae after intramuscular injection of nonsteroidal anti-inflammatory drugs: report of 2 cases and review. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;33:740–744. [DOI] [PubMed] [Google Scholar]
- 21.Furuichi M, Horikoshi Y. Sites of infection associated with Streptococcus anginosus group among children. J Infect Chemother Off J Jpn Soc Chemother. 2018;24:99–102. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert SG, Reuhl KR, Wong JH, Rice DC. Fatal pneumococcal meningitis in a colony-born monkey (Macaca fascicularis). J Med Primatol. 1987;16:333–338. [PubMed] [Google Scholar]
- 23.Good RC, May BD. Respiratory pathogens in monkeys. Infect Immun. 1971;3:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graczyk TK, Cranfield MR, Kempske SE, Eckhaus MA. Fulminant Streptococcus pneumoniae meningitis in a lion-tailed macaque (Macaca silenus) without detected signs. J Wildl Dis. 1995;31:75–78. [DOI] [PubMed] [Google Scholar]
- 25.Gryllos I, Cywes C, Shearer MH, Cary M, Kennedy RC, Wessels MR. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol Microbiol. 2001;42:61–74. [DOI] [PubMed] [Google Scholar]
- 26.Herman PH, Fox JG. Panophthalmitis associated with diplococcic septicemia in a rhesus monkey. J Am Vet Med Assoc. 1971;159:560–562. [PubMed] [Google Scholar]
- 27.Hubbard GB, Lee DR, Eichberg JW. Diseases and pathology of chimpanzees at the southwest foundation for biomedical research. J Med Primatol. 1991;24:273–282. [Google Scholar]
- 28.hms EA, Daniels JB, Koivisto CS, Barrie MT, Russell DS. Fatal Streptococcus anginosus-associated pneumonia in a captive Sumatran orangutan (Pongo abelii). J Med Primatol. 2014;43:48–51. [DOI] [PubMed] [Google Scholar]
- 29.Iovino F, Seinen J, Henriques-Normark B, van Dijl JM. How does Streptococcus pneumoniae invade the brain? Trends Microbiol. 2016;24:307–315. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs DM, Yung F, Hart E, Nguyen MNH, Shaver A. Trends in pneumococcal meningitis hospitalizations following the introduction of the 13-valent pneumococcal conjugate vaccine in the United States. Vaccine. 2017. 27;35:6160–6165. [DOI] [PubMed] [Google Scholar]
- 31.Jensen A, Scholz CFP, Kilian M. Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int J Syst Evol Microbiol. 2016;66:4803–4820. [DOI] [PubMed] [Google Scholar]
- 32.Johnson AL, Ducore RM, Colgin LM, Lewis AD. Hepatic abscesses in five outdoor-housed rhesus macaques (Macaca mulatta). J Med Primatol. 2014;43:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. [DOI] [PubMed] [Google Scholar]
- 34.Keeling M, McClure H. Pneumococcal meningitis and fatal enterobiasis in a chimpanzee. Lab Anim Sci. 1974;24:92–95. [Google Scholar]
- 35.Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum Reprod Oxf Engl. 2014;29:2446–2456. [DOI] [PubMed] [Google Scholar]
- 36.Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32–42. [DOI] [PubMed] [Google Scholar]
- 37.Kolaczkowska E, Jenne CN, Surewaard BGJ, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraft BD, Piantadosi CA, Benjamin AM, et al. Development of a novel preclinical model of pneumococcal pneumonia in nonhuman primates. Am J Respir Cell Mol Biol. 2014;50:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Fox B, Owston M, Hubbard GB, Dick EJ. Pathology of spontaneous air sacculitis in 37 baboons and seven chimpanzees and a brief review of the literature. J Med Primatol. 2012;41:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leong KM, Terrell SP, Savage A. Causes of mortality in captive cotton‐top tamarins (Saguinus oedipus). Zoo Biol. 2004;23:127–137. [Google Scholar]
- 41.Lugano SD, Nyerere KA, Kariuki WK, Samuel K, Joseph K, Apondi OJ. Gastrointestinal microbial flora in wild and captive olive baboons (Papio anubis). Am J Infect Dis Microbiol. 2018. March 23;6:30–37. [Google Scholar]
- 42.Madrid L, Seale AC, Kohli-Lynch M, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;65:S160–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Asplenic patients and invasive pneumococcal disease—how bad is it these days? Int J Infect Dis. 2016;51:27–30. [DOI] [PubMed] [Google Scholar]
- 44.Mätz-Rensing K, Winkelmann J, Becker T, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infection in a group of rhesus monkeys (Macaca mulatta). J Med Primatol. 2009;38:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obiero JA, Waititu KK, Mulei I, Omar FI, Jaoko W, Mwethera PG. Baboon vaginal microbial flora. J Med Primatol. 2016;45:147–155. [DOI] [PubMed] [Google Scholar]
- 46.Padovan D, Cantrell C. Causes of death of infant rhesus and squirrel monkeys. J Am Vet Med Assoc. 1983;183:1182–1184. [PubMed] [Google Scholar]
- 47.Paoletti LC, Pinel J, Kennedy RC, Kasper DL. Maternal antibody transfer in baboons and mice vaccinated with a group B streptococcal polysaccharide conjugate. J Infect Dis. 2000;181:653–658. [DOI] [PubMed] [Google Scholar]
- 48.Queiros da Mota V, Prodhom G, Yan P, Hohlfheld P, Greub G, Rouleau C. Correlation between placental bacterial culture results and histological chorioamnionitis: a prospective study on 376 placentas. J Clin Pathol. 2013;66:243‐248. [DOI] [PubMed] [Google Scholar]
- 49.Reyes LF, Restrepo MI, Hinojosa CA, et al. A non-human primate model of severe pneumococcal pneumonia. PloS One. 2016;11:e0166092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera-Hernandez T, Carnathan DG, Jones S, et al. An Experimental Group A Streptococcus Vaccine That Reduces Pharyngitis and Tonsillitis in a Nonhuman Primate Model. MBio. 2019. 30;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiller CA, Wolff MJ, Munson L, Montali RJ. Streptococcus zooepidemicus infections of possible horsemeat source in red-bellied tamarins and Goeldi’s monkeys. J Zoo Wildl Med. 1989;20:322–327. [Google Scholar]
- 52.Simmons J, Gibson S. Bacterial and mycotic diseases of nonhuman primates. In: Vol. 1, Nonhuman primates in biomedical research. 2012. [Google Scholar]
- 53.Skangalis M, Swenson CE, Mahoney CJ, O’Leary WM. The normal microbial flora of the baboon vagina. J Med Primatol. 1979;8:289–297. [DOI] [PubMed] [Google Scholar]
- 54.Solleveld HA, van Zwieten MJ, Heidt PJ, van Eerd PM. Clinicopathologic study of six cases of meningitis and meningoencephalitis in chimpanzees (Pan troglodytes). Lab Anim Sci. 1984;34:86–90. [PubMed] [Google Scholar]
- 55.Stevens DL, Bryant AE, Hackett SP, et al. Group A streptococcal bacteremia: the role of tumor necrosis factor in shock and organ failure. J Infect Dis. 1996;173:619–626. [DOI] [PubMed] [Google Scholar]
- 56.Sumby P, Tart AH, Musser JM. A Non-Human Primate Model of Acute Group A Streptococcus Pharyngitis In: DeLeo FR, Otto M, eds. Bacterial Pathogenesis: Methods and Protocols. Totowa, NJ: Humana Press; 2008:255–267. [DOI] [PubMed] [Google Scholar]
- 57.Swindle MM, Craft CF, Marriott BM, Strandberg JD, Luzarraga M. Ascending intrauterine infections in rhesus monkeys. J Am Vet Med Assoc. 1982;181:1367–1370. [PubMed] [Google Scholar]
- 58.Szentiks CA, Köndgen S, Silinski S, Speck S, Leendertz FH. Lethal pneumonia in a captive juvenile chimpanzee (Pan troglodytes) due to human-transmitted human respiratory syncytial virus (HRSV) and infection with Streptococcus pneumoniae. J Med Primatol. 2009;38:236–240. [DOI] [PubMed] [Google Scholar]
- 59.Taylor FB, Bryant AE, Blick KE, et al. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clin Infect Dis Off Publ Infect Dis Soc Am. 1999;29:167–177. [DOI] [PubMed] [Google Scholar]
- 60.Tezer Tekçe Y, Erbay A, Ünaldı Ö, Çabadak H, Şen S, Durmaz R. An outbreak of Streptococcus pyogenes surgical site infections in a cardiovascular surgery department. Surg Infect. 2015;16:151–154. [DOI] [PubMed] [Google Scholar]
- 61.Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;46:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varghese R, Jayaraman R, Veeraraghavan B. Current challenges in the accurate identification of Streptococcus pneumoniae and its serogroups/serotypes in the vaccine era. J Microbiol Methods. 2017;141:48–54. [DOI] [PubMed] [Google Scholar]
- 63.Wagner JD, Jayo MJ, Bullock BC, Washburn SA. Gestational diabetes mellitus in a cynomolgus monkey with group A streptococcal metritis and hemolytic uremic syndrome. J Med Primatol. 1992;21:371–374. [PubMed] [Google Scholar]
- 64.Zimmermann N, Pirovino M, Zingg R, et al. Upper respiratory tract disease in captive orangutans (Pongo sp.): prevalence in 20 European zoos and predisposing factors. J Med Primatol. 2011;40:365–375. [DOI] [PubMed] [Google Scholar]
- 65.Zou S, Luo Q, Chen Z, et al. Isolation, identification of Streptococcus pneumoniae from infected rhesus monkeys and control efficacy. J Med Primatol. 2010;39:417–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


