ABSTRACT
Background
There are few descriptions of the clinical presentation and evolution of consecutive SARS-CoV-2 infections with a long-enough follow up.
Methods
Description of the first consecutive 100 patients with microbiologically-proven COVID-19 in a large hospital in Madrid, Spain including a minimum of two-month follow up.
Results
The median age of the patients (52% males) was 61.5 years (IQR=39.5-82.0) and the median BMI was 28.8 kg/m2 (IQR=24.7-33.7). Overall 72% of the patients had one or more co-morbid conditions with a median age-adjusted Charlson index of 2 (IQR=0-5.7). Five patients (5%) were immunosup-pressed. The most common symptoms at the time of diagnosis were fever (80.0%), cough (53.0%) and dyspnea (23.0%). The median O2 saturation at the time of first examination was 94% (IQR=90-97). Chest X-ray on admission was compatible with pneumonia in 63% of the cases (bilateral in 42% and unilateral in 21%). Overall, 30% were managed at home and 70% were admitted to the hospital. Thirteen patients were admitted to the ICU with a median of 11 days of stay in the Unit (IQR=6.0-28.0). CALL score of our population ranged from 4 to 13. Overall, 60.0% of patients received antibiotic treatment and 66.0%, empirical antiviral treatment, mainly with lopina-vir/ritonavir (65%) or hydroxychloroquine (42%). Mortality, with a minimum of 60 days of follow up, was 23%. The median age of the deceased patients was 85 years (IQR=79-93).
Conclusions
We found a high mortality in the first 100 patients diagnosed with COVID-19 at our institution, associated with advanced age and the presence of serious underlying diseases.
Keywords: SARS-CoV-2, COVID-19, Coronavirus
RESUMEN
Antecedentes
Existen pocas descripciones de la presentación clínica y evolución de infecciones consecutivas por SARS-CoV-2 con un seguimiento lo suficientemente largo.
Métodos
Descripción de los primeros 100 pacientes consecutivos con COVID-19 probada microbiológicamente en un gran hospital de Madrid, incluyendo un seguimiento mínimo de dos meses.
Resultados
La mediana de edad de los pacientes (52% hombres) fue de 61,5 años (RIC=39,5-82,0) y la mediana de IMC fue de 28,8 kg/m2 (RIC=24,7-33,7). El 72% de los pacientes tuvieron una o más comorbilidades con un índice de Charlson ajustado a la edad de 2 (RIC=0-5,7). Cinco pacientes (5%) estaban inmunodeprimidos. Los síntomas más comunes al momento del diagnóstico fueron fiebre (80,0%), tos (53,0%) y disnea (23,0%). La mediana de saturación de O2 en el momento del primer examen fue del 94% (RIC=90-97). La radio-grafía de tórax al ingreso fue compatible con neumonía en el 63% de los casos (bilateral en el 42% y unilateral en el 21%). El 30% fueron manejados en su domicilio y el 70% ingresados en el hospital. Trece pacientes ingresaron en la UCI con una mediana de 11 días de estancia en la Unidad (RIC=6,0-28,0). El score CALL de nuestra población varió de 4 a 13. En general, el 60,0% de los pacientes recibió tratamiento antibiótico y el 66,0%, tratamiento antiviral empírico, principalmente con lopinavir/ritonavir (65%) o hidroxicloroquina (42%). La mortal-idad, con un mínimo de 60 días de seguimiento, fue del 23%. La mediana de edad de los pacientes fallecidos fue de 85 años (RIC=79-93).
Conclusiones
Encontramos una alta mortalidad en los primeros 100 pacientes diagnosticados con COVID-19 en nuestra institución, asociada con edad avanzada y presencia de enfermedades subyacentes graves.
Palabras clave: SARS-CoV-2, COVID-19, Coronavirus
INTRODUCTION
The COVID-19 epidemic is yielding highly variable data on incidence, evolution and mortality from one report to another, largely because the populations described are not comparable. On the other hand, the rush to provide valid scientific information on the epidemic means that many reports are preliminary and do not offer a sufficiently comprehensive perspective on the evolution of patients [1-10].
The first case of COVID-19 was confirmed in the Community of Madrid on February 27th, 2020 in a 24-year-old patient who had recently travelled to northern Italy. On March 1st, 2020, our institution (Hospital General Universitario Gregorio Marañón HGUGM) admitted the first confirmed case to the Center and within 10 days another 99 patients were diagnosed consecutively. During this period, the diagnosis of SARS-CoV-2 infection was offered exclusively to symptomatic patients.
The criteria for hospital admission was initially systematic but soon those who did not have severity criteria began to be treated at home.
Having these first 100 patients a follow-up of 60, or more days in all cases, our objective is to evaluate this first series, with a special perspective on its presentation, treatment, evolution, and mortality.
MATERIAL AND METHODS
Location of the study. The Hospital General Universitario Gregorio Marañón is a general and reference hospital, linked to the Universidad Complutense, with 1,350 beds, serving a population of approximately 350,000 inhabitants in the southeast area of Madrid. The Centre performs highly complex surgery, attends to patients with malignant diseases of both solid and haematological organs, has a very active transplant programme and is a reference center for many diseases. The Clinical Microbiology and Infectious Diseases Service is a multidisciplinary unit with a long history of care, teaching and research.
Type of study and population. This is a single-centre retrospective observational study that includes the first 100 consecutive patients with a proven diagnosis of COVID-19 in the HGUGM since the beginning of the epidemic, with a minimum follow-up of 60 days after etiological confirmation.
Procedures. The diagnosis of COVID-19 was performed in all cases from nasopharyngeal samples by reverse transcriptase polymerase chain reaction (Roche/Thermo Fisher RT-PCR) with prior extraction of viral RNA by NucliSENS® easyMag® (bioMérieux). A cycle threshold value (Ct-value) of less than 37 was considered positive. PCR (Roche/Thermo Fisher RT-PCR) from nasopharyngeal exudate was performed from the virus medium in which the samples are transported. The rest of the analytical determinations in blood followed the conventional methods established in our hospital.
Data collected. The following data were collected for each patient: demographic characteristics, underlying conditions, previous contact with suspected cases, days with symptoms prior to diagnosis by PCR, hospital stay, ICU stay, presence of pneumonia (unilateral/bilateral), oxygen saturation, laboratory analysis, severity of infection, antibiotic, antifungal and antiviral therapy, clinical evolution and mortality.
Definitions. Immunosuppressed patients were considered to be those with active solid organ tumor, malignant/hematological neoplasms under chemotherapy, HIV patients (<200 CD4), neutropenic individuals (<500 mm3), solid organ transplant recipients or those under corticosteroid therapy at doses equivalent to ≥15 mg of prednisone (or equivalent)/day in the 30 days prior to admission.
Proven infection by SARS-CoV-2 was considered when a patient had signs and symptoms compatible with COVID-19 and a positive PCR in nasopharyngeal exudate.
The severity of the patients’ disease was classified by the “CALL (comorbidity, age, lymphocyte and LDH) score” [11], which ranks 3 levels of risk according to their probability of progression. Those patients with 4-6 points have less than 10% chance of progression and are considered low risk (Class A). Patients with 7-9 points had a 10-40% chance of progression and are at intermediate risk (Class B) and patients with 10-13 points with more than 50% chance of progression are considered at high risk (Class C).
Statistical analysis. The median and interquartile range, were used for descriptive analysis of continuous variables. A value of p<0.05 was considered significant. Categorical variables were compared with the chi-square test and continuous variables using the Mann-Whitney test. A multivariate forward analysis including variables with p<0.01 in the univariate analysis was carried out to identify mortality risk factors. Statistical analysis was performed using IBM SPSS Statistics v.21 (IBM Corp., Armonk, NY).
Ethical aspects. This study was approved by the HGUGM Ethics Committee with the code MICRO.HGUGM.2020-020.
RESULTS
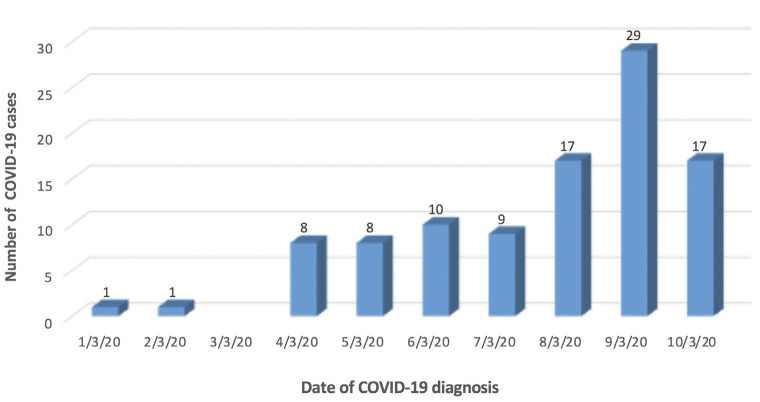
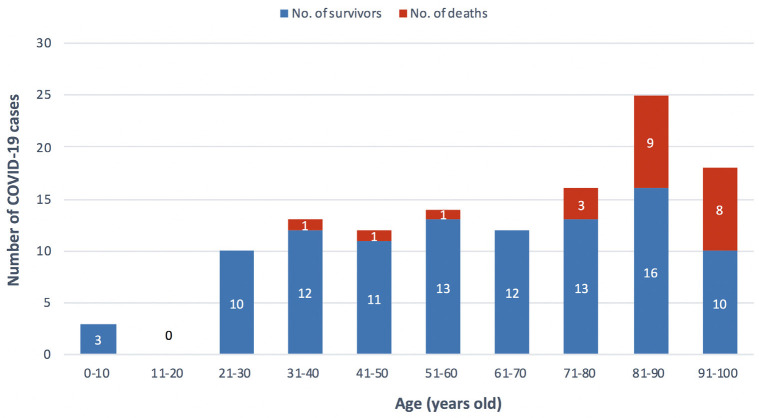
Of the first 100 proven patients with COVID-19 at the HGUGM, 52% were male. The age of the patients ranged from 3 months to 99 years with a median of 61.5 years (IQR=39.5-82.0). Figure 1 shows the speed at which the first 100 cases of COVID-19 were diagnosed in our institution and Figure 2 shows their distribution by decades of life and mortality rate.
Figure 1.
Evolution of admissions of the first 100 cases with confirmed COVID-19 at Hospital General Universitario Gregorio Marañón.
Figure 2.
Distribution of the first 100 COVID-19 cases (survivors/deaths) in the Hospital General Universitario Gregorio Marañón by decades of life.
The median patient weight was 75.8 kg (IQR=64.7-85.0) and the median Body Mass Index (BMI) was 28.8 kg/m2 (IQR=24.7-33.7 kg/m2). The characteristics of the patients and the main underlying diseases are shown in Table 1.
Table 1.
Demographic, clinical characteristics and evolution of patients.
| Cases (n=100) | |
|---|---|
| Age, median, IQR | 61.5 (39.5–82.0) |
| Sex, male, n (%) | 52 (52.0) |
| BMI, kg/m2 | 28.8 (24.7-33.7) |
| Underlying diseases, n (%) | |
| Cardiopathy | 22 (22.0) |
| Diabetes mellitus | 19 (19.0) |
| Malignant/Hematological neoplasia | 11 (11.0) |
| Chronic obstructive pulmonary disease | 10 (10.0) |
| Chronic renal disease | 8 (8.0) |
| Chronic hepatic disease | 7 (7.0) |
| Neurologic disease | 6 (6.0) |
| Solid tumor | 6 (6.0) |
| Psychiatric disease | 1 (1.0) |
| Hemodialysis | 0 |
| HIV | 0 |
| Solid organ trasplantation | 0 |
| Other | |
| Hypertension | 31 (31.0) |
| Hypothyroidism | 8 (8.0) |
| Asthma | 4 (4.0) |
| Cushing illness | 1 (1.0) |
| Celiac disease | 1 (1.0) |
| Lupus | 1 (1.0) |
| Sarcoidosis | 1 (1.0) |
| Myopathy | 1 (1.0) |
| Crohn disease | 1 (1.0) |
| Peptic esophagitis | 1 (1.0) |
| Osteoporosis | 1 (1.0) |
| Thyroiditis de Hashimoto | 1 (1.0) |
| Colon angiodysplasia | 1 (1.0) |
| Latent tuberculosis | 1 (1.0) |
| None, n (%) | 28 (28.0) |
| Inmunodepressed, n (%) | 5 (5.0) |
| Charlson Index adjusted to age, median, IQR | 2 (0-5.7) |
| Previous contact, n (%) | 47 (47.0) |
| Days with symptoms previous to PCR, median, IQR | 4.0 (2.0-7.0) |
| Symptoms, n, % | |
| Fever | 80 (80.0) |
| Cough | 53 (53.0) |
| Dyspnoea | 23 (23.0) |
| Myalgia | 7 (7.0) |
| Thoracic pain | 5 (5.0) |
| Asthenia | 4 (4.0) |
| Headache | 3 (3.0) |
| Confusional syndrome | 3 (3.0) |
| Vomiting | 3 (3.0) |
| Diarrhea | 3 (3.0) |
| Dizziness | 2 (2.0) |
| Odinofagia | 2 (2.0) |
| Rhinorrea | 1 (1.0) |
| Conjunctivitis | 1 (1.0) |
| Pleuritic pain | 1 (1.0) |
| Syncope | 1 (1.0) |
| Need of hospitalization, n (%) | 70 (70.0) |
| Days of hospital stay, median, IQR | 9.0 (7.0-15.2) |
| Need of ICU hospitalization, n (%) | 13 (13.0) |
| Days of ICU stay, median, IQR | 11.0 (6.0-28.0) |
| Pneumonia, n (%) | 63 (63.0) |
| Unilateral pneumonia | 21 (21.0) |
| Bilateral pneumonia | 42 (42.0) |
| Oxygen saturation at hospital admission, median, IQR | 94.0 (90.0-97.0) |
| Lower level of oxygen saturation during hospitalization, median, IQR | 92.0 (88.0-94.0) |
| Antiviral treatment, n (%) | 66 (66.0) |
| Antibiotic treatment, n (%) | 60 (60.0) |
| Antifungal treatment, n (%) | 5 (5.0) |
| Clinical outcome at 30 days, alive, n (%) | 77 (77.0) |
| Recovered at home | 72 (72.0) |
| Hospitalized | 2 (2.0) |
| At ICU | 3 (3.0) |
| Clinical outcome at 60 days, alive, n (%) | 77 (77.0) |
| Recovered at home | 75 (75.0) |
| Hospitalized | 1 1.0) |
| At ICU | 2 (2.0) |
| Mortality | 23 (23.0) |
| Related to COVID | 22 (22.0) |
| Not related to COVID | 1 (1.0) |
| Complications | |
| Cardiopathy | 18 (18.0) |
| Acute respiratory distress syndrome | 30 (30.0) |
| Sepsis syndrome | 17 (17.0) |
| Proven bacterial sepsis | 9 (9.0) |
| Acute kidney injury | 27 (27.0) |
| Acute liver injury | 25 (25.0) |
| Pulmonary embolism | 1 (1.0) |
The most common comorbidities were hypertension (31%), heart disease (22%) and diabetes mellitus (19%). The age-adjusted Charlson index [12] ranged from a minimum of 0 to a maximum of 10 (median 2, IQR=0-5.7). Five patients (5%) were immunosuppressed. Twenty-eight percent of the patients had no underlying disease.
The most common symptoms presented by patients at the time of diagnosis were fever (80.0%), cough (53.0%) and dyspnea (23.0%), followed by myalgia (7.0%), chest discomfort (5.0%) and asthenia (4.0%) (Table 1). The median number of days patients referred symptoms prior to performing the diagnostic PCR of COVID-19 was 4.0 (IQR= 2.0-7.0) with a mini-mum of 1 day and a maximum of 14 days. Forty-seven percent of the patients stated that they had had previous contact with a person diagnosed with COVID-19, either proven or probable.
O2 saturation at the time of first examination ranged from a minimum of 80% to a maximum of 99%, with a median of 94% (IQR=90-97). A total of 19.8% of patients had saturation <90% on admission.
Sixty-three percent of the patients presented alterations in the chest X-Ray on admission, compatible with the diagnosis of pneumonia (Table 1). Radiological images were classified as bilateral pneumonia in 42% of patients and as unilateral in 21%. The infiltrates generally had a ground glass pattern but on some occasions they were clear alveolar infiltrates (Figure 3).
Overall, 70% of COVID-19 cases were admitted to hospital. The length of hospital stay for those patients who remained hospitalized ranged from a minimum of 1 day to a maximum of 32 days, with a median of 9 days (IQR=7.0-15.2). Thirteen patients required admission to the ICU during the course of their hospitalization, with a minimum of 2 days and a maximum of 28 days of stay and a median of 11 days (IQR=6.0-28.0). The mortality rate of these patients admitted to ICU was 38.5% (5/13). The median time from the onset of symptoms associated with COVID-19 in these patients until they were admitted to the ICU was 5 days (IQR=1.5-9.5) with a minimum of 1 day and a maximum of 13 days. Patients admitted to the ICU were previously hospitalized on the ward for a median of 2 days (IQR=0-3) with a minimum of 0 days and a maximum of 6 days.
Fourteen patients of our study cohort also showed other type of infections (n=17). Three of them were coinfections present at the same time as the diagnosis of SARS-CoV-2 and 14 were nosocomially acquired. The origin of all of them was urinary (n=6), respiratory (n=5), bloodstream (n=4, 2 primary bacteremia and 2 catheter-related bacteremia), gastrointestinal (n=1) and catheter-related (n=1) infections. The most frequently isolated microorganisms were Escherichia coli (n=5), Staphylococcus epidermidis (n=2), Staphylococcus aureus (n=1), Enterobacter cloacae (n=1), Klebsiella pneumoniae (n=1), Micrococcus luteus (n=1), Enterococcus faecalis (n=1), Staphylococcus haemolyticus (n=1), Aspergillus fumigatus complex (n=1), Respiratory Syncytial Virus (n=1) and Clostridium difficile (n=1). Nine cases of proven bacterial sepsis were detected among 17 patients with sepsis syndrome.
Overall, 60.0% of patients received antibiotic treatment and 66.0% of patients received antiviral treatment, with lopinavir/ritonavir (65%) or hydroxychloroquine (42%) in an empirical basis. The main treatments are shown in Table 2. A greater use of antibiotics was observed in patients who died compared to survivors (91.3% vs 50.6%, p<0.01) but no significant differences were detected between both groups in terms of any type of antiviral or monoclonal antibody administered as treatment to the COVID-19.
Table 2.
Prognostic factors and clinical response to different treatments
| Total (n=100) |
Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Survivor (n=77) | Non-survivor (n=23) | p | OR (95% CI) | p | ||
| Age, years, median (IQR) | 61.5 (39.5-82.0) | 54 (35.5-70.5) | 85.0 (79.0-93.0) | <0.01 | ||
| 0-18 | 3 (3.0) | 3 (3.9) | 0 (0) | 0.584 | ||
| 19-44 | 29 (29.0) | 28 (36.4) | 1 (4.3) | <0.01 | ||
| 45-54 | 11 (11.0) | 9 (11.7) | 2 (8.7) | 0.737 | ||
| 55-64 | 11 (11.0) | 11 (14.3) | 0 (0) | 0.064 | ||
| 65-74 | 11 (11.0) | 11 (14.3) | 0 (0) | 0.064 | ||
| ≥75 | 35 (35.0) | 15 (19.5) | 20 (87.0) | <0.01 | ||
| Sex, male, n (%) | 52 (52.0) | 41 (53.2) | 11 (47.8) | 0.812 | ||
| BMI, kg/m2 | 28.8 (24.7-33.7) | 27.9 (24.4-30.5) | 30.8 (26.7-33.5) | 0.05 | ||
| BMI<30 | 42/69 (60.9) | 34/50 (68.0) | 8/19 (42.1) | 0.049 | ||
| BMI 30-40 | 24/69 (34.8) | 14/50 (28.0) | 10/19 (52.6) | 0.055 | ||
| BMI >40 | 3 /69 (4.3) | 2/50 (4.0) | 1/19 (5.3) | 0.818 | ||
| Smoker, n (%) | 20 (20.0) | 14 (18.2) | 6 (26.1) | 0.391 | ||
| Underlying diseases | ||||||
| Diabetes, n (%) | 19 (19.0) | 10 (13.0) | 9 (39.1) | <0.01 | ||
| Malignant/Hematological neoplasia, n (%) | 11 (11.0) | 5 (6.5) | 6 (26.1) | 0.017 | ||
| Solid tumor, n (%) | 6 (6.0) | 1 (1.3) | 5 (21.7) | <0.01 | ||
| COPD, n (%) | 10 (10.0) | 4 (5.2) | 6 (26.1) | <0.01 | ||
| Cardiopathy, n (%) | 22 (22.0) | 13 (16.9) | 9 (39.1) | 0.042 | ||
| Neurologic disease, n (%) | 6 (6.0) | 3 (3.9) | 3 (13.0) | 0.053 | ||
| Hypertension, n (%) | 31 (31.0) | 16 (20.8) | 15 (65.2) | <0.01 | 4.47 (1.36-14.66) | 0.013 |
| Symptoms | ||||||
| Disnea, n (%) | 23 (23.0) | 12 (15.6) | 11 (47.8) | <0.01 | ||
| Cough, n (%) | 53 (53.0) | 47 (61.0) | 6 (26.1) | <0.01 | ||
| Antibiotic treatment, n (%) | 60 (60.0) | 39 (50.6) | 21 (91.3) | <0.01 | ||
| Antiviral treatment, n (%) | 66 (66.0) | 48 (62.3) | 18 (78.3) | 0.212 | ||
| Lopinavir/ritonavir | 65 (65.0) | 47 (61.0) | 18 (78.3) | 1.0 | ||
| Hydroxycloroquine | 42 (42.0) | 33 (42.9) | 9 (39.1) | 0.250 | ||
| Interferon-beta | 27 (27.0) | 19 (24.7) | 8 (34.8) | 0.783 | ||
| Remdisivir | 7 (7.0) | 6 (7.8) | 1 (4.3) | 0.664 | ||
| Darunavir | 1 (1.0) | 1 (1.3) | 0 | 1.0 | ||
| Ritonavir | 1 (1.0) | 1 (1.3) | 0 | 1.0 | ||
| Oseltamivir | 2 (2.0) | 1 (1.3) | 1 (4.3) | 1.0 | ||
| Tocilizumab, n (%) | 11 (11.0) | 8 (10.4) | 3 (13.0) | 1.0 | ||
| Oxygen saturation on admission, median (IQR) | 94 (90-97) | 95 (93-97.2) | 90 (87-92) | <0.01 | ||
| Lower oxygen saturation during the hospitalization, median (IQR) | 92 (88-94) | 93 (90-95) | 83.5 (80.2-89) | <0.01 | ||
| Bilateral pneumonia, n (%) | 42 (42.0) | 23 (29.9) | 19 (82.6) | <0.01 | ||
| Age-adjusted Charlson Index, n (%) | 1.5 (0-5.7) | 0 (0-3) | 6 (5-8) | <0.01 | 1.55 (1.26-1.91) | <0.01 |
| CALL Score, median (IQR) | 10 (8-11) | 9 (7-11) | 11 (10-12) | <0.01 | ||
| Length of hospitalization, days, median (IQR) | 9.0 (7.0-15.2) | 9 (7-22) | 8 (5-14) | 0.109 | ||
| Days at ICU, median (IQR) | 11.0 (6.0-28.0) | 27 (8.7-28) | 9 (3-14.5) | 0.07 | ||
| Days of hospital stay previous to ICU admission, median (IQR) | 2 (0-3) | 2 (1.2-3.7) | 0 (0-2) | 0.05 | ||
| Complications | ||||||
| Cardiopathy | 18 (18.0) | 6 (7.8) | 12 (52.2) | <0.01 | ||
| ARDS | 30 (30.0) | 9 (11.7) | 21 (91.3) | <0.01 | ||
| Sepsis | 17 (17.0) | 8 (10.4) | 9 (39.1) | <0.01 | ||
| Kidney injury | 27 (27.0) | 12 (15.6) | 15 (65.2) | <0.01 | ||
| Liver injury | 25 (25.0) | 22 (28.6) | 3 (13.0) | 0.174 | ||
| Pulmonary embolism | 1 (1.0) | 0 | 1 (4.3) | 0.230 | ||
COPD: chronic obstructive pulmonary disease, ARDS: acute respiratory distress syndrome
Laboratory findings showed lymphopenia in 71% of patients diagnosed with COVID-19 with analytical determination on admission. Fourteen of the 23 patients with D-dimer determination (60.9%), had values greater than 250 ng/mL. IL-6 was only measured in 3 patients of our cohort but values were elevated in all the cases. Lymphocyte levels were lower in patients who died (p<0.01) compared to those who survived. C-reactive protein, procalcitonin, LDH, blood nitrogen-urea and NTproBNP values were significantly higher in non-survivors compared to survivors at 30-60 days after diagnosis of COVID-19 (Table 3).
Table 3.
Summary of main laboratory results.
| Total (n=100) |
Survivor (n=77) |
Non-survivor (n=23) |
P | |
|---|---|---|---|---|
| Lymphocyte count (10E3/μL), median, IQR | 0.9 (0.7-1.3) | 1 (0.7-1.4) | 0.7 (0.5-1.1) | <0.01 |
| <1.3 | 55/77 (71.4%) | 36/55 (65.5) | 19/22 (86.4) | 0.094 |
| <0.8 | 31/77 (40.3%) | 18/55 (32.7) | 13/22 (59.1) | 0.042 |
| Platelets (10E3/μL), median, IQR | 155 (136-205.2) | 159 (136-239.5) | 155 (100-188) | 0.189 |
| <140 | 24/78 (30.8%) | 15/56 (26.8) | 9/22 (40.9) | 0.278 |
| ≥140 | 54/78 (69.2%) | 41/56 (73.2) | 13/22 (59.1) | |
| C-reactive protein (mg/dL), median, IQR | 4 (2-9.6) | 2.7 (1.5-6.5) | 6.3 (3.3-17.6) | <0.01 |
| Procalcitonin (μg/L), median, IQR | 0.05 (0.03-0.13) | 0.04 (0.03-0.08) | 0.12 (0.1-0.5) | <0.01 |
| <0.1 | 53/79 (67.1%) | 44/56 (78.6) | 9/23 (39.1) | <0.01 |
| ≥0.1 a <0.25 | 12/79 (15.2%) | 5/56 (8.9) | 7/23 (30.4) | 0.022 |
| ≥0.25 a <0.5 | 6/79 (7.6%) | 4/56 (7.1) | 2/23 (8.7) | 1.0 |
| ≥0.5 | 8/79 (10.1%) | 3/56 (5.4) | 5/23 (21.7) | 0.042 |
| D-dimer (ng/mL), median, IQR | 284 (219-794) | 274 (208-680.5) | 2010 (565-) | 0.154 |
| ALT (U/L), median, IQR | 24 (15-37) | 24.5 (15-38.7) | 24 (17-34) | 0.754 |
| ≤41 | 64/79 (81.0%) | 44/56 (78.6) | 20/23 (87.0) | 0.533 |
| >41 | 15/79 (19.0%) | 12/56 (21.4) | 3/23 (13.0) | |
| LDH (U/L), median, IQR | 256 (192.5-345.7) | 226 (191-297) | 316 (224-434) | 0.047 |
| ≤250 | 32/66 (48.5%) | 28/49 (57.1) | 4/17 (23.5) | 0.024 |
| 250-500 | 28/66 (42.4%) | 17/49 (34.7) | 11/17 (64.7) | 0.046 |
| >500 | 6/66 (9.1%) | 4/49 (8.2) | 2/17 (11.8) | 1.0 |
| IL-6 (pg/mL), median, IQR | 166.3 (61.4-) | 166.3 (61.4-) | - | - |
| Blood urea nitrogen, (mg/dL), median, IQR | 31 (19-79) | 21 (17-31) | 106 (61.7-205.7) | <0.01 |
| Ferritin (μg/L), median, IQR | 598 (267-1048.5) | 494 (153-681) | 2922 (676-) | 0.222 |
| NTproBNP (ng/L), median, IQR | 1036 (274-3981) | 465 (74-2500.5) | 1520.5 (588.2-5900) | 0.012 |
The median CALL score of the total patient cohort was 10, corresponding to Class C severity with a probability of more than 50% high risk of disease progression. The minimum CALL score was 4 (Class A) and the maximum was 13 (Class C) (Table 2). A significantly higher CALL score (p<0.01) was observed in patients who died compared to those who did not die (11 vs 9). When comparing patients with low-intermediate severity (Class A and B CALL score) with patients with high severity (Class C, CALL score), it was observed that those with greater probability of disease progression (Class C) presented greater age (p<0. 01), higher Charlson index (p<0.01), more dyspnea (40.5% vs 13.8%, p<0.01), higher percentage of antibiotic use (89.2% vs 65.5%, p<0.05) and a greater presence of complications such as heart disease (p<0.04), respiratory distress (p<0.05) and kidney damage (p=0.01) (Table 4). The percentage of survivors was lower in this group (48.6% goes 89.7%, p<0.01). Regarding laboratory findings, lymphocyte, C-reactive protein, procalcitonin and LDH levels were significantly higher in these high severity patients (p<0.01).
Table 4.
Comparative study in terms of severity scale (CALL score)
| Class A and B (low and intermediate risk) n=29 |
Class C (high risk) n=37 |
p | |
|---|---|---|---|
| Age, median, IQR | 51 (34.5-71) | 76 (64-86) | <0.01 |
| Charlson Index, median, IQR | 0 (0-5) | 4 (2-6.5) | <0.01 |
| Oxygen saturation on admission, median, IQR | 95 (94-97) | 92 (88-95) | <0.01 |
| Dyspnoea, n, % | 4/29 (13.8%) | 15/37 (40.5%) | 0.027 |
| Antibiotic treatment, n, % | 19/29 (65.5%) | 33/37 (89.2%) | 0.032 |
| Complications | |||
| Cardiopathy, n, % | 2/29 (6.9%) | 3/37 (35.1%) | <0.01 |
| ARDS, n, % | 6/29 (20.7%) | 18/37 (48.6%) | 0.023 |
| Kidney injury, n, % | 5/29 (17.2%) | 28/37 (48.6%) | 0.01 |
| Survivor at 30-day follow-up (at home), n, % | 26/29 (89.7%) | 18/37 (48.6%) | <0.01 |
| Laboratory | |||
| Lymphocyte count, median, IQR | 1.3 (1.1-1.5) | 0.7 (0.4-0.9) | <0.01 |
| C-reactive protein, median, IQR | 2.3 (1.3-4.7) | 5.3 (2.8-11.8) | <0.01 |
| Procalcitonin, median, IQR | 0.04 (0.03-0.06) | 0.09 (0.04-0.3) | <0.01 |
| LDH, median, IQR | 212 (190.5-267.5) | 283 (211.5-399.5) | <0.01 |
ARDS: acute respiratory distress syndrome
At 30 days of follow-up after positive PCR test for SARSCoV-2, 23 patients (23%) had died, 72 (72%) were recovering at home, 2 (2%) remained hospitalized and 3 (3%) were in the ICU. After 60 days of follow-up, no more deaths were detected in our cohort, one of the 2 patients who had been hospitalized at 30 days remained hospitalized and the other was at home. As for the ICU patients, 2 remained hospitalized at 60 days and the third was discharged after 49 days of admission. Figure 2 shows the distribution of patients who survived and died by age groups (decades).
A comparative study between live and dead patients 30-60 days after diagnosis of COVID-19 showed that dead patients were significantly older (p<0.01), had a higher Charlson index (p<0.01) and had a higher percentage of bilateral pneumonias (p<0.01). The frequency of complications was higher in non-survivors than in survivors. Table 2 shows this comparison and the profile of the deceased patients in detail. By performing a multivariate analysis including variables with statistical significance in the univariate analysis less than 0.01, the presence of hypertension and age-adjusted Charlson Index were identified as risk factors associated with death.
The age of the deceased patients ranged from 39 to 99 years with a median of 85 years (IQR=79-93). Only 3 of the 23 deceased patients (13%) were under 75 years of age. One of them (51 years old) had diabetes mellitus, Cushing’s disease, high blood pressure, hepatitis C and SARS-CoV-2 coinfection with RSV. Another (45 years old) had diabetes mellitus, high blood pressure, morbid obesity, chronic renal disease, Clostridium difficile colitis and was a carrier of a biological mitral pros-thesis. The third, was a 39 years old patient with epidermoid carcinoma of the cervix treated with surgery in 2014 and currently with stage IV anal canal carcinoma in progression, with peritoneal carcinomatosis in treatment with palliative chemo-therapy. The 23 patients who died had a median Charlson Index of 6 (IQR=5-8). Eleven patients (47.8%) had a BMI greater than 30 and 3 were immunosuppressed. Nineteen patients (82.6%) had bilateral pneumonia and 4 (17.4%) had unilateral pneumonia.
DISCUSSION
Our study shows a high mortality of the first hundred patients treated with COVID-19 in our institution, associated with advanced age and the presence of serious underlying diseases in our population. Twenty of the 23 (23%) deceased patients were over 75 years of age and all had serious comorbidities.
The mortality in our series is similar to that reflected by Western countries such as Italy, UK, USA and others (19% to 39%) [3, 13-15] and differs significantly from that reported from China or Korea (0.9% to 7.5%) [4, 16-20]. It is clear that when calculating mortality the denominators matter [17, 21, 22].
The basic reason for these differences are to be found in the median age of the respective populations which was between 62 and 65.5 years in Western publications [3, 13, 15] and between 41 and 47 in the case of Chinese publications [4, 18]. In addition, comorbidity appears as a clear factor of poor prognosis as well as the level of care [23, 24].
Mortality in the case of China is even more surprising, since the initially reported cases considered pneumonia as a constant in clinical presentation [2, 25-28], to the extent that the presence of certain lesions in the thoracic CT was considered a diagnostic criterion in the early stages [29-31]. However, as demonstrated by our first 100 microbiologically confirmed patients, pneumonia was absent in a high proportion of cases and is even less frequent in subsequent series where diagnostic suspicion is spread to less severe or asymptomatic patients [4, 19, 32].
An interesting aspect of our series is the evaluation of the ability to predict and anticipate patients with poor clinical evolution. Our study has used the CALL score [11] and validated its usefulness. For example, all the patients sent home, evolved well during the follow-up and did not require admission in the two months of follow-up.
No antiviral treatment has been shown to be effective to date. Most patients in our cohort were treated with lopinavir/ ritonavir but none of the different types of antivirals administered during this first period have shown significant differences between patients who survived and those who died [33-39].
Our results and previous studies show that lymphopenia is common in COVID-19 cases, suggesting that SARS-CoV-2 infection causes an inhibition of the cellular immune response and a significant number of complications as seen in our patient cohort. We have also observed that elevation of markers such as C-reactive protein and procalcitonin is common in severe COVID-19 cases [4, 40].
Our study aims to contribute to a better understanding of the clinical evolution and mortality among the cases of COVID-19 in different continents, with a follow-up perspective of more than two months. The real mortality will not be known until the true dimension of the epidemic can be analyzed through population studies and the number of deceased cases can be related to the underlying diseases and the situation of the hospitals at different times of the epidemic.
ACKNOWLEDGEMENTS
Gregorio Marañón Microbiology-ID COVID 19 Study Group:
Alcalá (Luis), Aldámiz (Teresa), Alonso (Roberto), Álvarez (Beatriz), Álvarez-Uría (Ana), Andueza (Juan Antonio), Arias (Alexi), Arroyo (Luis Antonio), Berenguer (Juan), Bermúdez (Elena), Bouza (Emilio), Burillo (Almudena), Candela (Ana), Carrillo (Raquel), Catalán (Pilar), Cercenado (Emilia), Cobos (Alejandro), Díez (Cristina), Escribano (Pilar), Estévez (Agustín), Fanciulli (Chiara), Galar (Alicia), García (Mª Dolores), García de Vied-ma (Darío), Gijón (Paloma), González (Adolfo), Guerrero (José Eugenio), Guillén (Helmuth) Guinea (Jesús), Haces (Laura Vanessa), Kestler (Martha), López (Juan Carlos), Losada (Carmen Narcisa), Machado (Marina), Marín (Mercedes), Martín (Pab-lo), Montilla (Pedro), Moure (Zaira), Muñoz (Patricia), Olmedo (María), Padilla (Belén), Palomo (María), Parras (Francisco), Pérez-Granda (María Jesús), Pérez (Laura), Pérez (Leire), Pescador (Paula), Puente (Luis), Reigadas (Elena), Rincón (Cristina), Rodríguez (Belén), Rodríguez (Sara), Rojas (Adriana), Ruiz-Serrano (María Jesús), Sánchez (Carlos), Sánchez (Mar), Serrano (Julia), Tejerina (Francisco), Valerio (Maricela), Veintimilla (Mª Cristina), Vesperinas (Lara), Vicente (Teresa), de la Villa (Sofía).
FUNDING
This study was supported by internal funding. Alicia Galar and Teresa Aldamiz-Echevarría are supported by Juan Rodés contracts from the Instituto de Salud Carlos III, Madrid, Spain, partially financed by the European Social Fund (grant numbers JR18/00030 and JR17/00018, respectively).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest
References
- 1.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020;382(22):2081-90. DOI: 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemio-logical and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. DOI: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. DOI: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. DOI: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y, et al. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2020.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-2059. DOI: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061-1069. DOI: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-81. DOI: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488-1494. DOI: 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62. DOI: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for Progression Risk in Patients with COVID-19 Pneumonia: the CALL Score. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of clinical epidemiology. 1994;47(11):1245-51. DOI: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020;7(2):91-6. DOI: 10.1515/dx-2020-0046 [DOI] [PubMed] [Google Scholar]
- 14.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996 DOI: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. DOI: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang YJ. Mortality Rate of Infection With COVID-19 in Korea From the Perspective of Underlying Disease. Disaster Med Public Health Prep. 2020:1-3. DOI: 10.1017/dmp.2020.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55(4):105946 DOI: 10.1016/j.ijantimicag.2020.105946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;ciaa272 DOI: 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. DOI: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767-72. DOI: 10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaye B, Fanidi A, Jouven X. Denominator matters in estimating COVID-19 mortality rates. Eur Heart J. 2020;ehaa282 DOI: 10.1093/eurheartj/ehaa282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccininni M, Rohmann JL, Foresti L, Lurani C, Kurth T. Use of all cause mortality to quantify the consequences of covid-19 in Nembro, Lombardy: descriptive study. BMJ. 2020;369:m1835 DOI: 10.1136/bmj.m1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Analysis on 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132 DOI: 10.3346/jkms.2020.35.e132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Yao W, Wang Y, Long C, Fu X. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J Infect. 2020;81(1):147-178. DOI: 10.1016/j.jinf.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. DOI: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425-34. DOI: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020; DOI: 10.1002/jmv.25884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. DOI: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020:200642 DOI: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang EP, Sung CW, Chen CH, Fan CY, Lai PC, Huang YT. Can computed tomography be a primary tool for COVID-19 detection? Evidence appraisal through meta-analysis. Crit Care. 2020;24(1):193 DOI: 10.1186/s13054-020-02908-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoji H, Fonseca E, Teles G, Passos RBD, Yanata E, Silva MMA, et al. Structured thoracic computed tomography report for COVID-19 pandemic. Einstein (Sao Paulo). 2020;18:eED5720 DOI: 10.31744/einstein_journal/2020ED5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388-93. DOI: 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. DOI: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guastalegname M, Vallone A. Could chloroquine /hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020;ciaa321 DOI: 10.1093/cid/ciaa321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Intson K, Kumar S, Botta A, Neckles R, Leung C, Jawaid A. An independent appraisal and re-analysis of hydroxychloroquine treatment trial for COVID-19. Swiss Med Wkly. 2020;150:w20262 DOI: 10.4414/smw.2020.20262 [DOI] [PubMed] [Google Scholar]
- 36.Mahevas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844 DOI: 10.1136/bmj.m1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monzani A, Genoni G, Scopinaro A, Pistis G, Kozel D, Secco GG. QTc evaluation in COVID-19 patients treated with chloroquine/hydroxychloroquine. Eur J Clin Invest. 2020;50(6):e13258 DOI: 10.1111/eci.13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slomski A. No Benefit for Lopinavir-Ritonavir in Severe COVID-19. JAMA. 2020;323(20):1999 DOI: 10.1001/jama.2020.6793 [DOI] [PubMed] [Google Scholar]
- 39.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849 DOI: 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;ciaa243 DOI: 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]