Abstract
Mutations of the succinate dehydrogenase (SDHX) enzyme subunits commonly lead to a loss of function of the holoenzyme complex, and germline SDHX mutations lead to a genetic predisposition to SDH-deficient neoplasms, including renal cell carcinomas (RCC). Similarly, loss of function alterations of fumarate hydratase (FH) leads to a genetic predisposition to hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated RCC. Loss of FH leads to an accumulation of fumarate and aberrantly high levels of S-(2-succino)-cysteine (2SC).
Subtype-specific consecutively diagnosed renal cell neoplasms were selected for the study and cases were not otherwise selected based on clinicopathologic features. Tissue Microarrays were constructed from 1009 renal cell neoplasms [papillary: 400, clear cell: 203, chromophobe: 87, oncocytomas (original diagnosis): 273, unclassified: 46] and these cases were immunostained for SDHA/SDHB to screen for SDH loss. A smaller subset (n=730; oncocytomas, papillary and unclassified RCCs) were screened for FH-deficiency using immunohistochemistry for FH/2SC. Loss of SDHA/SDHB was seen in three of 273 tumors originally diagnosed as oncocytomas (1.1%). Diffuse nuclear and cytoplasmic 2SC staining, with retained FH expression was seen in one case (suggestive of dysfunctional FH protein), while absent FH was seen in 3 cases (2/400 papillary RCCs, 0.5% and 2/46 unclassified RCCs, 4.35%). No aberrant FH/2SC expression was noted in 273 cases originally diagnosed as oncocytomas.
SDH-deficient RCCs were identified only in the cases originally diagnosed as oncocytomas (1.1%), while FH-deficient RCCs were identified in the papillary (0.5%) and unclassified RCC cohorts (4.35%). These results can help guide immunohistochemistry-based screening strategies for these tumors.
Keywords: Succinate Dehydrogenase, SDHA, SDHB, Fumarate Hydratase, FH, S-(2-succino)-cysteine, 2SC, Renal Cell Carcinoma
1.0. Introduction
Recently described variants of renal cell carcinomas (RCC) include succinate dehydrogenase (SDH)-deficient RCC, as well as fumarate hydratase (FH)-deficient RCC (1–6).
The SDH holoenzyme subunits (SDHA, SDHB, SDHC, and SDHD) are assembled in association with SDHAF1 and SDHF2 proteins to form complex II which participates as a component of the mitochondrial electron transport system (7). SDH-deficient RCCs often occur in the setting of germline inactivating mutations of genes encoding SDH subunits and have frequently been reported in patients with a strong hereditary predisposition to SDH-deficient neoplasia (1, 2, 8). Immunophenotypically, these tumors are characterized by a loss of SDHB, secondary to molecular alterations of the SDH holoenzyme subunits (1, 2, 8). While most SDH-deficient RCCs are morphologically characterized by the presence of cells with eosinophilic cytoplasm and flocculent eosinophilic cytoplasmic inclusions, in a solid growth pattern, a subset of cases with variant histology have been reported (1, 2). Cases with variant histology include clear cell, papillary, chromophobe, and hybrid chromophobe-oncocytoma RCCs (7, 9–17).
Germline mutations of FH lead to a genetic predisposition to hereditary leiomyomatosis and renal cell cancer-associated RCC (HLRCC) (3–6). FH-deficient RCC can also occur in a sporadic setting (18). Inactivation of fumarate hydratase (FH) leads to an accumulation of its substrate fumarate, which leads to aberrant protein succination, high levels of covalent modifications of cysteine residues [S-(2-succino)-cysteine (2SC)], which may serve as epigenetic modifiers (19, 20). A combination of FH/2SC immunohistochemistry is useful in screening for FH-deficient RCC with a high sensitivity and specificity (3, 20). While the morphologic spectrum of these tumors commonly includes papillary architecture with prominent eosinophilic macronucleoli and perinucleolar halos, the spectrum of reported morphologic patterns has been expanding (3, 6, 20, 21).
In the present study, we sought to assess the incidence of both SDH-deficient and FH-deficient RCCs using an immunohistochemistry-based screening strategy involving the use of SDHA/SDHB (1009 cases screened) and FH/2SC (730 cases screened) in a series of consecutively diagnosed subtype-specific cases. In addition, we sought to assess the morphologic diversity of these tumors in the context of previously reported SDH-deficient tumors with variant histology and the expanding morphologic spectrum of FH-deficient tumors (3–7, 9–17).
2.0. Materials and Methods
2.1. Patient specimens
Following approval from the Institutional Review Board at Mayo Clinic, Rochester, Minnesota, 1009 renal neoplasms diagnosed and treated by partial/radical nephrectomy between 1970 and 2012, were retrieved from the archival files. Apart from having carried a diagnosis of renal cell neoplasia and tissue available for analysis, cases were not otherwise selected based on clinicopathologic features. These included 5 cohorts, each of consecutively diagnosed cases of a subtype of renal cell neoplasia, including 400 papillary RCCs, 203 clear cell RCCs, 87 chromophobe RCCs, 273 oncocytomas, and 46 unclassified RCCs. All 1009 renal tumors were screened with SDHA/SDHB immunohistochemistry, while a smaller subset (400 papillary RCCs, 273 oncocytomas, and 46 unclassified RCCs) were screened with FH/2SC immunohistochemistry.
In the cohort of oncocytomas a subset was reclassified on pathologic review as renal cell carcinomas with cytoplasmic eosinophilia. The mean duration of follow up for oncocytomas was 13.3 years (median: 14.1 years, interquartile range: 6.8 to 19.1 years, minimum: 15 days and maximum: 40.8 years) and only 11 of 273 patients had a follow up of less than 1 year. No recurrences were identified for any of these patients on follow up. Finally, in the cohort of papillary RCCs, the mean size was 5.6cm (median: 4.5cm, interquartile range: 3.0 to 7.0cm, minimum: 1.5cm and maximum: 24.5cm).
Follow-up information for cases with abnormal immunohistochemistry results was obtained by reviewing the medical record and accessing the Mayo Clinic Nephrectomy Registry.
Clinical and pathologic features collected at nephrectomy for this registry include year, age, sex, symptoms, smoking status, Eastern Cooperative Oncology Group performance status, Charlson score, body mass index, tumor size, histologic subtype, the 2018 primary tumor, regional lymph node, and distant metastases classifications (TNM), coagulative tumor necrosis, rhabdoid differentiation, and sarcomatoid differentiation. All clinical information by chart abstraction and yearly follow-up communications with patients is provided by a nurse abstractor and all pathology related data is provided by a slide review by a designated urologic pathologist using 2016 WHO/ISUP classification and grading criteria (22).
2.2. Immunohistochemistry
Four, 1.0 mm cores of representative formalin-fixed, paraffin-embedded tissues from each tumor were used to construct tissue microarrays (TMA) for immunophenotyping. Confirmation of aberrant SDHA/SDHB, FH/2SC immunohistochemistry results was performed on representative whole-slide sections. One case each of a papillary renal cell carcinoma and clear cell renal cell carcinoma with equivocal staining for SDHB were excluded from follow up analysis as they lacked appropriate internal control (vascular endothelial) staining. SDHA/SDHB antibodies (Abcam; clones 2E3GC12FB2AEZ and 21A11AE7) were used at a dilution of 1:200. A granular pattern of immunostaining for SDHA/SDHB in adjacent renal tubules and vascular endothelium was considered a positive internal control. FH/2SC IHC was performed as previously described (3, 20, 23). Briefly, IHC was conducted by an automated Ventana Discovery system using Optiview detection system (Ventana), with 3,3′-diaminobenzidine (DAB) visualization and counterstained with hematoxylin. FH antibody (Clone J-13, Santa Cruz Biotechnology) was used at a dilution of 1:1000. 2SC polyclonal antibody (Dr. Norma Frizzell, Univ. of South Carolina) was used at a dilution of 1:2000 (20). FH staining was scored qualitatively as negative or positive when compared with internal positive controls (endothelial/stromal cells). 2SC staining was assessed for intensity (1+ to 3+) and staining pattern (nuclear and cytoplasmic versus cytoplasmic only), and only 3+ intensity of nuclear-cytoplasmic staining was interpreted as positive, as reported previously (3).
2.3. Statistical analysis
Continuous clinicopathologic variables were analyzed with frequency counts and percentages.
3.0. Results
3.1. SDH-Deficient Renal Cell Carcinoma: Immunohistochemistry and Clinico-pathologic Features
Three tumors originally diagnosed as oncocytomas showed characteristic features of SDH-deficient renal tumors characterized by eosinophilic and flocculent cytoplasm and showed absence of SDHB expression, while SDHA was retained in the same cases (Figure 1 A–C). Clinico-pathologic features for these three cases are summarized in Table 1. The average age at diagnosis was 42 years (range: 28–65), the mean size of the tumors was 7.6cm (range: 2.5 to 10.7cm); all cases were pathologic stage pT2 or lower and none of the patients had documented regional or distant metastasis at presentation. One patient presented with bilateral kidney involvement. No disease recurrence, adverse outcomes or germline SDHX alteration-associated neoplasia were identified on follow up.
Figure 1: SDH-Deficient Renal Cell Carcinoma.
Representative images of an SDH-deficient renal cell carcinoma (Case 1) is depicted. An H&E stained image (A), corresponding results of SDHA (B) and SDHB (C) immunostaining is shown.
Table 1.
Renal Cell Carcinoma with Aberrant SDHA/SDHB Expression.
| Case No. | Age (years) | Gender | Follow Up (months) | Size (cm) | pT | N | M | SDHA IHC | SDHB IHC | Histologic Features | Outcome | Associated Neoplasia Including Pheochromocytoma/Paraganglioma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | 1 | 3 | pT1a | Nx | M0 | Retained | Absent | Eosinophilic with flocculent cytoplasm | Dead of other causes | No |
| 2 | 34 | M | 20 | 10.7 | pT2b | Nx | M0 | Retained | Absent | Eosinophilic with flocculent cytoplasm | Alive without disease | No |
| 3 | 28 | M | 37 | Bilateral; Right:9, Left:2.5 | pT2a | Nx | M0 | Retained | Absent | Eosinophilic with flocculent cytoplasm | Alive without disease | No |
3.2. FH-Deficient Renal Cell Carcinoma: Immunohistochemistry and Clinico-pathologic Features
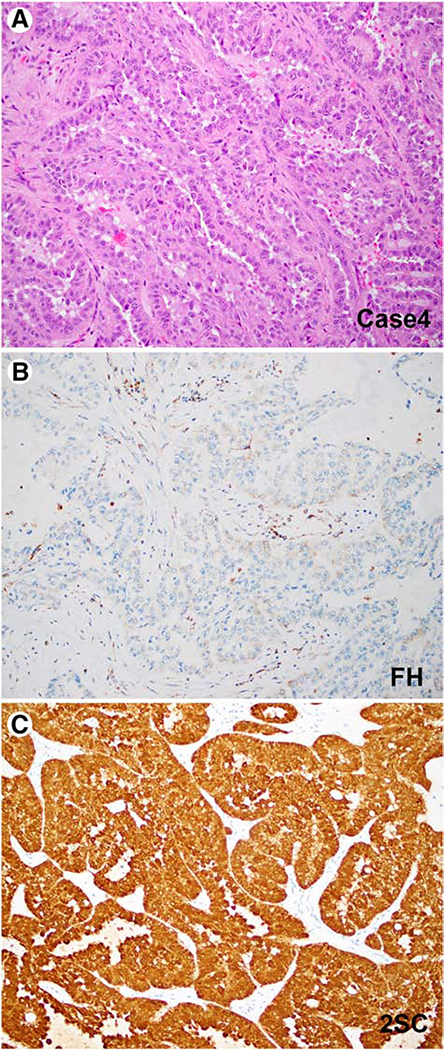
Four tumors with aberrant FH/2SC immunohistochemistry were identified (Figures 2–4). Clinico-pathologic features for all 4 cases are summarized in Table 2. Two cases showed classic morphologic features characterized by papillary architecture and prominent eosinophilic macronucleoli with perinucleolar clear halos (3). The first case was identified in a 19-year-old female who presented with bilateral renal tumors, with the largest measuring 12 cm, and regional lymph node involvement. This patient showed an absence of fumarate hydratase expression by immunohistochemistry, coupled with an accumulation of 2SC (Case 4, Figure 2). The second case, which was a 3cm tumor diagnosed in a 34-year-old male, showed retained FH expression, coupled with an accumulation of 2SC, suggestive of dysfunctional FH protein (Case 5, Figure 4).
Figure 2: FH-Deficient (Papillary) Renal Cell Carcinoma.
Representative images of an FH-deficient papillary renal cell carcinoma (Case 4) is depicted. An H&E stained image (A), corresponding results of FH (B) and 2SC (C) immunostaining is shown.
Figure 4: FH+/2SC+ Immunophenotype.
Representative images of an FH-deficient papillary renal cell carcinoma is depicted (Case 5, A-C). An H&E stained image (A), corresponding results of FH (B) and 2SC (C) immunostaining is shown.
Table 2.
Renal Cell Carcinoma with Aberrant FH/2SC Expression.
| Case No. | Age (years) | Gender | Follow Up (months) | Size (cm) | pT | N | M | FH IHC | 2SC IHC | Tumor Type | Outcome | Associated Neoplasia Including Leiomyomata |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 19 | F | 44 | Bilateral; Right:12, Left:2.5 | pT2b | N1 | M0 | Lost | Increased, with nuclear & cytoplasmic positivity | Papillary renal cell carcinoma (WHO/ISUP Grade3) | Dead of disease* | No |
| 5 | 34 | M | 19 | 3 | pT3a | Nx | M0 | Retained | Increased, with nuclear & cytoplasmic positivity | Papillary renal cell carcinoma (WHO/ISUP Grade3) | Dead of disease | No |
| 6 | 22 | M | 10 | 3.8 | NA | N1 | NA | Lost | Increased, with nuclear & cytoplasmic positivity | Unclassified renal cell carcinoma | Dead of disease** | No |
| 7 | 66 | M | 29 | 12.5 | pT3b | N1 | M0 | Lost | Increased, with nuclear & cytoplasmic positivity | Unclassified renal cell carcinoma | Dead of disease*** | No |
Metastasis to the liver was detected, for patient#4, at 11 months of follow up. In addition, family history of maternal death from unspecified cancer at age 40 was documented in this case.
Patient 6 developed lumbar metastases 1 month after surgery.
Patient#7 developed pulmonary metastases 13 months after surgery.
NA: Not available
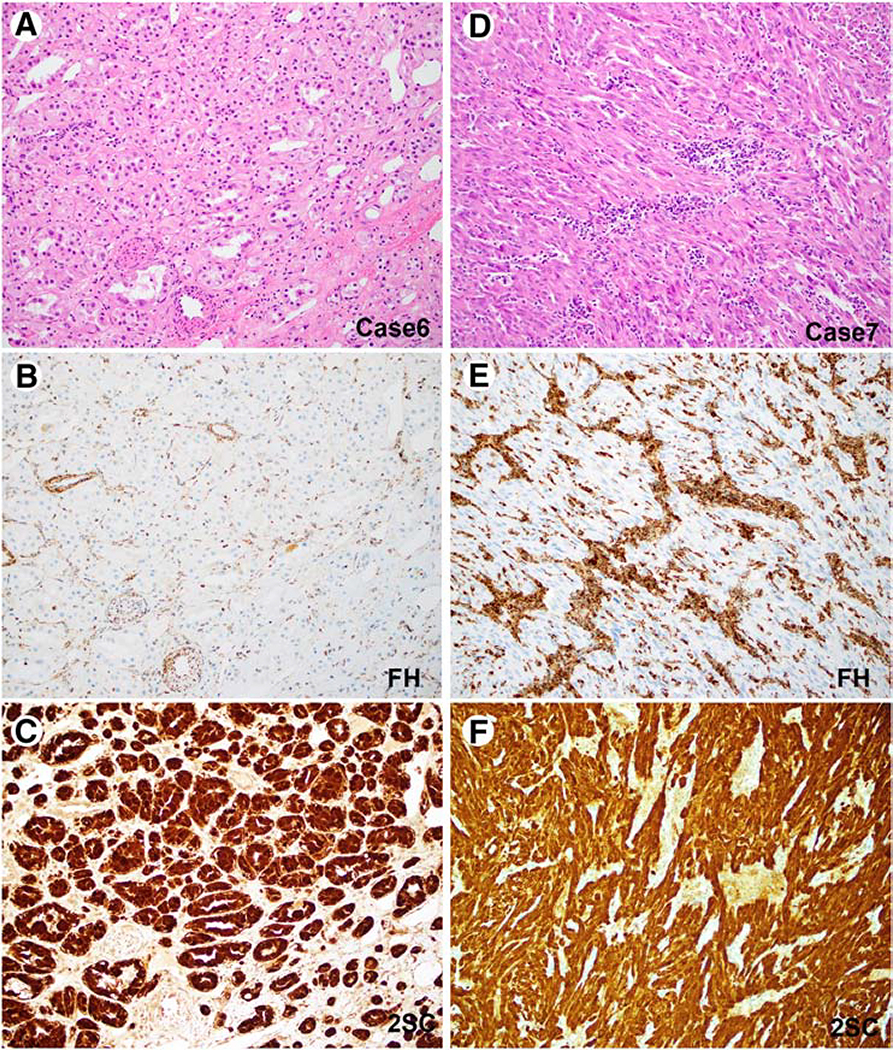
Two additional FH-deficient renal tumors were identified in the unclassified cohort. The first was seen in a 22-year-old male and exhibited low grade oncocytic features with tubular architecture and had documented lymph node metastasis at presentation (Case 6, Figure 3 A–C). This patient died of disease related complications at 10 months of follow up. The second unclassified tumor, in a 66-year-old male showed a predominant spindle cell component arranged in fascicles without significant cytologic atypia and was also associated with lymph node involvement at presentation (Case 7, Figure 3 D–F). This patient also died of disease related complications at 29 months of follow up.
Figure 3: FH-Deficient (Unclassified) Renal Cell Carcinoma.
Representative images of two FH-deficient (unclassified) renal cell carcinomas are depicted (Case 6, A-C; Case 7, D-F). H&E stained images (A, D), corresponding results of FH (B, E) and 2SC (C, F) immunostaining are shown.
Of note, confirmatory molecular analysis was either not pursued as the Institutional Review Board approval for this study did not allow for germline testing or when pursued, molecular analysis failed. The latter was due to the poor quality of nucleic acids extracted, likely due to the advanced age of the specimens (>30 years).
4.0. Discussion
Mutations of genes encoding SDH holoenzyme subunits such as SDHB, SDHC and SDHD lead to a loss of SDHB immunoreactivity; however, concurrent loss of SDHA immunoreactivity is only seen in tumors with SDHA mutations (24). The vast majority of reported cases of SDH-deficient RCCs are morphologically characterized by sheets of eosinophilic cells with flocculent eosinophilic cytoplasmic inclusions (1, 2). Therefore, diagnostic algorithms used to identify these tumors, for the most part, involve morphology-based screening followed by SDHA/SDHB immunohistochemistry or molecular studies for confirmation. However, multiple reports have documented the presence of SDH alterations in renal tumors with variant histology (7–17). We therefore sought to determine the incidence of SDH alterations using SDHA/SDHB immunohistochemistry in a large subtype-specific series of renal neoplasia.
In this study, all three cases with SDHB loss by immunohistochemistry were in the oncocytoma cohort (i.e. cases initially diagnosed as oncocytomas) and had characteristic histologic features of SDH-deficient renal cell carcinomas on retrospective review. Two cases with variant histology (clear cell and papillary RCC) had predominant loss of SDHB expression and only focal staining, in the absence of a convincing pattern of internal control staining; molecular analysis failed due to the degraded nature of the nucleic acids extracted from both specimens. The presence of a metastatic pheochromocytoma with retained SDHB expression argued against the presence of a germline alteration for one of these cases. Hence, our study recommends caution in the interpretation of negative SDHA/SDHB immunohistochemistry results in cases with variant histology, particularly for clear cell RCCs with optically clear cytoplasm, as has been suggested by others (25). In addition, SDH-deficient tumors associated with SDHD alterations have been reported to show a non-specific cytoplasmic blush when compared to tumors with SDHA/SDHB/SDHC pathogenic alterations, which normally demonstrate completely negative cytoplasmic staining (26). However, it must be noted that the majority of SDH-deficient RCCs harbor alterations of SDHB, with only rare reported cases that have documented alterations of SDHC/SDHA (26).
In addition, we document a low incidence of SDH-deficient RCCs in the cohort of cases originally diagnosed as oncocytomas (1.1%); in comparison, the estimated reported prevalence in unselected renal cell carcinomas ranges from 0.05% to 0.2% (1). A similar study by Cornejo et al involving 450 cases of sporadic renal epithelial neoplasia did not identify any cases with SDHB loss (25). In the study performed by Miettinen et al interrogating 711 RCCs, the investigators identified four cases (of 711), including one clear cell RCC (of 553, 0.2%), one papillary RCC (of 33, 3%) and two unclassified RCCs (of 16, 12.5%), one of which can likely be reclassified as having characteristic morphology of an SDH-deficient RCC (11). If these three studies are combined, the overall incidence of SDHB loss by immunostaining in clear cell RCCs is 0.1% (1 of 996), papillary RCCs is 0.2% (1 of 517) and no cases were identified in 160 chromophobe RCCs.
The morphologic spectrum of FH-deficient RCCs includes papillary, solid, tubulocystic, cribriform, cystic and the recently described low grade oncocytic forms where the latter are morphologically reminiscent of SDH-deficient RCC (3–5, 18, 21). In our series, two FH-deficient RCCs were identified in the papillary RCC cohort (0.5%, 2 of 400) and two additional cases were identified in the unclassified cohort (2 of 46, 4.35%). Interestingly, one of the FH-deficient tumors in the unclassified cohort exhibited morphologic features consistent with what has been described for FH-deficient RCC showing a low grade oncocytic morphology, reminiscent of SDH-deficient RCC, and this patient died of disease at 10 months of follow up (18). Our results suggest that FH-deficient RCCs with this morphology are likely to be exceptionally rare, given that only 1 such case was identified amongst 319 renal tumors initially classified as either oncocytomas (n=273) or unclassified renal tumors (n=46), with a prevalence of approximately and 0.3% in these groups.
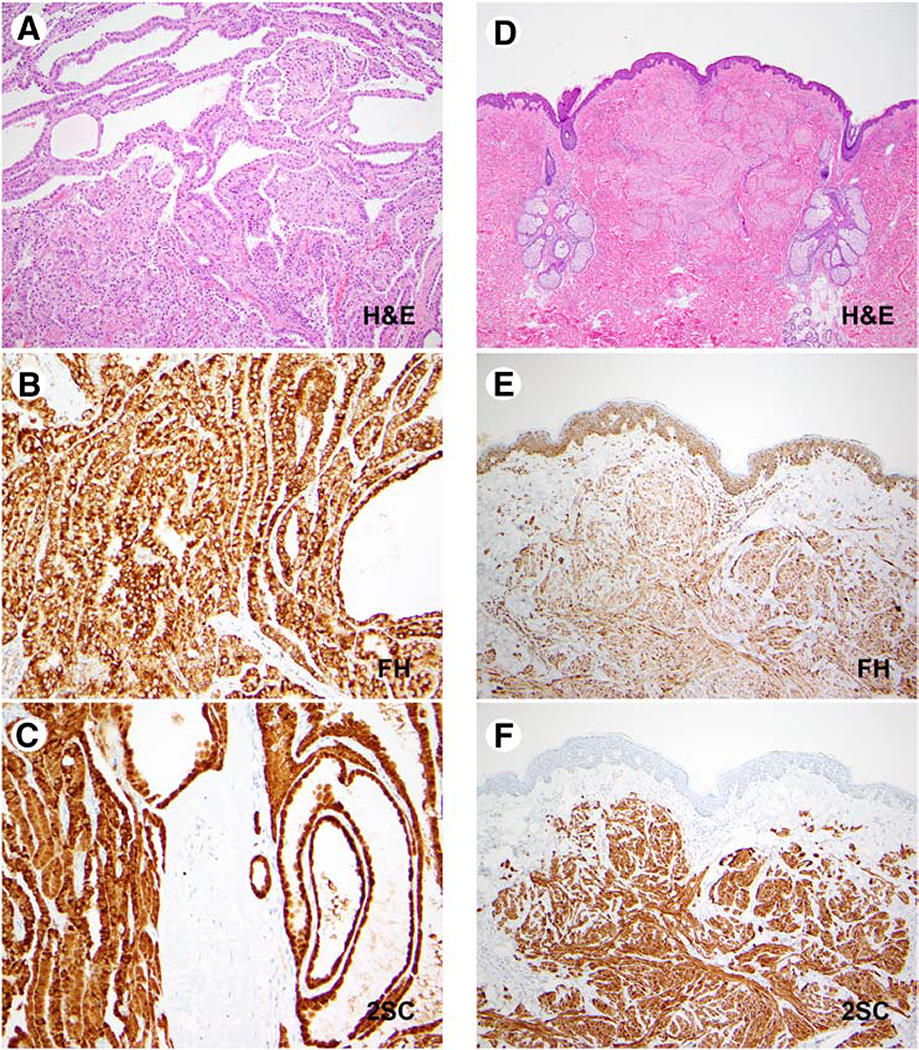
As previously reported, the common immunophenotypic pattern seen in FH-deficient RCCs is a lack of FH, coupled with positive 2SC staining (FH-/2SC+) (3–5). A positive 2SC immunostaining pattern is defined as diffuse, strong nuclear and cytoplasmic expression and in an initial validation study of 2SC immunohistochemistry in which clear cell RCCs were used as a negative control, this pattern of immunostaining was not appreciated in any of these controls (3). Follow-up studies have confirmed the lack of FH gene alterations by molecular testing in cases with retained FH, which exhibit a FH+/2SC− pattern of immunolabeling (4). Some FH-deficient RCCs demonstrate variable FH expression, coupled with 2SC positivity (FH±/2SC+) (4). We identified three tumors with the conventional FH-/2SC+ immunophenotype and a fourth case with a FH+/2SC+ immunophenotype. Of note, we have recently seen a case (not part of the cohort of patients included in the TMA) with an FH+/2SC+ phenotype (Figure 5) in a patient with confirmed hereditary leiomyomatosis associated RCC, secondary to a germline alteration affecting the FH gene (c.1097G>A, p.S366N). This 48-year-old male had a family history of cutaneous leiomyomas affecting his mother and maternal aunt. He presented with a 26.0 cm renal cell carcinoma with tubulocystic and poorly differentiated foci of infiltrative adenocarcinoma and developed peritoneal carcinomatosis 8 months after surgical resection. The occurrence of the FH+/2SC+ immunophenotype highlights the need for using the 2SC antibody in day-to-day clinical practice, and towards this end, additional efforts should be implemented to incorporate this antibody into mainstream use. Furthermore, this immunophenotype suggests that the terminology of “FH-deficient RCC” should not be interpreted strictly to only include tumors with negative FH staining and should also include the spectrum of cases with dysfunctional FH expression on immunohistochemistry (FH retained/2SC positive).
Figure 5: FH+/2SC+ Immunophenotype: Renal Cell Carcinoma & Cutaneous Leiomyoma.
Representative images of an FH-deficient papillary renal cell carcinoma and a cutaneous leiomyoma in a patient with a germline pathogenic FH alteration are depicted (FH c.1097G>A, p.S366N; renal cell carcinoma: A-C; cutaneous leiomyoma: D-F). H&E stained images (A, D), corresponding results of FH (B, E) and 2SC (C, F) immunostaining are shown.
In summary, SDH-deficient RCCs were identified only in the cohort of cases originally diagnosed as oncocytomas (1.1%) and FH-deficient RCCs were identified both in the papillary RCC (0.5%) and unclassified RCC cohorts (4.35%). This study helps ascertain the incidence of these tumors in a subtype-specific series of cases to help inform screening strategies for these rare tumors.
Highlights.
Renal neoplasms were screened for SDH (n=1009) and FH-deficiency (n=730) using IHC.
Screening limited to subtype-specific consecutively diagnosed renal cell neoplasms.
SDH-deficient RCCs in cases historically diagnosed as oncocytomas: 1.1% (n=273).
Incidence of FH-deficient RCCs in the papillary RCC cohort was 0.5% (n=400).
Incidence of FH-deficient RCCs in the unclassified RCC cohort was 4.35% (n=46).
6.0. Acknowledgement
The authors have no conflicts of interest or funding to disclose. SG, AAS, YBC, TL, DM, BRK, BCL, RHT, LHH, JCC and REJ performed the research, SG and REJ designed the research study, YBC assisted with experimental analysis involving FH/2SC immunohistochemistry, SG, YBC, LHH, JCC and REJ analyzed the data, SG and REJ wrote the paper. The authors would like to thank Christine M. Lohse, MS, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, for assistance with accessing the Mayo Clinic nephrectomy registry.
Sources of Support: None
Footnotes
Disclosures: The authors of this article have no relevant financial relationships with commercial interests to disclose. Preliminary results from this study were presented as an abstract at the 2017 USCAP annual meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 References
- 1.Gill AJ, Hes O, Papathomas T, Sedivcova M, Tan PH, Agaimy A, Andresen PA, Kedziora A, Clarkson A, Toon CW, Sioson L, Watson N, Chou A, Paik J, Clifton-Bligh RJ, Robinson BG, Benn DE, Hills K, Maclean F, Niemeijer ND, Vlatkovic L, Hartmann A, Corssmit EP, van Leenders GJ, Przybycin C, McKenney JK, Magi-Galluzzi C, Yilmaz A, Yu D, Nicoll KD, Yong JL, Sibony M, Yakirevich E, Fleming S, Chow CW, Miettinen M, Michal M, Trpkov K. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol 2014; 38, 1588–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson SR, Eble JN, Amin MB, Gupta NS, Smith SC, Sholl LM, Montironi R, Hirsch MS, Hornick JL. Succinate dehydrogenase-deficient renal cell carcinoma: detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod Pathol 2015; 28, 80–94. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, Brannon AR, Toubaji A, Dudas ME, Won HH, Al-Ahmadie HA, Fine SW, Gopalan A, Frizzell N, Voss MH, Russo P, Berger MF, Tickoo SK, Reuter VE. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol 2014; 38, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SC, Trpkov K, Chen YB, Mehra R, Sirohi D, Ohe C, Cani AK, Hovelson DH, Omata K, McHugh JB, Jochum W, Colecchia M, Amin M, Divatia MK, Hes O, Menon S, Werneck da Cunha I, Tripodi S, Brimo F, Gill AJ, Osunkoya AO, Magi-Galluzzi C, Sibony M, Williamson SR, Nesi G, Picken MM, Maclean F, Agaimy A, Cheng L, Epstein JI, Reuter VE, Tickoo SK, Tomlins SA, Amin MB. Tubulocystic Carcinoma of the Kidney With Poorly Differentiated Foci: A Frequent Morphologic Pattern of Fumarate Hydratase-deficient Renal Cell Carcinoma. Am J Surg Pathol 2016; 40, 1457–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trpkov K, Hes O, Agaimy A, Bonert M, Martinek P, Magi-Galluzzi C, Kristiansen G, Luders C, Nesi G, Comperat E, Sibony M, Berney DM, Mehra R, Brimo F, Hartmann A, Husain A, Frizzell N, Hills K, Maclean F, Srinivasan B, Gill AJ. Fumarate Hydratase-deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Am J Surg Pathol 2016; 40, 865–875. [DOI] [PubMed] [Google Scholar]
- 6.Muller M, Guillaud-Bataille M, Salleron J, Genestie C, Deveaux S, Slama A, de Paillerets BB, Richard S, Benusiglio PR, Ferlicot S. Pattern multiplicity and fumarate hydratase (FH)/S-(2-succino)-cysteine (2SC) staining but not eosinophilic nucleoli with perinucleolar halos differentiate hereditary leiomyomatosis and renal cell carcinoma-associated renal cell carcinomas from kidney tumors without FH gene alteration. Mod Pathol 2018. [DOI] [PubMed] [Google Scholar]
- 7.Ricketts CJ, Shuch B, Vocke CD, Metwalli AR, Bratslavsky G, Middelton L, Yang Y, Wei MH, Pautler SE, Peterson J, Stolle CA, Zbar B, Merino MJ, Schmidt LS, Pinto PA, Srinivasan R, Pacak K, Linehan WM. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J Urol 2012; 188, 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Zhang J, Milosevic D, Mills JR, Grebe SK, Smith SC, Erickson LA. Primary Renal Paragangliomas and Renal Neoplasia Associated with Pheochromocytoma/Paraganglioma: Analysis of von Hippel-Lindau (VHL), Succinate Dehydrogenase (SDHX) and Transmembrane Protein 127 (TMEM127). Endocr Pathol 2017; 28, 253–268. [DOI] [PubMed] [Google Scholar]
- 9.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, Maher ER. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 2008; 100, 1260–1262. [DOI] [PubMed] [Google Scholar]
- 10.Malinoc A, Sullivan M, Wiech T, Schmid KW, Jilg C, Straeter J, Deger S, Hoffmann MM, Bosse A, Rasp G, Eng C, Neumann HP. Biallelic inactivation of the SDHC gene in renal carcinoma associated with paraganglioma syndrome type 3. Endocr Relat Cancer 2012; 19, 283–290. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Sarlomo-Rikala M, McCue P, Czapiewski P, Langfort R, Waloszczyk P, Wazny K, Biernat W, Lasota J, Wang Z. Mapping of succinate dehydrogenase losses in 2258 epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014; 22, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozluk Y, Taheri D, Matoso A, Sanli O, Berker NK, Yakirevich E, Balasubramanian S, Ross JS, Ali SM, Netto GJ. Renal carcinoma associated with a novel succinate dehydrogenase A mutation: a case report and review of literature of a rare subtype of renal carcinoma. Hum Pathol 2015; 46, 1951–1955. [DOI] [PubMed] [Google Scholar]
- 13.Srirangalingam U, Walker L, Khoo B, MacDonald F, Gardner D, Wilkin TJ, Skelly RH, George E, Spooner D, Monson JP, Grossman AB, Akker SA, Pollard PJ, Plowman N, Avril N, Berney DM, Burrin JM, Reznek RH, Kumar VK, Maher ER, Chew SL. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf) 2008; 69, 587–596. [DOI] [PubMed] [Google Scholar]
- 14.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD, Lehtonen R, Januszewicz A, Jarvinen H, Juhola M, Mecklin JP, Pukkala E, Herva R, Kiuru M, Nupponen NN, Aaltonen LA, Neumann HP, Eng C. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 2004; 74, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill AJ, Pachter NS, Clarkson A, Tucker KM, Winship IM, Benn DE, Robinson BG, Clifton-Bligh RJ. Renal tumors and hereditary pheochromocytoma-paraganglioma syndrome type 4. N Engl J Med 2011; 364, 885–886. [DOI] [PubMed] [Google Scholar]
- 16.Papathomas TG, Gaal J, Corssmit EP, Oudijk L, Korpershoek E, Heimdal K, Bayley JP, Morreau H, van Dooren M, Papaspyrou K, Schreiner T, Hansen T, Andresen PA, Restuccia DF, van Kessel I, van Leenders GJ, Kros JM, Looijenga LH, Hofland LJ, Mann W, van Nederveen FH, Mete O, Asa SL, de Krijger RR, Dinjens WN. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: a clinicopathological and molecular analysis. Eur J Endocrinol 2014; 170, 1–12.24096523 [Google Scholar]
- 17.Yakirevich E, Ali SM, Mega A, McMahon C, Brodsky AS, Ross JS, Allen J, Elvin JA, Safran H, Resnick MB. A Novel SDHA-deficient Renal Cell Carcinoma Revealed by Comprehensive Genomic Profiling. Am J Surg Pathol 2015; 39, 858–863. [DOI] [PubMed] [Google Scholar]
- 18.Smith SC, Sirohi D, Ohe C, McHugh JB, Hornick JL, Kalariya J, Karia S, Snape K, Hodgson SV, Cani AK, Hovelson D, Luthringer DJ, Martignoni G, Chen YB, Tomlins SA, Mehra R, Amin MB. A distinctive, low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma, morphologically reminiscent of succinate dehydrogenase-deficient renal cell carcinoma. Histopathology 2017; 71, 42–52. [DOI] [PubMed] [Google Scholar]
- 19.Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, Drubbel AV, Theobald SJ, Abbo SR, Tran MG, Rajeeve V, Cardaci S, Foster S, Yun H, Cutillas P, Warren A, Gnanapragasam V, Gottlieb E, Franze K, Huntly B, Maher ER, Maxwell PH, Saez-Rodriguez J, Frezza C. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 2016; 537, 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, Howarth K, O’Flaherty L, Roberts I, Turner G, Taylor J, Giaslakiotis K, Macaulay VM, Harris AL, Chandra A, Lehtonen HJ, Launonen V, Aaltonen LA, Pugh CW, Mihai R, Trudgian D, Kessler B, Baynes JW, Ratcliffe PJ, Tomlinson IP, Pollard PJ. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol 2011; 225, 4–11. [DOI] [PubMed] [Google Scholar]
- 21.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol 2007; 31, 1578–1585. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Cheville JC, Jungbluth AA, Zhang Y, Zhang L, Chen YB, Tickoo SK, Fine SW, Gopalan A, Al-Ahmadie HA, Sirintrapun SJ, Blum KA, Lohse CM, Hakimi AA, Thompson RH, Leibovich BC, Berger MF, Arcila ME, Ross DS, Ladanyi M, Antonescu CR, Reuter VE. JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell carcinomas with sarcomatoid transformation: implications for clinical management. Mod Pathol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YB, Xu J, Skanderup AJ, Dong Y, Brannon AR, Wang L, Won HH, Wang PI, Nanjangud GJ, Jungbluth AA, Li W, Ojeda V, Hakimi AA, Voss MH, Schultz N, Motzer RJ, Russo P, Cheng EH, Giancotti FG, Lee W, Berger MF, Tickoo SK, Reuter VE, Hsieh JJ. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun 2016; 7, 13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papathomas TG, Oudijk L, Persu A, Gill AJ, van Nederveen F, Tischler AS, Tissier F, Volante M, Matias-Guiu X, Smid M, Favier J, Rapizzi E, Libe R, Curras-Freixes M, Aydin S, Huynh T, Lichtenauer U, van Berkel A, Canu L, Domingues R, Clifton-Bligh RJ, Bialas M, Vikkula M, Baretton G, Papotti M, Nesi G, Badoual C, Pacak K, Eisenhofer G, Timmers HJ, Beuschlein F, Bertherat J, Mannelli M, Robledo M, Gimenez-Roqueplo AP, Dinjens WN, Korpershoek E, de Krijger RR. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol 2015; 28, 807–821. [DOI] [PubMed] [Google Scholar]
- 25.Cornejo KM, Lu M, Yang P, Wu S, Cai C, Zhong WD, Olumi A, Young RH, Wu CL. Succinate dehydrogenase B: a new prognostic biomarker in clear cell renal cell carcinoma. Hum Pathol 2015; 46, 820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018; 72, 106–116. [DOI] [PubMed] [Google Scholar]